Abstract
Mesenchymal stromal cells (MSC) have shown potential efficacy in both animal and human trials of acute respiratory distress syndrome (ARDS). Especially during the COVID-19 pandemic, MSC was intensely studied for treating COVID-19-induced ARDS. The purpose of this study is to evaluate the safety and efficacy of MSC in ARDS via a meta-analysis of randomized controlled trials (RCTs). Therefore, a meta-analysis of RCTs of MSC as a therapy for ARDS was conducted. The protocol of this review was registered on Open Science Framework. With no language restriction and according to the “PICOs” principle, searches were conducted on Pubmed and Embase to retrieve any clinical literature on MSC for ARDS. Any RCT, which compared MSC to controls for ARDS, where MSC and controls were intravenously infused, of any dosage, was eligible for inclusion. A total of 13 RCTs, which evaluated MSC versus control for treating ARDS, enrolling a total of 655 cases, met the inclusion criteria and appeared in this meta-analysis. A heterogeneity assessment was carried out using the χ2 test, where a P value less than 0.05 was considered significant. The choice of a fixed-effect or a random-effect model was decided by the I2 value in each of the analyses. This meta-analysis indicated that there was no significant difference in terms of adverse events between MSC and control for ARDS (OR = 0.64, 95% CI [0.34, 1.20], P = 0.17, and I2 = 0%). In comparison with control, MSC could reduce the mortality of ARDS (OR = 0.66, 95% CI [0.46, 0.96], P = 0.03, and I2 = 10%). Based on the results of our meta-analysis, the safety of MSC was demonstrated to be non-inferior to that of standard treatment, and MSC may reduce the mortality rate of ARDS. Though the heterogeneity in the main results was low (I2 < 25%), more high-quality and large-scale clinical trials are needed to further confirm our findings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04287-4.
Keywords: Mesenchymal stromal cells, Acute respiratory distress syndrome, Acute lung injury, Cell transplantation, Coronavirus disease 2019
Introduction
Acute respiratory distress syndrome (ARDS) is a life-threatening clinical syndrome with high morbidity and mortality, which is featured by acute non-cardiogenic lung edema, hypoxia refractory to routine oxygenation, and severe respiratory distress [1]. According to the “Lung Safe” international epidemiological investigation, the mortality of ARDS ranged from 34.9 to 46.1% and the prevalence of it accounted for 10.4% of all ICU admissions around the globe [2]. Though lung-protective ventilation [3], controlling driving pressure [4], prone position [5], and ECMO [6] were identified as effective measures, the mortality of ARDS was still unacceptably high. Apart from low-dose corticosteroids (such as 6 mg/day dexamethasone) and remdesivir were recommended for treating COVID-19-induced ARDS [7], there is no other guideline-recommended therapy directly targeting the pathophysiology of this lethal clinical syndrome.
Mesenchymal stromal cells (MSC) belonging to a member of pluripotent stem cells, are of stromal origin and can be extracted from bone marrow, adipose tissue, umbilical cord, etc. [8]. MSCs are considered candidates for the treatment of ARDS because they can be deployed to the injured sites, where they are shown to repair tissue through its paracrine and anti-fibrosis effects in animal models of ARDS induced by endotoxin [9]. Additionally, MSC may transfer mitochondria into alveolar epithelium to improve bioenergetics of lung tissue and improve lung function [10]. The secretome released by MSC also is demonstrated to possess anti-inflammatory effects and is protective in animal models of ARDS [11]. Through the release of lipocalin-2 and LL-37, MSC has been shown to possess antimicrobial effects, possibly by enhancing the phagocytic activity of host immune cells [12]. In addition, MSC has been reported to preserve the integrity of vascular endothelial and alveolar epithelial barrier in preclinical models of ARDS [13]. Beyond that, in lung injuries induced by endotoxin, MSC is able to improve alveolar fluid clearance [14]. By exhibiting multipotent characteristics such as tissue repair, regeneration, antimicrobial, and anti-inflammation, MSC was widely investigated in ARDS animal models and was considered as a promising therapy for ARDS [15].
In the last decade, clinical trials have been conducted to investigate the safety and efficacy of MSC concerning ARDS [16–23]. However, due to the small sample size of these early clinical trials, the potency of MSC for ARDS is still subject to question and thus merits further discussion and investigation. Toward this end, we conducted a meta-analysis of randomized controlled trials of MSC in patients with ARDS to review the safety and efficacy of MSC for ARDS. The main outcomes of this meta-analysis were treatment-related adverse events (AEs) and all-cause mortality.
Materials and methods
Data sources
The protocol of this review was registered on Open Science Framework (OSF), registration https://doi.org/10.17605/OSF.IO/V74XA. PubMed and EMBASE (up to November 2022) were searched to identify relevant clinical trials with a tailored search strategy. Trials other than randomized controlled trials (RCT) were excluded from further screening. Search terms included ‘Mesenchymal Stromal Cells,’ ‘Mesenchymal Stem Cells’ ‘MSC,’ ‘Acute Respiratory Distress Syndrome,’ ‘ARDS,’ ‘Acute Lung Injury,’ and ‘ALI,’ and they were combined by patients, intervention, control, and outcomes (PICOs) principle. No language restriction was set in the database search. The search strategy is as follows: (((((Acute Respiratory Distress Syndrome[Title/Abstract]) OR (ARDS[Title/Abstract])) OR (acute lung injury[Title/Abstract])) OR (ALI[Title/Abstract])) AND ((((Mesenchymal Stem Cells[Title/Abstract]) OR (Mesenchymal Stromal Cells[Title/Abstract])) OR (MSC[Title/Abstract])) OR (MSCs[Title/Abstract]))) AND ((((((((control[Title/Abstract]) OR (randomized[Title/Abstract])) OR (randomly[Title/Abstract])) OR (controlled[Title/Abstract])) OR (RCT[Title/Abstract])) OR (placebo[Title/Abstract])) OR (sham[Title/Abstract])) OR (random[Title/Abstract])).
Study selection
Two authors (FYW and YML) independently searched and scrutinized literature on databases and read the title and abstract of each retrieved article to determine which of them required further assessment. Full texts of articles were retrieved when the information given in the titles and abstracts indicated that the study adopted a prospective design to compare MSC with control in patients with ARDS. When disputes existed, they were discussed thoroughly to reach a consensus. The inclusion criteria were (1) any RCTs that compared MSC with controls for ARDS, (2) included patients who were adults, of any gender, and had an established ARDS, (3) MSC intravenously infused, of any dosage; and controls or placebo intravenously infused, of any dosage.
Data extraction
Review authors (FYW and YML) independently extracted data with a customized data extraction form. The data extraction form included the following detailed information: (1) year of publication, (2) the number of included patients, (3) descriptions of dose, route, and timing of MSC and controls, (4) treatment-related AEs, all-cause mortality and other secondary outcomes.
Analyzed outcomes
The primary outcomes of this review were treatment-related AEs and all-cause mortality at 28 days. The secondary outcomes included clinical data such as ICU length of stay, PiO2/FiO2; and inflammatory biomarkers such as IL-6 and IL-8.
Data analysis and statistical methods
Data analyses of this review were performed by the Review Manager (Version: 5.4, Cochrane Collaboration, UK). Clinical heterogeneity was assessed in the population, methodology, and in interventions and outcomes of each study to assess whether the pooling of results was feasible. Values of I2 less than 25% were considered low in heterogeneity, for which the fixed-effect model of meta-analysis was used, whereas values of I2 between 25 and 75% were considered moderate in heterogeneity and a random-effects model was used. Values of I2 higher than 75% indicated high levels of heterogeneity, in which case no meta-analysis was performed. All statistical tests were two-sided and a P value less than 0.05 was considered statistically significant. Dichotomous variables such as treatment-related AEs and all-cause mortality expressed in ratios were extracted. Continuous variables such as inflammatory biomarkers IL-6 and IL-8 expressed in mean and standard deviation were extracted. Serum IL-6 and IL-8 examined 5 days or 7 days after trial drug or placebo administration were to be extracted in our review.
Heterogeneity exploration and quality assessment
A heterogeneity assessment was performed using the χ2 test, where a P value less than 0.1 was considered as the significance set. The funnel plot was utilized to detect any possible publication bias. The quality of the included literature was assessed by the Cochrane Collaboration tool for assessing risk of bias, which contains the following five aspects: sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting. The assessment of risk of bias was presented by using a “risk of bias summary figure,” which presents all of the judgments in a cross-tabulation of study by entry. This display of internal validity indicates the weight the writer may give to the results of each study.
Results
Study selection process
The whole search and selection process of the electronic databases was shown in the flow diagram (Fig. 1). Specifically, 170 articles were retrieved from Pubmed and 143 articles were retrieved from Embase. After duplicates were removed, a total of 259 articles were retrieved. After reading the titles and abstracts of each of the retrieved articles, the 166 retrieved articles were preserved and the full text of 23 of them was obtained for further examination. Seven papers were eliminated from consideration because they were either case series [23] or uncontrolled safety studies [22] or a study protocol [24–28]. Another three papers [29–31] were discarded because they reported the same trials as the included studies [17, 18, 32] did. These three excluded studies were only the secondary analysis of the three relevant studies included in our review and they didn’t report outcomes analyzed in our study. Finally, 13 papers met the inclusion criteria and were included in this meta-analysis [16–19, 32–40]. MSC or controls were initiated once the patients met the Berlin definition of ARDS or severe/critical COVID-19 in all the included studies after randomization.
Fig. 1.
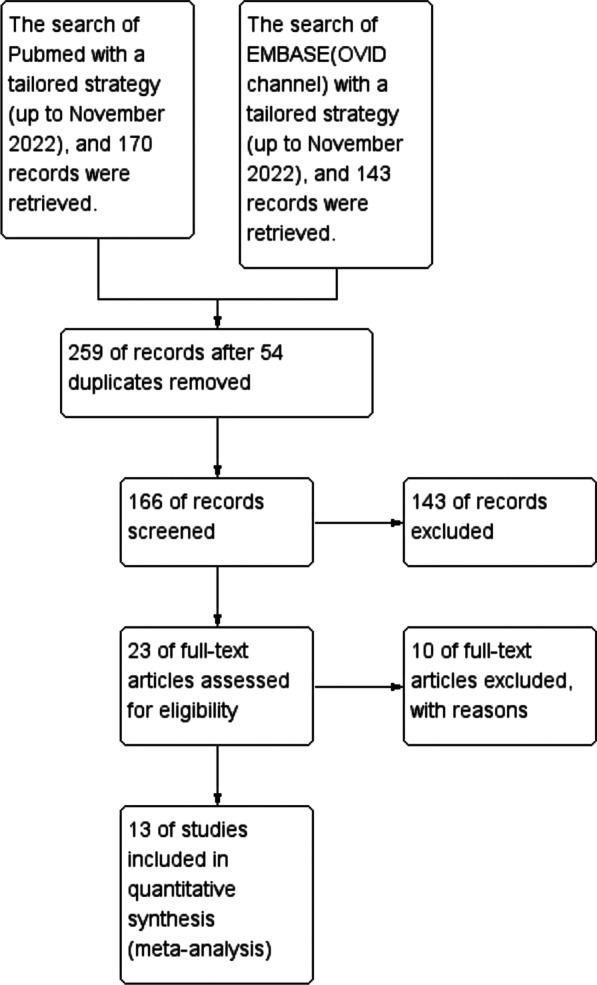
The flow diagram of the literature search process
Characteristics of the included studies
The main characteristics of the 13 studies including the type of study design, patients’ characteristics, dose and treatment duration of the studied medicine, population, and outcomes are presented in Table 1. The etiology of ARDS was not restricted to one specific disease in two included studies [16, 17], whereas, in the other 11 studies, ARDS was solely induced by COVID-19. The average age of the patients in the included studies ranged from 53 to 69.8 years old, and in terms of which, there was no significant difference between the MSC group and the Control group (P = 0.55, Additional file 1: Fig. S1A). Male patients accounted for 66.1% of the MSC group and 66.3% of the Control group (P = 0.77, Additional file 1: Fig. S1B). MSC was only used in patients with moderate-to-severe ARDS in six included studies, but in the other seven studies, either the severity was not defined, or MSC can be used for all patients with ARDS, regardless of the severity of the disease. Four included studies held a modality of multi-center RCT [17, 19, 34, 38], while the other nine studies were just one single-center RCTs. The method of randomization and allocation concealment was not thoroughly elucidated in four included trials [16, 18, 19, 37]. The source origins of the MSCs included adipose, bone marrow, umbilical cord, etc., and the dose of MSCs ranged from 1 × 106 to 100 ± 20 × 106 in included studies.
Table 1.
The main characteristics of the studies included in the meta-analysis
| Reference | Design | Age (MSC vs. Control, years) | Gender (male ratio) | Population | Group(dose, treatment duration) | ITT Population | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| MSC | Control | |||||||
| Guoping Zheng [16] | RCT | 66.7 ± 20.4 vs. 69.8 ± 9.1 | 6/6 vs. 5/6 | N = 12 (1:1), adult patients who met the Berlin definition of moderate and severe ARDS | AD MSCs, 1 × 106 cells/kg of body weight, one IV dose | NS | 12 (6 vs. 6) |
Adverse events Oxygenation index, length of hospital stay, ventilator-free days and ICU-free days at day 28, and SP-D, IL-6 or IL-8 levels in serum |
| Michael Matthay [17] | RCT | 55 ± 17 vs. 55 ± 20 | 23/40 vs. 10/20 | N = 60 (2:1), ventilated adult patients with moderate-to-severe ARDS | BM-MSCs, 10 × 106 MSC/kg (pbw), one IV dose | Placebo | 60 (40 vs. 20) |
Infusion-associated adverse events All-cause mortality, ventilator-free days, intensive-care-free days, days free from organ failure, SOFA score, oxygenation index, and lung injury score Angiopoietin 2, RAGE, IL-6, or IL-8 levels in serum |
| Giacomo Lanzoni [18] | RCT | 58.58 ± 15.93 vs. 58.83 ± 11.61 | 5/12 vs. 8/12 | N = 24 (1:1), adult patients hospitalized for severe COVID-19 |
UC-MSCs, 100 ± 20 × 106 UC-MSCs, 2 IV doses |
50 mL vehicle solution | 24 (12 vs. 12) | Infusion-associated adverse events, cardiac arrest or death within 24 h post-infusion, and incidence of AEs. Survival, time to recovery, and AEs within 31 days |
| Ismail Dilogo [19] | RCT | – | 15/20 vs. 15/20 | N = 40 (1:1), intubated patients with severe COVID-19 (moderate and severe ARDS) | UC-MSCs, 1 × 106 cells/kg body weight, one IV dose | 100 ml NS | 40 (20 vs. 20) |
Mortality rate, length of ventilation, Length of ICU stay, AE or serious AE (SAE) Routine blood count, differential count, CRP, D-dimer, fibrinogen, and procalcitonin, VEGF, ferritin, IL-6, LIF, CX-CR3 in serum |
| G Adas [33] | RCT | – | – | N = 20 (1:1), COVID-19 patients with severe ARDS | WJ MSCs, 3 × 106 cells/kg body weight, three IV doses | Placebo | 20 (10 vs. 10) | Adverse events, mortality, lymphocyte subgroups, and inflammation markers such as CRP, PCT, and Ferritin |
| Antoine Monsel [34] | RCT | 64 ± 10.4 vs. 63.2 ± 11.4 | 17/21 vs. 20/24 | N = 45, COVID-19 patients with ARDS | UC-MSCs, 3 × 106 cells/kg body weight, IV | 150 ml NS | 45 (21 vs. 24) | Respiratory improvement, SOFA scores, PaO2/FiO2 ratios, ventilation-free days and 28-day mortality; adverse events; and inflammatory biomarkers |
| Carmen Rebelatto [35] | RCT | 53 ± 15.3 vs. 61.7 ± 9.7 | 8/11 vs. 4/6 | N = 17, COVID-19 patients with moderate/severe ARDS | UC-MSCs, 5 × 106 cells/kg body weight, IV | Placebo | 17 (11 vs. 6) | Adverse events, mortality, PaO2/FiO2; ferritin, IL-6, CRP, D-dimer, and neutrophils |
| Hamid Aghayan [36] | RCT | 62.3 vs. 58.4 | 6/10 vs. 8/10 | N = 20 (1:1), COVID-19 patients with ARDS | PL-MSCs, 1 × 106 cells/kg body weight, IV | Placebo | 20 (10 vs. 10) | Adverse events, mortality, and lymphocyte subgroups |
| Lei Shi [32] | RCT | 60.72 ± 9.14 vs. 59.94 ± 7.79 | 37/65 vs. 19/35 | N = 100, COVID-19 patients with ARDS | UC-MSCs, 4 × 106 cells/kg body weight, IV | Placebo | 100 (65 vs. 35) | The proportion of whole lung lesion volumes, adverse events, and mortality |
| Lei Shu [37] | RCT | 61.00 ± 17.87 vs. 57.86 ± 15.79 | 8/12 vs. 16/29 | N = 41, COVID-19 patients with ARDS | UC-MSCs, 2 × 106 cells/kg body weight, IV | 100 ml NS | 41 (12 vs. 29) | Mortality, the time to clinical improvement, adverse events, and lab indexes |
| Michael Bowdish [38] | RCT | 61.8 ± 13.0 vs. 59.6 ± 13.8 | 79/112 vs. 75/110 | N = 222, COVID-19 patients with moderate/severe ARDS | BM-MSCs, 2 × 106 cells/kg body weight, IV | Placebo | 222 (112 vs. 110) | Mortality, mechanical ventilation days, clinical improvement, ICU length of stay, and adverse events |
| Najmeh Farkhad [39] | RCT | – | 7/10 vs. 6/10 | N = 20, COVID-19 patients with mild/moderate ARDS | UC-MSCs, 1 × 106 cells/kg body weight, IV | Placebo | 20 (10 vs. 10) | Mortality, PaO2/FiO2, lung imaging, and inflammatory biomarkers such as IL-1 beta, IL-6, and TNF-a |
| Xiaowei Xu [40] | RCT | 58.31 ± 12.49 vs. 61.11 ± 11.03 | 17/26 vs. 13/18 | N = 44, COVID-19 patients with ARDS | UC-MSCs, 9 × 106 cells/kg body weight, IV | Placebo | 44 (26 vs. 18) | Mortality, clinical improvement, PaO2/FiO2, inflammatory indices (including CRP and IL-6), and adverse events |
ITT Intention to treat; AD MSCs Adipose-derived MSCs; BM-MSCs Bone Marrow MSCs; UC-MSCs Umbilical cord MSCs; IV Intravenous; SOFA Sequential Organ Failure Assessment; NS Normal saline; COVID-19 Coronavirus disease 2019
The meta-analysis of the primary outcomes
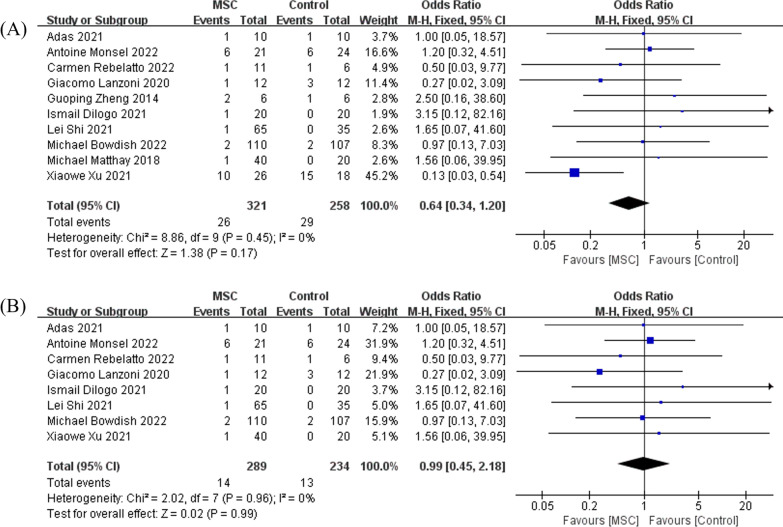
Regarding treatment-related AEs, the pooling results of 10 RCTs, enrolling a total of 579 patients, suggested that in comparison with control, MSC infusion did not increase any pre-defined AEs in treating ARDS (OR = 0.64, 95% CI [0.34, 1.20], P = 0.17, and I2 = 0%), Fig. 2A. For the COVID-19-induced ARDS subgroup, the pooled results of eight RCTs indicated that when compared with control, MSC did not increase any treatment-related AEs (OR = 0.99, 95% CI [0.45, 2.18], P = 0.99, and I2 = 0%), Fig. 2B. When the random-effects model was adopted, the results remained unchanged (Additional file 1: Fig. S2A and B).
Fig. 2.
The meta-analyses of adverse events, comparing MSC with the control: A the comparison of MSC with control in general ARDS; B the comparison of MSC with control in COVID-19-induced ARDS. The size of each square represents the proportion of information given by each trial. Crossing with the vertical line suggests no difference between the two groups
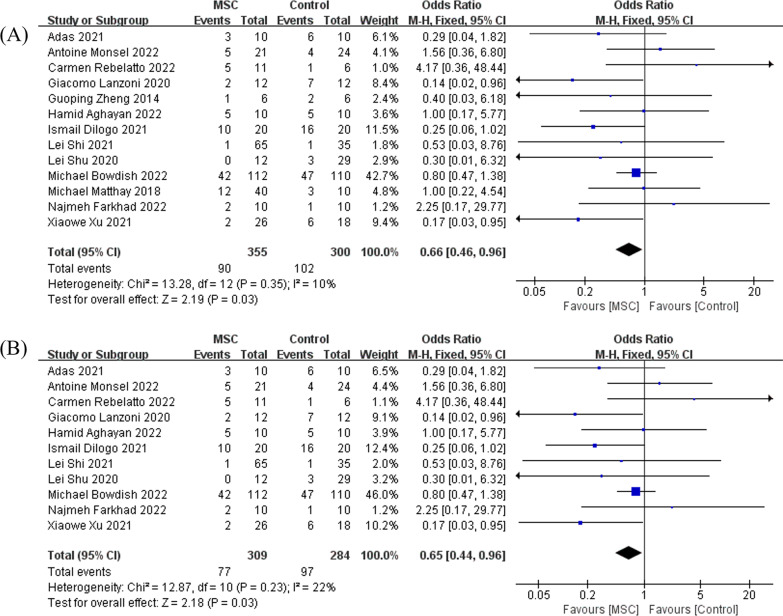
As for 28 days all-cause mortality, 13 studies with a total of 655 patients enrolled, the synthesized data indicated that compared with control, MSC reduced the mortality rate in adult patients with ARDS (OR = 0.66, 95% CI [0.46, 0.96], P = 0.03, and I2 = 10%), Fig. 3A. When the model of meta-analysis was adjusted to a random-effects model, the difference remained significant and the P value was 0.05 (Additional file 1: Fig. S3A). For the COVID-19-induced ARDS subgroup, 11 studies with a total of 593 patients were included, and the pooled results proved that compared with controls, MSC reduced mortality in COVID-19 patients with ARDS (OR = 0.65, 95% CI [0.44, 0.96], P = 0.03, and I2 = 22%), Fig. 3B. Of note, when the random-effects model was adopted, the P value was 0.07 (Additional file 1: Fig. S3B).
Fig. 3.
The meta-analyses of mortality, comparing MSC with the control: A the comparison of MSC with control in general ARDS; B the comparison of MSC with control in COVID-19-induced ARDS. The size of each square represents the proportion of information given by each trial. Crossing with the vertical line suggests no difference between the two groups
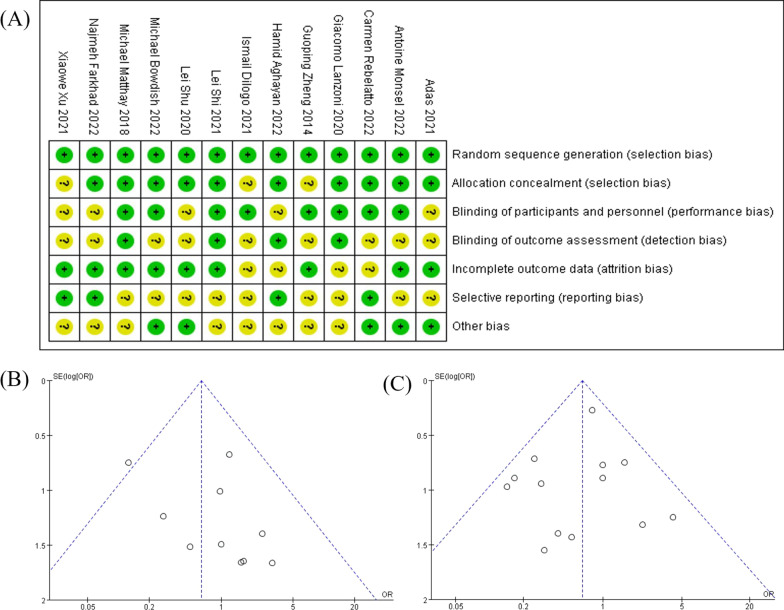
The risk of bias summary for the included trials is presented in Fig. 4A. The general heterogeneity is low among these studies, and therefore, it is possible to pool them for meta-analyses. The funnel plot is utilized to detect any possible publication bias. As expressed in Fig. 4B, C the majority of the studies included in the meta-analyses are distributed symmetrically. Therefore, the publication bias in the present analysis is low and acceptable.
Fig. 4.
The assessment of possible bias: A The risk of bias summary graph: review authors' judgements about each risk of bias item for each included study. B The funnel plot for adverse events. C The funnel plot for mortality
The summarization of secondary outcomes
The meta-analysis of secondary outcomes was not conducted either because the data were not extractable or not presented. Six included studies reported the effect of MSC on oxygenation. Though three included studies implied that MSC may increase PaO2/FiO2 ratio [16, 37, 39], the other three studies suggested that MSC did not have much impact on PaO2/FiO2 ratio at any timepoints [17, 19, 34]. On ventilation-free days to 28 days in ARDS, five studies didn’t detect any significant difference between MSC and controls [16, 17, 19, 34, 38]. In terms of ICU-free days, although five studies discovered no significant difference between the two groups [16, 19, 34, 38, 40], one study revealed that MSC may reduce ICU-free days in ARDS [17]. Meanwhile, the effects of MSC on serum IL-6 in ARDS were also controversial, as while three studies suggested no significant difference detected [16, 17, 40], four others implied that MSC may downregulate serum IL-6 [18, 19, 35, 39]. Additionally, three included studies reported no significant impact of MSC on serum IL-8 in ARDS [16, 17, 35].
Discussion
Our meta-analysis summarized the results of currently available RCT studies focused on MSC for ARDS and determined that the safety of MSC was not inferior to that of standard treatment. Second, with the treatment of MSC, the short-term survival of ARDS was improved. Third, the impact of MSC on oxygenation, ventilation-free days, ICU-free days, and systemic inflammation was still inconclusive thus far because no meta-analysis was done for these important outcomes.
No discrepancy regarding treatment-related adverse events was observed between MSC and controls in the 10 included RCTs, indicating the safety of MSC is reliable and further studies are warranted. In the COVID-19-induced ARDS subgroup analysis, of AEs, there are still no significant differences between MSC and control. Thus, MSC is safe for treating severe COVID-19. Since our meta-analyses showed that mortality is reduced in both general ARDS and COVID-19-induced ARDS, MSC can be further investigated as a promising therapy for ARDS. Though I2 < 25%, when the random-effects model of meta-analysis was used, the P value of the subgroup analysis of COVID-19-induced ARDS exceeded 0.05 (P = 0.07). Although the subtle difference in random-effects model would not undermine the findings of mortality, more MSC studies are needed to consolidate its protective effect in COVID-19-induced ARDS. In our meta-analysis, albeit improved survival with the treatment of MSC, three included studies indicated that compared with control, oxygenation was not improved, this may suggest that the improvement of survival by MSC was not primarily dependent on oxygenation for its effectiveness. The paracrine of growth factors, promotion of tissue repair and regeneration, and the anti-inflammatory effects of MSC [41, 42] may comprehensively alter the pathophysiological progress of ARDS. However, the particular mechanism awaits future studies to decode.
Regarding secondary outcomes, because of the different modalities used in data presentation, not enough data can be extracted. For this reason, no meta-analysis was conducted for secondary outcomes. Of note, despite no difference reported in the incidence of AEs and ventilation-free days, the study by Michael Matthay et al. [17] revealed that ICU-free days were reduced in the MSC group. They also detected nonsignificant elevated mortality with the treatment of MSC for ARDS (12/40 in the MSC and 3/20 in the control died). However, they acknowledged that mortality, as expected, was higher in the group of MSC than in the control group and that this was due to higher severity of the disease in the first group than in the latter group [17].
So far, due to a lack of effective targeted treatments, ARDS is still one of the most deadly clinical syndromes in the critical care field even after more than half a century of its discovery [43, 44]. Even for patients who survived this purgatory, their quality of life inevitably and dramatically declined because of their substantially damaged and not fully recovered lung function [45]. Especially after COVID-19 had swept all over the globe in the last three years and caused millions of deaths [46, 47], effective and available therapies for ARDS are quite needed.
In the last decade, cell therapy including MSC has been clinically investigated in a variety of pulmonary diseases. In 2013, Daniel Weiss et al. investigated the safety and efficacy of MSC in COPD. Though they didn’t observe any significant differences in pulmonary function or life-quality indicators, the safety of MSC was found to be satisfying and an anti-inflammatory effect of MSC was detected as it can decrease circulating CRP [48]. For preterm infants with bronchopulmonary dysplasia, intratracheal transplantation of allogeneic UC-MSC was also found to be safe and feasible [49, 50]. In the phase 1 clinical trial conducted by Jennifer Wilson et al., the dose-escalation of MSC from 1 × 106 to 10 × 106 MSC/kg was well tolerated by patients with moderate-to-severe ARDS, and no infusion-associated AEs and serious AEs were observed during the trial [22]. A compassionate treatment trial of COVID-19-induced ARDS with UC-MSC was demonstrated to be safe, yet the improvement of oxygenation may have been attributable to the effects of MSC or the evolution of the course of the disease itself. This needs to be validated by more controlled trials [23]. Furthermore, not only was MSC clinically investigated for treating ARDS but MSC-derived therapies such as exosomes of MSC were also considered for treating this syndrome [11]. In a cohort study, BM-MSC-derived exosomes were demonstrated to be safe and could restore oxygenation and downregulate cytokines for the treatment of severe COVID-19 [51].
Though MSC may be a promising therapy for ARDS, how to use it correctly in ARDS is still an issue that many clinicians are concerned about. According to the summary of the dosage of MSC in our study, one dose or several doses of 1 × 106 cells/kg of MSC seems to be safe in ARDS since this dosage didn’t increase any treatment-related AEs. Umbilical cord (UC) MSC was used in 8 of the 13 included studies, and given its high availability, it may be one of the most promising MSCs in the area of ARDS. Diana Islam et al. discovered that the effect of MSC in ARDS was determined by the microenvironment at the time of administration [52]. They proved that MSC might worsen ARDS in a microenvironment of high levels of IL-6 and fibronectin along with low antioxidant capacity. Correcting this adverse microenvironment with anti-oxidants or anti-inflammatory factors can reverse the detrimental effects of MSC. The aforementioned findings might guide us to use MSC in ARDS correctly. A combination of MSC with anti-oxidants and anti-inflammatory factors may be more beneficial for the treatment of ARDS.
There are several limitations within our meta-analysis. First, the sample size is small because the clinical investigation of MSC in ARDS is still at an early stage. Second, not enough data on secondary outcomes were extracted and no related meta-analysis was conducted. Third, because 11 of the 13 included studies were focused on COVID-19-induced ARDS, the evidence for non-COVID-19 ARDS is still scarce. Finally, male patients constituted about 66% of the total population, leading to the imbalance of the female-to-male ratio, which might be a source of clinical heterogeneity and limit the interpretation of the effects of MSC on female patients.
Conclusion
Though 13 studies were included, the sample size (655 cases) was small. According to the results of our meta-analysis, the administration of MSC in adult patients with ARDS tended to be safe and feasible, and that MSC may possess the potential to improve the survival of ARDS. However, more high-quality, well-designed studies aiming to engineer and explore the beneficiary effects of MSC in ARDS are necessary and expected.
Supplementary Information
Additional file 1: Fig. S1. The meta-analyses of age and gender (male patients ratio), comparing MSC with the control: A the comparison of MSC with control regarding age; B the comparison of MSC with control regarding gender. The size of each square represents the proportion of information given by each trial. Crossing with the vertical line suggests no difference between the two groups. Fig. S2. The meta-analyses of adverse events, comparing MSC with the control in the random-effects model: A the comparison of MSC with control in general ARDS; B the comparison of MSC with control in COVID-19-induced ARDS. The size of each square represents the proportion of information given by each trial. Crossing with the vertical line suggests no difference between the two groups. Fig. S3. The meta-analyses of mortality, comparing MSC with the control in the random-effects model: A the comparison of MSC with control in general ARDS; B the comparison of MSC with control in COVID-19-induced ARDS. The size of each square represents the proportion of information given by each trial. Crossing with the vertical line suggests no difference between the two groups
Acknowledgements
We thank all the authors for their meticulous work.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ALI
Acute lung injury
- BM
Bone marrow
- UC
Umbilical cord
- AD
Adipose-derived
- MSCs
Mesenchymal stem cells
Author contributions
FYW and YML contributed equally to this work; they conceived the idea and analyzed the medical files together. BQW and JGL made supportive contributions to this work. ZYP was involved in drafting the manuscript and revising it critically for important intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant 81971816, 81772046, 82102273).
Availability of data and materials
Data sharing does not apply to this article as no new data were created or analyzed in this study.
Declarations
Ethics approval and consent to participate
Ethical Approval is not applicable to this article.
Human and animal rights
This article does not contain any studies with human or animal subjects.
Informed consent
There are no human subjects in this article and informed consent is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fengyun Wang and Yiming Li contributed equally to this work.
References
- 1.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 4.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 5.Mancebo J, Fernandez R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173:1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 6.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 7.Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49:e219–e234. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]
- 8.Huss R. Isolation of primary and immortalized CD34-hematopoietic and mesenchymal stem cells from various sources. Stem Cells. 2000;18:1–9. doi: 10.1634/stemcells.18-1-1. [DOI] [PubMed] [Google Scholar]
- 9.Maron-Gutierrez T, Silva JD, Asensi KD, et al. Effects of mesenchymal stem cell therapy on the time course of pulmonary remodeling depend on the etiology of lung injury in mice. Crit Care Med. 2013;41:e319–e333. doi: 10.1097/CCM.0b013e31828a663e. [DOI] [PubMed] [Google Scholar]
- 10.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F, Fang B, Qiang X, Shao J, Zhou L. The efficacy of mesenchymal stromal cell-derived therapies for acute respiratory distress syndrome-a meta-analysis of preclinical trials. Respir Res. 2020;21:307. doi: 10.1186/s12931-020-01574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pati S, Khakoo AY, Zhao J, et al. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/beta-catenin signaling. Stem Cells Dev. 2011;20:89–101. doi: 10.1089/scd.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fengyun W, LiXin Z, Xinhua Q, Bin F. Mesenchymal stromal cells attenuate infection-induced acute respiratory distress syndrome in animal experiments: a meta-analysis. Cell Transplant. 2020;29:963689720969186. doi: 10.1177/0963689720969186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dilogo IH, Aditianingsih D, Sugiarto A, et al. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med. 2021;10:1279–1287. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashemian SR, Aliannejad R, Zarrabi M, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12:91. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Oliveira HG, Cruz FF, Antunes MA, et al. Combined bone marrow-derived mesenchymal stromal cell therapy and one-way endobronchial valve placement in patients with pulmonary emphysema: a phase I clinical trial. Stem Cells Transl Med. 2017;6:962–969. doi: 10.1002/sctm.16-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iglesias M, Butron P, Torre-Villalvazo I, et al. Mesenchymal stem cells for the compassionate treatment of severe acute respiratory distress syndrome due to COVID 19. Aging Dis. 2021;12:360–370. doi: 10.14336/AD.2020.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu KD, Wilson JG, Zhuo H, et al. Design and implementation of the START (STem cells for ARDS Treatment) trial, a phase 1/2 trial of human mesenchymal stem/stromal cells for the treatment of moderate-severe acute respiratory distress syndrome. Ann Intensive Care. 2014;4:22. doi: 10.1186/s13613-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord-derived mesenchymal stem cells for COVID-19 patients with acute respiratory distress syndrome (ARDS) CellR4 Repair Replace Regen Reprogr. 2020;8:e2839. doi: 10.32113/cellr4_20204_2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorman E, Shankar-Hari M, Hopkins P, et al. Repair of acute respiratory distress syndrome by stromal cell administration (REALIST): a structured study protocol for an open-label dose-escalation phase 1 trial followed by a randomised, triple-blind, allocation concealed, placebo-controlled phase 2 trial. Trials. 2022;23:401. doi: 10.1186/s13063-022-06220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payares-Herrera C, Martinez-Munoz ME, Vallhonrat IL, et al. Double-blind, randomized, controlled, trial to assess the efficacy of allogenic mesenchymal stromal cells in patients with acute respiratory distress syndrome due to COVID-19 (COVID-AT): a structured summary of a study protocol for a randomised controlled trial. Trials. 2021;22:9. doi: 10.1186/s13063-020-04964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkham AM, Monaghan M, Bailey AJM, et al. Mesenchymal stromal cells as a therapeutic intervention for COVID-19: a living systematic review and meta-analysis protocol. Syst Rev. 2021;10:249. doi: 10.1186/s13643-021-01803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouroupis D, Lanzoni G, Linetsky E, et al. Umbilical cord-derived mesenchymal stem cells modulate TNF and soluble TNF receptor 2 (sTNFR2) in COVID-19 ARDS patients. Eur Rev Med Pharmacol Sci. 2021;25:4435–4438. doi: 10.26355/eurrev_202106_26156. [DOI] [PubMed] [Google Scholar]
- 30.Wick KD, Leligdowicz A, Zhuo H, Ware LB, Matthay MA. Mesenchymal stromal cells reduce evidence of lung injury in patients with ARDS. JCI Insight. 2021;6:e148983. doi: 10.1172/jci.insight.148983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L, Yuan X, Yao W, et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2022;75:103789. doi: 10.1016/j.ebiom.2021.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi L, Huang H, Lu X, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6:58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adas G, Cukurova Z, Yasar KK, et al. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transplant. 2021;30:9636897211024942. doi: 10.1177/09636897211024942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monsel A, Hauw-Berlemont C, Mebarki M, et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care. 2022;26:48. doi: 10.1186/s13054-022-03930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rebelatto CLK, Senegaglia AC, Franck CL, et al. Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Res Ther. 2022;13:122. doi: 10.1186/s13287-022-02796-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aghayan HR, Salimian F, Abedini A, et al. Human placenta-derived mesenchymal stem cells transplantation in patients with acute respiratory distress syndrome (ARDS) caused by COVID-19 (phase I clinical trial): safety profile assessment. Stem Cell Res Ther. 2022;13:365. doi: 10.1186/s13287-022-02953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu L, Niu C, Li R, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:361. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowdish ME, Barkauskas CE, Overbey JR, et al. A Randomized trial of mesenchymal stromal cells for moderate to severe ARDS from COVID-19. Am J Respir Crit Care Med. 2022. [DOI] [PMC free article] [PubMed]
- 39.Kaffash Farkhad N, Sedaghat A, Reihani H, et al. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful phase 1, control-placebo group, clinical trial. Stem Cell Res Ther. 2022;13:283. doi: 10.1186/s13287-022-02920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu X, Jiang W, Chen L, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin Transl Med. 2021;11:e297. doi: 10.1002/ctm2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 42.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 43.Sweeney RM, McAuley DF. Acute respiratory distress syndrome. Lancet. 2016;388:2416–2430. doi: 10.1016/S0140-6736(16)00578-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yadav H, Thompson BT, Gajic O. Fifty years of research in ARDS. Is acute respiratory distress syndrome a preventable disease? Am J Respir Crit Care Med. 2017;195:725–736. doi: 10.1164/rccm.201609-1767CI. [DOI] [PubMed] [Google Scholar]
- 45.Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Meng Q, Rao X, et al. Corticosteroid therapy in critically ill patients with COVID-19: a multicenter, retrospective study. Crit Care. 2020;24:698. doi: 10.1186/s13054-020-03429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr. 2014;164(966–72):e6. doi: 10.1016/j.jpeds.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Powell SB, Silvestri JM. Safety of intratracheal administration of human umbilical cord blood derived mesenchymal stromal cells in extremely low birth weight preterm infants. J Pediatr. 2019;210(209–13):e2. doi: 10.1016/j.jpeds.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 51.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Islam D, Huang Y, Fanelli V, et al. Identification and modulation of microenvironment is crucial for effective mesenchymal stromal cell therapy in acute lung injury. Am J Respir Crit Care Med. 2019;199:1214–1224. doi: 10.1164/rccm.201802-0356OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. The meta-analyses of age and gender (male patients ratio), comparing MSC with the control: A the comparison of MSC with control regarding age; B the comparison of MSC with control regarding gender. The size of each square represents the proportion of information given by each trial. Crossing with the vertical line suggests no difference between the two groups. Fig. S2. The meta-analyses of adverse events, comparing MSC with the control in the random-effects model: A the comparison of MSC with control in general ARDS; B the comparison of MSC with control in COVID-19-induced ARDS. The size of each square represents the proportion of information given by each trial. Crossing with the vertical line suggests no difference between the two groups. Fig. S3. The meta-analyses of mortality, comparing MSC with the control in the random-effects model: A the comparison of MSC with control in general ARDS; B the comparison of MSC with control in COVID-19-induced ARDS. The size of each square represents the proportion of information given by each trial. Crossing with the vertical line suggests no difference between the two groups
Data Availability Statement
Data sharing does not apply to this article as no new data were created or analyzed in this study.