Abstract
OBJECTIVE:
To assess the presence of sociodemographic and clinical disparities in fertility-sparing treatment and assisted reproductive technology (ART) use among patients with a history of cervical, endometrial, or ovarian cancer.
METHODS:
We conducted a population-based cohort study of patients aged 18–45 years who were diagnosed with cervical (stage IA, IB), endometrial (grade 1, stage IA, IB), or ovarian (stage IA, IC) cancer between January 1, 2000, and December 31, 2015, using linked data from the California Cancer Registry, the California Office of Statewide Health Planning and Development, and the Society for Assisted Reproductive Technology. The primary outcome was receipt of fertility-sparing treatment, defined as surgical or medical treatment to preserve the uterus and at least one ovary. The secondary outcome was fertility preservation, defined as ART use after cancer diagnosis. Multivariable logistic regression analysis was used to estimate odds ratios and 95% confidence intervals for the association between fertility-sparing treatment and exposures of interest: age at diagnosis, race and ethnicity, health insurance, socioeconomic status, rurality, and parity.
RESULTS:
We identified 7,736 patients who were diagnosed with cervical, endometrial, or ovarian cancer with eligible histology. There were 850 (18.8%) fertility-sparing procedures among 4,521 cases of cervical cancer, 108 (7.2%) among 1,504 cases of endometrial cancer, and 741 (43.3%) among 1,711 cases of ovarian cancer. Analyses demonstrated non-uniform patterns of sociodemographic disparities by cancer type for fertility-sparing treatment and ART. Fertility-sparing treatment was more likely among young patients overall, and of those in racial and ethnic minority groups among survivors of cervical and ovarian cancer. ART use was low (n=52) and was associated with non-Hispanic White race and ethnicity, younger age (18–35 years), and private insurance.
CONCLUSION:
This study demonstrates that clinical and sociodemographic disparities exist in the receipt of fertility-sparing treatment and ART use among patients with a history of cervical, endometrial, or ovarian cancer.
Precis
Disparities exist in fertility-sparing treatment and the use of assisted reproductive technology among patients with gynecologic malignancies.
INTRODUCTION
Gynecologic malignancies account for over 10,000 cancer diagnoses among reproductive-aged women in the United States each year.1 Fertility preservation may improve the ability of reproductive-aged patients to cope with cancer2–5 and improve survivors’ quality of life.6 While definitive surgical resection is indicated for many, national guidelines recommend that physicians discuss fertility at the time of cancer diagnosis to allow for counseling about options.7–10 Fertility-sparing treatment is underused in early-stage gynecologic malignancies, despite being a reasonable and safe alternative to hysterectomy or bilateral-salpingooophorectomy.11–14
Prior studies demonstrate disparities in cancer-related treatment by geography,15–17 race and ethnicity,15,18–21 and insurance status;22,23 however, few examine disparities specifically related to fertility-sparing treatment.24 Reproductive-age patients face disparities accessing assisted reproductive technology (ART) services due to socioeconomic status (SES), geographic location, and race and ethnicity despite state mandates to offer insurance coverage for ART services, 25–27 however there are few studies of cancer survivors’ use of ART.28
This study sought to assess the presence of disparities in fertility-sparing treatment and ART use among patients with a history of cervical, endometrial, or ovarian cancer. We hypothesized that fertility-sparing treatments are more likely to be covered by insurance than ART, resulting in fewer disparities. Disparity was defined using the Institute of Medicine definition: a difference in health care quality not due to differences in the health care needs of the patient.29
METHODS
This population-based study used data linked between the California Cancer Registry (CCR), the California Office of Statewide Health Planning and Development (OSHPD), and the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS). We obtained approval for this study from the MD Anderson Institutional Review Board, the California OSHPD, CCR, the State of California Committee for the Protection of Human Subjects, and SART. The linked data set included CCR data from January 1, 2000, to December 31, 2015, and OSHPD data files for patients treated from January 1, 2000, through December 31, 2012 (Appendix 1, available online at http://links.lww.com/xxx). The data files included diagnostic and procedure codes using the International Classification of Diseases, 9th Clinical Modification and 10th Revisions.30
The data from CCR and OSHPD were linked to SART CORS to identify patients with a gynecologic malignancy who underwent ART treatment between January 1, 2004 (earliest available data from SART), and December 31, 2015, using the woman’s birth date, first and last name, social security number, and infant’s birth date (when applicable). SART CORS includes more than 80% of clinics that provide ART, 90% of ART cycles in the United States, and is subject to annual review and verification.31,32
In the linked database, we identified patients aged 18–45 years at the time of diagnosis with cervical cancer (stage IA, IB), endometrial cancer (grade 1, stage IA or IB), and ovarian cancer (stage IA, IC) between January 1, 2000, and December 31, 2015. The primary outcome analysis used the maximal date range of data available, 2000–2015. Secondary analyses utilized date ranges pending availability of data, described below. All stages were based on available data and defined by the American Joint Committee on Cancer (3rd edition for 2000–2004, 6th edition for 2004–2009, and 7th edition for 2010–2015).33 We included histologies eligible for fertility-sparing procedures using the International Classification of Diseases for Oncology codes (Appendix 2, available online at http://links.lww.com/xxx).34
The primary outcome, receipt of fertility-sparing treatment, was defined as interventions that allowed for the retention of at least one ovary and the uterus for all three cancer types. Treatment included: loop electrosurgical excisional procedure, conization, and trachelectomy, with or without ovarian transposition or lymph node evaluation for cervical cancer;11 hormonal management with progestin via intrauterine devices or oral medications for endometrial cancer;35 and unilateral oophorectomy without hysterectomy, with or without additional biopsies for ovarian cancer.11 This outcome was investigated using data from 2000–2015.
The secondary outcome, ART use following cancer diagnosis, was defined as one or more autologous embryo- or oocyte-freeze cycles or embryo transfer cycles after the date of cancer diagnosis. This outcome was investigated using ART data from 2004–2015 for individuals diagnosed from 2000–2015.
The exposures of interest included age (18–35 or 36–45 years) at diagnosis, race and ethnicity (American Indian, Asian or Pacific Islander, Hispanic, non-Hispanic Black, non-Hispanic White, and none of the above), insurance status at diagnosis (public, private, uninsured or self-pay, and other or unknown), rurality (urban or rural, according to medical service study area by census tract), and socioeconomic status (SES, Yost SES index at the census tract level,36 by quintiles). The additional variables collected for multivariable analysis included parity (0, 1, or 2 or more prior births), Charlson comorbidity scores at the time of diagnosis, marital status, year of diagnosis (2000–2005, 2006–2010, 2011–2015), cancer stage, and receipt of adjuvant chemotherapy, radiotherapy, or hormonal therapy. Histology was collected for all cancer types but only included in multivariate analysis for ovarian cancer due to exclusion of histologies contraindicated for fertility-sparing treatment limiting this variable’s impact on endometrial and cervical cancer outcomes. Race and ethnicity data were used as exposures of interest given the results of prior studies demonstrating differences in fertility-sparing treatment among patients with a history of gynecologic cancer24 and access to ART overall25 by race and ethnicity.
Descriptive statistics of sociodemographic variables were performed among those who received fertility-sparing treatment and had a subsequent live birth after excluding those who received radiation. Live births were defined as the first birth whose fertilization occurred 3 or more months following a cancer diagnosis and fertility-sparing treatment. The fertilization date for each live birth was estimated using the infant’s date of birth and gestational age at delivery.37 Live birth outcomes were available through the OSHPD database for births between January 1, 2000, and December 31, 2012, and were assessed for diagnoses from the same time period.
Categorical variables were assessed using Chi-squared tests or Fisher’s exact tests. An analysis of the primary outcome was performed using multivariable logistic regression to assess the association between fertility-sparing treatment and the exposures of interest by cancer type. Due to the limited number of ART instances in the linked dataset, the variables of interest were collapsed to binary categories. Given overall limited number of ART procedures, univariable logistic regression analysis was performed for the secondary outcome to assess the association of exposures of interest and ART use by cancer type. Odds ratios were calculated with 95% confidence intervals.
All statistical tests were two-sided, and differences were considered statistically significant at P<0.05 and a 95% confidence interval not inclusive of the null (1.0). SAS Enterprise Guide software version 7.11 (SAS Institute, Inc.) was used for all statistical analyses.
We performed a sensitivity analysis of our primary outcome using the E-value, a measure which describes the robustness of estimates to possible unmeasured confounders using minimal assumptions.38 We used the E-value to quantify the magnitude of the association an unmeasured confounder would need with the exposures (sociodemographic and clinical variables) and primary outcome (fertility-sparing treatment) to explain away the calculated estimate, the E-value required to explain the confidence limit that was closest to the null, and to shift the confidence limit to a significant result.
RESULTS
We identified 7,736 patients aged 18–45 years who were diagnosed with a gynecologic malignancy in California between January 1, 2000, and December 31, 2015, and were eligible by histology to receive fertility-sparing treatment. Of these, 4,521 (58.4%) were diagnosed with cervical cancer (stage IA or IB), 1,504 (19.4%) with endometrial cancer (stage IA, IB), and 1,711 (22.1%) with ovarian cancer (stage IA, IC) (Figure 1).
Figure 1.
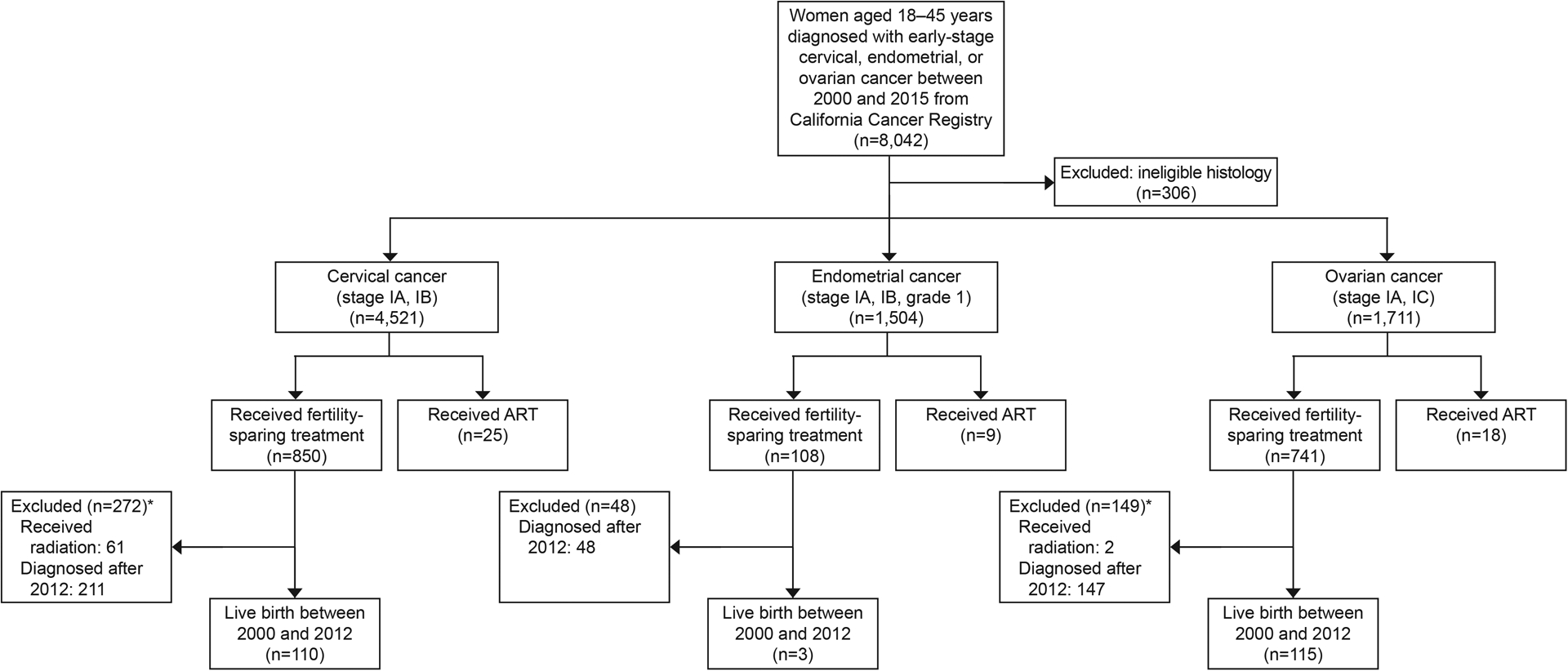
Selection of cases. Assisted reproductive technology (ART) use was assessed for women diagnosed 2000–2015, based on available ART data from 2004 to 2015. Live births were assessed for those with available data (diagnoses from 2000–2012). Included histologies are listed in Appendix 2 (http://links.lww.com/xxx) *Not mutually exclusive.
There were 1,699 (22.0%) fertility-sparing procedures. Of the 4,521 patients with a history of cervical cancer 850 (18.8%) received fertility-sparing procedures, of the 1,504 patients with a history of endometrial cancer 108 (7.2%) received fertility-sparing treatment, and of the 1,711 patients with a history of ovarian cancer 741 (43.3%) received fertility-sparing procedures (Table 1). There were 52 (0.7%) instances of ART, and 228 (17.6%) patients had a live birth between 2000 and 2012 following fertility-sparing treatment (Table 2).
Table 1.
Patient Characteristics by Fertility-Sparing Treatment and Cancer Type
| Sociodemographic and clinical characteristics | Cervical (n=4,521) | Endometrial (n=1,504) | Ovarian (n=1,711) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fertility-Sparing (n=850) | Not Fertility-Sparing (n=3,671) | P Value | Fertility-Sparing (n=108) | Not Fertility-Sparing (n=1,396) | P Value | Fertility-Sparing (n=741) | Not Fertility-Sparing (n=970) | P Value | |
| Age (years), n (%) | <0.01 | <0.01 | <0.01 | ||||||
| 18–35 | 573 (31.3) | 1,260 (68.7) | 66 (18.2) | 296 (81.8) | 552 (70.5) | 231 (29.5) | |||
| 36–45 | 277 (10.3) | 2,411 (89.7) | 42 (3.7) | 1,100 (96.3) | 189 (20.4) | 739 (79.6) | |||
| Diagnosis year, n (%) | <0.01 | 0.04 | 0.22 | ||||||
| 2000–2005 | 296 (16.4) | 1508 (83.6) | 17 (6.8) | 233 (93.2) | 255 (42.6) | 344 (57.4) | |||
| 2006–2010 | 292 (19.5) | 1208 (80.5) | 32 (5.3) | 571 (94.7) | 229 (41.2) | 327 (58.8) | |||
| 2011–2015 | 262 (21.5) | 955 (78.5) | 59 (9.1) | 592 (90.9) | 257 (46.2) | 299 (53.8) | |||
| SES, n (%) | <0.01 | 0.52 | 0.96 | ||||||
| Lowest | 160 (17.8) | 738 (82.2) | 24 (7.2) | 309 (92.8) | 106 (42.1) | 146 (57.9) | |||
| Lower-middle | 154 (16.5) | 780 (83.5) | 21 (5.6) | 355 (94.4) | 151 (43.8) | 194 (56.2) | |||
| Middle | 156 (16.7) | 778 (83.5) | 20 (6.8) | 274 (93.2) | 165 (44.7) | 204 (55.3) | |||
| Upper-middle | 206 (20.6) | 794 (79.4) | 26 (8.2) | 293 (91.8) | 163 (43.1) | 215 (56.9) | |||
| Highest | 174 (23.0) | 581 (77.0) | 17 (9.3) | 165 (90.7) | 156 (42.5) | 211 (57.5) | |||
| Charlson comorbidity score, n (%) | <0.01 | <0.01 | 0.01 | ||||||
| Score=0 | 621 (16.6) | 3121 (83.4) | 70 (6.8) | 966 (93.2) | 648 (44.4) | 813 (55.6) | |||
| Score≥1 | 63 (14.0) | 386 (86.0) | 22 (5.2) | 399 (94.8) | 70 (33.8) | 137 (66.2) | |||
| Unknown | 166 (50.3) | 164 (49.7) | 16 (34.0) | 31 (66.0) | 23 (53.5) | 20 (46.5) | |||
| Insurance, n (%) | 0.01 | 0.31 | 0.92 | ||||||
| Public | 162 (15.5) | 880 (84.5) | 21 (9.5) | 199 (90.5) | 110 (44.5) | 137 (55.5) | |||
| Private | 558 (19.4) | 2313 (80.6) | 74 (6.9) | 997 (93.1) | 519 (42.8) | 694 (55.6) | |||
| Uninsured/self-pay | 28 (24.6) | 86 (75.4) | * | * | 25 (45.5) | 30 (54.5) | |||
| Other or unknown | 102 (20.6) | 392 (79.4) | 11 (7.4) | 137 (92.6) | 87 (44.4) | 109 (55.6) | |||
| Marital status, n (%) | <0.01 | 0.34 | <0.01 | ||||||
| Single | 397 (26.9) | 1079 (73.1) | 49 (8.8) | 510 (91.2) | 396 (53.4) | 345 (46.6) | |||
| Married | 337 (14.0) | 2064 (86.0) | 49 (6.2) | 738 (93.8) | 283 (34.7) | 532 (65.3) | |||
| Other† | 68 (13.8) | 425 (86.2) | * | * | 45 (39.1) | 70 (60.9) | |||
| Unknown | 48 (31.8) | 103 (68.2) | * | * | 17 (42.5) | 23 (57.5) | |||
| Race or ethnicity, n (%) | <0.01 | 0.01 | <0.01 | ||||||
| American Indian | * | * | * | * | * | * | |||
| Asian or Pacific Islander | 110 (22.1) | 388 (77.9) | 34 (11.4) | 263 (88.6) | 161 (43.8) | 207 (56.3) | |||
| Hispanic | 246 (15.4) | 1348 (84.6) | 44 (6.9) | 597 (93.1) | 262 (49.0) | 273 (51.0) | |||
| Non-Hispanic Black | 46 (19.3) | 192 (80.7) | * | * | 46 (53.5) | 40 (46.5) | |||
| Non-Hispanic White | 416 (19.8) | 1688 (80.2) | 22 (4.5) | 464 (95.5) | 264 (37.3) | 444 (62.7) | |||
| None of the above | 23 (50.0) | 23 (50.0) | 0 (0.0) | 10 (100.0) | * | * | |||
| Rurality, n (%) | 0.02 | 0.67 | <0.01 | ||||||
| Rural | 91 (15.2) | 506 (84.8) | 11 (6.4) | 161 (93.6) | 49 (29.2) | 119 (70.8) | |||
| Urban | 759 (19.3) | 3165 (80.7) | 97 (7.3) | 1235 (92.7) | 692 (44.8) | 851 (55.2) | |||
| Parity, n (%)‡ | <0.01 | 0.22 | 0.19 | ||||||
| 0 | 632 (19.6) | 2590 (80.4) | 102 (7.4) | 1276 (92.6) | 597 (43.2) | 785 (56.8) | |||
| 1 | 89 (31.2) | 196 (68.8) | * | * | 64 (50.0) | 64 (50.0) | |||
| ≥2 | 129 (12.7) | 883 (87.3) | * | * | 80 (39.8) | 121 (60.2) | |||
| Cancer stage, n (%) | <0.01 | <0.01 | <0.01 | ||||||
| 1A | 641 (30.4) | 1469 (69.6) | 66 (6.3) | 987 (93.7) | 545 (47.0) | 614 (53.0) | |||
| 1B | 209 (8.7) | 2202 (91.3) | * | * | NA | NA | |||
| 1C | NA | NA | NA | NA | 196 (35.5) | 356 (64.5) | |||
| 1, unknown | NA | NA | 38 (18.7) | 165 (81.3) | NA | NA | |||
| Histology, n (%)§ | <0.01 | 0.08 | <0.01 | ||||||
| Adenocarcinoma | 215 (15.7) | 1158 (84.3) | 23 (9.9) | 210 (90.1) | NA | NA | |||
| Adenosquamous carcinoma | 15 (6.9) | 201 (93.1) | NA | NA | NA | NA | |||
| Squamous carcinoma | 577 (21.5) | 2110 (78.5) | NA | NA | NA | NA | |||
| Endometrioid adenocarcinoma | NA | NA | 85 (6.7) | 1186 (93.3) | NA | NA | |||
| Germ cell | NA | NA | NA | NA | 246 (74.5) | 84 (25.5) | |||
| Sex cord stromal | NA | NA | NA | NA | 50 (50.0) | 50 (50.0) | |||
| Epithelial | NA | NA | NA | NA | 445 (34.7) | 836 (65.3) | |||
| None of the above | 43 (17.6) | 202 (82.4) | 0 (0.0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Chemotherapy, n (%) | <0.01 | 1.00 | <0.01 | ||||||
| Yes | 47 (9.6) | 444 (90.4) | *) | * | 245 (35.8) | 440 (64.2) | |||
| No | 799 (19.9) | 3,216 (80.1) | 107 (7.2) | 1370 (92.7) | 482 (48.7) | 508 (51.3) | |||
| Unknown | * | * | * | * | 14 (38.9) | 22 (61.1) | |||
| Radiotherapy, n (%)‡ | <0.01 | 0.25 | 0.84 | ||||||
| Yes | 61 (8.2) | 682 (91.8) | 0 (0.0) | 26 (100.0) | * | * | |||
| No | 789 (20.9) | 2,988 (79.1) | 108 (7.3) | 1370 (92.7) | 739 (43.4) | 965 (56.6) | |||
| Hormonal therapy, n (%)‡ | 0.20 | <0.01 | 0.72 | ||||||
| Yes | * | * | 108 (100.0) | 0 (0.0) | * | * | |||
| No | 848 (18.8) | 3,656 (81.2) | 0 (0.0) | 1393 (100.0) | 736 (43.3) | 965 (56.7) | |||
Abbreviations: SES, socioeconomic status, NA, not applicable.
Cells are n (row percentage). P values were calculated by chi-squared and Fisher’s exact tests.
Values are not shown to protect confidentiality of the individuals summarized in the data.
”Other” marital status included separated, divorced, widowed, unmarried, or in a domestic partnership.
There were 2 unknown cases of parity, 2 unknown cases of radiotherapy, and 4 unknown cases of hormonal therapy among patients who received non-fertility sparing treatment in the dataset. These rows were excluded from the table for conciseness.
Specific histology codes are listed in Appendix 2 (http://links.lww.com/xxx).
Table 2.
Analysis of live birth outcomes after cancer diagnosis and fertility-sparing surgery by cancer type
| Sociodemographic and clinical characteristics | Cervical Cancer (n=639) | Ovarian Cancer (n=594) | ||||
|---|---|---|---|---|---|---|
| Live Birth (n=110) | No Live Birth (n=529) | P Value | Live Birth (n=115) | No Live Birth (n=479) | P Value | |
| Age (years), n (%) | <0.01 | <0.01 | ||||
| 18–35 | 99 (21.9) | 354 (78.1) | 109 (24.4) | 338 (75.6) | ||
| 36–45 | 11 (5.9) | 175 (94.1) | * | * | ||
| Diagnosis year, n (%) | <0.01 | <0.01 | ||||
| 2000–2005 | 63 (23.3) | 207 (76.7) | 75 (29.4) | 180 (70.6) | ||
| 2006–2012 | 47 (12.7) | 322 (87.3) | 40 (11.8) | 299 (88.2) | ||
| SES, n (%) | 0.42 | 0.05 | ||||
| Lowest | 14 (12.3) | 100 (87.7) | 22 (28.9) | 54 (71.1) | ||
| Lower-middle | 18 (16.4) | 92 (83.6) | 29 (23.8) | 93 (76.2) | ||
| Middle | 22 (18.5) | 97 (81.5) | 19 (13.7) | 120 (86.3) | ||
| Upper-middle | 33 (21.2) | 123 (78.8) | 21 (16.4) | 107 (83.6) | ||
| Highest | 23 (16.4) | 117 (83.6) | 24 (18.6) | 105 (81.4) | ||
| Charlson comorbidity score, n (%) | <0.01 | 0.35 | ||||
| Score=0 | 62 (13.7) | 389 (86.3) | 105 (20.2) | 414 (79.8) | ||
| Score≥1 | * | * | * | * | ||
| Unknown | 44 (28.8) | 109 (71.2) | * | * | ||
| Insurance, n (%) | 0.90 | 0.88 | ||||
| Public | 17 (16.3) | 87 (83.7) | 16 (20.8) | 61 (79.2) | ||
| Private | 72 (16.8) | 356 (83.2) | 79 (19.0) | 337 (81.0) | ||
| Uninsured/self-pay | * | * | * | * | ||
| Other/unknown | 17 (20.0) | 68 (80.0) | 14 (18.2) | 63 (81.8) | ||
| Marital status, n (%) | 0.02 | 0.40 | ||||
| Single | 41 (14.1) | 250 (85.9) | 53 (17.1) | 257 (82.9) | ||
| Married | 57 (22.4) | 198 (77.6) | 51 (22.1) | 180 (77.9) | ||
| Other† | * | * | * | * | ||
| Unknown | * | * | * | * | ||
| Race or ethnicity, n (%) | 0.03 | 0.23 | ||||
| American Indian | * | * | 0 (0.0) | * | ||
| Asian or Pacific Islander | * | * | 29 (22.5) | 100 (77.5) | ||
| Hispanic | 37 (21.0) | 139 (79.0) | 45 (22.8) | 152 (77.2) | ||
| Non-Hispanic Black | * | * | * | * | ||
| Non-Hispanic White | 54 (16.5) | 273 (83.5) | 33 (14.6) | 193 (85.4) | ||
| None of the above | * | 13 (65.0) | * | * | ||
| Rurality, n (%) | 0.46 | 0.11 | ||||
| Rural | 12 (20.7) | 46 (79.3) | * | 37 (90.2) | ||
| Urban | 98 (16.9) | 483 (83.1) | 111 (20.1) | 442 (79.9) | ||
| Parity, n (%) | <0.01 | 0.01 | ||||
| 0 | 71 (14.8) | 409 (85.2) | 100 (20.2) | 396 (79.8) | ||
| 1 | 24 (34.3) | 46 (65.7) | 13 (27.7) | 34 (72.3) | ||
| ≥2 | 15 (16.9) | 74 (83.1) | * | * | ||
| Cancer stage, n (%) | 0.03 | 0.37 | ||||
| 1A | 96 (18.8) | 414 (81.2) | 91 (20.2) | 360 (79.8) | ||
| 1B | 14 (10.9) | 115 (89.1) | NA | NA | ||
| 1C | NA | NA | 24 (16.8) | 119 (83.2) | ||
| Histology, n (%)‡ | 0.97 | 0.01 | ||||
| Adenocarcinoma | 29 (17.6) | 136 (82.4) | NA | NA | ||
| Adenosquamous carcinoma | * | * | NA | NA | ||
| Squamous carcinoma | 74 (17.1) | 358 (82.9) | NA | NA | ||
| Endometrioid adenocarcinoma | NA | NA | NA | NA | ||
| Germ cell | NA | NA | 54 (26.1) | 153 (73.9) | ||
| Sex cord stromal | NA | NA | * | * | ||
| Epithelial | NA | NA | 53 (15.4) | 291 (84.6) | ||
| None of the above | * | * | 0 (0.0) | 0 (0.0) | ||
| Chemotherapy, n (%) | 1.00 | 0.97 | ||||
| Yes | 0 (0.0) | * | 38 (19.5) | 157 (80.5) | ||
| No | 110 (17.4) | 522 (82.6) | 75 (19.2) | 315 (80.8) | ||
| Unknown | 0 (0.0) | * | * | * | ||
| Hormonal therapy, n (%) | 0.83 | 0.48 | ||||
| Yes | 0 (0.0) | * | * | * | ||
| No | 110 (17.2) | 528 (82.8) | 114 (19.3) | 477 (80.7) | ||
| Unknown | 0 (0.0) | * | 0 (0.0) | 0 (0.0) | ||
SES, socioeconomic status; NA, not applicable.
Live birth was defined as a live birth that had been conceived 3 months after the cancer diagnosis and fertility-sparing surgery. The three births that occurred among patients with a history of endometrial cancer are not presented because of the low number of results to protect the confidentiality of individuals represented in these data. Those who had prior radiation or were diagnosed after 2012 were excluded from analysis, due to contraindication to attempting a live birth and lack of data, respectively.
Values are not shown to protect the confidentiality of the individuals summarized in the data.
”Other” marital status included separated, divorced, widowed, unmarried, or in a domestic partnership.
Specific histology codes are listed in Appendix 2 (http://links.lww.com/xxx).
Among patients with cervical cancer who received fertility-sparing surgery versus those who did not, there were significant differences in all clinical and sociodemographic variables (Table 1). Multivariable regression (Table 3, Figure 2) demonstrated that younger age (18–35 vs 36–45 years), Asian or Pacific Islander (vs non-Hispanic White), single status (vs married), year of diagnosis (2006–2010 and 2011–2015 vs 2000–2005), and cancer stage IA (vs 1B) were associated with higher odds of fertility-sparing treatment.
Table 3.
Multivariable* logistic regression of fertility-sparing treatment by cancer type
| Cervical | Endometrial | Ovarian | ||||
|---|---|---|---|---|---|---|
| Sociodemographic and clinical characteristics | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| Age, years | ||||||
| 18–35 | Reference | Reference | Reference | Reference | Reference | Reference |
| 36–45 | 0.25 (0.22–0.30) | 0.25 (0.21–0.31) | 0.17 (0.11–0.26) | 0.11 (0.06–0.21) | 0.11 (0.09–0.13) | 0.11 (0.09–0.15) |
| Diagnosis year | ||||||
| 2000–2005 | Reference | Reference | Reference | Reference | Reference | Reference |
| 2006–2010 | 1.23 (1.03–1.47) | 2.36 (1.83–3.04) | 0.77 (0.42–1.41) | 1.70 (0.54–5.32) | 0.95 (0.75–1.19) | 0.98 (0.71–1.35) |
| 2011–2015 | 1.40 (1.16–1.68) | 2.86 (2.20–3.72) | 1.37 (0.78–2.39) | 1.55 (0.49–4.94) | 1.16 (0.92–1.46) | 1.29 (0.93–1.78) |
| SES | ||||||
| Lowest | Reference | Reference | Reference | Reference | Reference | Reference |
| Lower-middle | 0.91 (0.71–1.16) | 0.81 (0.59–1.13) | 0.76 (0.42–1.39) | 1.34 (0.56–3.18) | 1.07 (0.77–1.49) | 1.16 (0.75–1.80) |
| Middle | 0.93 (0.73–1.18) | 0.75 (0.54–1.04) | 0.94 (0.51–1.74) | 0.89 (0.31–2.61) | 1.11 (0.81–1.54) | 1.75 (1.11–2.77) |
| Upper-middle | 1.20 (0.95–1.51) | 1.12 (0.81–1.55) | 1.14 (0.64–2.04) | 2.25 (0.89–5.68) | 1.04 (0.76–1.44) | 1.63 (1.03–2.60) |
| Highest | 1.38 (1.09–1.76) | 1.28 (0.90–1.81) | 1.33 (0.69–2.54) | 1.94 (0.62–6.08) | 1.02 (0.74–1.41) | 1.90 (1.17–3.09) |
| Charlson comorbidity score | ||||||
| Score=0 | Reference | Reference | Reference | Reference | Reference | Reference |
| Score≥1 | 0.82 (0.62–1.09) | 0.94 (0.67–1.32) | 0.76 (0.47–1.25) | 1.13 (0.58–2.20) | 0.64 (0.47–0.87) | 0.79 (0.53–1.17) |
| Insurance | ||||||
| Public | Reference | Reference | Reference | Reference | Reference | Reference |
| Private | 1.31 (1.08–1.59) | 1.13 (0.88–1.45) | 0.70 (0.42–1.17) | 1.03 (0.47–2.24) | 0.93 (0.71–1.23) | 1.12 (0.78–1.63) |
| Uninsured or self-pay | 1.77 (1.12–2.80) | 1.28 (0.68–2.40) | 0.30 (0.07–1.32) | 0.65 (0.13–3.27) | 1.04 (0.58–1.87) | 1.23 (0.60–2.55) |
| Marital status | ||||||
| Single | Reference | Reference | Reference | Reference | Reference | Reference |
| Married | 0.44 (0.38–0.52) | 0.51 (0.41–0.63) | 0.69 (0.46–1.04) | 0.62 (0.32–1.20) | 0.46 (0.38–0.57) | 0.80 (0.60–1.06) |
| Other† | 0.44 (0.33–0.58) | 0.72 (0.51–1.02) | 0.71 (0.32–1.62) | 0.72 (0.20–2.64) | 0.56 (0.38–0.84) | 1.65 (0.98–2.80) |
| Race or ethnicity | ||||||
| Non-Hispanic White | Reference | Reference | Reference | Reference | Reference | Reference |
| American Indian | 1.41 (0.54–2.41) | 1.03 (0.37–2.89) | 2.22 (0.49–10.14) | 1.99 (0.34–11.64) | 2.52 (0.42–15.20) | 0.98 (0.08–11.97) |
| Asian or Pacific Islander | 1.15 (0.91–1.46) | 1.52 (1.12–2.08) | 2.73 (1.56–4.76) | 2.50 (0.97–6.43) | 1.31 (1.01–1.69) | 1.30 (0.92–1.82) |
| Hispanic | 0.74 (0.62–0.88) | 0.83 (0.66–1.06) | 1.55 (0.92–2.63) | 1.70 (0.72–4.02) | 1.61 (1.29–2.03) | 1.44 (1.04–1.99) |
| Non-Hispanic Black | 0.97 (0.69–1.37) | 0.60 (0.37–0.99) | 2.94 (1.13–7.65) | 3.88 (0.97–15.51) | 1.93 (1.23–3.03) | 2.26 (1.22–4.17) |
| Rurality | ||||||
| Rural | Reference | Reference | Reference | Reference | Reference | Reference |
| Urban | 1.33 (1.05–1.69) | 0.96 (0.70–1.33) | 1.14 (0.60–2.19) | 0.48 (0.19–1.21) | 1.97 (1.39–2.79) | 1.37 (0.86–2.17) |
| Parity | ||||||
| 0 | Reference | Reference | Reference | Reference | Reference | Reference |
| 1 | 1.86 (1.43–2.43) | 0.93 (0.65–1.33) | 1.22 (0.43–3.48) | 0.39 (0.05–3.12) | 1.32 (0.92–1.89) | 0.77 (0.48–1.24) |
| ≥2 | 0.60 (0.49–0.73) | 0.45 (0.34–0.58) | 0.38 (0.08–1.31) | 0.66 (0.15–2.99) | 0.87 (0.64–1.18) | 1.01 (0.67–1.52) |
| Cancer stage | ||||||
| 1A | Reference | Reference | Reference | Reference | Reference | Reference |
| 1B | 0.22 (0.18–0.26) | 0.23 (0.19–0.28) | 0.25 (0.09–0.68) | 0.37 (0.12–1.11) | NA | NA |
| IC | NA | NA | NA | NA | 0.62 (0.50–0.76) | 0.72 (0.55–0.95) |
| Histology | ||||||
| Epithelial | NA | NA | NA | NA | Reference | Reference |
| Germ cell | NA | NA | NA | NA | 5.50 (4.18–7.23) | 2.29 (1.62–3.25) |
| Sex cord stromal | NA | NA | 1.88 (1.25–2.83) | 1.73 (1.01–2.97) | ||
Abbreviations: OR, odds ratio; CI, confidence interval; SES, socioeconomic status; NA, not applicable.
Histology was included only for ovarian cancer. All other variables presented in the table were included in the multivariable logistic regression for all three cancer types.
”Other” marital status included separated, divorced, widowed, unmarried, or in a domestic partnership.
Figure 2.
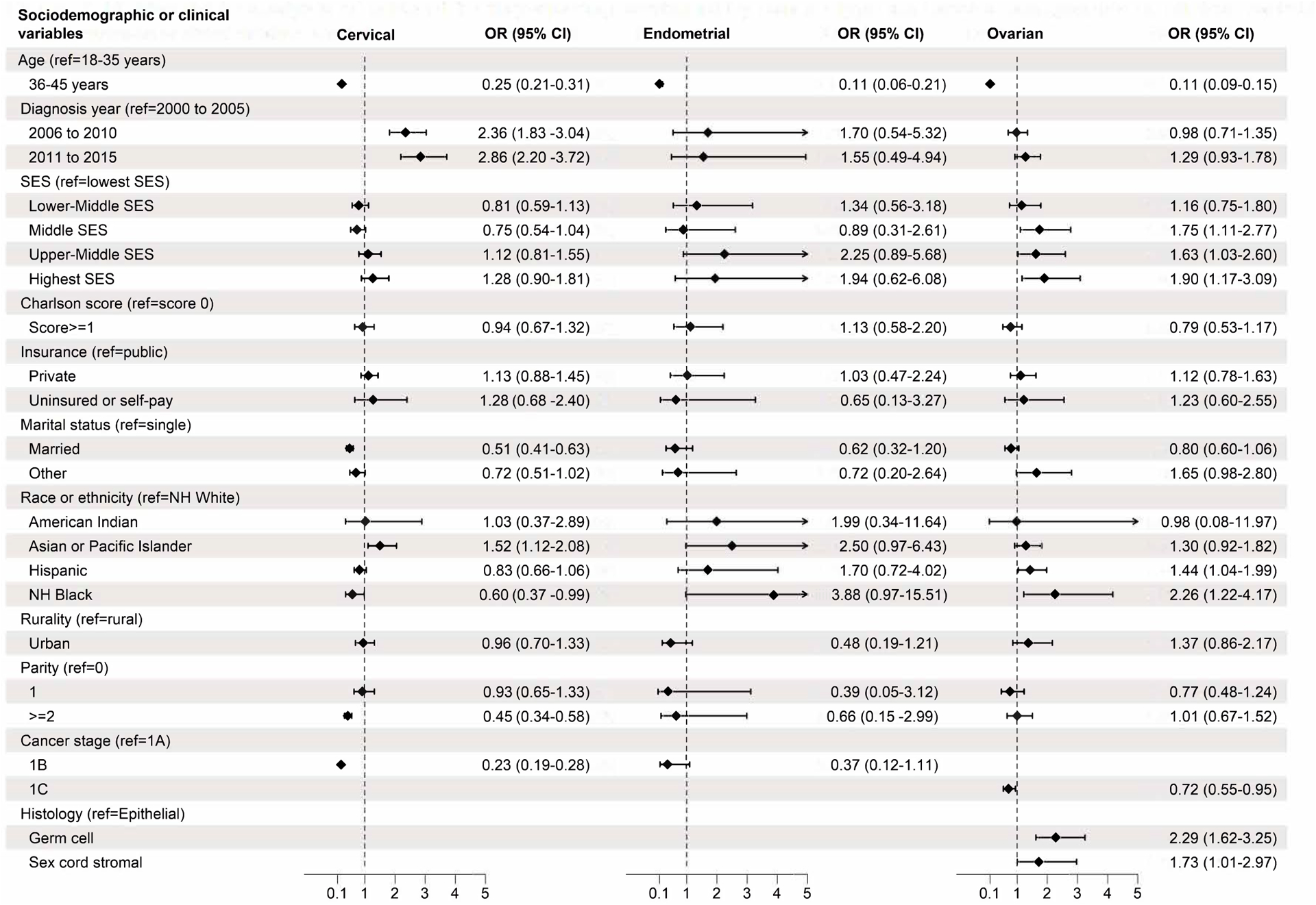
Multivariable analysis of adjusted odds ratio of fertility-sparing treatment by cancer type and variables of interest. All variables presented were included in multivariable analysis. Rows without adjusted odds ratios presented were not applicable to that cancer type (stage IB for ovarian, stage IC and histology type for cervical, endometrial). aOR, adjusted odds ratio; SES, socioeconomic status; NH, non-Hispanic.
There were 25 (0.6%) instances of ART among patients with a history of cervical cancer. There were no instances of ART among non-Hispanic Black patients, American Indian patients, those in the lowest SES quintile, or those with public insurance. Univariable logistic regression demonstrated higher odds of ART among patients who were younger (18–35 vs 36–45 years), non-Hispanic White (vs all non-White races or ethnicities), nulliparous (vs ≥1), of high SES (vs low) or diagnosed between 2000–2007 (vs 2008–2015) (Figure 3). Women with a history of cervical cancer accounted for 48.2% (n=110) of patients who had a live birth outcome following cancer diagnosis and fertility-sparing treatment (Table 2).
Figure 3.
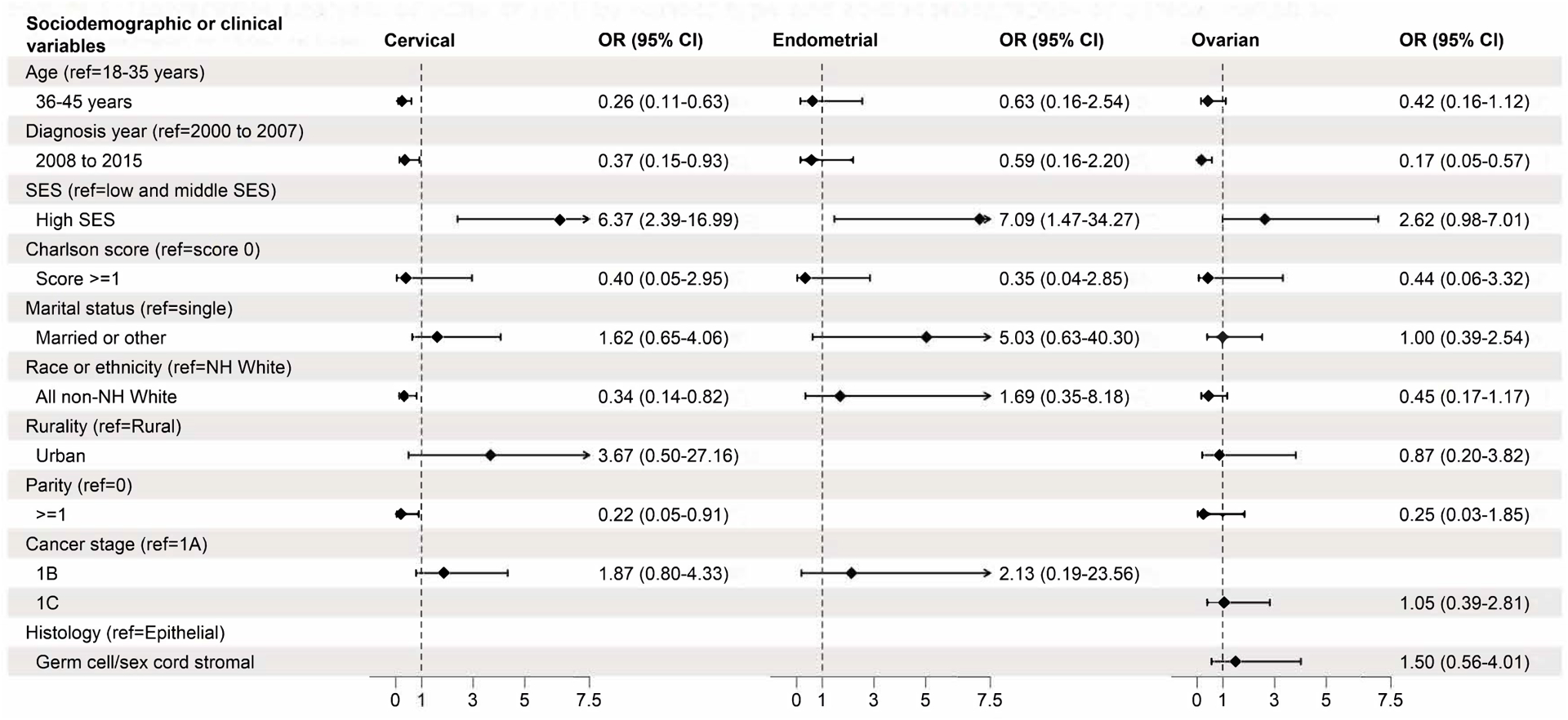
Univariable analysis of odds ratio of assisted reproductive technology by cancer type and variables of interest. Rows without odds ratios presented were not applicable to that cancer type (stage IB for ovarian, stage IC and histology type for cervical, endometrial), or unable to be calculated (rurality and parity for endometrial cancer). OR, odds ratio; SES, socioeconomic status; NH, non-Hispanic.
There were significant differences in age, year of diagnosis, Charlson comorbidity score, race or ethnicity, and cancer stage between patients with endometrial cancer who received fertility-sparing treatment versus those who did not (Table 1). Multivariable regression analysis (Table 3, Figure 2) found that age 18–35 years (vs 36–45 years) was associated with increased odds of fertility-sparing treatment.
There were 9 instances (0.6%) of ART, which was associated with high SES (vs low and middle) on univariable analysis (Figure 3). There were no instances of ART among non-Hispanic Black patients, American Indian patients, or publicly insured patients. Patients with a history of endometrial cancer accounted for 1.3% (3) of those who had a live birth outcome following their cancer diagnosis and fertility-sparing treatment.
Among patients with ovarian cancer who received fertility-sparing treatment versus those who did not, significant differences existed in age, Charlson comorbidity scores, marital status, race or ethnicity, rurality, cancer stage, histology, and receipt of chemotherapy (Table 1). Multivariable logistic regression models identified age 18–35 years (vs 36–45 years), middle, upper-middle, and highest SES (vs lowest SES), stage IA (vs IC), non-Hispanic Black and Hispanic (vs non-Hispanic White), and germ cell or sex cord stromal histology (vs epithelial) were associated with increased odds of fertility-sparing treatment (Table 3, Figure 2).
A total of 18 instances (1.1%) of ART occurred after diagnosis. On univariable analysis, ART use was associated with diagnosis between 2000–2007 (vs 2008–2015) (Figure 3). No ART instances were observed among non-Hispanic Black, American Indian, or publicly insured patients. Patients with a history of ovarian cancer accounted for the half of those who experienced a live birth following a cancer diagnosis and fertility-sparing treatment (115 of 228 [50.4%]) (Table 2).
Sensitivity analysis demonstrated our data were robust to moderate unmeasured confounders related to both exposure and outcome for most exposure variables. Our results appear to be robust to strong confounders with respect to age and diagnosis year (all cancer types) (Appendix 3, available online at http://links.lww.com/xxx).
DISCUSSION
We found differing patterns of sociodemographic disparities by cancer type for fertility-sparing treatment and ART that did not always follow anticipated patterns based on prior studies. Fertility-sparing treatment was more likely among patients in racial and ethnic minority groups for cervical and endometrial cancers and younger patients for all three cancers. ART was more likely among those diagnosed earlier in the cohort and those with private insurance across all three cancer types, and also among younger and non-Hispanic White patients with a history of cervical cancer.
Patients in racial and ethnic minority groups had higher odds of receiving fertility-sparing treatment than did non-Hispanic White patients among those with cervical and ovarian cancer, with no difference among those with a history of endometrial cancer. This result contrasts prior studies demonstrating racial disparities in access to, and receipt of, guideline-based treatments for ovarian,19 endometrial,21 and cervical cancer.23 Although the baseline cohort could be affected by higher rates of prior hysterectomy or unilateral salpingo-oophorectomy among patients in racial and ethnic minority groups compared to non-Hispanic White patients, the overall rate of these procedures in the ages selected for this study is low.39–41 Furthermore, while differences in ovarian histology by race or ethnicity could explain our findings,42,43 race and ethnicity remained significant predictors of fertility-sparing procedures after controlling for histology. Lastly, incidental diagnoses of cancer at the time of surgery, while not different by race or ethnicity, accounted for the majority of cases for cervical and ovarian cancer in our study, which could affect interpretation of our findings.
The fertility-sparing treatments investigated in this analysis should be covered by both public and private insurance as they are guideline-based therapies; this is reflected in our findings of no significant disparities by insurance status. In contrast, prior studies have demonstrated that patients with public or no insurance are less likely to receive guideline-based treatment for gynecologic malignancies.22,44–46 Our study had a high overall percentage of patients with insurance, thus possibly limiting generalizability and partially explaining the findings.
The lack of geographic disparities in fertility-sparing treatments differ from prior studies among patients with gynecologic malignancies that demonstrated a lower likelihood of receiving guideline-adherent care if they live farther from urban centers.16 It is possible our study found different results due to a high percentage of fertility-sparing treatments (LEEP, cone, unilateral oophorectomy) being feasible in rural settings and by non-gynecologic oncologists.
There was overall low utilization of ART among patients in our study. The majority of instances of ART were noted in the earlier half of the study years, which may reflect lack of follow-up time for those diagnosed after 2007. The type of treatment patients in this cohort received may have variably impacted their fertility, from limited impact after a LEEP to more substantial impact after trachelectomy or oophorectomy, particularly if they had received prior gynecologic surgery.
Unlike trends seen in fertility-sparing treatment, we found that racial and ethnic minority patients were less likely to use ART after a diagnosis of cervical cancer. This finding is consistent with prior studies’ findings of lower ART use in the general non-Hispanic Black and Hispanic populations.47
Insurance type may mediate the race and ethnicity disparity as Medicaid does not cover fertility treatment, disproportionately affecting patients in racial and ethnic minority groups, as the program covers 30% of Black patients and 25% of Hispanic patients versus 15% of non-Hispanic White patients.48 Additionally, during the study period, California had a mandate to offer coverage for infertility services that applied differentially to private insurances, did not apply to Medicaid or public insurances, and specifically excluded in vitro fertilization.49
In the general population, geographic disparity is demonstrated by the unequal distribution of access to ART services nationwide, as nearly 30% of reproductive-age patients do not have access to a local ART clinic based on census data.50 The lack of geographic disparity in ART services in this study may be due to low overall numbers, California’s above-national average ART use rates, or the abundance of ART clinics.51 Despite the high use of ART in California among the general population, our findings suggest that ART use among patients after treatment for cervical, endometrial, or ovarian cancers is low.
Live births were overall infrequent (228 births among 1,293 cases from 2000–2012). Half of live births were to patients with a history of ovarian cancer. This may be related to increased rates of infertility52 and increased likelihood of preterm birth53 among those undergoing fertility-sparing treatment for endometrial and cervical cancer, respectively. In comparison, prior studies of fertility-sparing treatment for ovarian cancer did not find an association with adverse pregnancy outcomes.37 The low number of live births may be related to recurrence or ongoing disease, as patients who receive fertility-sparing treatment are at risk for further infertility-inducing procedures or treatments in these circumstances.
The strengths of this study include a large database created by unique linkages that were robust in sample size and granularity. California is a state with a large population and relatively high access to medical centers, which allowed for a large number of outcomes compared to prior studies. Restricting the analysis to one state decreased possible confounding by insurance mandates or practice patterns. Additionally, though state demographics changed during the study period, the demographics of our cohort remained constant.
The limitations of our study include inability to verify registry data, data misclassification or underreporting, and inaccuracies in the linkage process. Women may have received fertility-sparing treatment or ART in California and subsequently left the state. A lack of knowledge of the surgeon’s intent was mitigated by selecting a cohort for whom fertility-sparing treatment represented guideline-concordant care. We could not address patient-, health care professional-, or intuition-specific barriers to fertility-sparing treatment or ART, nor individuals’ desire for such interventions. We were limited by the years of data available in each dataset, with possible ART use or live births following a cancer diagnosis not yet published, and by missing or unknown data regarding oncologic or sociodemographic variables. Lastly, there were very few outcomes of interest for ART and live births, limiting the certainty of the statistical conclusions.
The results of this study for the primary outcome of fertility-sparing treatment did not follow anticipated race or ethnicity disparities. In addition, the results demonstrated no insurance-based disparities for fertility-sparing treatments, in contrast to the significant findings of insurance-based disparities for ART. There were few ART instances overall and zero instances among non-Hispanic Black and publicly insured patients, highlighting a need to address these disparities in clinical practice. The results of this study will improve health care professionals’ awareness of existing disparities and emphasize the need for equitable access to fertility-related care among survivors of gynecologic malignancies.
Supplementary Material
Acknowledgements:
Editorial support was provided by Anne Sutton, scientific editor, of the Research Medical Library at The University of Texas MD Anderson Cancer Center. Ms. Sutton’s sole compensation for this work was her salary, which is paid by The University of Texas MD Anderson Cancer Center.
The authors thank the Society for Assisted Reproductive Technology (SART) for the dataset, as well as all SART members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of SART members, this research would not have been possible.
Sources of funding:
This work was supported by grants from the National Institutes of Health National Cancer Institute (Jose Alejandro Rauh-Hain: K08 CA234333; Jose Alejandro Rauh-Hain, Kirsten Jorgensen, Roni Nitecki, Chi-Fang Wu: P30 CA016672; Kirsten Jorgensen, Roni Nitecki T32 CA101642; Clare Meernik: F31 CA260787). The funding sources were not involved in the development of the research hypothesis or in the study design, data analysis, or manuscript writing.
Financial Disclosure
Caitlin C. Murphy reports receiving payment from Freenome. Jose Alejandro Rauh-Hain reports receiving payment from the Schlesinger Group and Guidepoint. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
Footnotes
Prior presentation:
Presented at the Society of Gynecologic Oncology Annual Meeting in Phoenix, Arizona, March 21, 2022, and the OncLive Fellow’s Forum in Denver, Colorado, October 13–15, 2022.
Contributor Information
Kirsten Jorgensen, The University of Texas MD Anderson Cancer Center, Department of Gynecologic Oncology and Reproductive Medicine, Houston, TX.
Clare Meernik, Duke University School of Medicine, Department of Public Health Sciences, Durham, NC.
Chi-Fang Wu, The University of Texas MD Anderson Cancer Center, Department of Health Services Research, Houston, TX.
Caitlin C. Murphy, Department of Health Promotion and Behavioral Sciences, School of Public Health, University of Texas Health Science Center at Houston, Houston, TX.
Valerie L. Baker, Johns Hopkins University School of Medicine, Department of Gynecology and Obstetrics, Division of Reproductive Endocrinology and Infertility, Baltimore, MD.
Peiton Jarmon, Tulane University School of Medicine, New Orleans, LA.
Paula C. Brady, Columbia University Irving Medical Center, Columbia University Fertility Center, New York, NY.
Roni Nitecki, The University of Texas MD Anderson Cancer Center, Department of Gynecologic Oncology and Reproductive Medicine, Houston, TX.
Hazel B. Nichols, University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Chapel Hill, NC.
Jose-Alejandro Rauh-Hain, The University of Texas MD Anderson Cancer Center, Department of Gynecologic Oncology and Reproductive Medicine and Department of Health Services Research, Houston, TX.
REFERENCES
- 1.U.S. Cancer Statistics Working Group. U.S. cancer statistics data visualizations tool, based on 2021 submission data. Published 2021. Accessed December 21, 2021. https://gis.cdc.gov/Cancer/USCS/#/AtAGlance/
- 2.Deshpande NA, Braun IM, Meyer FL. Impact of fertility preservation counseling and treatment on psychological outcomes among women with cancer: a systematic review. Cancer. 2015;121(22):3938–3947. doi: 10.1002/cncr.29637 [DOI] [PubMed] [Google Scholar]
- 3.Peate M, Meiser B, Hickey M, Friedlander M. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat. 2009;116(2):215–223. doi: 10.1007/s10549-009-0401-6 [DOI] [PubMed] [Google Scholar]
- 4.Peate M, Meiser B, Friedlander M, Zorbas H, Rovelli S, Sansom-Daly U, et al. It’s now or never: fertility-related knowledge, decision-making preferences, and treatment intentions in young women with breast cancer—an Australian fertility decision aid collaborative group study. J Clin Oncol. Published online March 28, 2011. doi: 10.1200/JCO.2010.31.2462 [DOI] [PubMed] [Google Scholar]
- 5.Chan JL, Letourneau J, Salem W, Cil AP, Chan SW, Chen LM, et al. Regret around fertility choices is decreased with pre-treatment counseling in gynecologic cancer patients. J Cancer Surviv Res Pract. 2017;11(1):58–63. doi: 10.1007/s11764-016-0563-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letourneau JM, Ebbel EE, Katz PP, Katz A, Chien AJ, Melisko ME, et al. Pre-treatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118(6):1710–1717. doi: 10.1002/cncr.26459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Adolescent and Young Adult (AYA) Oncology (version 2.2022). Accessed May 8, 2022. https://www.nccn.org/guidelines/guidelines-detail [DOI] [PubMed]
- 8.National Comprehensive Cancer Network. Cervical Cancer (version 1.2022). Accessed May 8, 2022. https://www.nccn.org/guidelines/guidelines-detail
- 9.National Comprehensive Cancer Network. Ovarian Cancer (version 1.2022). Accessed May 8, 2022. https://www.nccn.org/guidelines/guidelines-detail
- 10.National Comprehensive Cancer Network. Uterine Neoplasms (version 1.2022). Accessed May 8, 2022. https://www.nccn.org/guidelines/guidelines-detail
- 11.Angarita AM, Johnson CA, Fader AN, Christianson MS. Fertility preservation: a key survivorship issue for young women with cancer. Front Oncol. 2016;6:102. doi: 10.3389/fonc.2016.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melamed A, Rizzo AE, Nitecki R, Gockley AA, Bregar AJ, Schorge JO, et al. All-cause mortality after fertility-sparing surgery for stage I epithelial ovarian cancer. Obstet Gynecol. 2017;130(1):71–79. doi: 10.1097/AOG.0000000000002102 [DOI] [PubMed] [Google Scholar]
- 13.Obermair A, Baxter E, Brennan DJ, McAlpine JN, Muellerer JJ, Amant F, et al. Fertility-sparing treatment in early endometrial cancer: current state and future strategies. Obstet Gynecol Sci. 2020;63(4):417–431. doi: 10.5468/ogs.19169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Li W, Kanis MJ, Qi G, Li M, Yang X, et al. Oncologic and obstetrical outcomes with fertility-sparing treatment of cervical cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(28):46580–46592. doi: 10.18632/oncotarget.16233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu L. Rural–urban and racial/ethnic disparities in invasive cervical cancer incidence in the United States, 2010–2014. Prev Chronic Dis. 2019;16. doi: 10.5888/pcd16.180447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villanueva C, Chang J, Bartell SM, Ziogas A, Bristow R, Vieira VM. Contribution of geographic location to disparities in ovarian cancer treatment. J Natl Compr Canc Netw. 2019;17(11):1318–1329. doi: 10.6004/jnccn.2019.7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo W, Kim S, Huh WK, Dilley S, Coughlin SS, Partridge EE, et al. Recent trends in racial and regional disparities in cervical cancer incidence and mortality in United States. PLOS ONE. 2017;12(2):e0172548. doi: 10.1371/journal.pone.0172548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Letourneau JM, Smith JF, Ebbel EE, Craig A, Katz PP, Cedars MI, et al. Racial, socioeconomic, and demographic disparities in access to fertility preservation in young women diagnosed with cancer. Cancer. 2012;118(18):4579–4588. doi: 10.1002/cncr.26649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan JK, Zhang M, Hu JM, Shin JY, Osann K, Kapp DS. Racial disparities in surgical treatment and survival of epithelial ovarian cancer in United States. J Surg Oncol. 2008;97(2):103–107. doi: 10.1002/jso.20932 [DOI] [PubMed] [Google Scholar]
- 20.Liu F, Randall L, Tewari K, Bristow R. Racial disparities and patterns of ovarian cancer surgical care in California. Gynecol Oncol. 2014;132(1):221–226. doi: 10.1016/j.ygyno.2013.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allard JE, Maxwell GL. Race disparities between black and white women in the incidence, treatment, and prognosis of endometrial cancer. Cancer Control. 2009;16(1):53–56. doi: 10.1177/107327480901600108 [DOI] [PubMed] [Google Scholar]
- 22.Levinson KL, Bristow RE, Donohue PK, Kanarek NF, Trimble CL. Impact of payer status on treatment of cervical cancer at a tertiary referral center. Gynecol Oncol. 2011;122(2):324–327. doi: 10.1016/j.ygyno.2011.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markt SC, Tang T, Cronin AM, Katz IT, Howitt BE, Horowitz NS, et al. Insurance status and cancer treatment mediate the association between race/ethnicity and cervical cancer survival. PLoS ONE. 2018;13(2):e0193047. doi: 10.1371/journal.pone.0193047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutaria T, Sparks AD, Rao YJ, Lopez-Acevedo M, Long B. Trends in guideline-adherent fertility-sparing surgery for early-stage cervical cancer before and after the Affordable Care Act. Gynecol Oncol. 2020;158(2):424–430. doi: 10.1016/j.ygyno.2020.05.027 [DOI] [PubMed] [Google Scholar]
- 25.The Ethics Committee of the American Society for Reproductive Medicine. Disparities in access to effective treatment for infertility in the United States: an Ethics Committee opinion. Fertil Steril. 2021;116(1):54–63. doi: 10.1016/j.fertnstert.2021.02.019 [DOI] [PubMed] [Google Scholar]
- 26.Meernik C, Engel SM, Wardell A, Baggett CD, Gupta P, Rodriguez-Ormaza N, et al. Disparities in fertility preservation use among adolescent and young adult women with cancer. J Cancer Surviv. Published online February 16, 2022. doi: 10.1007/s11764-022-01187-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bitler MP, Schmidt L. Utilization of Infertility Treatments: The Effects of Insurance Mandates. Demography. 2012;49(1):125–149. doi: 10.1007/s13524-011-0078-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zapardiel I, Cruz M, Diestro MD, Requena A, Garcia-Velasco JA. Assisted reproductive techniques after fertility-sparing treatments in gynaecological cancers. Hum Reprod Update. 2016;22(3):281–305. doi: 10.1093/humupd/dmv066 [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. (Smedley BD, Stith AY, Nelson AR, eds.). National Academies Press; (US: ); 2003. Accessed December 21, 2021. http://www.ncbi.nlm.nih.gov/books/NBK220358/ [PubMed] [Google Scholar]
- 30.ICD - Classification of Diseases, Functioning, and Disability. Published July 23, 2021. Accessed July 19, 2022. https://www.cdc.gov/nchs/icd/index.htm
- 31.Toner JP, Coddington CC, Doody K, Voorhis BV, Seifer DB, Ball GD, et al. Society for Assisted Reproductive Technology and assisted reproductive technology in the United States: a 2016 update. Fertil Steril. 2016;106(3):541–546. doi: 10.1016/j.fertnstert.2016.05.026 [DOI] [PubMed] [Google Scholar]
- 32.Luke B, Brown MB, Missmer SA, Spector LG, Leach RE, Williams M, et al. Assisted reproductive technology use and outcomes among women with a history of cancer. Hum Reprod Oxf Engl. 2016;31(1):183–189. doi: 10.1093/humrep/dev288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.California Cancer Registry. California Cancer Registry Data Dictionary. Published online June 13, 2016. Accessed May 8, 2022. https://www.ccrcal.org/?s=data+dictionary
- 34.World Health Organization. International Classification of Diseases for Oncology (ICD-O). 3rd ed., 1st revision. World Health Organization; 2013. Accessed September 25, 2022. https://apps.who.int/iris/handle/10665/96612 [Google Scholar]
- 35.Corzo C, Barrientos Santillan N, Westin SN, Ramirez PT. Updates on conservative management of endometrial cancer. J Minim Invasive Gynecol. 2018;25(2):308–313. doi: 10.1016/j.jmig.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 36.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control CCC. 2001;12(8):703–711. doi: 10.1023/a:1011240019516 [DOI] [PubMed] [Google Scholar]
- 37.Nitecki R, Clapp MA, Fu S, Lamiman K, Melamed A, Brady P, et al. Outcomes of the first pregnancy after fertility-sparing surgery for early-stage ovarian cancer. Obstet Gynecol. 2021;137(6):1109–1118. doi: 10.1097/AOG.0000000000004394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 39.Temkin SM, Rimel BJ, Bruegl AS, Gunderson CC, Beavis AL, Doll KM. A contemporary framework of health equity applied to gynecologic cancer care: A Society of Gynecologic Oncology evidenced-based review. Gynecol Oncol. 2018;149(1):70–77. doi: 10.1016/j.ygyno.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 40.Simms KT, Yuill S, Killen J, Smith MA, Kulasingam S, de Kok I, et al. Historical and projected hysterectomy rates in the USA: Implications for future observed cervical cancer rates and evaluating prevention interventions. Gynecol Oncol. 2020;158(3):710–718. doi: 10.1016/j.ygyno.2020.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erickson Z, Rocca WA, Smith CY, Gazzuola Rocca L, Steward EA, Laughlin-Tommaso S, et al. Time trends in unilateral and bilateral oophorectomy in a geographically defined American population. Obstet Gynecol. 2022;139(5):724–734. doi: 10.1097/AOG.0000000000004728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinchcliff E, Rauh-Hain JA, Clemmer JT, Diver E, Hall T, Stall J, et al. Racial disparities in survival in malignant germ cell tumors of the ovary. Gynecol Oncol. 2016;140(3):463–469. doi: 10.1016/j.ygyno.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 43.Schultz KAP, Harris AK, Schneider DT, Young RH, Brown J, Gershenson DM, et al. Ovarian Sex Cord-Stromal Tumors. J Oncol Pract. 2016;12(10):940–946. doi: 10.1200/JOP.2016.016261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Churilla T, Egleston B, Dong Y, Shaikh T, Murphy C, Mantia-Smaldone G, et al. Disparities in the management and outcome of cervical cancer in the United States according to health insurance status. Gynecol Oncol. 2016;141(3):516–523. doi: 10.1016/j.ygyno.2016.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bodurtha Smith AJ, Pena D, Ko E. Insurance-mediated disparities in gynecologic oncology care. Obstet Gynecol. 2022;139(2):305–312. doi: 10.1097/AOG.0000000000004643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parikh-Patel A, Morris CR, Kizer KW. Disparities in quality of cancer care: The role of health insurance and population demographics. Medicine (Baltimore). 2017;96(50):e9125. doi: 10.1097/MD.0000000000009125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dieke AC, Zhang Y, Kissin DM, Barfield WD, Boulet SL. Disparities in assisted reproductive technology utilization by race and ethnicity, United States, 2014: A commentary. J Womens Health 2002. 2017;26(6):605–608. doi: 10.1089/jwh.2017.6467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weigel G, Ranji U, Long M, 2020. Coverage and use of fertility services in the U.S. KFF. Published September 15, 2020. Accessed May 8, 2022. https://www.kff.org/womens-health-policy/issue-brief/coverage-and-use-of-fertility-services-in-the-u-s/
- 49.National Conference of State Legislatures. State laws related to insurance coverage for infertility treatment. Published 2022. Accessed September 23, 2022. https://www.ncsl.org/research/health/insurance-coverage-for-infertility-laws.aspx
- 50.Harris JA, Menke MN, Haefner JK, Moniz MH, Perumalswami CR. Geographic access to assisted reproductive technology health care in the United States: a population-based cross-sectional study. Fertil Steril. 2017;107(4):1023–1027. doi: 10.1016/j.fertnstert.2017.02.101 [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. State-specific assisted reproductive technology surveillance. Published December 23, 2021. Accessed May 8, 2022. https://www.cdc.gov/art/state-specific-surveillance/index.html
- 52.Park JY, Seong SJ, Kim TJ, Kim JW, Kim SM, Bae DS, et al. Pregnancy outcomes after fertility-sparing management in young women with early endometrial cancer. Obstet Gynecol. 2013;121(1):136–142. doi: 10.1097/aog.0b013e31827a0643 [DOI] [PubMed] [Google Scholar]
- 53.Nitecki R, Floyd J, Lamiman K, Clapp MA, Fu S, Jorgensen K, et al. Outcomes of the first pregnancy after fertility-sparing surgery for early-stage cervical cancer. Obstet Gynecol. 2021;138(4):565–573. doi: 10.1097/AOG.0000000000004532 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


