Abstract
Background
Soil-transmitted helminthes (STHs) are the major public health problems that affect the health of pregnant women and their incoming newborns. In Ethiopia, about 33.35% of pregnant women were affected by these infections. Utilization of deworming medication during pregnancy is the main strategy endorsed by the World Health Organization (WHO) to reduce the burden of STH-induced anemia and its related complications. However, information related to the coverage and its individual as well as community-level factors on the utilization of deworming medication among pregnant mothers with at least one antenatal care (ANC) visit is limited in Ethiopia.
Methods
A nationwide population-based cross-sectional study was conducted from January 18 to June 27, 2016. The information was obtained from the 2016 Ethiopian Demographic Health Survey (EDHS 2016), which can be accessed at: https://www.dhsprogram.com. A weighted sample of 4690 pregnant women selected using a two-stage stratified cluster sampling technique was included in the final analysis. A Multi-variable multilevel binary logistic regression model was fitted to identify the determinants of the utilization of deworming medication during pregnancy. Log-likelihood ration (LLR), deviance and Akaike’s Information Criterion (AIC) were used to select the best fitted model in the multilevel analysis. Statistical significance was declared at p-value <0.05.
Result
From a total of 4690 mothers included in the final analysis, only 365 (7.8%) of them utilized deworming medication in pregnancy. After controlling for confounding effects, having four or more Antenatal care (ANC) visits, having functional working status, intake of iron folic acid (IFA) tablets and coming from a community with a low poverty level increases the odds of utilization of deworming medication during pregnancy.
Conclusion and recommendation
In this study, less than one in ten pregnant mothers takes deworming medication. Mothers with less than four ANC visits, who did not receive IFA tablets, who came from a community with a high poverty level, and mothers with no good functional status were at the greatest risk of not receiving deworming medication during pregnancy. Sustained efforts need to be undertaken to increase the socioeconomic status of the community and to scale up the health care utilization behaviors of pregnant mothers.
1. Introduction
Soil transmitted helminthes (STH) infections are common public health problems that impacts the health of reproductive age women’s and children’s [1, 2]. More than 1.5 billion people (24%) of the world’s population were infected by STH infection, with the greatest number occurring in low and middle-incomes [1–3]. Women of reproductive age, preschool-age children, and school-age children have been identified as the three highest risk groups for STH-related morbidities and death [2, 4]. In this aspect, pregnant women are particularly vulnerable to hookworms, and it is estimated that each year, approximately 44 million pregnancies are affected by STH globally [5]. Similarly, an estimated 30% and 70% of pregnant women in Sub-Saharan Africa and in Ethiopia, respectively are also affected by soil-transmitted helminthes infections [6, 7].
Helminthiasis infection during pregnancy poses a serious threat to the health of the mother, the fetus, and their newborns [6, 8–11]. For instance, STHs infections are the leading cause of iron deficiency anemia (IDA) during pregnancy [5, 12]. Over 50% of pregnant women in low and middle income countries (LMIC) suffer from IDA [13–15]. Anemia during pregnancy simultaneously leads to infection, stillbirth/miscarriage, and adverse poor feto-maternal outcomes including preterm birth, low birth weight, and increased neonatal or child mortality [13, 14, 16–18]. In this regard, Ethiopia has been registered as one of the top five countries in the world with the highest neonatal mortality rate (33/1000 live births), which in turn accounts for 58% of Ethiopia’s disability-adjusted life years (DALYs) in 2022 [19, 20]. A lack of iron also hinder the babies’ mental abilities and development as well as their physical growth [21, 22].
The World Health Organization (WHO), therefore, highly recommends administration of a single dose of albendazole (400 mg) or mebendazole (500 mg) coupled with hygiene education to pregnant mothers in order to prevent STH-related complications for the mother as well as the incoming neonate [23–25]. In this aspect, WHO had set a target to attain 75% coverage of deworming for pregnant women in 2030 [26, 27]. Deworming drugs are cheap, safe for pregnancy, and effective in reducing morbidity and mortality related to soil-transmitted helminthes [28–30]. Similarly, a large-scale follow-up study also noted that deworming can result in a 14% and 11% decrease in the risk of neonatal mortality and low birth weight, respectively [31]. In addition, deworming pregnant women and children significantly increases their hemoglobin level and nutritional status, which indirectly results in a huge reduction in maternal and neonatal mortality [32–34]. Large-scale anti-helminthic drug distribution programs, on the other hand, were entirely focused on deworming preschool and school-age children’s. In line with this, currently available research shows that the majority of pregnant women did not receive deworming medication throughout their pregnancy. For instance, only 23% (95% CI 19–28%) of pregnant women in 49 STH endemic countries were dewormed during their routine antenatal care follow-up time [35]. Similar studies conducted in Cameroon also reported that only 29.8% of pregnant married women received deworming medications [36]. Indeed, a number of factors affect the utilization of deworming medication during pregnancy [35–38]. Women’s educational status, age, occupation, economic status, exposure to media, and having optimal ANC visits were a few of them.
Although deworming is the mainstay control strategy for STH-induced anemia and its related complications in pregnancy, data regarding the coverage and individual as well as community-level factors affecting the utilization of deworming medication among pregnant mothers with antenatal care (ANC) in Ethiopia is very limited. Determining the coverage as well as the individual and community level predictors of utilization of deworming medication in pregnant mothers with at least one ANC visit in Ethiopia will have a paramount role in improving national deworming status and averting the complications of STH in pregnancy.
2. Methods
2.1. Study design, area and period
A nationally representative population-based cross-sectional study was conducted from January 18 to June 27, 2016, in Ethiopia using the fourth round of the Demographic Health Survey (EDHS 2016). Ethiopia is the second most populous country in Africa, with a total population of over 117 million [39]. It has a total area of 1,100,00 km2 and lies between latitudes 3° and 15° N and longitudes 33° and 48° E. Ethiopia has nine administrative regions and two administrative cities (Fig 1). The country is also subdivided into 68 zones, 817 districts, and 16,253 kebeles (the lowest administrative unit in the country). Keble’s are further subdivided into census Enumeration Areas (EAs) or clusters, which serve as a sampling frame for surveys. The 2016 survey includes all nine regions and two town administrations in Ethiopia.
Fig 1. ArcMap of the study areas used to assess utilization status of deworming medication among pregnant mothers in Ethiopia, 2022.
2.2. Data sources and populations
This study used data from the 2016 Ethiopia Demographic Health Survey (EDHS), which can be obtained from the DHS database www.meauredhs.com. All pregnant women in the last 05 years preceding the survey were the source population, whereas all pregnant mothers with ANC follow up in the last 05 years preceding the survey in the selected EAs were the study populations.
2.3. Sample size determination and sampling technique
In the EDHS 2016 survey, a total of 15,683 households (HHs) had eligible women in reproductive groups, and 10,641 under-five children were found in these HHs. In the case of two or more under-five children per household, the most recent births were only considered. Mothers with no ANC follow-up and unknown records of utilization of deworming medication were excluded in the study. Finally, a total of 4658(weighted sample of 4690) women were included in the final analysis.
Regarding the sampling technique, a two-stage stratified cluster sampling design was used to select the final eligible participants. In the first stage, 645 (202 from urban and 443 from rural areas) EAs were randomly selected using a probability proportional to size (PPS) method from the lists of 84915 EAs generated from the 2007 population and housing census. In the second stage, a fixed number of 28 households per cluster were chosen with an equal probability systematic random sampling technique. The detailed procedure regarding the sampling procedure can be obtained from the EDHS, 2016 PDF report [40].
2.4. Variables of the study
2.4.1. Dependent/outcome variable
The outcome variable in this study was deworming utilization status of pregnant women’s with at least one ANC follow up. It was categorized into binary (yes/ no) categories.
2.4.2. Explanatory/independent variables
The factors of deworming utilization status were broadly categorized into individual and community-level factors. The individual level variables includes age of the mother, educational status of women and husband, marital status, education of, working, household wealth index, distance to health facility, media exposure, sex of the head of the HH, decision maker status of the women, number of ANC, birth order, IFA intake status and total number of children’s in the HHs.
On the other hand, factors including region, residence, distance to health facility, community poverty level, community women’s education status, and community media exposure status were considered as community level variables.
The community level factors such as community media exposure status, community educational status, community poverty were obtained by aggregating individual-level characteristics at the community (cluster) level. Categorization of the aggregate variables was done as high or low based on the nature of the distribution of the data (using either median or mean).
2.5. Operational definitions
Deworming of pregnant mothers was categorized as yes when the pregnant mother takes at least a single dose of albendazole (400 mg) or mebendazole (500 mg) [24]. In the current study, ANC utilization is considered "yes" when women visit a health facility at least once during their last pregnancy, otherwise not. Community poverty status is defined as the proportion of households in the poorer or poorest quintile of each cluster [40]. Community women’s educational level is also defined as the proportion of women who have completed at least a primary level of education in each cluster [40]. Community women’s educational level is also defined as the proportion of women who have completed at least a primary level of education in each cluster [40]. In addition, distance to health facility was defined as the proportion of households with big problem of distance to health facility with each cluster [40]
A decision maker is defined as a person who usually decides on a respondent’s health care utilization. These may include the respondents themselves, their partner/husband, mother-in-law, father-in-law, and someone else.
2.6. Data processing and analysis
After the extraction of relevant data, further cleaning, coding, and analysis were done using Microsoft Excel 2013 and STATA V.16 software. A weighted sample using the recommended weighting factor was used to ensure the representativeness of the survey by reducing sampling variability. Summary measures of the variables were obtained using frequency as well as mean or median, depending on the nature of the data. Since the EDHS data had a hierarchical nature (pregnant women’s were nested within a cluster), a model which handles the dependence of observations within a cluster was fitted to identify predictors of deworming among pregnant mothers. Therefore, a multilevel binary logistic regression analysis was computed after observing the value of intra-class correlation (ICC > 5%). First, bi-variable multilevel binary logistic regression models were fitted, and all variables with a p-value less than or equal to 0.25 were fitted to the final model. After recruiting variables for multi-variable analysis, four models were fitted, sequentially: the null model (a model without explanatory variables is used to test random variability in the intercept and to estimate the intra-class correlation coefficient); model 1(containing only individual-level factors); model 2 (containing only community-level factors); and model 3 (which comprises both individual and community-level factors). The log likelihood ratio (LLR), device, and Akaike Information Criterion (AIC) were used to compare and select the best model. The model that has the highest log likelihood or lowest deviance and AIC was taken as the best model.
In addition, the total measure of variation (random effect) of utilization of deworming medication was assessed using ICC, proportional change in variance (PCV), and the median odds ratio (MOR). The MOR was used to determine the heterogeneity between clusters (the second-level variation) by comparing two people from two randomly selected clusters. Proportional change in variance (PCV) is used to assess the total variation attributed to individual and community level factors in the multilevel model as compared to the null model.
Finally, as adjusted odds ratios (AOR) with 95% confidence intervals (CI) was reported in the multi-variable multilevel-logistic regression model and statistical significance was declared at p-value< 0.05. The presence of multi-collinearity among independent variables was checked using a variance inflation factor (VIF>10) through running a pseudo-linear regression analysis.
2.7. Ethical considerations
Informed consent from the participants was waived because the study used secondary (2016 EDHS data). Permission to get access to download DHS data was obtained from the major DHS data archivist through a reasonable online request using http://www.dhsprogram.com. In the DHS data, there are no names of individuals or household addresses. The information retrieved was only used for statistical reporting and analysis of our registered research. The EDHS report included detailed information on the procedure for ensuring the ethical issue.
3. Result
3.1. Individual and community level socio-demographic variables
Related to the socio-demographic profiles of the participants, the majority of them were married (4360, or 93.0%), aged between 25 and 34 (2439, or 52%), lived in rural areas (3823, or 81.5%), and did not attend formal education (2519, or 53.7%). The median age of the mothers was 28 years, with an inter-quartile range (IQR) of 33–24 years. Regarding community level variables, 2,833 (60.4%) of the respondents were from the community with low educational status. In addition, nearly half (2,115, or 45.1%) of the respondents came from the community with a high community poverty level (Table 1).
Table 1. Socio-demographic and economic characteristics of respondents in 2016 EDHS, Ethiopia (weighted N = 4690).
| Variables | Category | Deworming utilization status | Total weighted frequency | % | |
|---|---|---|---|---|---|
| (N = 4690) | Yes (N = 365) | No (4325) | |||
| Head of the HHs | Male | 311 | 3695 | 4006 | 84.4 |
| Female | 54 | 630 | 684 | 14.6 | |
| Age category | Age 15–24 | 82 | 1143 | 1,225 | 26.1 |
| Age 25–34 | 219 | 2,229 | 2,439 | 52.0 | |
| Age 35 and above | 74 | 952 | 1,026 | 21.9 | |
| Median age = 28 years (IQR = 33–24) | |||||
| Religion | Orthodox | 179 | 1,819 | 1,998 | 42.6 |
| Moslem | 103 | 1,423 | 1,526 | 32.5 | |
| Protestant | 73 | 974 | 1,047 | 22.3 | |
| catholic/traditional/other | 10 | 109 | 119 | 2.6 | |
| Marital status | Married/ in union | 333 | 4,027 | 4,360. | 93.0 |
| Not-married/not in union | 32 | 298 | 330 | 7.0 | |
| Educational status of the respondent | No formal education | 180 | 2,339 | 2519 | 53.7 |
| Primary education | 136 | 1,423 | 1559 | 33.3 | |
| Secondary | 34 | 351 | 385 | 8.2 | |
| Tertiary and above | 15 | 212 | 227 | 4.8 | |
| Educational status of the husband | No formal education | 125 | 1,622 | 1,747 | 37.2 |
| Primary education | 150 | 1,649 | 1,799 | 38.3 | |
| Secondary | 41 | 467 | 508 | 10.8 | |
| Tertiary and above | 25 | 310 | 335 | 7.2 | |
| I don’t know | 3 | 20 | 23 | 0.5 | |
| Working status of the respondent | Working | 214 | 2,982 | 3,196 | 68.1 |
| Not working | 151 | 1,343 | 1,494 | 31.9 | |
| Region | Tigray | 46 | 433 | 479 | 10.2 |
| Afar | 4 | 33 | 37 | 0.8 | |
| Amhara | 96 | 973 | 1,069 | 22.8 | |
| Oromia | 122 | 1,450 | 1,572 | 33.5 | |
| Somali | 3 | 112 | 115 | 2.5 | |
| Benishangul | 6 | 50 | 56 | 1.2 | |
| SNNPR | 72 | 1,043 | 1,115 | 23.8 | |
| Gambela | 1 | 14 | 15. | 0.3 | |
| Harari | 1 | 12 | 1 3 | 0.3 | |
| Addis Abeba | 11 | 181 | 191 | 4.1 | |
| Dire Dewa | 3 | 25 | 28 | 0.6 | |
| Residence | Urban | 74 | 793 | 867 | 18.5 |
| Rural | 291 | 3,532 | 3,823 | 81.5 | |
| Poverty status of the HHs | Poor | 111 | 1,582 | 1,693 | 36.1 |
| Middle | 84 | 884 | 968 | 20.6 | |
| Rich | 170 | 1,859 | 2,029 | 43.3 | |
| Media exposure status | Not exposed | 160 | 2,527 | 2,687 | 57.3 |
| Exposed | 205 | 1,798 | 2,003 | 42.7 | |
| Community poverty level | Poor | 110 | 2,005 | 2,115 | 45.1 |
| Rich | 255 | 2,320 | 2,575 | 54.9 | |
| Community education Status | Low | 205 | 2,628 | 2,833 | 60.4 |
| High | 160 | 1,697 | 1,857 | 39.6 | |
| Community distance to Health | Big problem | 195 | 2,520 | 2,715 | 57.9 |
| Not big problem | 170 | 1,805 | 1,975 | 42.1 | |
| Community media Exposure | Low | 169 | 2,491 | 2660 | 52.5 |
| High | 195 | 1,833 | 2,229 | 47.5 | |
IQR = Inter-quartile range, HH- Households.
3.2. Maternal characteristics
Regarding the ANC utilization status of the mothers, only half (2354 or 50.2%) of them had four or more ANC visits throughout their pregnancy. Additionally, more than one third (1890, or 40.3%) of mothers didn’t receive IFA tablets during pregnancy. One fourth (669, or 14.3%) of the mothers were able to decide solely on their own health care needs, while the rest depended on their husbands or someone else (Table 2).
Table 2. Maternal characteristics result of respondents in 2016 EDHS, Ethiopia.
| Variables | Category | Deworming utilization status | Total weighted frequency | % | |
|---|---|---|---|---|---|
| (N = 4690) | |||||
| Yes (N = 365) | No (4325) | ||||
| ANC visit | ANC1 | 16 | 371 | 387 | 7.6 |
| ANC 2 and 3 | 130 | 1847 | 1,977 | 42.2 | |
| ≥ ANC4 | 219 | 2,106 | 2,325 | 50.2 | |
| IFA intake status | Yes | 297 | 2503 | 2,800 | 59.7 |
| No | 68 | 1822 | 1,890 | 40.3 | |
| Birth order | 1st | 82 | 1023 | 1,105 | 23.8 |
| 2nd -3rd | 114 | 1397 | 1,511 | 32.2 | |
| 4+ | 159 | 1915 | 2,074 | 44.0 | |
| Total living children | 0–3 | 232 | 2575 | 2807 | 59.9 |
| ≥ 04 | 133 | 1,750. | 1883 | 40.1 | |
| Decision maker | Respondent | 67 | 602 | 669 | 14.3 |
| Other | 298 | 3723 | 4,021 | 85.7 | |
Others- Partner/ husband, mother in law, father in law, someone else: IFA- Iron Folic Acid intake.
3.3. Deworming utilization status
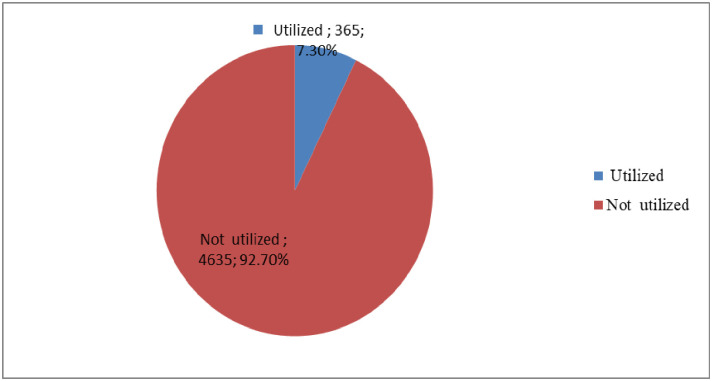
In the current study, less than one in ten (7.8%: 95% CI 5.1–10.5%) pregnant women’s utilize deworming medication (Fig 2).
Fig 2. Utilization status of deworming medication among pregnant mothers with at least one ANC visit in Ethiopia, 2022.
3.4. Multilevel binary logistic regression model analysis
As shown in the table below (Table 3), the results of intra class correlation (ICC) in the empty mode showed 23% of the total variation in utilization of deworming medication during pregnancy was attributed to the difference in the community or cluster. Therefore, multilevel (two-level) binary logistic regression model which handles the dependency or correlation of observation with in a cluster was fitted In the multilevel binary logistic regression model, the full model (model III) containing both the individual and community level variables was considered as the best fit model because it has the lowest deviance and AIC value (deviance = 2308, AIC = 2338.237, p-value = 0.0001). The presence of heterogeneity between clusters was also explained by the Median Odds Ratio (MOR). In the full model, the MOR was 2.5, which implies that the odds of utilization of deworming medications was 2.5 among mothers who came from a community with high to low proportion of deworming utilization status. The proportional change in variance (PCV) in the final model also indicates that 2.4% of the variation in the utilization of deworming medication among pregnant mothers observed was explained by both community and individual-level variables.
Table 3. Multi-variable multilevel binary logistic regression analysis result of both community and individual level factors associated with utilization of deworming medication in pregnant mothers in Ethiopia, EDHS 2016.
| Community and individual level factors | Models | |||
|---|---|---|---|---|
| Null model | Model I | Model II | Model III | |
| (AOR 95%CI) | (AOR 95%CI) | (AOR 95%CI) | (AOR 95%CI) | |
| Marital status | ||||
| Married/ in union | 1 | 1 | ||
| Not married | 1.24 (0.69–2.24) | 1.28 (0.71–2.30) | ||
| Religion | ||||
| Orthodox | 1 | 1 | ||
| Muslim | 1.23 (0.77–1.98) | 1.20 (0.76–1.89) | ||
| Protestant | 1.12 (0.69–1.84) | 1.10 (0.67–1.78) | ||
| catholic/traditional/other | 1.83(0.52–6.51) | 1.77 (0.51–6.13) | ||
| Working status of the respondent | ||||
| Not working | 1 | 1 | ||
| Working | 1.56 (1.09–2.24) | 1.57 (1.09–2.26)* | ||
| Media exposure | ||||
| Yes | 1.49 (1.01–2.03) | 1.6 (0.97–2.19) | ||
| No | 1 | 1 | ||
| ANC visit | ||||
| ANC 1 | 1 | 1 | ||
| ANC 2 to 3 | 1.99 (0.76–5.21) | 1.983 (0.75–6.51) | ||
| ≥ ANC 4 | 2.85 (1.05–7.74) | 2.83 (1.03–7.79) * | ||
| IFA status | ||||
| No | 1 | 1 | ||
| Yes | 3.35 (2.11–5.33) | 3.42 (2.15–5.43)* | ||
| Decision maker | ||||
| Respondent | 1 | 1 | ||
| Others | 0.67 (0.41–1.11) | 0.67 (0.41–1.83) | ||
| Community education level | ||||
| Low | 1 | 1 | ||
| High | 0.91(0.65–1.28) | 0.76 (0.51–1.13) | ||
| Distance to Health facility | ||||
| Big problem | 1 | 1 | ||
| Not Big problem | .89(0.64–1.24) | 0.72 (0.50–1.52) | ||
| Community poverty level | ||||
| High | 1 | 1 | ||
| Low | 1.95 (1.35–2.84) | 1.84 (1.23–2.75)* | ||
| Measure of random effect (variation) | ||||
| Variance | .9913999 | 0.97654 | 0.8624397 | 0.9688124 |
| ICC (%) | 23.2% | 23.1% | 20.8% | 22.8% |
| PVC (%) | Reference | 1.5% | 13% | 2.3% |
| MOR | 2.57 | 2.55 | 2.4 | 2.5 |
| Model fitness | ||||
| Log likelihood | -1226.4761 | -1159.74 | -1218.96 | -1154.1187 |
| Deviance | 2452.95 | 2319.48 | 2437.92 | 2308 |
| AIC | 2456.952 | 2343.489 | 2449.92 | 2338.237 |
1- Reference category, AOR-adjusted odds ratio, CI- confidence interval, * and old letter—statistically significant variables in the full model.
3.4.1. Determinants of deworming utilization status
Variables with a P-value of <0.25 in the bi-variable multilevel binary logistic regression model were fitted into the final multivariable multilevel binary logistic regression model. In this regard, variables like religion, marital status, working status, ANC visit level, IFA intake status, decision maker on women’s health care needs, household media exposure status, community poverty level, community education level, and distance to the health facility level were computed in the full model (model III). Finally, working status, ANC visit level, IFA intake status, and community poverty level were identified as significant predictors of utilization of deworming among pregnant mothers.
In comparison to their counterparts, pregnant women who took IFA tablets were 3.42 (AOR 3.42, 95% CI: 2.15–5.4) times more likely to use deworming medication In addition, the odds of utilization of deworming medication among pregnant mothers with functional working status was 1.57 times higher (AOR 1.57, 95%CI: 1.09–2.26) when compared with mothers not working status. The utilization of deworming medication in pregnant mothers was 2.83 times (AOR 2.83, 95%CI: 1.03–7.79) more likely in mothers with four or more ANC visits compared with those mothers with only a single ANC visit. The odds of deworming in pregnant mothers who came from a community with a low poverty level was 1.84 (95%CI: 1.23–2.75) times higher than their counterparts.
4. Discussion
Deworming pregnant women is the main strategy endorsed by world health organization to reduce the burden and associated complications of STHs. This study was undertaken to investigate the coverage and its community as well as individual level factors of utilization of deworming medication among pregnant mothers with ANC follow-up in Ethiopia. In this study, the coverage of utilization of deworming medication among pregnant mothers was found to be 7.8%. This figure is significantly lower when compared with prior studies conducted in 49 STH endemic countries in the world, in Ghana, Cameron and Tanzania [35–38]. The discrepancy in the proportion of utilization of deworming medication in pregnancy could be due to variation in the time when the DHS data is collected [37, 40, 41], the difference in socioeconomic status, and/or variation in terms of maternal health care utilization status across these countries [42–46]. The very low figure in Ethiopia will further challenge the country’s efforts to reduce neonatal and mortality rates to the WHO threshold level.
In this study, the intake of IFA tablet during pregnancy was found to be significantly associated with the utilization of deworming medication. Those pregnant mothers who received IFA tablets were 3.42 (AOR 3.42, 95% CI: 2.15–5.4) times more likely to utilize deworming medications compared to their counter parts. The possible elucidation for the observed association might be due to the fact that those mothers took IFA were more likely be educated, receive intensive counseling and had optimal ANC visits which all affects the utilization of deworming medication [30, 47].
Deworming medication use during pregnancy is also 2.83 times more likely in mothers who had four or more ANC visits compared to those who had only one ANC visit. This suggests that mothers who had more ANC visits will be more likely to receive adequate information about the utilization of deworming medication during pregnancy, and they may also have enough time to access the medication during their second and third trimesters if they had not taken it during their first ANC visit. This finding is in line with a prior study conducted in Tanzania, Cameroon and in 49 STH endemic countries [35, 36, 38]. Therefore, the quality and duration of ANC services were found to have a significant role in increasing coverage of deworming medication during pregnancy.
In the current study, we also identified that women who came from a community with a low poverty level were more likely to receive deworming medications when compared with their counterparts. The observed association is in line with prior research conducted in Ghana, Tanzania and in 49 STH endemic countries [36–38]. This association could be explained by the fact that women with higher socioeconomic status have better access to health institutions, which increases their health care demands and utilization [45, 48]. Besides, women from the wealthiest communities are more likely to have optimal ANC visits, which further enhance utilization of deworming medications [49–51]. On the other hand, soil transmitted helminthic infections are more prevalent in this economically disadvantaged communities, which necessities a strong and well-organized interventions [52].
Finally, this study also identified that pregnant women with working functional status are almost twice more likely to take deworming medication than their counterparts. This could be explained by the fact that women who do have functional work experience are more likely to perform their daily living activities and to visit health institutions whenever they are in need of health services [53]. In addition, women with working status can easily earn money so that they can afford any payments for their healthcare needs.
4.1. Strengths and limitations of study
The major strength of this study is the use of nationally representative data. In addition, the methodology used in the DHS survey increases the generalizability and representativeness of the data. Using multilevel logistic regression analysis, which can easily identify both individual and community-level variables, also plays a pivotal role in designing interventions that target such factors. On the other hand, we would like to remind you that our findings should be considered in light of the following limitations: First, the effect of important variables like the availability of deworming drugs, the level of counseling, and the perception and attitude of mothers towards deworming medications were not investigated. Secondly, due to the retrospective nature of the response, recall bias might be introduced.
4.2. Implication of the result of this study
The very low uptake of deworming medication among pregnant mother in this study implies that a substantial number of mothers were affected by STH-related complications, which further increase neonatal and maternal mortality. Therefore, maternal and newborn health programs should give great emphasis towards improving the utilization of deworming medication during pregnancy.
5. Conclusions
The finding of this study shows that less than one in ten pregnant mothers with at least one ANC visit utilize deworming medication, which is too far from WHO 2030 target. Mothers with less than four ANC visits, not taking IFA tablets, came from a community with high poverty level and mothers with not good functional status were at most risk for not receiving deworming medication during pregnancy. Sustained effort need to be undertaken to increase the socioeconomic status of the community and to scale up health care utilization behaviors of pregnant mothers.
Acknowledgments
The authors would like to provide our profound thanks to the Demographic and Health Survey (DHS) data archivist who allows accessing the EDHS 2016 data.
Abbreviations
- AIC
Akakies information criteria
- ANC
Antenatal care
- AOR
Adjusted odds ratio
- CI
Confidence interval
- DHS
Demographic Health Survey
- EDHS
Ethiopia Demographic Health Survey
- HHs
Households
- ICC
Intra cluster correlation
- IFA
Iron Folic Acid tablet
- IQR
inter quartile range
- MOR
Median Odds Ratio
- PCV
Proportion of change
- STH
Soil-transmitted helminthes
- WH0
World Health Organization
Data Availability
All data that have been used to support the conclusion of this study are available in the manuscript the manuscript.
Funding Statement
The authors did not receive any funds to undergo this study.
References
- 1.World Health Organizations(WHOs), health topics on Soli transmitted infections, https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections, Cited on [11/28/2022].
- 2.Jourdan PM, Lamberton PH, Fenwick A, Addiss DG: Soil-transmitted helminth infections. The Lancet 2018, 391(10117):252–265. doi: 10.1016/S0140-6736(17)31930-X [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Health of India, National Guidelines for Deworming in Pregnancy, December 2014, http://www.nrhmorissa.gov.in/writereaddata/Upload/Documents/_National_Guidelines_for_Deworming_in_Pregnancy.pdf.
- 4.Amare Y. Umbilical cord care in Ethiopia and implications for behavioral change: a qualitative study. BMC international health and human rights 2014, 14(1):1–8. doi: 10.1186/1472-698X-14-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salam RA, Das JK, Bhutta ZA. Effect of mass deworming with antihelminthics for soil‐transmitted helminths during pregnancy. Cochrane Database of Systematic Reviews 2021(5). doi: 10.1002/14651858.CD005547.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrison A, Boivin M, Khoshnood B, Courtin D, Alao J, Mireku M, et al. Soil-transmitted helminth infection in pregnancy and long-term child neurocognitive and behavioral development: a prospective mother-child cohort in Benin. PLoS neglected tropical diseases 2021, 15(3):e0009260. doi: 10.1371/journal.pntd.0009260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feleke BE, Jember TH. Prevalence of helminthic infections and determinant factors among pregnant women in Mecha district, Northwest Ethiopia: a cross sectional study. BMC infectious diseases 2018, 18(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steketee RW. Pregnancy, nutrition and parasitic diseases. The Journal of nutrition 2003, 133(5):1661S–1667S. doi: 10.1093/jn/133.5.1661S [DOI] [PubMed] [Google Scholar]
- 9.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. The lancet 2006, 367(9521):1521–1532. doi: 10.1016/S0140-6736(06)68653-4 [DOI] [PubMed] [Google Scholar]
- 10.Parija SC, Chidambaram M, Mandal J. Epidemiology and clinical features of soil-transmitted helminths. Tropical parasitology 2017, 7(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil transmitted helminths during pregnancy. Cochrane Database of Systematic Reviews 2009(2). doi: 10.1002/14651858.CD005547.pub2 [DOI] [PubMed] [Google Scholar]
- 12.Gyorkos TW, Gilbert NL. Blood drain: soil-transmitted helminths and anemia in pregnant women. PLoS neglected tropical diseases 2014, 8(7):e2912. doi: 10.1371/journal.pntd.0002912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Renzo GC, Spano F, Giardina I, Brillo E, Clerici G, Roura LC. Iron deficiency anemia in pregnancy. Women’s Health 2015, 11(6):891–900. doi: 10.2217/whe.15.35 [DOI] [PubMed] [Google Scholar]
- 14.Garzon S, Cacciato PM, Certelli C, Salvaggio C, Magliarditi M, Rizzo G. Iron deficiency anemia in pregnancy: novel approaches for an old problem. Oman Medical Journal 2020, 35(5):e166. doi: 10.5001/omj.2020.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goonewardene M, Shehata M, Hamad A. Anaemia in pregnancy. Best practice & research Clinical obstetrics & gynaecology 2012, 26(1):3–24. doi: 10.1016/j.bpobgyn.2011.10.010 [DOI] [PubMed] [Google Scholar]
- 16.Teshale AB, Tesema GA, Worku MG, Yeshaw Y, Tessema ZT. Anemia and its associated factors among women of reproductive age in eastern Africa: A multilevel mixed-effects generalized linear model. Plos one 2020, 15(9):e0238957. doi: 10.1371/journal.pone.0238957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-Ouf NM, Jan MM. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi medical journal 2015, 36(2):146–149. doi: 10.15537/smj.2015.2.10289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mawani M, Ali SA, Bano G, Ali SA. Iron deficiency anemia among women of reproductive age, an important public health problem: situation analysis. Reproductive System & Sexual Disorders: Current Research 2016, 5(3):1. [Google Scholar]
- 19.Ethiopian Public Health Institute (EPHI), ICF, Ethiopia mini demographic and health survey 2019: Final Report, 2021, https://dhsprogram.com/pubs/pdf/FR363/FR363.pdf.
- 20.World Health organizations (WHO), Global health estimates: Leading causes of DALYs, Top 10 causes of DALY in Ethiopia for both sexes aged all ages, https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/global-health-estimates-leading-causes-of-dalys.
- 21.Moos T, Skjørringe T, Thomsen LL. Iron deficiency and iron treatment in the fetal developing brain–a pilot study introducing an experimental rat model. Reproductive Health 2018, 15(1):117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annual review of nutrition 2002, 22(1):35–59. doi: 10.1146/annurev.nutr.22.120501.134539 [DOI] [PubMed] [Google Scholar]
- 23.Organization WH. WHO recommendations on newborn health: guidelines approved by the WHO Guidelines Review Committee. In.: World Health Organization; 2017.
- 24.Organization WH. Guideline: preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups: World Health Organization; 2017. [PubMed]
- 25.Organization WH. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee: World Health Organization; 2002. [PubMed]
- 26.World Health organization(WHO), Neglected tropical diseases. 2030 targets for soil-transmitted helminthiases control programmes. Geneva: World Health Organization; 2019. License: CC BY-NC-SA 3.0IGO.
- 27.WHO. Soil-transmitted helminth infections, 2020. https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections. cited on 11/28/2022.
- 28.Bangert M, Bancalari P, Mupfasoni D, Mikhailov A, Gabrielli AF, Montresor A. Provision of deworming intervention to pregnant women by antenatal services in countries endemic for soil-transmitted helminthiasis. PLoS neglected tropical diseases 2019, 13(5):e0007406. doi: 10.1371/journal.pntd.0007406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Organization WH: Guideline: preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups: World Health Organization; 2017. [PubMed]
- 30.Lau R, Chris RB, Phuong MS, Khatib A, Kopalakrishnan S, Bhasker S, et al. Treatment of soil-transmitted helminth infections in pregnancy: a systematic review and meta-analysis of maternal outcomes. Journal of Travel Medicine 2020, 27(2):taz079. doi: 10.1093/jtm/taz079 [DOI] [PubMed] [Google Scholar]
- 31.Walia B, Kmush BL, Lane SD, Endy T, Montresor A, Larsen DA. Routine deworming during antenatal care decreases risk of neonatal mortality and low birthweight: a retrospective cohort of survey data. PLOS Neglected Tropical Diseases 2021, 15(4):e0009282. doi: 10.1371/journal.pntd.0009282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salam R, Cousens S, Welch V, Gaffey M, Middleton P, Makrides M, et al. Mass deworming for soil‐transmitted helminths and schistosomiasis among pregnant women: A systematic review and individual participant data meta‐analysis. Campbell Systematic Reviews 2019, 15(3):e1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larocque R, Gyorkos TW. Should deworming be included in antenatal packages in hookworm-endemic areas of developing countries? Canadian journal of public health 2006, 97(3):222–224. doi: 10.1007/BF03405590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atukorala T, De Silva L, Dechering W, Dassenaeike T, Perera RS. Evaluation of effectiveness of iron-folate supplementation and anthelminthic therapy against anemia in pregnancy—a study in the plantation sector of Sri Lanka. The American journal of clinical nutrition 1994, 60(2):286–292. doi: 10.1093/ajcn/60.2.286 [DOI] [PubMed] [Google Scholar]
- 35.Zegeye B, Keetile M, Ahinkorah BO, Ameyaw EK, Seidu A-A, Yaya S. Utilization of deworming medication and its associated factors among pregnant married women in 26 sub-Saharan African countries: a multi-country analysis. Tropical Medicine and Health 2021, 49(1):53. doi: 10.1186/s41182-021-00343-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zegeye B, Ahinkorah BO, Ameyaw EK, Seidu A-A, Yaya S. Utilization of deworming drugs and its individual and community level predictors among pregnant married women in Cameroon: a multilevel modeling. BioMed Research International 2021, 2021. doi: 10.1155/2021/6645336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lumor O, Dzabeng F, Adanu RM. Factors influencing the use of anemia preventing measures among antenatal clinic attendees in the Kintampo North Municipality, Ghana. African journal of reproductive health 2019, 23(2):35–43. [DOI] [PubMed] [Google Scholar]
- 38.Bankanie V, Moshi FV. Factors associated with the use of deworming drugs during pregnancy in Tanzania; an analysis from the 2015–16 Tanzanian HIV and malaria indicators survey. BMC Pregnancy and Childbirth 2022, 22(1):1–9. doi: 10.1186/s12884-021-04291-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demographics of Ethiopia, Total population in Ethiopia, https://en.wikipedia.org/wiki/Demographics_of_Ethiopia.
- 40.Agency(Csa) Cs. Central Statistical Agency (CSA) [Ethiopia] and ICF. Ethiopia Demographic and Health Survey, Addis Ababa. Central Statistical Agency 2016.
- 41.National Institute of Statistics (Cameroon) and ICF. Cameroon DHS Summary Report. Rockville: NIS and ICF; 2018. p. 2020.
- 42.Addisu D, Mekie M, Melkie A, Abie H, Dagnew E, Bezie M, et al. Continuum of maternal healthcare services utilization and its associated factors in Ethiopia: A systematic review and meta-analysis. Women’s Health 2022, 18:17455057221091732. doi: 10.1177/17455057221091732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nuamah GB, Agyei-Baffour P, Mensah KA, Boateng D, Quansah DY, Dobin D, et al. Access and utilization of maternal healthcare in a rural district in the forest belt of Ghana. BMC pregnancy and childbirth 2019, 19(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orwa J, Mantel M, Mugerwa M, Brownie S, Pallangyo ES, Mwasha L, et al. Maternal healthcare services use in Mwanza region, Tanzania: a cross-sectional baseline survey. BMC pregnancy and childbirth 2019, 19(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obiyan MO, Kumar A. Socioeconomic inequalities in the use of maternal health care services in Nigeria: trends between 1990 and 2008. Sage Open 2015, 5(4):2158244015614070. [Google Scholar]
- 46.African Development Bank group, African Economic Outlook 2022, https://www.afdb.org/en/knowledge/publications/african-economic-outlook, Cited on [11/29/2022].
- 47.Sendeku FW, Azeze GG, Fenta SL. Adherence to iron-folic acid supplementation among pregnant women in Ethiopia: a systematic review and meta-analysis. BMC pregnancy and childbirth 2020, 20(1):1–9. doi: 10.1186/s12884-020-2835-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarekegn SM, Lieberman LS, Giedraitis V. Determinants of maternal health service utilization in Ethiopia: analysis of the 2011 Ethiopian Demographic and Health Survey. BMC pregnancy and childbirth 2014, 14(1):1–13. doi: 10.1186/1471-2393-14-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arthur E. Wealth and antenatal care use: implications for maternal health care utilisation in Ghana. Health economics review 2012, 2(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yehualashet DE, Seboka BT, Tesfa GA, Mamo TT, Seid E. Determinants of optimal antenatal care visit among pregnant women in Ethiopia: a multilevel analysis of Ethiopian mini demographic health survey 2019 data. Reproductive Health 2022, 19(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fagbamigbe AF, Idemudia ES. Wealth and antenatal care utilization in Nigeria: policy implications. Health care for women international 2017, 38(1):17–37. doi: 10.1080/07399332.2016.1225743 [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization(WHO), Health topics, Soil-transmitted helminthiases, Avaliableon https://www.who.int/health-topics/soil-transmitted-helminthiases#tab=tab_1, Accessed on 9/19/2022.
- 53.Birmeta K, Dibaba Y, Woldeyohannes D. Determinants of maternal health care utilization in Holeta town, central Ethiopia. BMC health services research 2013, 13(1):1–10. doi: 10.1186/1472-6963-13-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that have been used to support the conclusion of this study are available in the manuscript the manuscript.