Abstract
Many antimicrobial peptides permeabilize the bacterial cytoplasmic membrane. However, it is unclear how membrane permeabilization and antimicrobial activity are related for distinct peptides. This study investigated the relationship between Staphylococcus aureus membrane permeabilization and cell death due to the following antistaphylococcal peptides: thrombin-induced platelet microbicidal protein 1 (tPMP-1), gramicidin D, and protamine. Isogenic S. aureus strains ISP479C and ISP479R (tPMP-1 susceptible and resistant, respectively), were loaded with the fluorochrome calcein and exposed to a range of concentrations of each peptide. Flow cytometry was then used to monitor membrane permeabilization by quantifying the release of preloaded calcein. Killing was determined by quantitative culture at time points simultaneous to measurement of membrane permeabilization. Membrane permeabilization and killing caused by tPMP-1 occurred in a time- and concentration-dependent manner, reflecting the intrinsic tPMP-1 susceptibilities of ISP479C and ISP479R. In comparison, gramicidin D killed both S. aureus strains to equivalent extents in a concentration-dependent manner between 0.5 to 50 μg/ml, but cell permeabilization only occurred at the higher peptide concentrations (25 and 50 μg/ml). Protamine permeabilized, but did not kill, either strain at concentrations up to 10 mg/ml. Regression analyses revealed different relationships between membrane permeabilization and staphylocidal activity for the distinct antimicrobial peptides. Taken together, these findings demonstrate that permeabilization, per se, does not invariably result in staphylococcal death due to distinct antimicrobial peptides. Thus, although each of these peptides interacts with the S. aureus cytoplasmic membrane, diversity exists in their mechanisms of action with respect to the relationship between membrane permeabilization and staphylocidal activity.
Naturally occurring antimicrobial peptides (APs) are believed to play a major role in innate host defense against infection in species ranging from prokaryotes to humans (10, 18, 22, 35, 36). However, the mechanism(s) of action of APs, and their selective microbicidal activities for some organisms but not others, is not well understood.
Despite considerable variation in composition, structure, charge, and putative mechanism of action, many APs initially interact with and perturb microbial membranes (1, 4, 5, 16, 17, 32). In general, bacterial membrane perturbation by APs leads to a sequence of events which includes (i) loss of intracellular solutes (e.g., K+ and amino acids); (ii) dissipation of the transmembrane potential (ΔΨ); (iii) inhibition of respiration; (iv) a reduction in ATP pools; and (v) inhibition of DNA, RNA, and protein synthesis (7, 19, 24; Y.-Q. Xiong, M. R. Yeaman, S.-P. Koo, K. Gank, and A. S. Bayer, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2287, p. 132, 2000). Although the loss of metabolites probably results in the inhibition of key intracellular functions, membrane permeabilization per se has been viewed as a lethal mechanism of many APs. However, it is not yet established whether membrane permeabilization alone is sufficient to kill susceptible microorganisms or if other events and/or sites of action (such as intracellular targets beyond the membrane) are involved. Moreover, certain APs, such as buforins from bullfrogs, exhibit microbicidal activity via mechanisms that are independent of membrane permeabilization (25). Therefore, the present study was carried out to compare the relationships between membrane permeabilization and killing of Staphylococcus aureus by three diverse membrane-targeting APs: thrombin-induced platelet microbicidal protein 1 (tPMP-1), gramicidin D, and protamine.
tPMP-1 from rabbit platelets is an 8.5-kDa cationic AP that is rich in the basic residues lysine, arginine, and histidine (24% of total mass; detailed in reference 35). tPMP-1 exhibits potent microbicidal activity against common blood-borne pathogens, including Staphyococcus aureus, Staphylococcus epidermidis, viridans group streptococci, Candida albicans, and Crytococcus neoformans (29, 35). The mechanism of tPMP-1 activity appears to involve initial cytoplasmic membrane permeabilization of microorganisms in a voltage-dependent manner (15–17, 36). Furthermore, intracellular targets have been indicated to be involved in its mechanism of staphylocidal activity (33; Xiong et al., 40th ICAAC). Gramicidin D is a neutral, linear pentadecapeptide antibiotic produced by Bacillus brevis. Possessing mainly anti-gram-positive activity, this AP forms channels with specificity for monovalent metal and ammonium cations (6). Gramicidin D represents a heterogeneous mixture of six components, with gramicidin A being the most predominant (5); the structure of gramicidin A is NH2-Val-Gly-Ala-dLeu-Ala-dVal-Val-Val-Trp-dLeu-Trp-dLeu-Trp-COOH. Although several double-stranded, α-helical models have been proposed for the structure of active gramicidin D channels, there is no clear understanding of the relationship between membrane permeabilization and the bactericidal activity of this AP (30, 31). Protamine sulfate is a 34-amino-acid, highly cationic (21 arginine residues), quaternary amine isolated from fish sperm nuclei. This polypeptide has the following sequence of amino acids: NH2-Pro-Arg-Arg-Arg-Arg-Arg-Ala-Ser-Arg-Pro-Val-Arg-Arg-Arg-Arg-Arg-Ala-Arg-Arg-Arg-Ser-Thr-Ala-Arg-Arg-Arg-Arg-Arg-Val-Val-Arg-Arg-Arg-COOH. Protamine has been reported to exert broad-spectrum activity against gram-positive and gram-negative bacteria but lacks an amphiphilic structure believed necessary for channel formation (14, 20, 23). However, protamine induces cell envelope permeabilization in gram-positive and gram-negative bacteria and disrupts several membrane-associated processes (e.g., nutrient uptake) in gram-negative bacteria (1, 12, 13). Since tPMP-1, gramicidin D, and protamine display a diverse range of origins, antimicrobial activities, charges, structural profiles, and putative mechanisms of action, they were selected to facilitate the comparison of the relationship between membrane permeabilization and the killing of S. aureus.
(This study was presented, in part, at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 17 to 20 September 2000.)
MATERIALS AND METHODS
Staphylococcus aureus strains.
A stable, well-defined, and isogenic pair of tPMP-1-susceptible and -resistant S. aureus strains, ISP479C and ISP479R, were used in the present study. ISP479C and ISP479R are derived from the parental strain ISP479 (tPMP-1 susceptible), which carries the temperature-sensitive plasmid pl258, containing transposon Tn551, which encodes erythromycin resistance. ISP479C is a spontaneous, plasmid-cured variant that is tPMP-1 susceptible, while ISP479R is the stable tPMP-1-resistant, erythromycin-resistant transposon mutant derived from ISP479. Extensive phenotypic and genotypic analyses have demonstrated that these strains are identical, except for their differences in tPMP-1 susceptibility profiles (8). Cells were cultured from a −70°C stock when required and grown to mid-logarithmic phase in brain heart infusion medium (BHI; Difco Laboratories, Detroit, Mich.) at 37°C with shaking. Cells were washed once with phosphate-buffered saline (pH 7.2), sonicated briefly to ensure singlet cells (4 s), and then adjusted spectrophotometrically to an optical density at 600 nm of 1.0 (∼109 CFU/ml).
Antistaphylococcal peptides.
The antistaphylococcal APs selected for the present study were tPMP-1, gramicidin D, and protamine. tPMP-1 was purified from thrombin-stimulated rabbit platelets, as described in detail elsewhere (35). Briefly, fresh rabbit platelets were collected and stimulated with bovine thrombin in glutamine-free Eagle's minimal essential medium (pH 7.4; Irvine Scientific, Irvine, Calif.), yielding a tPMP-1-rich supernatant. Previous studies have shown that tPMP-1 is the predominant cationic staphylocidal peptide present in thrombin-stimulated rabbit platelet preparations (34, 35). tPMP-1 was then homogeneously purified by reversed-phase high-pressure liquid chromatography (RP-HPLC) from such platelet supernatants, as described previously (35). The purity of tPMP-1 was confirmed by acid-urea polyacrylamide gel electrophoresis and analytical RP-HPLC. The microbicidal activity of purified tPMP-1 was confirmed by 100% killing of 103 CFU of Bacillus subtilis ATCC 6633 (an indicator organism highly susceptible to this peptide) per ml at 37°C within 30 min, as described elsewhere (34, 35). Concentrations of purified tPMP-1 in assay buffer were determined by integration of RP-HPLC analytic chromatograms and modified Lowry protein assays (Pierce, Rockford, Ill.). Gramicidin D (purity, >95%) and protamine sulfate (from salmon; grade X) were purchased from Sigma Chemicals (St. Louis, Mo.). Stock solutions of the last two APs (10 mg/ml) were prepared in dimethyl sulfoxide (DMSO) and HEPES buffer (20 mM HEPES, 132 mM NaCl, 3.5 mM KCl, 1 mM CaCl2, and 0.5 mM MgCl2; pH 7.25), respectively. All final AP concentrations were achieved by dilution of stock solutions in HEPES buffer; assay buffer controls exerted no activity against the two strains of S. aureus used.
It is likely that pathogens such as S. aureus encounter a range of AP concentrations during infection. Therefore, we subjected S. aureus to a range of AP concentrations encompassing sub- and supra-MIC levels. Based on previous data, ISP479C and ISP479R can be differentially distinguished by their tPMP-1 susceptibility and resistance profiles, respectively, at 1 μg of tPMP-1 per ml (8). Furthermore, preliminary data from our laboratory indicate that the minimum staphylocidal concentration of gramicidin D is <1 μg/ml versus an inoculum of 107 CFU/ml. For protamine, previous growth inhibition experiments indicated that the MICs for strains ISP479C and ISP479R were 0.5 and 10 mg/ml, respectively (8). Therefore, the membrane permeabilization and staphylocidal assays described below were conducted by exposing S. aureus to the following concentrations of APs: 0.5, 1.0, and 2.0 μg/ml for tPMP-1; 0.5, 5, 25, and 50 μg/ml for gramicidin D; and 0.5, 2, 5, and 10 mg/ml for protamine.
S. aureus membrane permeabilization by APs.
Membrane permeabilization of S. aureus by APs was detected and quantified by flow cytometry, via the release of the preloaded fluorophore calcein (excitation wave length, 494 nm; emission wavelength, 517 nm). Previous studies with eukaryotic cells indicated that membrane-impermeable calcein could be loaded into intact cells using calcein acetoxymethyl ester (calcein AM) (9, 11). Calcein AM is a nonfluorescent derivative of calcein that is lipid soluble and, therefore, can readily diffuse across membranes. Once within the cytoplasms of target cells, calcein AM is hydrolyzed by cytoplasmic esterases, yielding fluorescent calcein (9, 11). In the present study, a protocol was developed to load intact S. aureus cells with calcein using the calcein AM strategy described above. Our pilot experiments using fluorometry and a standard laboratory S. aureus strain, 502A, demonstrated that S. aureus accumulation of calcein increased with time and reached a maximum level after ∼2 h of incubation in calcein AM, as detected by a progressive increase in total cell fluorescence (data not shown). Intracellular calcein accumulation, optimal when calcein AM was introduced in the presence of BHI (10% [vol/vol]), did not affect cell viability. Further, the fluorophore was well retained by S. aureus for at least 4 h following accumulation. Membrane permeabilization leads to calcein leakage, which is detectable via flow cytometry as a decrease in total cell fluorescence (S. Koo, A. S. Bayer, J. P. Adler-Moore, and M. R. Yeaman, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. A-117, p. 29, 2000); our pilot experiments have shown that antimicrobial peptides that kill S. aureus through cytoplasmic membrane permeabilization (e.g., gramicidin S) are associated with complete leakage of calcein. In contrast, antimicrobial peptides that have poor staphylocidal activities (e.g., polymyxin B) cause relatively little or no calcein release (Koo et al., Abstr. 100th Gen. Meet. Am. Soc. Microbiol.). Finally, cell wall-active agents (e.g., penicillin G and vancomycin) cause no calcein release (Xiong et al., 40th ICAAC). Thus, this simple method has proven to be rapid, sensitive, and reproducible in our pilot studies, yielding membrane permeabilization data that are consistent with those obtained via other methods (e.g., uptake of propidium iodide) (36).
S. aureus cells were first loaded with calcein prior to AP exposure. Stock solutions of calcein AM (1 mM; Molecular Probes, Eugene, Oreg.) were prepared in DMSO and stored at −20°C under desication: such solutions were used within 10 days. Based on the pilot experiments discussed above, S. aureus cells (109 CFU/ml) were incubated with 2 to 4 μM calcein AM in HEPES buffer containing 10% (vol/vol) BHI for 2 h at 37°C. Calcein-loaded cells were collected by centrifugation (3,000 × g, 10 min), resuspended in HEPES buffer, and briefly sonicated before being adjusted spectrophotometrically to an optical density at 600 nm of ∼1.0 (109 CFU/ml), as before. Each experiment included a quantitative culture to ensure that calcein loading did not alter S. aureus viability.
Calcein-loaded S. aureus cells were diluted 100-fold to achieve a final inoculum of 107 CFU/ml within the test AP solutions described above. Cells were incubated at 37°C for 2 h. Previous data showed that membrane permeabilization by tPMP-1 occurred within minutes of peptide addition, while cell death was generally detected after longer exposures (≥1 h) (17, 36). Thus, S. aureus cells exposed to tPMP-1 were sampled more frequently than cells exposed to gramicidin D or protamine. At the predetermined sample times (i.e., 6 s and 15, 30, 60, 90, and 120 min for tPMP-1; and 6 s and 60 and 120 min for gramicidin D or protamine), cells were briefly sonicated and duplicate aliquots were removed. One sample was analyzed for the release of preloaded calcein via flow cytometry (FACSCalibur; Becton-Dickinson, San Jose, Calif.) as a measure of membrane permeabilization; a second sample was used for the assessment of staphylocidal activity (see below). A total of 10,000 cells was acquired for each flow cytometry analysis. Based on an extensive pilot study, cells at or above a threshold of 10 fluorescence units (FL1 units) were considered to have retained calcein, indicative of an intact cytoplasmic membrane; those cells exhibiting <10 FL1 units were interpreted to have lost calcein as a result of AP-induced membrane permeabilization. Controls for membrane permeabilization consisted of cells in HEPES buffer lacking AP but containing the appropriate amount of the proper AP diluent (i.e., RP-HPLC buffer for tPMP-1, DMSO for gramicidin D, and HEPES buffer for protamine). Experiments were repeated at least two times independently on separate days. Differences in levels of membrane permeabilization were defined as the percentages of difference in cell fluorescence (in fluorescence channel 1 [FL1] units) between AP-treated and control samples.
Staphylocidal activities of APs.
In parallel to assessment of membrane permeabilization, samples were evaluated for the extent of S. aureus killing by quantitative culture. Controls for staphylocidal activity consisted of cells in HEPES buffer lacking AP but containing the appropriate amount of the proper AP diluent, as for membrane permeabilization assays. Experiments were repeated at least two times independently on separate days. Differences in changes in log10 CFU per milliliter (Δlog10 CFU per milliliter) were calculated as differences in CFU per milliliter between AP-treated and control samples.
Statistical analyses.
Differences in mean levels of permeabilization (± standard errors) or killing of S. aureus by the different APs were compared by Kruskal-Wallis rank-sum analysis for nonparametric data. P values of ≤0.05 were considered significant in all experiments. Regression analyses compared the relationships between staphylococcal membrane permeabilization and killing by the distinct APs. The degrees of AP-induced permeabilization and the staphylocidal activities of the peptides at different concentrations were compared over time with those of the respective controls. Correlations of degrees of time- and/or concentration-dependent membrane permeabilization (percentages) and staphylocidal activity (−Δlog10 CFU per milliliter) were performed by linear-regression analysis using Microsoft Excel software. Correlation coefficients (r2) of at most −0.5 or ≥0.5 were considered significant.
RESULTS
Effect of tPMP-1 on S. aureus membrane permeabilization and viability.
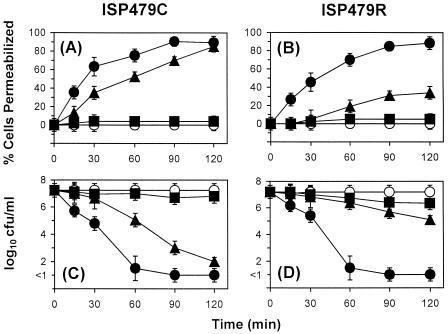
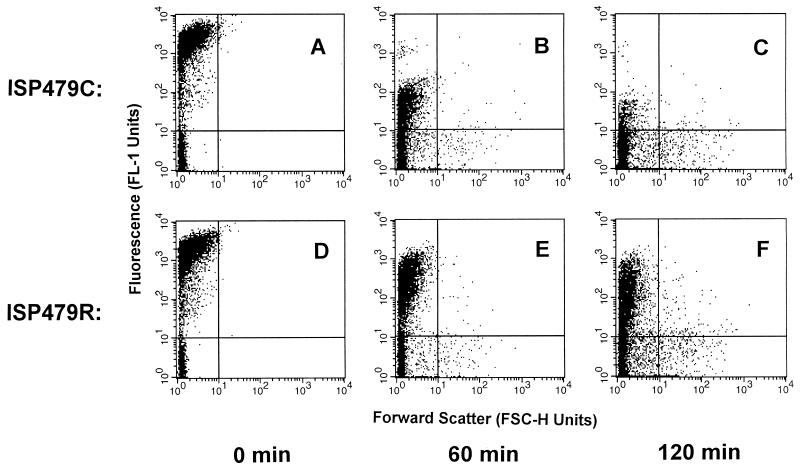
Membrane permeabilization by tPMP-1 was exposure time and peptide concentration dependent. At 0.5 μg/ml, tPMP-1 did not significantly permeabilize either strain, compared to the levels of permeabilization of the respective controls (P > 0.05) (Fig. 1A and B). In contrast, 1 μg of tPMP-1 per ml significantly permeabilized the cell membranes of both strains in a time-dependent manner, unlike results observed for the controls (P < 0.05) (Fig. 1A and 1B). However, ISP479C was significantly more susceptible to membrane permeabilization than ISP479R (90% versus 34% of cells were permeabilized after 120 min of incubation, respectively; P < 0.05) (Fig. 1A and 1B). Distributions of the levels of fluorescence of ISP479C and ISP479R treated with tPMP-1 are shown in Fig. 2 (1 μg of tPMP-1 per ml; range, 0 to 120 min; 10,000 cells were analyzed via flow cytometry). The fluorescence of ISP479C cells decreased more rapidly over the 120-min incubation time than did that of ISP479R cells, indicating an increased susceptibility to membrane permeabilization by tPMP-1 (Fig. 2). Exposure to 2.0 μg of tPMP-1 per ml caused equivalent degrees of membrane permeabilization for both strains (∼90%) after 90 min of incubation (P < 0.05 versus the levels for respective controls) (Fig. 1A and B). There was no significant loss of calcein observed in control cells over the 120-min experimental period.
FIG. 1.
tPMP-1 staphylocidal activity and membrane permeabilization. ISP479C (A and C) and ISP479R (B and D; 107 CFU/ml) were exposed to 0.5 (■), 1.0 (▴), or 2.0 (●) μg of tPMP-1 per ml for 2 h at 37°C. Membrane permeabilization (A and B) and cell viability (C and D) were determined in parallel at each sample time, as outlined in Materials and Methods. Controls (○) represent cells exposed to buffer alone.
FIG. 2.
Membrane permeabilization of S. aureus by tPMP-1. S. aureus ISP479C and ISP479R were analyzed for differences in their susceptibilities to membrane permeabilization by 1 μg of tPMP-1 per ml. A total of 10,000 cells was analyzed by flow cytometry for the loss of calcein fluorescence. Cells with FL1 units of ≥10 were considered to possess intact membranes, while those with FL1 units of ≤10 were considered to be permeabilized by APs, as discussed in Materials and Methods. The density plots indicate the fluorescence distribution (FL1 units) versus the forward scatter (FSC-H units) of ISP479C (A to C) or ISP479R (D to F) treated with tPMP-1 over the following times: 0 (A and D), 60 (B and E), and 120 (C and F) min. The increasing number of cells with an increase in forward scatter (an indication of particle size) over time was not significantly different from the overall population analyzed for either strain. (P ≥ 0.05).
In parallel with the above data regarding membrane permeabilization, tPMP-1 also caused killing of both S. aureus strains in a time- and concentration-dependent manner (Fig. 1C and D). For example, 0.5 μg of tPMP-1 per ml did not significantly affect the viability of either strain, compared to that of untreated controls (P > 0.05). In contrast, 1 μg of tPMP-1 per ml exerted significantly greater staphylocidal effects against ISP479C than against ISP479R (reductions of 5 versus 2.1 log10 CFU/ml within 120 min, respectively; P < 0.05) (Fig. 1C and D). At 2.0 μg/ml, tPMP-1 killed both strains of S. aureus to equivalent extents and was maximal within 90 min of exposure (reductions of >7 log10 CFU/ml at 90 min; P > 0.05 for ISP479C values versus ISP479R values). There was no loss in the viability of cells exposed to control buffer over the 120-min experiment (Fig. 1C and D).
Effect of gramicidin D on S. aureus membrane permeabilization and viability.
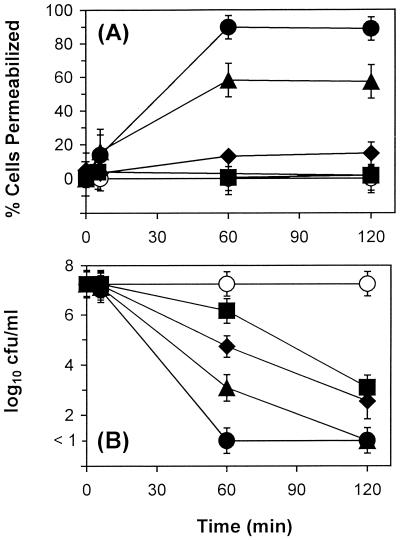
Across the concentration range tested, the extent of gramicidin D-induced membrane permeabilization of the study strains was concentration dependent and was maximal at 60 min of peptide exposure (Fig. 3A). Therefore, in contrast to tPMP-1, the extent of membrane permeabilization by gramicidin D did not appear to be time dependent beyond the 60-min incubation time. At the lower gramicidin D concentrations of 0.5 or 5 μg/ml, no significant membrane permeabilization of ISP479C was observed over 120 min, compared to that of controls (P > 0.05) (Fig. 3A). In contrast, significant membrane permeabilization was observed at gramicidin D concentrations of 25 and 50 μg/ml (55% and 90% of ISP479C cells were permeabilized after 60 min of incubation, respectively; P < 0.05) (Fig. 3A). Data for ISP479R (not shown) were not significantly different from those for ISP479C.
FIG. 3.
Gramicidin D staphylocidal activity and membrane permeabilization. ISP479C was exposed to 0.5 (■), 5 (⧫), 25 (▴), or 50 (●) μg of gramicidin D per ml for 2 h at 37°C. Membrane permeabilization (A) and cell viability (B) were determined in parallel at each sample time, as outlined in Materials and Methods. Controls (○) were cells exposed to buffer alone.
Unlike results observed with tPMP-1, the degree of S. aureus membrane permeabilization induced by gramicidin D did not parallel its staphylocidal activity. For example, 0.5 and 5 μg of gramicidin D per ml significantly decreased the viability of ISP479C, compared to that of controls (reductions of 4.2 and 4.7 log10 CFU/ml at 120 min, respectively; P < 0.05) (Fig. 3B). S. aureus killing by gramicidin D occurred in a time-dependent manner, despite the lack of significant membrane permeabilization at these peptide concentrations, over the 120-min exposure period. In contrast, 25 or 50 μg of gramicidin D per ml reduced the viability of ISP479C to levels below detection (i.e., there was a reduction of ≥7 log10 CFU/ml within 120 or 60 min of incubation, respectively) (Fig. 3B). As for membrane permeabilization, results pertaining to the effect of gramicidin D on the viability of ISP479R were not significantly different from those described for ISP479C (data not shown).
Effect of protamine on S. aureus membrane permeabilization and viability.
Unlike tPMP-1 or gramicidin D, protamine induced S. aureus membrane permeabilization in a peptide concentration-dependent but exposure time-independent manner. For example, 0.5 and 2 mg of protamine per ml did not induce significant membrane permeabilization in either ISP479C or ISP479R, compared to that of controls (P > 0.05; data not shown). However, 5 mg of protamine per ml induced rapid, significant (P < 0.05 versus control values), and equivalent degrees of membrane permeabilization in both S. aureus strains (30 to 40% of cells permeabilized within 1 min; data not shown; P > 0.05 for ISP479C values versus ISP479R values). At 10 mg/ml, the induction of membrane permeabilization by protamine was even more pronounced, with 70 to 80% of cells being permeabilized within 1 min of incubation. However, despite its capacity to permeabilize staphylococcal membranes, protamine failed to exert staphylocidal effects against either S. aureus strain under any condition tested (data not shown).
Relationship between membrane permeabilization and staphylocidal activity of antimicrobial peptides.
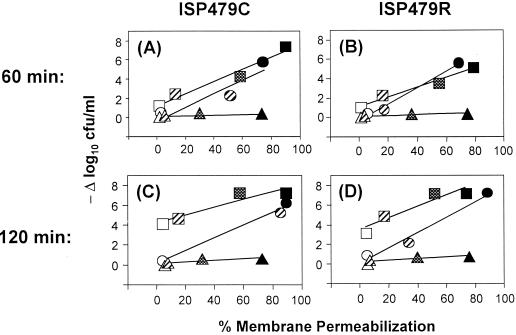
Regression analyses were performed to further characterize the relationship between membrane permeabilization and staphylocidal activity induced by tPMP-1, gramicidin D, or protamine (Fig. 4). For tPMP-1, the extents of peptide-induced membrane permeabilization and staphylocidal activity, which were directly related to peptide concentration, demonstrated a significant and positive correlation (r2 ≥ 0.5). The progressive increases in both of these parameters were parallel over the 120-min exposure time. Thus, these results suggested that the relationship between the membrane permeabilization and staphylocidal activity of tPMP-1 is constant over the duration of peptide exposure. Moreover, despite intrinsic differences in susceptibilities to tPMP-1-induced killing, this direct relationship was observed for both ISP479C and ISP479R.
FIG. 4.
Relationship of peptide exposure time and concentration to membrane-permeabilizing and staphylocidal effects of APs. Regression analyses were performed to determine the relationship between the membrane permeabilization and killing of ISP479C (A and C) or ISP479R (B and D) exposed to tPMP-1 (circles), gramicidin D (squares), or protamine (triangles) for 60 min (A and B) or 120 min (C and D). Standard error bars were intentionally excluded for clarity. Increasing AP concentrations are represented by different symbols patterns: open symbols indicate the lowest AP concentration used (i.e., 0.5 μg of tPMP-1 or gramicidin D per ml or 0.5 mg of protamine per ml); hatched symbols indicate the next higher concentration used (i.e., 1.0 μg of tPMP-1 per ml, 5 μg of gramicidin D per ml, or 2 mg of protamine) per ml); checkerboard symbols indicate 25 μg of gramicidin D per ml or 5 mg of protamine per ml; and solid symbols indicate the highest concentration used (i.e., 2.0 μg of tPMP-1 per ml, 50 μg of gramicidin D per ml, or 10 mg of protamine) per ml). For ISP479C treated with tPMP-1 or gramicidin D r2 obtained for the various plots were as follows: 0.870 or 0.955, respectively, after 60 min of treatment and 0.986 or 0.892 respectively, after 120 min of treatment. For ISP479R treated with tPMP-1 or gramicidin D, r2 were as follows: 0.979 or 0.959, respectively, after 60 min of treatment and 0.974 or 0.882, respectively, after 120 min of treatment.
Regression analyses of the effects of gramicidin D on S. aureus revealed a relationship between membrane permeabilization and staphylocidal activity that differed from that of tPMP-1. During the initial 60-min exposure of both strains to gramicidin D, there appeared to be a direct relationship between membrane permeabilization and staphylocidal activity (Fig. 4). However, at 120 min and at low concentrations of gramicidin D (0.5 or 5 μg/ml), the degree of staphylocidal activity increased without concomitant increases in membrane permeabilization. In contrast, at higher gramicidin D concentrations (25 or 50 μg/ml), significant increases in membrane permeabilization and killing of both ISP479C and ISP479R were observed at 120 min. The latter findings suggest that a direct relationship between membrane permeabilization and staphylocidal activity by gramicidin D is fulfilled only when S. aureus is exposed to relatively high concentrations of the peptide (e.g., ≥25 μg/ml) for extended exposure times.
As noted above, protamine permeabilized S. aureus in a concentration-dependent manner but did not exert any detectable staphylocidal effect. Thus, in contrast to what occurred with tPMP-1 or gramicidin D, there was no apparent correlation between the membrane permeabilization and staphylocidal activity of protamine against either S. aureus strain studied under any condition tested (Fig. 4).
DISCUSSION
Many APs have been shown to evoke permeabilizing effects in target microbial membranes. However, the relationship between AP-induced membrane effects and the overall mechanisms of microbicidal activity remains largely undefined. In the present study, we examined this relationship using S. aureus as a prototypic microbial target of three well-characterized, yet structurally distinct, APs: tPMP-1, gramicidin D, and protamine.
Numerous lines of evidence from our laboratories have identified the staphylococcal membrane as a key initial target triggering subsequent tPMP-1-induced lethality (15–17, 36). Moreover, structural and functional perturbations of the staphylococcal membrane substantially influence tPMP-1 activity. For example, tPMP-1 permeabilizes artificial planar lipid bilayers designed to mimic the compositions of staphylococcal membranes in a fashion modulated by both ΔΨ and peptide concentration (16, 17). Furthermore, the staphylocidal activity of tPMP-1 is significantly reduced against those strains exhibiting either a reduction in intrinsic Δψ (e.g., small colony variants [SCVs]) or an alteration in their membrane fluidity characteristics (3, 15, 36).
The observations described above clearly implicate the structural and functional status of the cytoplasmic membrane as a key determinant influencing the eventual staphylocidal activity of tPMP-1. In the present study, we delineated a direct correlation between membrane permeabilization and the staphylocidal activity of tPMP-1 against isogenic S. aureus strains, despite intrinsic differences in their tPMP-1 susceptibility profiles (8). Thus, tPMP-1 likely initiates its staphylocidal mechanism via permeabilization of the cytoplasmic membrane in a peptide concentration- and exposure-time-dependent manner. However, is should be noted that we have recently demonstrated that membrane permeabilization alone is likely insufficient to achieve the staphylocidal effects of tPMP-1 (33). Instead, downstream, intracellular targets beyond the cytoplasmic membrane appear to play an important role in the mechanism of action of tPMP-1. Therefore, we hypothesize that the cytoplasmic membrane is not the exclusive or final target involved in the mechanism of tPMP-1-induced staphylocidal activity. From this perspective, membrane permeabilization by tPMP-1 may facilitate its subsequent entry into the bacterial cytoplasm to access putative key intracellular targets. Studies to define the relative contributions of membrane permeabilization and intracellular targets in the overall staphylocidal action of tPMP-1 are in progress.
Gramicidin D is a linear, neutral, and hydrophobic channel-forming peptide (6). The principal mode of action proposed for gramicidin D involves the formation of a negatively charged, electrostatic pore (30, 31). Our current data indicate that the staphylocidal mechanism(s) of this AP varies significantly depending upon peptide concentration and exposure time. This variable relationship between membrane permeabilization and staphylocidal activity suggests that gramicidin D achieves a potent staphylocidal effect through multiple pathways and/or targets. Several possibilities may be proposed to explain these findings. First, it is conceivable that low gramicidin D concentrations evoke transient or reversible permeabilization of the S. aureus membrane. In concept, under these conditions, gramicidin D may still access a putative intracellular target(s) required for its staphylocidal activity. Alternatively, it is possible that low gramicidin D concentrations may create selective, calcein-impermeable channels in the staphylococcal membrane. Presumably, this scenario would also allow gramicidin D to enter the cell and inhibit intracellular targets. In contrast, as gramicidin D concentration increases, a threshold level necessary for peptide organization in the staphylococcal membrane (e.g., oligomerization and/or channel formation) that lacks such selectivity may be achieved (30, 31). These events may, in turn, cause extensive and durable membrane permeabilization, contributing further to the antimicrobial activity of this AP.
Protamine is a cationic AP that permeabilizes the cell membranes of both gram-positive and gram-negative bacteria (1, 12, 13, 28). However, protamine differs from many other cationic APs (including gramicidin D) in that it lacks an amphiphilic amino acid profile believed to be important for pore formation in target microbial membranes (14, 20, 23). In addition to having the capacity to perturb microbial membranes, protamine also impacts a number of intracellular events, including energy transduction and nutrient accumulation (1, 12, 21). We have previously observed the differential growth inhibitory effects of protamine against the tPMP-1-susceptible and -resistant S. aureus pair used in this study (MICs, 0.5 and 10 mg/ml, respectively) (8). However, in contrast to what occurred with tPMP-1 and gramicidin D, neither of these strains was killed by protamine in the present study at concentrations up to 10 mg/ml. Unexpectedly, despite this lack of staphylocidal activity, protamine caused significant, dose-dependent membrane permeabilization in both S. aureus strains. The mechanism(s) underlying this apparent disparity between the microbiostatic and microbicidal effects of protamine is likely to be multifactorial. (i) It is possible that S. aureus is inherently tolerant of the microbicidal effects of protamine (i.e., high minimal bactericidal concentration-to-MIC ratio) (27). (ii) Conversely, our microbicidal assays were performed over a shorter protamine exposure time than that used in the microbiostatic assays (2 versus 18 h). This reduced exposure time likely contributed to the apparent tolerance of S. aureus to protamine-induced killing. (iii) Cationic peptides such as protamine may induce S. aureus to undergo phenotypic switching to an SCV morphotype (2, 26). As noted previously, SCVs frequently display increased resistance to the microbicidal effects of APs (15, 36). (iv) Finally, protamine appears to differentially permeabilize the cell envelopes of gram-positive and gram-negative bacteria (1, 12, 13). Thus, it is conceivable that protamine may selectively permeabilize the S. aureus membrane to cause growth inhibition and membrane permeabilization, without facilitating peptide access to intracellular targets required for staphylocidal activity. Studies to further characterize the apparent mechanisms of protamine tolerance in S. aureus are in progress.
In conclusion, our present data strongly suggest that the relationships between S. aureus membrane permeabilization and killing are distinct for individual APs, indicating that their antistaphylococcal mechanisms of action are likely different. The distinct antistaphylococcal effects of these peptides are likely attributable to differing peptide compositions and structures. Further, these effects appear to be dynamically influenced by the duration of exposure and peptide concentration, as well as target cell status.
ACKNOWLEDGMENTS
This research was supported by grants from the National Institutes of Health (NIH) (A139108 to A.S.B. and A139001 to M.R.Y.); the flow cytometer was supported by a grant from the NIH Center for Research Resources (RR14857-01 to M.R.Y.).
We thank Kimberly Gank for her excellent technical support in purifying tPMP-1.
REFERENCES
- 1.Aspedon A, Groisman E A. The antibacterial action of protamine: evidence for disruption of cytoplasmic membrane energization in Salmonella typhimurium. Microbiology. 1996;142:3389–3397. doi: 10.1099/13500872-142-12-3389. [DOI] [PubMed] [Google Scholar]
- 2.Balwit J M, Van Langevelde P, Vann J M, Proctor R A. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J Infect Dis. 1994;170:1033–1037. doi: 10.1093/infdis/170.4.1033. [DOI] [PubMed] [Google Scholar]
- 3.Bayer A S, Prasad R P, Chandra J, Koul A, Smriti M, Varma A, Skurray R A, Firth N, Brown M H, Koo S-P, Yeaman M R. In vitro resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein associated with alterations in cytoplasmic membrane fluidity. Infect Immun. 2000;68:3548–3553. doi: 10.1128/iai.68.6.3548-3553.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J Membr Biol. 1997;156:197–211. doi: 10.1007/s002329900201. [DOI] [PubMed] [Google Scholar]
- 5.Burkhart B M, Li N, Langs D A, Pangborn W A, Duax W L. The conducting form of gramicidin A is a right-handed double-stranded double helix. Proc Natl Acad Sci USA. 1998;95:12950–12955. doi: 10.1073/pnas.95.22.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkhart B M, Gassman R M, Langs D A, Pangborn W A, Duax W L, Pletnev V. Gramicidin D conformation, dynamics and membrane ion transport. Biopolymers. 1999;51:129–144. doi: 10.1002/(SICI)1097-0282(1999)51:2<129::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 7.Cociancich S, Ghazi A, Hetru C, Hoffman J A, Letellier L. Insect defensins, an inducible antibacterial peptide, forms voltage-dependent channels in Micrococcus luteus. J Biol Chem. 1993;268:19239–19245. [PubMed] [Google Scholar]
- 8.Dhawan V K, Yeaman M R, Cheung A L, Kim E, Sullam P M, Bayer A S. Phenotypic resistance to thrombin-induced platelet microbicidal protein in vitro is correlated with enhanced virulence in experimental endocarditis due to Staphylococcus aureus. Infect Immun. 1997;65:3293–3299. doi: 10.1128/iai.65.8.3293-3299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Essodaïgui M, Broxterman H J, Garnier-Suillerot A. Kinetic analysis of calcein and calcein-acetoxymethylester efflux mediated by the multidrug-resistance protein and P-glycoprotein. Biochemistry. 1998;37:2243–2250. doi: 10.1021/bi9718043. [DOI] [PubMed] [Google Scholar]
- 10.Ganz T, Selsted M E, Szklarek D, Harwig S S L, Daher K, Lehrer R I. Defensins: natural peptide antibiotics of human neutrophils. J Clin Investig. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollö Z, Homolya L, Davis C W, Sarkadi B. Calcein accumulation as a fluorometric functional assay of the multidrug transporter. Biochim Biophys Acta. 1994;1191:384–388. doi: 10.1016/0005-2736(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 12.Johansen C, Gill T, Gram L. Changes in cell morphology of Listeria monocytogenes and Shewanella putrefaciens resulting from the action of protamine. Appl Environ Microbiol. 1996;62:1058–1064. doi: 10.1128/aem.62.3.1058-1064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansen C, Verheul A, Gram L, Gill T, Abee T. Protamine-induced permeabilization of cell envelopes of gram-positive and gram-negative bacteria. Appl Environ Microbiol. 1997;63:1155–1159. doi: 10.1128/aem.63.3.1155-1159.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser E T, Kézdy F J. Peptides with affinity for membranes. Annu Rev Biophys Chem. 1987;16:561–581. doi: 10.1146/annurev.bb.16.060187.003021. [DOI] [PubMed] [Google Scholar]
- 15.Koo S-P, Bayer A S, Sahl H-G, Proctor R A, Yeaman M R. Staphylocidal action of thrombin-induced platelet microbicidal protein is not solely dependent on transmembrane potential. Infect Immun. 1996;64:1070–1074. doi: 10.1128/iai.64.3.1070-1074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo S-P, Yeaman M R, Nast C C, Bayer A S. The cytoplasmic membrane is a primary target for the staphylocidal action of thrombin-induced platelet microbicidal protein. Infect Immun. 1997;65:4795–4800. doi: 10.1128/iai.65.11.4795-4800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo S-P, Kagan B L, Bayer A S, Yeaman M R. Membrane permeabilization by thrombin-induced platelet microbicidal protein-1 is modulated by transmembrane voltage orientation and magnitude. Infect Immun. 1999;67:2475–2481. doi: 10.1128/iai.67.5.2475-2481.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krijgsveld J, Zaat S A J, Meeldijk J, Van Veelan P A, Fang G, Poolman B, Brandt E, Ehlert J E, Kuijpers A J, Engber G H M, Feijen J, Dankert J. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC-chemokines. J Biol Chem. 2000;226:497–509. doi: 10.1074/jbc.275.27.20374. [DOI] [PubMed] [Google Scholar]
- 19.Lehrer R I, Barton A, Daher K A, Harwig S S L, Ganz T, Selsted M E. Interaction of human defensins with Escherichia coli. Mechanisms of bactericidal activity. J Clin Investig. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louie A J, Dixon G H. Enzymatic modifications of the protamines. II Separation and characterization of phosphorylated species of protamines from trout testis. Can J Biochem. 1974;52:536–546. doi: 10.1139/o74-078. [DOI] [PubMed] [Google Scholar]
- 21.MacMillan W G, Hibbitt K G. The effect of antimicrobial proteins on the fine structure of Staphylococcus aureus. J Gen Microbiol. 1969;56:373–377. doi: 10.1099/00221287-56-3-373. [DOI] [PubMed] [Google Scholar]
- 22.Mathews M, Jia H P, Guthmiller J M, Losh G, Graham S, Johnson G K, Tack B F, McCray P B., Jr Production of β-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun. 1999;67:2740–2745. doi: 10.1128/iai.67.6.2740-2745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller B F, Abrams R, Dorfman A, Klein M. Antimicrobial properties of protamines and histones. Science. 1942;96:428–429. doi: 10.1126/science.96.2497.428. [DOI] [PubMed] [Google Scholar]
- 24.Muller W E, Echert G P, Scheuer K, Cairns N J, Maras A, Gattaz W F. Effects of β-amyloid peptides on the fluidity of membranes from frontal and parietal lobes of human brains. High potencies of Aβ (1–42) and Aβ (1–43) Amyloid. 1998;5:10–15. doi: 10.3109/13506129809007284. [DOI] [PubMed] [Google Scholar]
- 25.Park C B, Kim H S, Kim S C. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem Biophys Res Commun. 1998;244:253–257. doi: 10.1006/bbrc.1998.8159. [DOI] [PubMed] [Google Scholar]
- 26.Proctor R A. Microbial pathogenic factors: small colony variants. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C.: American Society for Microbiology; 1994. pp. 79–94. [Google Scholar]
- 27.Sabath L D, Mokhbat J. What is the clinical significance of tolerance to β-lactam antibiotics? In: Remington J S, Swartz M N, editors. Current clinical topics in infectious diseases. New York, N.Y: McGraw-Hill Book Company; 1983. pp. 358–377. [Google Scholar]
- 28.Stumpe S, Bakker E P. Requirement of a large K+-uptake capacity and of extracytoplasmic protease activity for protamine resistance of Escherichia coli. Arch Microbiol. 1997;167:126–136. [PubMed] [Google Scholar]
- 29.Sullam P M, Frank U, Tauber M G, Yeaman M R, Bayer A S, Chambers H F. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J Infect Dis. 1993;168:910–914. doi: 10.1093/infdis/168.4.910. [DOI] [PubMed] [Google Scholar]
- 30.Urry D W, Goodall M C, Glickson J D, Mayers D F. The gramicidin A transmembrane channel: a proposed π(L,D) helix. Proc Natl Acad Sci USA. 1971;68:672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veatch W R, Fossel E T, Blout E R. The conformation of gramicidin A. Biochemistry. 1974;13:5249–5256. doi: 10.1021/bi00723a001. [DOI] [PubMed] [Google Scholar]
- 32.White S H, Wimley W C, Selsted M E. Structure, function, and membrane integration of defensins. Curr Opin Struct Biol. 1995;5:521–527. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 33.Xiong Y-Q, Yeaman M R, Bayer A S. In vitro activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob Agents Chemother. 1999;43:1111–1117. doi: 10.1128/aac.43.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeaman M R, Puentes S M, Norman D C, Bayer A S. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect Immun. 1992;60:1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeaman M R, Tang Y-Q, Shen A J, Bayer A S, Selsted M E. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect Immun. 1997;65:1023–1031. doi: 10.1128/iai.65.3.1023-1031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeaman M R, Bayer A S, Koo S-P, Foss W, Sullam P M. Platelet microbicidal protein and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J Clin Investig. 1998;101:178–187. doi: 10.1172/JCI562. [DOI] [PMC free article] [PubMed] [Google Scholar]