Abstract
Background
Contrast-associated acute kidney injury (CA-AKI) after percutaneous coronary intervention (PCI) is associated with increased mortality. We assessed the effectiveness of an electronic health records (EHR) safe contrast limit tool in predicting CA-AKI risk and reducing contrast use and CA-AKI.
Methods
We created an alert displaying the safe contrast limit to cardiac catheterization laboratory staff prior to PCI. The alert used risk factors automatically extracted from the EHR. We included procedures from 6/1/2020–10/1/2021; the intervention went live 2/10/2021. Using difference-in-differences analysis, we evaluated changes in contrast volume and CA-AKI rates after contrast limit tool implementation compared to control hospitals. Cardiologists were surveyed prior to and 9 months after alert implementation on beliefs, practice patterns, and safe contrast estimates for example patients.
Results
At the one intervention site there were 508 PCIs before and 531 after tool deployment. At 15 control sites there were 3550 and 3979 PCIs, respectively. The contrast limit predicted CA-AKI with an accuracy of 64.1%, negative predictive value of 93.3%, and positive predictive value of 18.7%. After implementation, in high/modifiable risk patients (defined as having a calculated contrast limit <500ml) there was a small but significant −4.60ml/month (95% CI −8.24,−1.00) change in average contrast use but no change in CA-AKI rates (OR 0.96 (0.84,1.10)). Low risk patients had no change in contrast use (−0.50ml/month (−7.49,6.49)) or CA-AKI (OR 1.24 (0.79,1.93)). In assessing CA-AKI risk, clinicians heavily weighted age and diabetes but often did not consider anemia, cardiogenic shock, and heart failure.
Conclusions
Clinicians often used a simplified assessment of CA-AKI risk that did not include important risk factors, leading to risk estimations inconsistent with established models. Despite clinician skepticism, an EHR-based contrast limit tool more accurately predicted CA-AKI risk and was associated with a small decrease in contrast use during PCI but no change in CA-AKI rates.
Keywords (MeSH terms): Acute Kidney Injury/ chemically induced, Contrast Media/ adverse effects, Percutaneous Coronary Intervention/ adverse effects, Risk Assessment/ methods, Clinical Decision Support
Introduction
Approximately 4.1 million invasive cardiac procedures are performed annually in the United States.1 The majority require radiocontrast, which, when used in excess, has been associated with the development of contrast-associated acute kidney injury (CA-AKI).2 Of these cardiac procedures, percutaneous coronary interventions (PCI) are associated with some of the highest rates of CA-AKI, with up to 14% of all PCIs resulting in this complication.3–7 Patients who develop CA-AKI are more likely to stay longer in the hospital and experience a 36% chance of dying during their procedural hospitalization and a 12% chance of dying within 1 year after hospital discharge.3,4,8,9
Given the morbidity and mortality associated with CA-AKI, numerous models have been developed to help risk stratify patients prior to PCI, although their routine clinical use has been limited.10–12 We recently developed a new approach to conveying CA-AKI risk information by presenting to clinicians the contrast volume limit prior to the procedure.13 Our model calculates the safe contrast volume limit automatically based on patient risk factors extracted from the electronic health record (EHR). This provides an actionable, individualized number that can be used by clinicians to (1) decide whether PCI is appropriate given competing CA-AKI risks (2) choose proportionate CA-AKI risk-mitigation strategies (3) guide intraprocedural contrast use.
We implemented this safe contrast limit tool into our medical center’s EHR and evaluated its ability to predict CA-AKI as well as its effects on contrast usage and CA-AKI rates. We additionally surveyed clinicians to better understand their beliefs, attitudes, and practice patterns concerning CA-AKI and the contrast limit tool.
Methods
Data disclosure
A limited de-identified subset of the data that support the findings of this study are available from the corresponding author upon reasonable request.
Study cohort
We included all PCI procedures from 6/1/2020 to 10/1/2021 performed at Cedars-Sinai Medical Center which serves a large, urban patient population. For a control group, we included all PCI procedures during the same time period from 15 hospitals sharing data with Biome Analytics, a cardiovascular data analytics firm in San Francisco, California. We ensured that there were no ongoing CA-AKI reduction initiatives at control sites. Patient characteristics, contrast use, and CA-AKI events were all derived from data submitted to the National Cardiovascular Disease Registry (NCDR). CA-AKI was defined according to the NCDR definition of a post-PCI serum creatinine increase of ≥50% or ≥0.3 mg/dl from baseline.13 Consistent with NCDR adjudication criteria for CA-AKI events, PCIs were excluded from the analysis if the patient was missing pre- or post-PCI serum creatinine values, was on dialysis at the time of PCI, was discharged on the same day as the PCI procedure, and/or had a prior left heart catheterization during the same hospitalization (non-index PCI). Based on these criteria, 54.2% of PCIs at the intervention site and a mean of 29.0% of PCIs at the control sites (95% CI 23.2, 34.8) were excluded, mostly due to patients being discharged on the same day of their PCI and/or not having a measured post-PCI creatinine (Supplemental Table 1).
Contrast limit tool
We created a Best Practice Advisory (BPA) alert in our Epic EHR system, which displayed the patient’s safe contrast limit along with CA-AKI risk reduction strategies (Figure 1). The BPA went live on 2/10/2021. The alert required no clinician data entry, automatically extracting data from the EHR and calculating the patient’s safe contrast limit according to our previously published model.13 Risk factors included age, sex, body mass index, creatinine clearance, hemoglobin, and use of intra-aortic balloon pump pre-procedure. Based on the calculated contrast limit, a patient was categorized into one of three risk groups (high, modifiable, or low risk) aimed at helping determine when the contrast limit tool would be most useful for pre-procedure and intra-procedural guidance. These categories were previously defined with the original model.13 Patients in the modifiable risk group (defined as a calculated contrast limit between 20–500mL) are most likely to have a meaningful change in CA-AKI risk if PCI operators stay under the contrast limit. Patients in the high risk group (calculated contrast limit < 20mL) are likely to continue to have a high risk of CA-AKI regardless of the amount of contrast used. Patients in the low risk group (calculated contrast limit > 500mL) are unlikely to have CA-AKI given typical contrast usage during PCI.
Figure 1.

EHR implementation of the safe contrast limit tool*
*Figure adapted from Yuan et al. 2020
At our institution, all PCIs require a pre-procedure order in the EHR to document patient consent after discussion of procedural risks and benefits. The contrast limit BPA was triggered for cardiologists as well as catheterization laboratory nurses and technicians as soon as the order to obtain patient consent for PCI was placed. The alert triggered once more when the patient’s location was updated to the catheterization laboratory and then every 30 minutes while the patient remained in the catheterization laboratory.
Cardiologists were educated on the contrast limit tool during multiple information sessions held at the medical center’s weekly cardiac catheterization conference. Catheterization laboratory staff were also provided education on the tool during morning staff huddles. Informational emails and fliers were also used. The tool was furthermore supported as one of the cardiology department’s major annual quality initiatives.
Outcomes
Our primary outcome was change in contrast volume after implementation of the contrast limit tool compared to change in contrast volume over the same time period at control hospitals (difference-in-differences). Our secondary outcome was change in CA-AKI rates.
Sample size estimates
For our primary outcome, assuming 80% power and a significance threshold of alpha = 0.05, it was estimated that a total sample size of 2000 patients would be needed to detect a decrease in contrast volume usage of 25 ml or more. This would mean sampling at least 500 patients in each of four groups: PCI patients at the intervention site before contrast limit tool implementation, PCI patients at intervention site after implementation, PCI patients at control sites before implementation, PCI patients at control sites after implementation. These estimates were determined from a simulation with 5000 iterations that assumed a standard deviation in contrast use of 95 ml and a constant contrast volume usage at control hospitals. Simulations were run in R using package paramtest v0.1.0.
Statistical analysis
For the intervention hospital site and the non-intervention control sites, we described patient characteristics, PCI contrast usage, and CA-AKI rates both before and after implementation of the contrast limit tool, expressed as frequency counts and percentages. The differences in discrete variables between groups were evaluated by the chi-squared test. Differences in continuous variables were evaluated using the t-test. We also described the percentages of patients falling into each of the three CA-AKI risk categories: high risk (contrast limit < 20 ml), modifiable risk (contrast limit 20–500 ml), and low risk (contrast limit > 500 ml). We assessed the sensitivity, specificity, negative predictive value, and positive predictive value of the safe contrast limit in predicting subsequent CA-AKI. We visualized the mean PCI contrast use over the study period at the intervention site and at the control sites, grouping procedures by 2-month time periods. We also graphed the mean PCI contrast use for each PCI operator at the intervention site before and after the intervention and compared change in contrast usage by two-sided paired t-testing. We used a difference-in-differences analysis with adjustment for CA-AKI risk factors (age, sex, body mass index, creatinine clearance, hemoglobin, use of intra-aortic balloon pump pre-procedure) to model the effects of the contrast limit tool on contrast usage and rates of CA-AKI. We conducted an additional difference-in-differences analysis with the same adjustments to study the effects of the contrast limit tool on the proportion of PCIs in which the contrast limit was exceeded. All analyses were performed using R software (version 3.4.1; R Foundation for Statistical Computing, Vienna, Austria)
Clinician surveys
We surveyed all interventional cardiologists who performed PCI at the intervention site catheterization laboratory. Clinicians were surveyed prior to and 9 months after the BPA implementation. Survey questions were developed in consultation with 3 clinicians with expertise in implementation science. Questions were aimed at addressing the major domains of the GUIDES checklist: a guideline for evaluating computerized clinical decision support.14 Pre-implementation questions covered beliefs about CA-AKI, practice patterns, and knowledge of CA-AKI risk factors. The survey also asked clinicians to consider 4 example patients and estimate their safe contrast ranges (0–25, 25–50, 50–75, 75–100, 100–125, 125–150, 150–175, 150–200, or > 200 ml). The “true” safe contrast limit for these patients was determined by using the previously published pragmatic full contrast model.13 We also compared the relative CA-AKI risk assumptions embedded in operators’ safe contrast estimates to the CA-AKI risks calculated for these 4 sample patients according to two widely available online CA-AKI risk calculators on the website QxMD. These calculators are based on models published by Mehran et al. and Tsai et al.11,15
Post-implementation questions assessed clinician practice behaviors and perceptions as to the BPA’s accuracy, efficacy, and utility. The post-implementation survey also contained a free response section that solicited clinicians for additional feedback or comments. Survey questions and responses are presented in the results and supplemental sections.
The study protocol was approved by the institutional review board at Cedars–Sinai Medical Center and was in accordance with data‐sharing agreements signed by hospitals working with Biome Analytics.
Results
A total of 1039 PCI procedures performed at the intervention site were included for analysis: 508 PCIs prior to implementation of the EHR-based safe contrast limit tool and 531 PCIs after (Table 1). At the 15 control medical centers (9 academic and 6 community hospitals) where the safe contrast limit tool was not implemented, a total of 3550 and 3979 PCI procedures were included during the same respective time periods. Compared to the control sites, patients included from the intervention site were on average older (70.84 [SD=11.98] vs. 67.63 [12.19] years, p<0.01) and had lower body mass index (BMI) (27.54 [5.58] vs. 29.04 [6.50] kg/m2, p<0.01), pre-procedure creatinine clearance (73.96 [35.73] vs. 83.30 [39.86] ml/min, p<0.01), and hemoglobin values (12.81 [2.24] vs. 13.21 [2.08] g/dl, p<0.01). They were also more likely to have diabetes (44.2% vs. 41.0%, p=0.05), hypertension (86.7% vs. 83.2%, p<0.01), and heart failure (31.9% vs. 23.2%, p<0.01). The indication for PCI at the intervention site was more often non-acute coronary syndrome and less often ST-elevation myocardial infarction (38.3% vs. 31.0% and 13.8% vs. 20.4% respectively, p<0.01). CA-AKI occurred more frequently at the intervention site than control sites (11.7 vs. 8.3%, p<0.01) although the average volume of contrast used during PCI procedures was lower (143.28 [63.19] vs. 168.61 ml [83.85], p<0.01). At the intervention site, compared to patents before the implementation of the contrast limit tool, patients after tool implementation had similar characteristics.
Table 1.
Patient risk factors, PCI contrast usage, CA-AKI rates
| Intervention Site | Control Sites | Intervention vs. Control Sites (all periods) (p-value) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Before Contrast Limit Tool | After Contrast Limit Tool | Before vs. After Tool (p-value) |
All | Before Contrast Limit Tool | After Contrast Limit Tool | Before vs. After Tool (p-value) |
||
| n | 1039 | 508 | 531 | 7529 | 3550 | 3979 | |||
| Age (SD) | 70.84 (11.98) | 70.09 (11.69) | 71.56 (12.21) | 0.05 | 67.63 (12.19) | 67.42 (11.96) | 67.83 (12.39) | 0.14 | <0.01 |
| Male (%) | 746 (71.8) | 378 (74.4) | 368 (69.3) | 0.08 | 5378 (71.4) | 2555 (72.0) | 2823 (70.9) | 0.34 | 0.83 |
| BMI (SD) | 27.54 (5.58) | 27.74 (5.84) | 27.34 (5.31) | 0.26 | 29.04 (6.50) | 28.98 (5.99) | 29.10 (6.92) | 0.41 | <0.01 |
| Pre-PCI IABP (%) | 8 (0.8) | 6 (1.2) | 2 (0.4) | 0.26 | 43 (0.6) | 18 (0.5) | 25 (0.6) | 0.59 | 0.57 |
| CrCl (SD) | 73.96 (35.73) | 75.59 (38.26) | 72.40 (33.08) | 0.15 | 83.30 (39.86) | 82.88 (39.61) | 83.67 (40.08) | 0.39 | <0.01 |
| Hemoglobin (SD) | 12.81 (2.24) | 12.80 (2.29) | 12.83 (2.18) | 0.84 | 13.21 (2.08) | 13.26 (2.11) | 13.16 (2.06) | 0.03 | <0.01 |
| Diabetes (%) | 459 (44.2) | 225 (44.3) | 234 (44.1) | 0.99 | 3084 (41.0) | 1441 (40.6) | 1643 (41.3) | 0.55 | 0.05 |
| Hypertension (%) | 901 (86.7) | 445 (87.6) | 456 (85.9) | 0.47 | 6261 (83.2) | 2945 (83.0) | 3316 (83.3) | 0.68 | <0.01 |
| Heart Failure (%) | 331 (31.9) | 163 (32.1) | 168 (31.6) | 0.93 | 1750 (23.2) | 799 (22.5) | 951 (23.9) | 0.16 | <0.01 |
| Cardiogenic shock (%) | 17 (1.6) | 12 (2.4) | 5 (0.9) | 0.12 | 93 (1.2) | 44 (1.2) | 49 (1.2) | 1.00 | 0.35 |
| ACS (%) | 0.11 | 0.18 | <0.01 | ||||||
| Non-ACS | 398 (38.3) | 178 (35.0) | 220 (41.4) | 2335 (31.0) | 1100 (31.0) | 1235 (31.0) | |||
| NSTEMI/UA | 498 (47.9) | 257 (50.6) | 241 (45.4) | 3656 (48.6) | 1694 (47.7) | 1962 (49.3) | |||
| STEMI | 143 (13.8) | 73 (14.4) | 70 (13.2) | 1538 (20.4) | 756 (21.3) | 782 (19.7) | |||
| Pre-PCI MCS (%) | 17 (1.6) | 12 (2.4) | 5 (0.9) | 0.12 | 93 (1.2) | 44 (1.2) | 49 (1.2) | 1.00 | 0.35 |
| Contrast volume (SD) | 143.28 (63.19) | 146.50 (63.57) | 140.20 (62.72) | 0.11 | 168.61 (83.85) | 170.37 (85.30) | 167.04 (82.51) | 0.09 | <0.01 |
| CA-AKI (%) | 122 (11.7) | 60 (11.8) | 62 (11.7) | 1.00 | 626 (8.3) | 283 (8.0) | 343 (8.6) | 0.33 | <0.01 |
Abbreviations: PCI = percutaneous coronary intervention, CA-AKI = contrast associated acute kidney injury, SD = standard deviation, BMI = body mass index, IABP = intra-aortic balloon pump, ACS = acute coronary syndrome, NSTEMI = non-ST elevation myocardial infarction, UA = unstable angina, STEMI = ST elevation myocardial infarction, MCS = mechanical circulatory support
Using the contrast limit tool, 33.6% of patients at the intervention site were classified as high risk for CA-AKI (contrast limit < 20 ml), 45.2% were modifiable risk (contrast limit 20–500 ml), and 21.2% were low risk (contrast limit > 500 ml). The contrast limit predicted CA-AKI rates using real-time EHR data at the intervention site with an overall accuracy of 64.1%, negative predictive value of 93.3%, and positive predictive value of 18.7%. When applied retrospectively to control sites, the accuracy was 63.5% with a negative predictive value of 95.4% and positive predictive value of 15.3%. The observed CA-AKI rates for the high, modifiable, and low risk categories were similar to the expected CA-AKI rates across these categories from the original model validation (Expected: 21.4% (95% CI 18.8–23.9%), 8.2% (7.1–9.2%), 3.5% (2.6–4.3%); Intervention site: 20.4%, 9.4%, 4.4%; Control sites: 17.8%, 6.8%, 3.0%).13
After implementation of the contrast limit tool there was a decline over time in average contrast volume use during PCI procedures at the intervention site for patients with high or modifiable CA-AKI risk (contrast limit < 500 ml) but not for patients with low risk (contrast limit > 500) (Figure 2A). There was little change over time in patients at control sites. Using a difference-in-differences analysis with multivariable adjustment for CA-AKI risk factors, we found that across all patients there was a significant −3.86 ml/month (95% CI −7.07, −0.64) change in average contrast use over time. In patients with high or modifiable risk there was a significant −4.60 ml/month (8.24, −1.00) change, but in just patients with low risk there was no significant change (−0.50 ml/month (−7.49, 6.49)). We visualized contrast volume usage on an individual PCI operator level and found that 8 out of 10 clinicians decreased their contrast use after contrast limit tool implementation when performing PCI in patients at high or modifiable risk (average decrease 26.5 ml; 95% CI 5.57, 47.50; p=0.02 for paired t-test) (Figure 2B). For rates of CA-AKI, there was no significant change over time across patients at the intervention site in difference-in-differences analyses (OR 0.96 (0.84, 1.10)). This was true both in modifiable and high risk patients (OR 0.94 (0.82, 1.07)) as well as low risk patients (OR 1.24 (0.79, 1.93)). There was no significant decrease in the odds of exceeding the contrast limit during a PCI after the intervention over time (OR 0.99 (0.89, 1.10)).
Figure 2.
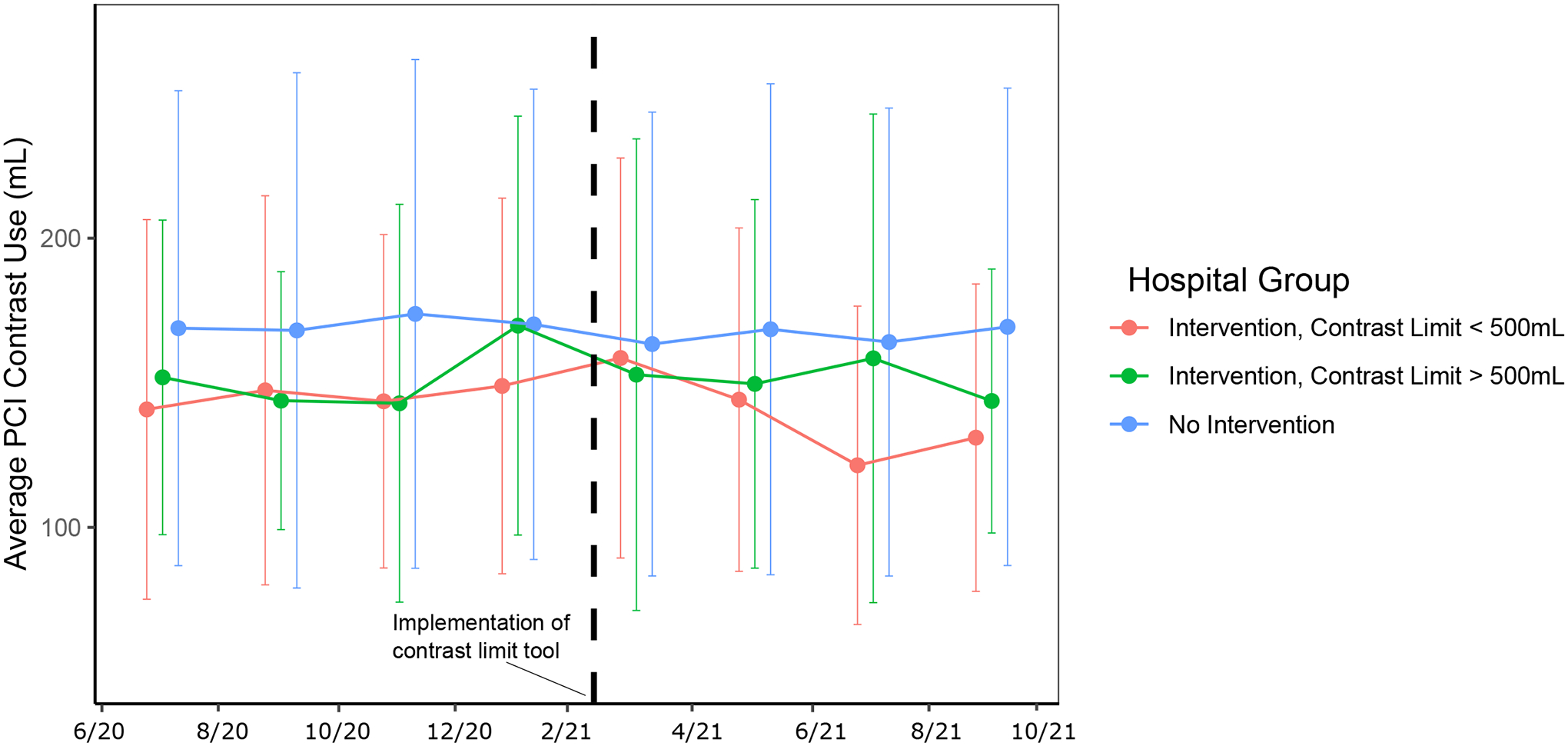
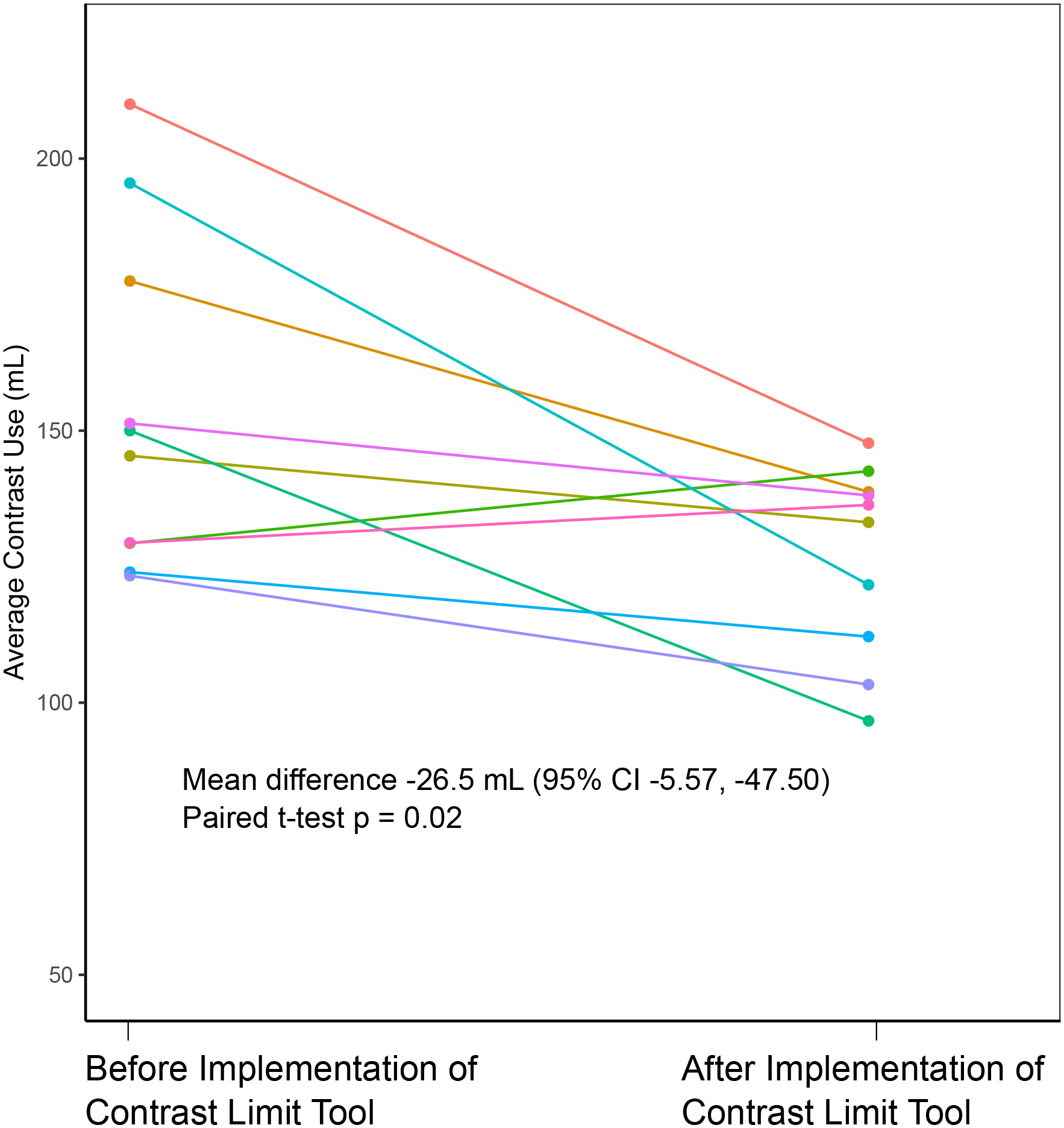
Contrast use before and after contrast limit tool implementation
A. Contrast use stratified by intervention vs. control group. Bars represent one standard deviation.
B. Contrast use stratified by PCI operator at the intervention site.
At the intervention site, we surveyed 8 interventional cardiologists pre-implementation and 10 post-implementation. Prior to implementation of the contrast limit tool, while 75% of clinicians agreed that CA-AKI after PCI remained a serious problem, only 12.5% believed that they could improve their CA-AKI rates (Figure 3A). We found that 25% reported using a contrast limit to make decisions about PCI and only 50% felt that knowing the safe contrast limit for a patient would substantially change how they practice. In their assessment of CA-AKI risk, respondents always considered creatinine/eGFR, diabetes, and age (Figure 3B). However, a substantial proportion of clinicians did not consider risk factors such as shock (25% of clinicians), history of heart failure (50%), and anemia (75%) despite these risk factors having higher contributions to CA-AKI risk in prior models than either diabetes or age.
Figure 3.
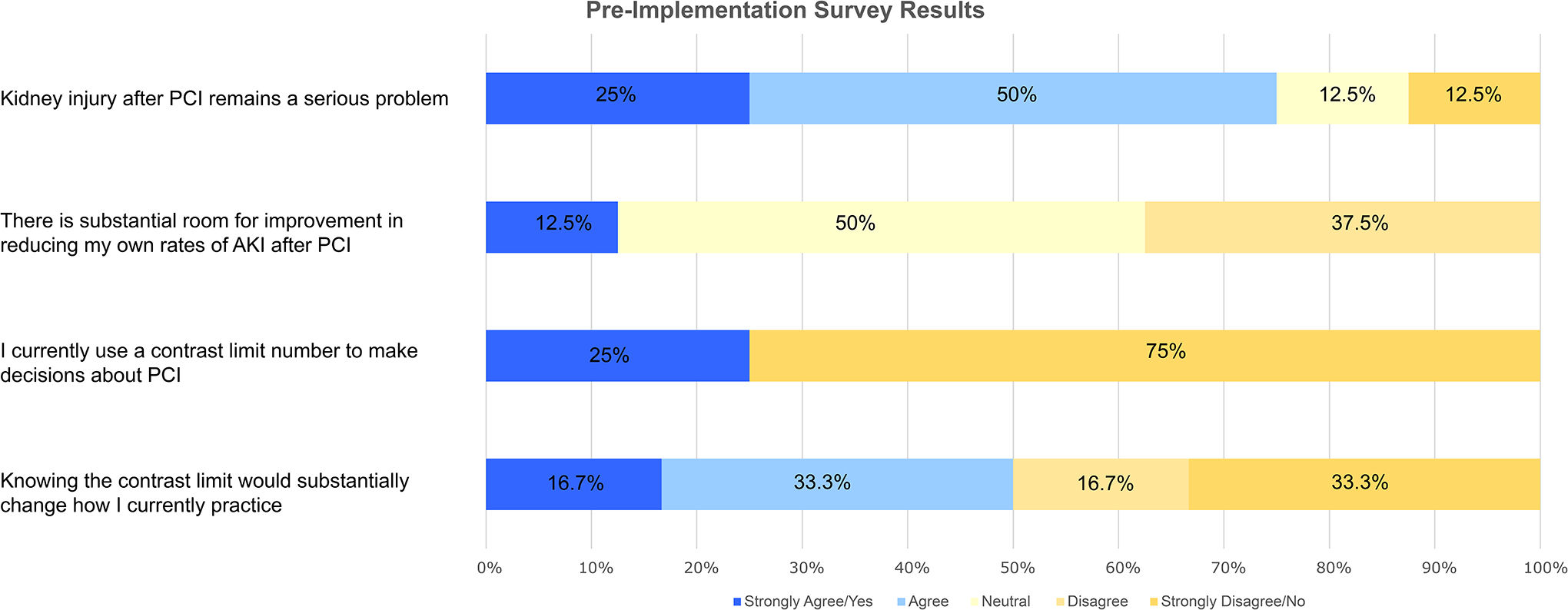
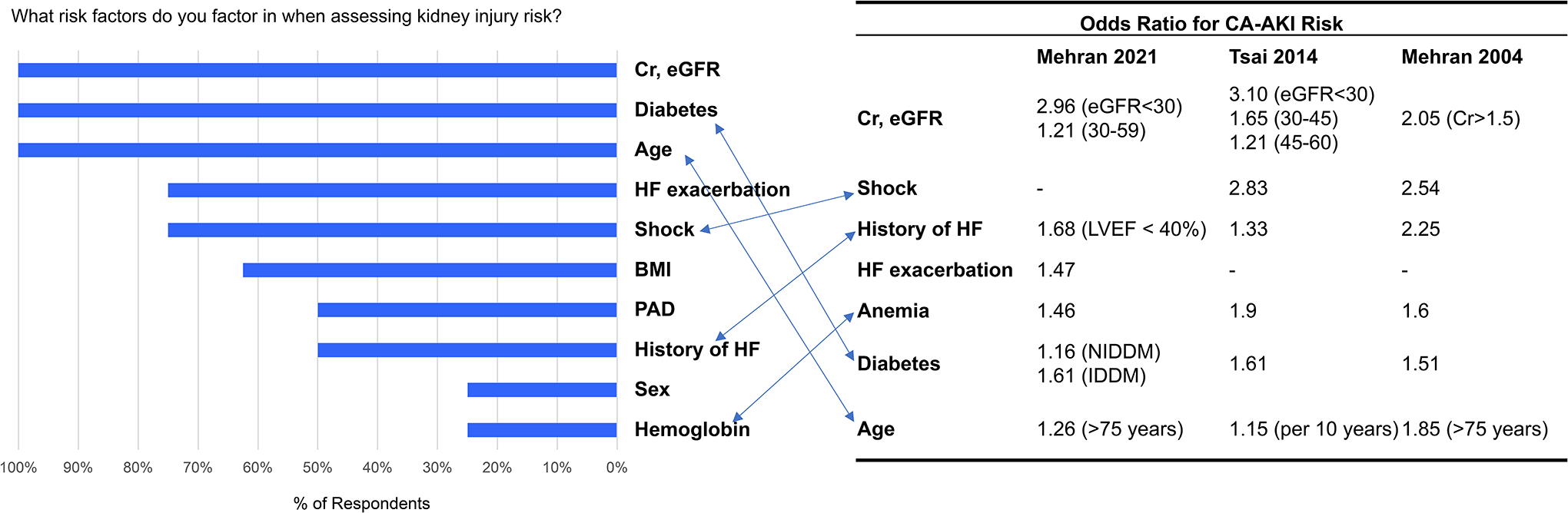
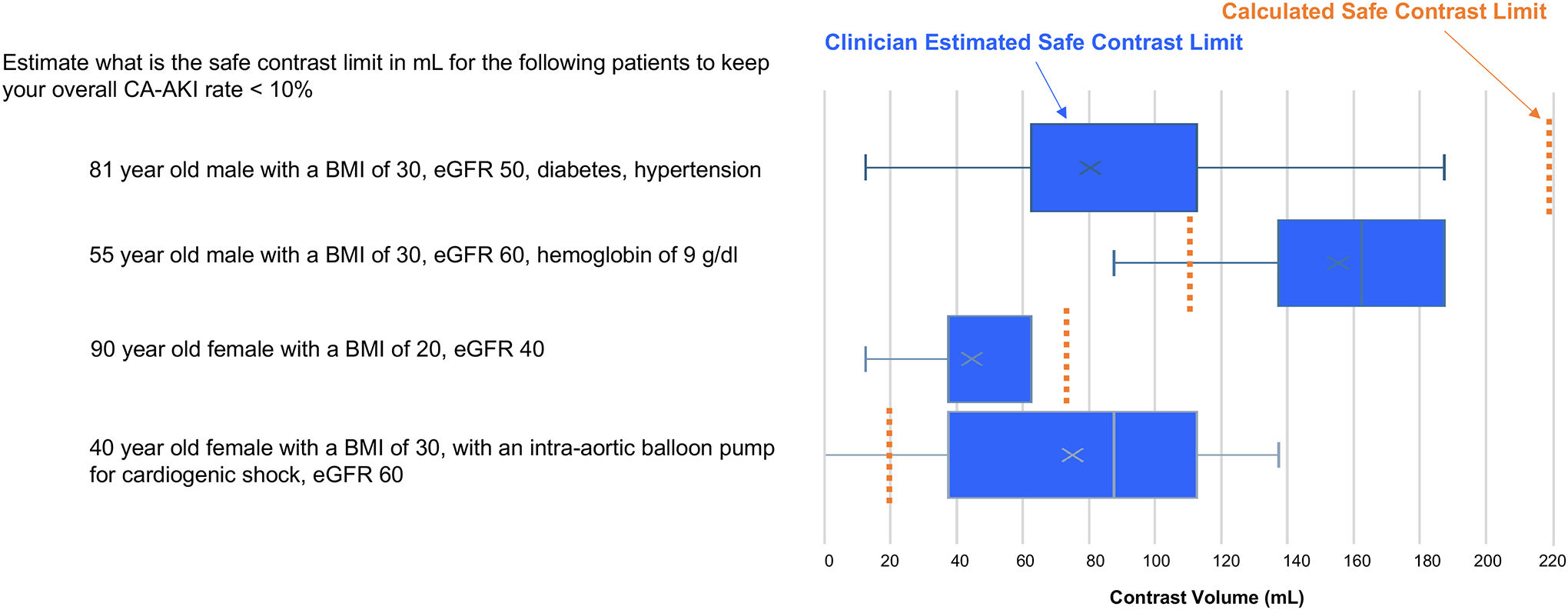
Clinician survey responses before implementation of contrast limit tool
A. Clinician beliefs
B. Clinician risk factor assessment compared to established CA-AKI risk models
C. Safe contrast limit estimations for example patients compared with contrast limits calculated from a multivariable CA-AKI risk model.
In their estimation of safe contrast limits for the 4 example patients, we found that compared to safe contrast limits calculated by our models, clinicians underestimated the contrast limit for the 81-year-old male with a BMI of 30 kg/m2, eGFR 50 ml/min, diabetes, and hypertension (IQR of clinician estimations 62.5–112.5 ml, calculated safe contrast limit 219 ml) as well as the 90-year-old female with a BMI of 20 kg/m2, and eGFR 40 ml/min (estimated 37.5–62.6, actual 97) (Figure 3C). Clinicians overestimated the safe contrast limit for the 55-year-old male with a BMI of 30 kg/m2, eGFR 60 ml/min, and hemoglobin of 9 g/dl (estimated 137.5–187.5, actual 115) as well as the 40-year-old female with a BMI of 30 kg/m2, an intra-aortic balloon pump for cardiogenic shock, and eGFR 60 ml/min (estimated 37.5–112.5, actual 20).
We additionally compared the relative risk predictions embedded in the PCI operators’ contrast limit estimations to the CA-AKI risk estimations from two commonly available CA-AKI risk online risk calculators.11,15 When comparing the 81-year-old with a BMI of 30 kg/m2, eGFR 50 ml/min, diabetes, and hypertension to the 55-year-old with a BMI of 30 kg/m2, eGFR 60 ml/min, and hemoglobin of 9 g/dl, for both calculators, the patients had similar CA-AKI risks (Mehran: 14%, 14% respectively; Tsai: 4.9%, 4.9%). This was discrepant with the embedded risk estimation by PCI operators who predicted that the former patient would have a much higher risk (and hence lower contrast limit) than the latter patient. For the 90-year-old female with a BMI of 20 kg/m2, and eGFR 40 ml/min as well as the 40-year-old female with a BMI of 30 kg/m2, an intra-aortic balloon pump for cardiogenic shock, and eGFR 60 ml/min, both calculators gave a substantially lower CA-AKI risk for the former patient compared to the latter (Mehran: 14%, 26.1%; Tsai 4.9%, 9.2%). PCI operators, however, estimated that the former patient had a similar or higher CA-AKI risk (and hence similar or lower contrast limit).
In the post-implementation survey, all clinicians reported seeing the contrast limit alert in the EHR and 80% said that the catheterization lab staff discussed the contrast limit with them before or during procedures (Supplemental Figure 1A). With regards to the alert implementation, 60% found the contrast limit clear and understandable and 70% agreed that the contrast limit alert did not significantly interfere with their clinical workflow. With respect to clinician beliefs about CA-AKI, 20% were surprised by the calculated contrast limit, 40% felt that the contrast limit accurately identified a patient’s true contrast limit, and 40% felt that the contrast limit helped them reduce their patient’s rates of CA-AKI. Half of clinicians believed that the contrast limit was useful information that they would want to continue to have access to. Eight of 10 clinicians reported considering the contrast limit when making PCI-related decisions, including reconsidering performing PCI (20%), staging a PCI procedure (50%), using aggressive hydration (70%), minimizing contrast use more than normal (50%), using a Dye ACIST system (10%), and diluting the contrast concentration (10%). Clinicians also gave free-response feedback through the survey. Their comments mainly concerned the usability of the contrast limit tool and the clinician’s underlying beliefs about CA-AKI and the utility of using a contrast limit (Supplemental Figure 1B).
We used the GUIDES checklist in conjunction with responses from our clinician surveys to systematically review the implementation of our contrast limit intervention (Supplemental Table 2). Areas we identified to focus on for the future included improving stakeholder and user acceptance of the tool, minimizing added perceived work burden, formalizing use of tool in pre-procedure time outs, and providing feedback to clinicians about their contrast use and CA-AKI rates.
Discussion
In this study we found that an EHR-based safe contrast limit tool using automatically derived patient data performed well in predicting CA-AKI and reduced the average amount of contrast used during PCI procedures over an 8 month follow-up period. We did not observe a significant difference in rates of CA-AKI. Surveys of interventional cardiologists before and after the contrast limit implementation showed that clinicians often overemphasized the importance of certain CA-AKI risk factors (age and diabetes) while underemphasizing others (anemia, heart failure, shock). This resulted in estimations of safe contrast limits that were frequently inconsistent with predictions from prior published CA-AKI risk models. However, clinicians, often believed that a more accurate auto-calculated contrast limit was unnecessary and that there was little room for improvement in their contrast usage and CA-AKI rates. Despite this initial skepticism, the safe contrast limit tool was frequently used by clinicians after implementation and was associated with small reductions in contrast use.
In prior work, we discussed the potential benefits of using a safe contrast limit tool, which can provide actionable information to clinicians to help risk stratify patients and also provide intraprocedural guidance for contrast use.13 We prospectively confirmed that the contrast limit model predicted CA-AKI with good accuracy, consistent with previously published performance characteristics.13 By design, the model was conservative to ensure that cases of CA-AKI were not missed, thus explaining the high negative predictive value but lower positive predictive value. Nearly half of the patients were in the modifiable risk group (contrast limit 20–500 ml), meaning that their CA-AKI risk could be potentially meaningfully influenced by efforts to reduce contrast.
At baseline there was a higher rate of CA-AKI at the intervention site compared to the control sites despite the average contrast use being less per PCI. This most likely reflects the higher risk patient population seen at the intervention hospital, which is an academic referral center specializing in advanced interventional procedures. Given that surveyed providers heavily weighted the influence of creatinine, age, and diabetes in determining CA-AKI risk, intervention site operators may have more actively limited their PCI contrast usage in this higher risk population compared to operators at control sites. However, their baseline efforts to reduce contrast usage appeared to be insufficient to reduce CA-AKI rates to those rates observed at the control hospitals.
We found that at the control sites, there was little change in PCI contrast volume usage during the study period. In comparison, at the intervention site, there was a small but significant decrease in average PCI contrast usage over time after implementation of the contrast limit tool, a difference that was also confirmed when looking at contrast use on an individual clinician basis. As might be expected, this decrease in contrast use was seen only in the high and modifiable CA-AKI risk patient groups where PCI operators would be motivated to actively limit their contrast use. There was no significant decrease in contrast use in the low CA-AKI risk patients, as limiting contrast use in these patients would be less necessary. Despite a decrease in contrast usage, we did not observe an appreciable difference in the proportion of PCIs where the contrast limit was exceeded or in rates of CA-AKI, likely due to the modest contrast volume reduction and being underpowered to detect a difference in CA-AKI rates. Given our sample size and a baseline CA-AKI rate of 12%, within the modifiable risk group of patients, we would have been able to detect with 80% power a fall in the CA-AKI rate by 7.4% or more. However, the difference in average contrast use before versus after the contrast limit intervention was 11.6 mL which would be expected to result in only a 2.3% decrease in CA-AKI.13 Longer follow-up including more patients would be helpful for ultimately clarifying the association between implementation of the contrast limit tool and CA-AKI rates.
Surveys of interventional cardiologists revealed that most clinicians did not think about CA-AKI risk in terms of a contrast limit when performing PCI. Instead, most chose to rely on a general assessment of a patient’s risk based most often on a patient’s creatinine, age, and diabetes status. Notably, when compared to the actual influence of such factors on CA-AKI risk per previously published models, clinicians often overestimated the contribution of age and diabetes to CA-AKI risk while often neglecting to consider other substantial risk factors such as anemia, cardiogenic shock, and history of heart failure.10,11,15 This pattern was paralleled in the contrast limit estimations for the example patients. In the 81-year-old male with diabetes and the 90-year-old female, clinicians underestimated the safe contrast limit relative to the calculated contrast limit, possibly because they overemphasized the influence of age and diabetes on CA-AKI risk. In the patient with a hemoglobin of 9 g/dl and the patient with an intra-aortic balloon pump, clinicians overestimated the safe contrast limit, likely because they underappreciated the contributions of anemia and shock to CA-AKI risk. These over- and underestimations were also apparent when comparing the embedded relative CA-AKI risk assumptions of the example patients to CA-AKI risks calculated by two widely available online CA-AKI risk calculators. These results suggest that an “eyeball” approach to CA-AKI risk assessment employed by some PCI operators may oversimplify CA-AKI risk assessment, resulting in both over- and underestimation of CA-AKI risk. Such errors are not surprising given the difficulty of accounting for multiple risk factors, which may naturally lead to simplified decision-making heuristics such as only considering kidney function and age.16 An automated risk tool such as the EHR-based contrast limit helps overcome these cognitive errors and can help standardize more precise risk-informed decisions.
We used the GUIDES checklist, a guideline for evaluating computerized clinical decision support, to help envision future improvements to the contrast limit tool.14 Some of the biggest challenges to our tool’s success came from clinicians’ pre-existing beliefs. Clinicians largely did not believe that there was room for improvement in their own CA-AKI rates. Less than half of surveyed clinicians believed that the contrast limit helped them reduce their patients’ rates of CA-AKI. In their free text responses, multiple clinicians felt that the contrast limit was not valuable because they were already “very conscientious of the amount of contrast used”. As such, improving implementation of the contrast limit tool will require greater focus on clinician education of the tool’s ability to improve upon current practices. This might be accomplished by presenting some of the aforementioned data showing the inaccuracies of an “eyeball” approach to CA-AKI risk assessment. It also may be useful to emphasize that despite clinicians feeling already highly attuned to the need to minimize contrast use, there is still room to do more as evidenced by the low rates of clinicians deciding to stage procedures, use more aggressive pre-and post-PCI hydration, or dilute contrast. Studies have shown that it is even possible to complete near-zero contrast studies using intravascular ultrasound imaging.17 Admittedly, these strategies are time and resource-intensive, which is why a contrast limit can help clinicians decide when such tradeoffs may be indicated. The contrast limit is also not always a restrictive parameter. In low risk patients, it may help clinicians liberalize their contrast use and/or convince patients and their providers that PCI is safe. As one provider noted, the contrast limit was “important for nephrologists and PCPs to let patients know how low the risk is, so they don’t defer lifesaving procedures”.
The GUIDES checklist additionally evaluates the implementation of the decision support tool. In designing the contrast limit tool, we conscientiously introduced an alert that required no additional clinician data entry, was simple in its recommendations (i.e. a single contrast limit number), and would only fire at the time of decision about PCI (i.e. when the patient PCI consent order was placed) or when the PCI was occurring (i.e. when the patient changed locations to the catheterization lab). In the post-implementation survey, the majority of clinicians felt that the contrast limit tool was clear and understandable and did not interfere with their clinical workflow. Nevertheless, some clinicians did find that having to see an additional alert in the EHR was bothersome. Decreasing this perceived burden in the future could include reducing the friction of action by providing linked interventions. Examples include connecting an auto-generated printout about the patient’s CA-AKI risk for shared-decision making, linking tailored pre- and post-procedure hydration orders, and developing a protocol for preparing dilute contrast for high risk patient PCIs. While 80% of clinicians reported that the catheterization lab staff discussed the contrast limit with them before or during procedures, this could be further standardized by incorporating the contrast limit into a pre-procedure Time-Out checklist. Providing both positive and negative feedback to clinicians with regards to how often they exceed the contrast limit and their rates of CA-AKI could also be helpful motivating factors.
Several study limitations warrant consideration. The contrast limit tool was implemented at one healthcare site, which may limit the generalizability of these results to other settings with different patient populations and clinician beliefs. Nevertheless, our site had many interventional cardiologists from both academic and private practice backgrounds. Our contrast limit tool was also implemented in an Epic-based EHR, one of the most prevalent EHR systems. Based on NCDR-defined criteria for CA-AKI events, a substantially higher proportion of PCIs at the intervention site compared to the control sites were excluded from the analysis, mainly because of a high rate of same-day discharge and missingness of post-procedure creatinine values. This would be expected to skew the intervention site cohort towards higher risk patients. Indeed, about a third of the intervention site patients fell into the high CA-AKI risk category (i.e. calculated contrast limit < 20mL), meaning that many patients may have been prone to developing CA-AKI regardless of how little contrast was used. This in turn could have blunted any observed reductions in CA-AKI by the contrast limit tool. It would be helpful to consider studying the intervention in lower risk patients. Model coefficients also could be re-derived within individual hospital systems to better reflect local populations.
We observed a small sacrifice in the accuracy of our contrast limit in predicting CA-AKI when using the most limited contrast limit equation (the pragmatic minimum model) from our initial publication.13 However, it was felt that this would make for the easiest EHR implementation and guarantee that no additional clinician-side data entry was needed. An expanded model from our prior publication (the pragmatic full model) is more accurate and could still be conceivably implemented from automatically derived EHR fields but would require more imputation from patient record information (E.g. identifying whether a patient is in cardiogenic shock based off of blood pressure, vasopressor usage, and/or presence of mechanical circulatory support). Using NCDR registry data, this model increased CA-AKI prediction accuracy modestly from 64.1% to 65.5% in the intervention cohort and from 63.5% to 66.6% in the control cohort. An even more expanded model (the full model) would be more accurate still, but would likely require additional clinician-side input that could hamper clinical adoption of the tool. Nevertheless, implementing a more accurate model could increase the efficacy of the safe contrast limit in preventing CA-AKI. Longer term follow up would be helpful to study whether the observed lower average contrast use persists and whether there is an observable effect on rates of CA-AKI.
Conclusion
In this study, we describe the implementation of an EHR-based contrast limit tool that was associated with a small but significant decrease in average contrast use during PCI procedures over an 8-month follow-up period when compared to control medical centers that did not implement the tool. In surveys of interventional cardiologists before and after implementation of the intervention, we found that clinicians often relied on a simplified assessment of CA-AKI risk that neglected important risk factors such as anemia, heart failure, and shock. This led to both over- and underestimation of contrast limits. While many clinicians remained skeptical of the utility of the contrast limit, the safe contrast limit tool was frequently used after its implementation and was associated with small reductions in contrast use.
Supplementary Material
Table S1. Percentage of total percutaneous coronary interventions (PCIs) excluded from study stratified by reasons for exclusion.
Figure S1. Clinician survey after implementation of contrast limit tool
A. Likert scale responses
B. Free text responses
Abbreviations: EHR = electronic health records, AKI = acute kidney injury, CA-AKI = contrast associated acute kidney injury
Table S2: GUIDES checklist evaluation of contrast limit tool
What is known.
Contrast-associated acute kidney injury (CA-AKI) is a frequent complication of percutaneous coronary intervention (PCI)
Reducing contrast usage can help mitigate CA-AKI risk, but the amount of contrast use that is safe depends on a patient’s individualized risk profile
What the study adds.
Surveys of interventional cardiologists revealed that clinicians often relied on a simplified, “eyeball” assessment of CA-AKI risk using creatinine, age, and/or diabetes status. Clinicians often did not consider other important risk factors such as anemia, cardiogenic shock, and heart failure, which led to risk estimations inconsistent with established CA-AKI risk models.
An electronic health records (EHR)-based tool that automatically calculates and displays the safe contrast limit using information from the EHR predicted CA-AKI events with moderate accuracy and resulted in small but significant decreases in contrast usage over time.
Funding
This works was supported by the following grants:
NIH T32 5T32HL116273-07
CSMC/UCLA CTSI Eigler-Whiting-Mann Research Grant
Funding sources were not involved in study design, data collection, or analysis
Non-standard Abbreviations and Acronyms
- CA-AKI
Contrast-associated acute kidney injury
- PCI
Percutaneous coronary intervention
- EHR
Electronic health records
- NCDR
National Cardiovascular Disease Registry
- BPA
Best practice advisory
- IABP
Intra-aortic balloon pump
Footnotes
Disclosures
RK works for Biome Analytics, a company contracted by Cedars‐Sinai Medical Center to conduct data analytics work using methods that are unrelated to the clinical content area of this manuscript and, thus, involving no direct or indirect financial or other interests. The remaining authors have no disclosures to report.
References
- 1.Mettler FA, Mahesh M, Bhargavan-Chatfield M, Chambers CE, Elee JG, Frush DP, Miller DL, Royal HD, Milano MT, Spelic DC, Ansari AJ, Bolch WE, Guebert GM, Sherrier RH, Smith JM, Vetter RJ. Patient Exposure from Radiologic and Nuclear Medicine Procedures in the United States: Procedure Volume and Effective Dose for the Period 2006–2016. Radiology. 2020;295:418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012;33:2007–2015. [DOI] [PubMed] [Google Scholar]
- 3.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–375. [DOI] [PubMed] [Google Scholar]
- 4.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. [DOI] [PubMed] [Google Scholar]
- 5.Finn WF. The clinical and renal consequences of contrast-induced nephropathy. Nephrol Dial Transplant. 2006;21:i2–10. [DOI] [PubMed] [Google Scholar]
- 6.Giacoppo D, Madhavan MV, Baber U, Warren J, Bansilal S, Witzenbichler B, Dangas GD, Kirtane AJ, Xu K, Kornowski R, Brener SJ, Généreux P, Stone GW, Mehran R. Impact of Contrast-Induced Acute Kidney Injury After Percutaneous Coronary Intervention on Short- and Long-Term Outcomes: Pooled Analysis From the HORIZONS-AMI and ACUITY Trials. Circ Cardiovasc Interv. 2015;8:e002475. [DOI] [PubMed] [Google Scholar]
- 7.Gupta R, Gurm HS, Bhatt DL, Chew DP, Ellis SG. Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv. 2005;64:442–448. [DOI] [PubMed] [Google Scholar]
- 8.Weisbord SD, Chen H, Stone RA, Kip KE, Fine MJ, Saul MI, Palevsky PM. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol. 2006;17:2871–2877. [DOI] [PubMed] [Google Scholar]
- 9.Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, Grines CL, O’Neill WW. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515–1519. [DOI] [PubMed] [Google Scholar]
- 10.Mehran R, Owen R, Chiarito M, Baber U, Sartori S, Cao D, Nicolas J, Pivato CA, Nardin M, Krishnan P, Kini A, Sharma S, Pocock S, Dangas G. A contemporary simple risk score for prediction of contrast-associated acute kidney injury after percutaneous coronary intervention: derivation and validation from an observational registry. Lancet. 2021;398:1974–1983. [DOI] [PubMed] [Google Scholar]
- 11.Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Weintraub WS, Curtis JP, Messenger JC, Rumsfeld JS, Spertus JA. Validated Contemporary Risk Model of Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Interventions: Insights From the National Cardiovascular Data Registry Cath‐PCI Registry. J Am Heart Assoc [Internet]. 2014. [cited 2018 Dec 13];3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4338731/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel Z. Risk prediction models for contrast induced nephropathy: systematic review. BMJ. 2015;351:h4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan N, Latif K, Botting PG, Elad Y, Bradley SM, Nuckols TK, Cheng S, Ebinger JE. Refining Safe Contrast Limits for Preventing Acute Kidney Injury After Percutaneous Coronary Intervention. J Am Heart Assoc. 2021;10:e018890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van de Velde S, Kunnamo I, Roshanov P, Kortteisto T, Aertgeerts B, Vandvik PO, Flottorp S, GUIDES expert panel. The GUIDES checklist: development of a tool to improve the successful use of guideline-based computerised clinical decision support. Implement Sci. 2018;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 16.Tversky A, Kahneman D. Judgment under Uncertainty: Heuristics and Biases. Science. 1974;185:1124–1131. [DOI] [PubMed] [Google Scholar]
- 17.Mariani J, Guedes C, Soares P, Zalc S, Campos CM, Lopes AC, Spadaro AG, Perin MA, Filho AE, Takimura CK, Ribeiro E, Kalil-Filho R, Edelman ER, Serruys PW, Lemos PA. Intravascular ultrasound guidance to minimize the use of iodine contrast in percutaneous coronary intervention: the MOZART (Minimizing cOntrast utiliZation With IVUS Guidance in coRonary angioplasTy) randomized controlled trial. JACC Cardiovasc Interv. 2014;7:1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Percentage of total percutaneous coronary interventions (PCIs) excluded from study stratified by reasons for exclusion.
Figure S1. Clinician survey after implementation of contrast limit tool
A. Likert scale responses
B. Free text responses
Abbreviations: EHR = electronic health records, AKI = acute kidney injury, CA-AKI = contrast associated acute kidney injury
Table S2: GUIDES checklist evaluation of contrast limit tool


