Abstract
The immunomodulatory role of neutrophils during infection with Toxoplasma gondii was investigated. Monoclonal antibody-mediated depletion revealed that neutrophils are essential for survival during the first few days of infection. Moreover, neutrophil depletion was associated with a weaker type 1 immune response as measured by decreased levels of gamma interferon, interleukin-12 (IL-12) and tumor necrosis factor alpha. IL-10 was also decreased in depleted animals. Additionally, splenic populations of CD4+ T cells, CD8+ T cells, and NK1.1+ cells were decreased in depleted mice. Neutrophil-depleted mice exhibited lesions of greater severity in tissues examined and a greater parasite burden as determined by histopathology and reverse transcription-PCR. We conclude that neutrophils are critical near the time of infection because they influence the character of the immune response and control tachyzoite replication.
The immune system is a complex nonlinear system, involving the coordination of multiple cell types. Disease often results from an insufficient immune response. Lack of protection can occur when any one or more of the components of the immune system are impaired. Neutropenia is a risk factor associated with Aspergillus fumigatus, Candida albicans, Strongyloides ratti, Yersinia enterocolitica, Chlamydia trachomatis, Mycobacterium tuberculosis, Listeria monocytogenes, and Toxoplasma gondii infections (2, 3, 5, 7, 8, 22, 27, 31, 41). In some cases, neutropenia has been correlated with impaired protective acquired immunity, suggesting that neutrophils function as immunomodulators of acquired immunity (27, 31). The list of microbes affected by the actions of neutrophils is ever-growing, and it is evident that these cells are involved in the immune response to a highly diverse array of both intracellular and extracellular pathogens.
We examined the development of immunity during T. gondii infection in mice depleted of neutrophils by monoclonal antibody (MAb) administration. T. gondii is an obligate intracellular protozoan parasite that poses an important public health risk. Congenital infections may result in severe birth defects, and reactivation of chronic infection can lead to development of encephalitis, a particular problem for persons who are immunosuppressed (25, 28). We found that neutrophil depletion at the time of infection led to development of lesions in multiple organs, including the spleen, lung, liver, and brain, and was associated with an impaired ability to produce early gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α) and interleukin-12 (IL-12). Splenic populations of T cells and NK cells were decreased in neutrophil-depleted infected mice. Moreover, neutrophil-depleted mice harbored an increased parasite burden. We conclude that neutrophils are important immunomodulators early in the course of T. gondii infection and play a critical role in protecting the host from uncontrolled tachyzoite replication.
MATERIALS AND METHODS
Mice.
C57BL/6 female mice (6 to 12 weeks of age) were obtained from The Jackson Laboratory (Bar Harbor, Maine). The animals were housed under specific-pathogen-free conditions at the College of Veterinary Medicine animal facility at Cornell University. The college maintains an animal facility that is accredited by the American Association for Accreditation of Laboratory Animal Care.
Parasites and infections.
ME49 bradyzoite cysts were maintained in Swiss Webster mice as described previously (5). Mice were rendered neutropenic with an anti-Gr-1 MAb (RB6C6.8C5 hybridoma originally provided by R. L. Coffman, DNAX Research Institute, Palo Alto, Calif.) or a control rat immunoglobulin G (IgG) (Accurate, Westbury, N.Y.) and infected intraperitoneally (i.p.) with 100 ME49 cysts as described previously (3).
Soluble tachyzoite antigen (STAg) was prepared as previously described (5). Briefly, tachyzoites were sonicated in the presence of protease inhibitors (0.2 mM phenylmethlysulfonyl fluoride, 0.2 mM aprotinin, 1 mM leupeptin, and 1 mM EDTA), dialyzed into phosphate-buffered saline, filter sterilized through a 0.2-μm-pore-size membrane. (Corning Costar Corp., Cambridge, Mass.), assayed for protein concentration, and stored at −70°C. Parasite extracts were found to be free of endotoxin as measured by the Limulus amebocyte assay.
Splenocyte cultures.
Mice were depleted of neutrophils by MAb adminstration (depleted infected mice) or given a control rat IgG (control infected mice) on days −2, 0, +2, and +4 and were infected i.p. with 100 ME49 cysts on day 0. Mice were euthanatized on days +2, +4, +6, and +8, and spleens were harvested. Plasma was also obtained at the time of euthanasia. Splenocytes from uninfected, control infected, and depleted infected mice were cultured at 5 × 106 cells/ml at 37°C and 5% CO2 or stained for flow cytometry (see below). Cells were stimulated with medium or STAg at 2, 20, or 200 μg/ml. After 3 days, cell-free supernatants were collected and stored at −20°C until cytokine analysis.
Cytokine measurement.
IL-12 p40 and IL-10 were measured in cell-free supernatants by enzyme-linked immunosorbent assay (ELISA) as described in detail previously (5, 39). To measure IFN-γ, the protocol for determining p40 levels was followed, except that clone HB170 (American Type Culture Collection, Manassas, Va.) was used as the coating antibody (Ab) at 10 μg/ml and clone XMG-biotin (PharMingen, San Diego, Calif.) was used as the secondary Ab at 1:4,000 dilution. TNF-α levels were determined using a murine-specific kit according to the manufacturer's instructions (R & D Systems, Minneapolis, Minn.). Detection sensitivities were 10 pg/ml for IL-12 p40, 20 pg/ml for TNF-α, 30 pg/ml for IL-10, and 75 pg/ml for IFN-γ.
Flow cytometric analysis.
Splenocytes at 5 × 106/ml were immediately stimulated ex vivo for 4 hours with phorbol myristate acetate and ionomycin (5 and 500 ng/ml, respectively; Sigma) according to the PharMingen protocol for intracellular murine IFN-γ detection. Brefeldin A (GolgiPlug) was added for the last 2 hours of culture. After blocking with 10% normal mouse serum in phosphate-buffered saline, cells were stained for surface markers with fluorescein isothiocyanate-conjugated Ab directed against CD4, CD8, and NK1.1 (PharMingen). Splenocytes were then permeabilized and subjected to intracellular IFN-γ staining with a phycoerythrin-conjugated anti-IFN-γ Ab, employing a commercially available kit (Cytofix/Cytoperm; PharMingen). Data were acquired on a FACSCalibur system and analyzed with CellQuest software (Beckton Dickinson Immunocytometry Systems, San Jose, Calif.). To obtain absolute cell numbers for a given subset, percentages determined by flow cytometry were multiplied by the mean total number of leukocytes in the spleens of a given group.
Histopathology.
Tissues were fixed in 10% (wt/vol) buffered formaldehyde following euthanasia. Samples were then progressively dehydrated in ethanol, cleared with xylene, and embedded in paraffin according to standard laboratory procedures. Six-micrometer sections were stained with hematoxylin and eosin for routine histopathological examination.
Quantitation of parasite burden.
Samples of brain, lung, liver, and spleen tissue were collected from uninfected, control infected, and depleted infected mice when the last group became clinically ill (usually day 8). RNA was extracted as previously described (21) and used to quantitate parasite burden with an ABI 7700 Sequence Detector (PE Biosystems, Foster City, Calif.) according to the manufacturer's instructions. Briefly, equal amounts of RNA from each mouse within a group were pooled, and quantitation of mRNA for p22 (a parasite surface protein) and hypoxanthine phosphoribosyltransferase (HPRT) (an endogenous control) was determined using the one-step reverse transcription RT-PCR kit (PE Biosystems). Levels of HPRT were used to normalize p22 values. No amplification of p22 was detected from uninfected controls. The data are expressed as the fold increase of normalized p22 expression in depleted infected samples over that of control infected samples, which were arbitrarily assigned a value of 1 according to the manufacturer's instructions.
Statistics.
A two-tailed paired t test was employed to assign statistical significance to cell depletion experiments.
RESULTS
Neutrophils are required for resistance during early- but not late- stage infection.
It has been demonstrated that neutrophils are essential at the time of infection for survival in a murine model (5, 33, 35). To better define when they are required during the acute stage of disease, we depleted mice of granulocytes by MAb administration at different times. Mice infected with the ME49 strain of T. gondii and administered a control rat IgG survived infection (Fig. 1). Similarly, mice depleted of granulocytes later during the acute stage (days +6, +8, and +10) also were resistant, indicating that neutrophils are not required after day 6 of infection. In contrast, mice depleted of granulocytes before day 6 showed increased susceptibility (Fig. 1). We also depleted mice of granulocytes during the chronic stage of disease (beginning at 66 days postinfection and continuing for 2 weeks) and found no differences in survival or clinical signs compared to mice treated with a control rat IgG (data not shown). Taken together, the data indicate that granulocytes are required for survival only during the initial days of infection.
FIG. 1.
Granulocytes are necessary for survival of T. gondii infection only during the first few days of infection. C57BL/6 mice were injected i.p. either with 200 μg of the granulocyte-depleting Ab RB6C6.8C5 on days −2, 0, and +2, days +2, +4, and +6, or days +6, +8, and +10 or with a control rat IgG on days −2, 0, and +2. Mice (four per group) were infected i.p. with 100 ME49 cysts on day 0 (four mice per group). Survival was monitored daily. Results represent three experiments.
Neutrophil-depleted mice display a weaker type 1 immune response.
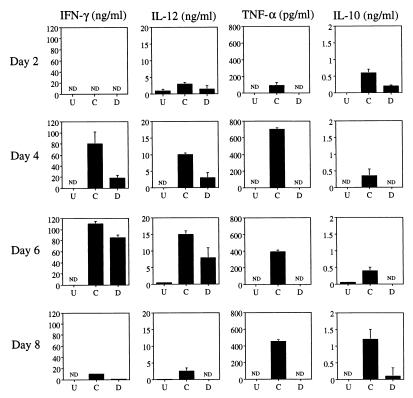
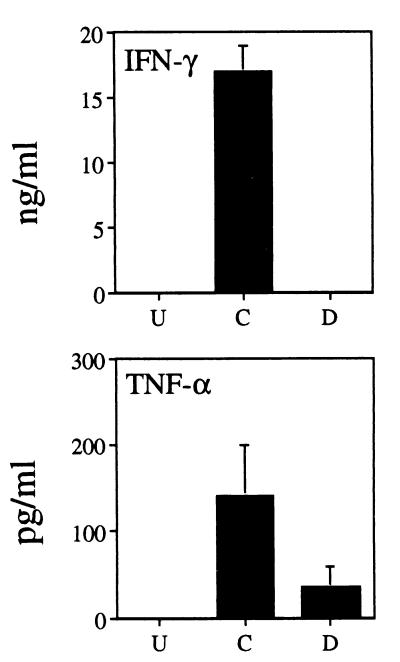
Equal numbers of splenocytes from uninfected, control infected, and depleted infected mice were cultured in medium for 3 days. When constitutive cytokine levels in cell-free supernatants were assessed, we found profound decreases using cells from neutrophil-depleted animals. IFN-γ was undetectable at 2 days postinfection from any group of mice (Fig. 2). However, by day 4 of infection, splenocytes from control infected mice produced a vigorous IFN-γ response. An approximately fivefold-weaker response was noted in cultures derived from depleted infected mice (Fig. 2). Nevertheless, IFN-γ production by splenocytes from these mice recovered by day 6 postinfection. Levels of IL-12 in splenocyte supernatants from depleted infected animals were substantially lower than levels in the control group at all time points tested (Fig. 2). Similarly, levels of TNF-α from depleted infected splenocyte cultures were always lower than levels in control samples, and indeed, TNF-α was not detected at any time point (Fig. 2). The basis for this dramatic effect is under investigation. Expression of IL-10 typically follows production of proinflammatory cytokines and is thought to control the tissue-destructive activities encountered in an inflammatory reaction (23, 26). We found that IL-10 levels in splenocyte cultures from depleted infected animals were consistently lower than those measured in control samples (Fig. 2). Levels of IL-4 and IL-5 were below the level of detection (data not shown). IFN-γ and TNF-α levels in plasma were also measured (Fig. 3). In contrast to the nondepleted group, IFN-γ was not detected in the plasma from depleted infected mice, while levels of TNF-α were approximately one-third that in plasma of control infected mice (Fig. 3). It has also been previously shown that plasma IL-12 levels are substantially lower in depleted infected animals (3). Taken together, the data indicate that depletion of neutrophils at the time of infection is associated with production of a weaker type 1 immune response.
FIG. 2.
Constitutive expression of cytokines from cultured splenocytes of neutrophil-depleted mice is impaired. C57BL/6 mice were administered either the anti-Gr-1 MAb or a control rat IgG on days −2, 0, +2, and +4. Mice (four per group) were infected i.p. with 100 ME49 cysts on day 0 as described in Materials and Methods (three mice per group). On days +2, +4, +6, and +8 postinfection, mice were euthanatized and spleens were collected for culture. Additionally, spleens from uninfected mice were cultured. Splenocytes were cultured in medium for 3 days, and cell-free supernatants were harvested for cytokine level determination by ELISA. U, cells from uninfected mice; C, cells from animals administered control Ab; D, mice depleted of neutrophils by anti-Gr-1 Ab injection; ND, not detected. Results are expressed as means + standard deviations and represent three experiments.
FIG. 3.
Levels of IFN-γ and TNF-α in plasma of neutrophil-depleted mice are decreased at 2 days postinfection. Plasma was obtained from mice (four per group) treated as described in the legend to Fig. 2 at 2 days postinfection. Cytokine levels were determined by ELISA. U, cells from uninfected animals; C, cells from mice injected with control Ab; D, animals depleted of neutrophils by anti-Gr-1 MAb administration. Results are expressed as means + standard deviations and represent three experiments.
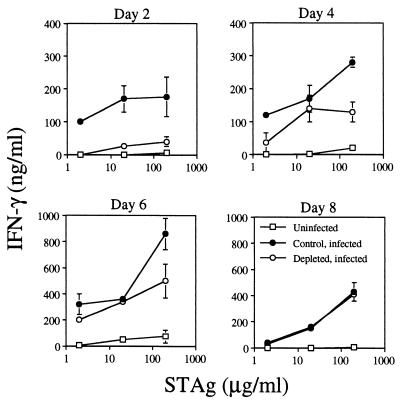
Since IFN-γ is an essential component of a protective immune response against T. gondii, we tested the ability of splenocytes to respond to parasite antigen by releasing this cytokine. Splenocytes from uninfected, control-infected, and depleted-infected mice were cultured in the presence of 2, 20, or 200 μg of STAg/ml for 3 days. After that time, cell-free supernatants were collected for cytokine level quantitation. Figure 4 demonstrates a virtually complete inability of splenocytes from depleted-infected mice to produce IFN-γ early in infection. Their ability to respond slowly recovered over time and was equivalent to that for control splenocytes by day 8 postinfection (Fig. 4). These data establish a link between neutrophil function and the ability of splenocytes to release IFN-γ upon antigen stimulation.
FIG. 4.
Ability of splenocytes from depleted infected mice to produce IFN-γ in response to parasite antigen stimulation is profoundly impaired at 2 days postinfection. Spleens were obtained from uninfected, control infected (control rat IgG given on days −2, 0, +2, and +4), and depleted infected (anti-Gr-1 MAb given on days −2, 0, +2, and +4) mice on days +2, +4, +6, and +8 postinfection. Four mice were used in each group. Splenocyte cultures were stimulated with STAg at 2, 20, or 200 μg/ml for 3 days. Cell-free supernatants were collected, and levels of IFN-γ were determined by ELISA as described in Materials and Methods. Results are expressed as means + standard deviations and represent three experiments.
Spleen cell populations are altered in neutrophil-depleted infected mice.
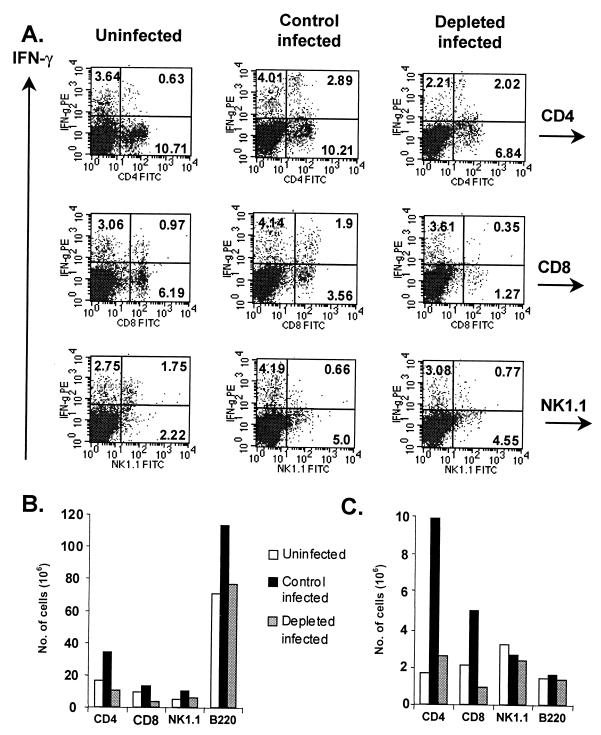
Given the noted decreases in IFN-γ production in splenocyte cultures derived from neutrophil-depleted mice, we wondered if there were phenotypic differences in the spleens of these animals. Splenocytes from uninfected, control infected, and depleted-infected mice were stained for CD4, CD8, NK1.1, B220, and IFN-γ and analyzed by flow cytometry. Figure 5A demonstrates a lower frequency of CD4+ and CD8+ cells at 6 days postinfection in depleted mice. While the percentages of IFN-γ+ CD4+ cells out of the total CD4+ population were similar in depleted and control groups (22 and 23%, respectively), fewer CD8+ cells were IFN-γ+ relative to the total CD8+ population in the depleted group than in the control group (22 and 35%, respectively; P < 0.05). In general, effects on the CD8+ subset were more pronounced at all time points (data not shown). In contrast, the frequencies of NK1.1+ cells and IFN-γ+ NK1.1+ cells were similar between the control-infected and depleted-infected groups (Fig. 5A).
FIG. 5.
Neutrophil-depleted mice display decreased numbers of IFN-γ+ T cells during infection. (A) IFN-γ expression in CD4+, CD8+, B220+ and NK1.1+ subsets at 6 days postinfection. Numbers in quadrants reflect the percentages of cells. PE, phycoerythrin; FITC, fluorescein isothiocyanate. (B) Total numbers of cells in each subset. (C) Numbers of IFN-γ+ cells in each subset. Splenocytes were obtained and stimulated ex vivo before staining as described in Materials and Methods. Cells were then analyzed by flow cytometry. To obtain absolute cell numbers, percentages in each population were multiplied by the mean total cell number in corresponding spleens (three mice per group). Results represent three separate experiments.
At the time of euthanasia, it was noted that the spleens from depleted mice were grossly smaller than those from either uninfected or control-infected mice. The differences in size were reflected in total cell counts. In Fig. 5B, the total numbers of cells in each subset are shown. By day 6 postinfection, there was an expansion in the numbers of CD4+, CD8+, B220+, and NK1.1+ cells from control mice. When the number of IFN-γ+ cells in each subset was plotted, a similar expansion was noted for CD4+ and CD8+ cells (Fig. 5C). There were approximately one-fifth the number of IFN-γ+ CD4+ cells and IFN-γ+ CD8+ cells in the spleens of depleted animals. At this stage of infection, there were similar levels of NK1.1+ and B220+ cells staining for IFN-γ+. It has been noted that the Ab used to deplete mice of neutrophils, which recognizes Gr-1 or Ly-6G, may cross-react with Ly-6C, an epitope found on CD8+ T cells (9, 24). Montes de Oca et al. (24) reported an approximately 25 to 30% loss of CD8+ T cells in uninfected spleen cell populations upon administration of RB6C6.8C5, a figure consistent with our own studies (data not shown). While this Ab may contribute to the loss in CD8+ T cells, it is unlikely to account for the nearly 80% loss of these cells in infected populations. As demonstrated by the data in Fig. 5, the total numbers of IFN-γ-producing cells differed greatly by group. Overall, these data indicate a decrease in the number of cell types known to be important in mediating protection against T. gondii when mice are depleted of neutrophils at the time of infection. The loss of CD8+ T cells itself is unlikely to account for the inability to survive early infection, since mice negative for this T-lymphocyte subset, through either Ab treatment or gene deletion, are capable of surviving acute Toxoplasma infection (10, 34).
Neutrophil-depleted mice display extensive pathologic changes in lymphoid and nonlymphoid tissue.
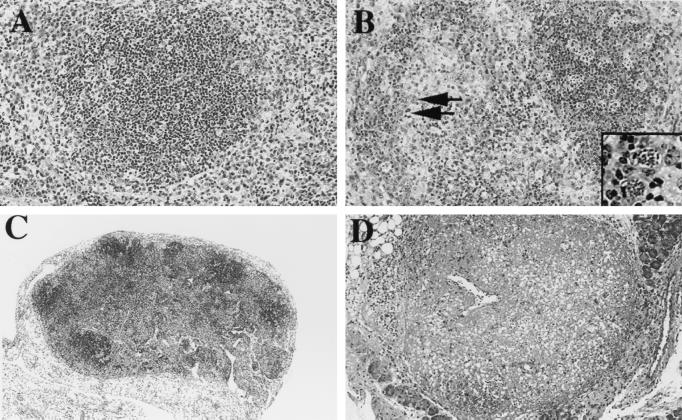
Tissues from all major organs were collected from depleted-infected, control-infected, and uninfected mice at the time when neutropenic mice became clinically ill (usually day 8). Tissues were fixed, and sections were stained with hematoxylin and eosin. Figure 6 demonstrates typical lesions found in the mice. In the spleens of neutrophil-depleted animals, there was extensive lymphoid follicular depletion due to marked lymphoid necrosis (Fig. 6B). In addition, there was moderate myeloid hyperplasia in perifollicular areas and red pulp (data not shown). Intracellular and extracellular tachyzoites were abundant (Fig. 6B). In contrast, lesions present in infected control mice were much less severe, and parasites were less numerous (Fig. 6A). Severe granulomatous and necrotizing lymphadenitis with marked disruption of architecture and small numbers of organisms were found in the mesenteric lymph nodes of these animals (Fig. 6D). Lesions in the mesenteric lymph nodes from control infected mice were less severe (Fig. 6C). The uninfected mice displayed no evidence of pathology (data not shown).
FIG. 6.
Depleted infected mice suffer from extensive lesions in lymphoid tissue. Tissues from spleens (A and B) and mesenteric lymph nodes (C and D) were collected from control infected (A and C) and depleted infected (B and D) mice (four per group) on day 8 postinfection. Samples were fixed and stained with hematoxylin and eosin. Arrows in panel B point to infected cells; this area is enlarged in the inset. Original magnifications, ×20 (A and B) and ×10 (C and D). Results represent two experiments.
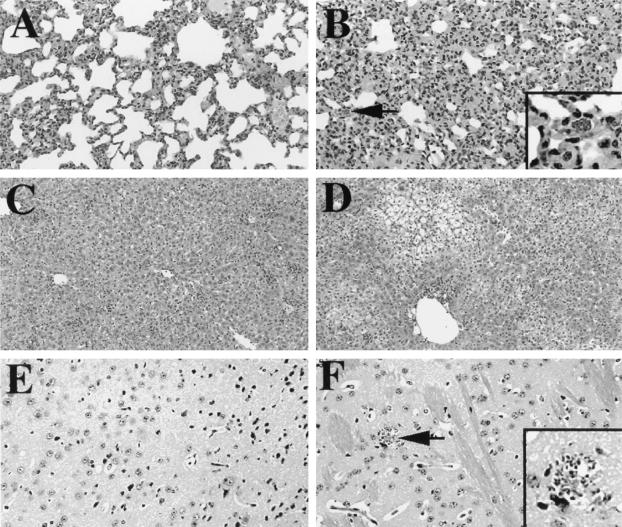
In general, lesions in nonlymphoid tissues of depleted infected mice were more extensive and severe than in infected control mice. There was moderate to locally-extensive alveolitis with moderate numbers of tachyzoites in the pulmonary tissue of depleted mice (Fig. 7B). Pulmonary tissue from control infected mice exhibited less severe inflammation, and tachyzoites were less abundant (Fig. 7A). The livers from depleted infected mice demonstrated moderate to marked hepatocyte cord dissociation with moderate cytoplasmic vacuolar degeneration (Fig. 7D). Additionally, there were small, multifocal areas of lymphoplasmacytic infiltrates accompanied by few macrophages (i.e., granulomas). The livers from control infected mice had similar multifocal granulomas and individual cell necrosis (Fig. 7C). In the brains of infected depleted mice, there were locally extensive areas of gliosis and a few parasites (Fig. 7F). Lesions in the control nervous system were rare in control infected mice (Fig. 7E). No pathologic changes were noted in uninfected mice (data not shown).
FIG. 7.
Depleted infected mice exhibit extensive lesions in nonlymphoid tissue. Tissues from lung (A and B), liver (C and D), and brain (E and F) were collected from control infected (A, C, and E) and depleted infected (B, D, and F) mice (four per group) on day 8 postinfection. Samples were fixed and stained with hematoxylin and eosin. The arrow in panel B points to an infected cell; the arrow in panel F points to tachyzoites within a focal area of necrosis. These regions are enlarged in the panel insets. Original magnifications, ×20 (A, B, E, and F) and ×10 (C and D). Results represent of two experiments.
Uncontrolled tachyzoite replication occurs in neutrophil-depleted mice.
Quantitation of parasite burden in the brain, lung, liver, and spleen was performed by RT-PCR using an ABI Sequence Detector. Levels of p22 (SAG 2) and HPRT (housekeeping gene) mRNA were measured. Normalized levels of p22 in control infected mice were arbitrarily set at 1. Levels of normalized p22 from depleted-infected mice were then compared with control levels (Fig. 8). In all organs examined, there was a 1.6- to 14-fold increase in p22 expression in tissues from depleted infected mice, indicating a greater parasite burden in neutrophil-depleted animals.
FIG. 8.
Neutrophil-depleted mice have a higher parasite burden in tissues from liver, spleen, lung, and brain. Four mice were used for each treatment group. RNA was extracted from tissue at 8 days postinfection from uninfected, control infected, and depleted-infected mice. RT-PCR was performed using an ABI 7700 Sequence Detector for p22 (a parasite antigen) and HPRT (an endogenous control). p22 was not detected in uninfected tissue. HPRT mRNA levels were used to normalize p22 levels. A value of 1 was arbitrarily set for control infected samples, and levels of normalized p22 from depleted infected samples were expressed as the fold increase over control samples. C, mice administered a control Ab; D, animals depleted of neutrophils by administration of anti-Gr-1 MAb.
DISCUSSION
Within the context of toxoplasmosis, the ability of neutrophils to release cytokines upon infection (3–5, 19), the occurrence of neutrophilia in vivo (19), the inability of neutropenic mice to survive infection (5, 33, 35), and the presence of increased numbers of neutrophils at the site of infection (3) all suggest that these cells play an important role in vivo. Neutrophils have also been implicated as immunomodulators in other infections. Perhaps the best-studied model is that of C. albicans, where neutrophil IL-12 production is associated with a protective type 1 response, while IL-10 production leads to a type 2 response and exacerbated disease (30–32). An immunomodulatory role for neutrophils has also been proposed for infections with M. tuberculosis and L. monocytogenes (7, 9, 27, 29). A recent paper by Tacchini-Cottier et al. (38) demonstrated that neutrophil depletion at the time of infection abrogated the early burst of IL-4 that otherwise occurs in the draining lymph nodes of mice infected with Leishmania major. Subsequently, development of a type 2 response was inhibited and there was partial resolution of footpad lesions, suggesting an early nonprotective role for neutrophils in susceptible BALB/c mice. Depletion of neutrophils 1 day after infection had no effect. Similarly, neutrophil depletion after day 3 of infection with a C. albicans vaccine strain did not enhance disease (31). In fact, mice appeared to benefit from late depletion, and this was attributed to an inhibition of inflammation. In accord with these results, we found that neutrophils are required for survival only during the first few days of T. gondii infection (Fig. 1). In the case of Plasmodium berghei infection, neutrophil depletion improves the clinical course of disease. Prevention of cerebral malaria with decreased central nervous system hemorrhages and sequestration of monocytes was attributed to impaired type 1 immune response development in the brain when neutrophils were absent (6). While neutrophils appear to be generally protective during infections with T. gondii and C. albicans, they may exacerbate disease in other situations, highlighting the paradoxical nature of neutrophil function. Thus, neutrophil function can be harmful or beneficial to the host, depending upon the character and magnitude of the response induced by these cells.
Depletion of neutrophils at the time of infection with T. gondii was associated with decreased levels of IFN-γ, TNF-α, and IL-12, cytokines that are well known to be important in parasite control (Fig. 2, 3, and 4) (1, 10, 15, 36, 42). Indeed, in our studies, neutrophil-depleted mice displayed greatly increased parasite levels in tissue as determined by histopathology and RT-PCR (Fig. 6, 7, and 8). The majority of organs examined revealed lesions of enhanced severity in depleted infected mice, suggesting that these mice succumb to infection because of multiorgan system disease due to uncontrolled tachyzoite replication and associated tissue destruction. In particular, there was extensive destruction of splenic white pulp in depleted animals. This finding corresponded to decreases in the numbers of CD4+, CD8+, and NK1.1+ subsets (Fig. 5).
Previous studies have demonstrated that neutrophils can produce cytokines such as IL-12 and TNF-α (5, 11, 12, 32). Nevertheless, we think it unlikely that decreased cytokine levels in the neutrophil-depleted mice are a direct consequence of the loss of these cells. Thus, our hypothesis is that during early T. gondii infection, neutrophils play an instructive role in the development of immunity to the parasite.
Classically, naive T helper cells are thought to be activated in secondary lymphoid tissue (14). Previous studies show rapid neutrophil accumulation at the site of infection (3). Our hypothesis is that neutrophils exert their protective effect during toxoplasmosis at least in part, through their ability to produce T-cell immunoregulatory cytokines. How do neutrophils exert their effects on T cells? One possibility is that neutrophils act indirectly through the actions of antigen-presenting cells, particularly dendritic cells. Numerous reports indicate a need for priming by various stimuli in order for dendritic cells to activate T cells (17, 37, 40). Neutrophils may provide these priming signals. Another possibility is that neutrophils enter secondary lymphoid tissue with phagocytosed antigen. Once there, they might influence T-cell differentiation by releasing instructive cytokines. In support of this hypothesis, Tacchini-Cottier et al. (38) found neutrophils in the subcapsular space of the draining lymph nodes after infection with L. major. Moreover, Harmsen et al. (18) demonstrated neutrophil antigen acquisition in the lung and subsequent migration to tracheobronchial lymph nodes. Antigen-containing neutrophils were found in lymphatic vessels and paracortical areas of the lymph nodes. Finally, major histocompatibility complex class II expression on human neutrophils has been demonstrated, and it is possible that neutrophils act as antigen-presenting cells themselves (13, 16, 20).
The studies presented here and elsewhere clearly demonstrate a previously unappreciated role for neutrophils as cytokine-producing immunoregulatory cells. Nevertheless, neutrophils are also well known for their phagocytic activity and release of microbicidal molecules during infection. Therefore, it is probable that both general functions contribute to the ability of these cells to protect against infection or, in certain situations, to exacerbate disease. The relative contributions of these activities to the outcome of infection likely depend upon the particular pathogen and, in the case of toxoplasmosis, are an area of ongoing study in our laboratory.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI47888. S.K.B. was supported by NIH grant F32 HD08654-01.
REFERENCES
- 1.Alexander J, Hunter C A. Immunoregulation during toxoplasmosis. Chem Immunol. 1998;70:81–102. doi: 10.1159/000058701. [DOI] [PubMed] [Google Scholar]
- 2.Barteneva N, Theodor I, Peterson E M, de la Maza L M. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64:4830–4833. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bliss S K, Butcher B A, Denkers E Y. Rapid recruitment of neutrophils with prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–4521. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 4.Bliss S K, Marshall A J, Zhang Y, Denkers E Y. Human polymorphonuclear leukocytes produce IL-12, TNF-α, and the chemokines macrophage-inflammatory protein-1 α and −1β in response to Toxoplasma gondii antigens. J Immunol. 1999;162:7369–7375. [PubMed] [Google Scholar]
- 5.Bliss S K, Zhang Y, Denkers E Y. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-γ-independent IL-12. J Immunol. 1999;163:2081–2088. [PubMed] [Google Scholar]
- 6.Chen L, Zhang Z H, Sendo F. Neutrophils play a critical role in the pathogenesis of experimental cerebral malaria. Clin Exp Immunol. 2000;120:125–133. doi: 10.1046/j.1365-2249.2000.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlan J W. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conlan J W, North R J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czuprynzki C J, Brown J F, Maroushek N, Wagner R D, Steinberg H. Administration of anti-granulocyte mAb RB6–8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- 10.Denkers E Y, Gazzinelli R T. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djeu J Y, Serbousek D, Blanchard D K. Release of tumor necrosis factor by human polymorphonuclear leukocytes. Blood. 1990;76:1405–1409. [PubMed] [Google Scholar]
- 12.Dubravec D B, Spriggs D R, Mannick J A, Rodrick M L. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor α. Proc Natl Acad Sci USA. 1990;87:6758–6761. doi: 10.1073/pnas.87.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanger N A, Liu C, Guyre P M, Wardwell K, O'Neil J, Guo T L, Chritian T P, Mudzinski S P, Gosselin E J. Activation of human T cells by major histocompatibility class II expressing neutrophils: proliferation in the presence of superantigen but not tetanus toxoid. Blood. 1997;89:4128–4135. [PubMed] [Google Scholar]
- 14.Flavell R. The molecular basis of T cell differentiation. Immunol Res. 1999;19:159–168. doi: 10.1007/BF02786484. [DOI] [PubMed] [Google Scholar]
- 15.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 16.Gosselin E J, Wardwell K, Rigby W F, Guyre P M. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol. 1993;151:1482–1490. [PubMed] [Google Scholar]
- 17.Grohmann U, Belladonna M L, Bianchi R, Orabona C, Ayroldi E, Fioretti E M C, Puccetti P. IL-12 acts directly on DC to promote nuclear localization of NF-κB and primes DC for IL-12 production. Immunity. 1998;9:315–323. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 18.Harmsen A G, Mason M J, Muggenburg B A, Gillett N A, Jarpe M A, Bice D E. Migration of neutrophils from lung to tracheobronchial lymph node. J Leukoc Biol. 1987;41:95–103. doi: 10.1002/jlb.41.2.95. [DOI] [PubMed] [Google Scholar]
- 19.Jebbari H, Roberts C W, Ferguson D J P, Bluethmann H, Alexander J. A protective role for IL-6 during early infection with Toxoplasma gondii. Parasite Immunol. 1998;20:231–239. doi: 10.1046/j.1365-3024.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenberger C, Zakeri S, Baier K, Willheim M, Holub M, Reinisch W. A novel high-purity isolation method for human peripheral blood neutrophils permitting polymerase chain reaction-based mRNA studies. J Immunol Methods. 1999;30:75–84. doi: 10.1016/s0022-1759(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 21.Marshall A J, Rosa Brunet L, van Gessel Y, Alcaraz A, Bliss S K, Pearce E J, Denkers E Y. Toxoplasma gondii and Schistosoma mansoni synergize to promote hepatocyte dysfunction associated with high levels of plasma TNF-α and early death in C57BL/6 mice. J Immunol. 1999;163:2089–2097. [PubMed] [Google Scholar]
- 22.Mehrad B, Moore T A, Standiford T J. Macrophage inflammatory protein-1 alpha is a critical mediator of host defense against invasive pulmonary aspergillosis in neutropenic hosts. J Immunol. 2000;165:962–968. doi: 10.4049/jimmunol.165.2.962. [DOI] [PubMed] [Google Scholar]
- 23.Mencacci A, Cenci E, Del Sero G, d'Ostiani C, Mosci P, Trinchieri G, Adorini L, Romani L. IL-10 is required for development of protective Th1 responses in IL-12 deficient mice upon Candida albicans infection. J Immunol. 1998;161:6228–6237. [PubMed] [Google Scholar]
- 24.Montes de Oca R, Buendía A J, Del Río L, Sánchez J, Salinas J, Navarro J A. Polymorphonuclear neutrophils are necessary for the recruitment of CD8+ T cells in the liver in a pregnant mouse model of Chlamydophila abortus (Chlamydia psittaci serotype 1) infection. Infect Immun. 2000;68:1746–1751. doi: 10.1128/iai.68.3.1746-1751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navia B A, Petito C K, Gold J W M, Cho E S, Jordon B D, Price J W. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol. 1986;19:224–238. doi: 10.1002/ana.410190303. [DOI] [PubMed] [Google Scholar]
- 26.Neyer L E, Grünig G, Fort M, Remington J S, Rennick D, Hunter C A. Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infect Immun. 1997;65:1675–1682. doi: 10.1128/iai.65.5.1675-1682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedrosa J, Saunders B M, Appelberg R, Orme I M, Silva M T, Cooper A M. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577–583. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remington J S, Desmonts G. Toxoplasmosis, P. 89–195. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. W. B. Philadelphia, Pa: Saunders Co.; 1990. [Google Scholar]
- 29.Rogers H W, Unanue E R. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romani L, Bistoni F, Puccetti P. Initiation of T-helper cell immunity to Candida albicans by IL-12: the role of neutrophils. Chem Immunol. 1997;68:110–135. doi: 10.1159/000058688. [DOI] [PubMed] [Google Scholar]
- 31.Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol. 1997;158:2356–2362. [PubMed] [Google Scholar]
- 32.Romani L, Mencacci A, Cenci E, Spaccapelo R, Del Sero G, Nicoletti I, Trinchieri G, Bistoni F, Puccetti P. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–5356. [PubMed] [Google Scholar]
- 33.Sayles P C, Johnson L L. Exacerbation of toxoplasmosis in neutrophil depleted mice. Nat Immun. 1997;15:249–258. [PubMed] [Google Scholar]
- 34.Sayles P C, Johnson L L. Intact immune defenses are required for mice to resist the ts-4 vaccine strain of Toxoplasma gondii. Infect Immun. 1996;64:3088–3092. doi: 10.1128/iai.64.8.3088-3092.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scharton-Kersten T, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1–13. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scharton-Kersten T M, Wynn T A, Denkers E Y, Bala S, Showe L, Grunvald E, Hieny S, Gazzinelli R T, Sher A. In the absence of endogenous IFN-γ mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 37.Snijders A, Kalinski P, Hilkens C M U, Kapsenberg M L. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 38.Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, Launois P, Milon G, Louis J. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol. 2000;165:2628–2636. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 39.Vella A T, Pearce E J. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly through an early, transient, Th0-like stage. J Immunol. 1992;148:2283–2290. [PubMed] [Google Scholar]
- 40.Vieira P L, De Jong E C, Wierenga E A, Kapsenberg M L, Kalinski P. Development of Th1-inducing capacity of myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe K, Noda K, Hamano S, Koga M, Kishihara K, Nomoto K, Tada I. The crucial role of granulocytes in the early host defense against Strongyloides ratti infection in mice. Parasitol Res. 2000;86:188–193. doi: 10.1007/s004360050030. [DOI] [PubMed] [Google Scholar]
- 42.Yap G S, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J Immunol. 1998;160:1340–1345. [PubMed] [Google Scholar]