Abstract
Background
The COVID-19 pandemic, caused by SARS-CoV-2 infection, has become the most devastating zoonotic event in recent times, with negative impacts on both human and animal welfare as well as on the global economy. Although SARS-CoV-2 is considered a human virus, it likely emerged from animals, and it can infect both domestic and wild animals. This constitutes a risk for human and animal health including wildlife with evidence of SARS-CoV-2 horizontal transmission back and forth between humans and wild animals.
Aim
Molecular surveillance in different wildlife rehabilitation centers and wildlife associated institutions in Chile, which are critical points of animal-human interaction and wildlife conservation, especially since the aim of wildlife rehabilitation centers is to reintroduce animals to their original habitat.
Materials and Methods
The survey was conducted in six WRCs and three wildlife associated institutions. A total of 185 samples were obtained from 83 individuals belonging to 15 different species, including vulnerable and endangered species. Each specimen was sampled with two different swabs: one oropharyngeal or nasopharyngeal according to the nostril diameter, and/or a second rectal sample. RNA was extracted from the samples and two different molecular assays were performed: first, a conventional RT-PCR with pan-coronavirus primers and a second SARS-CoV-2 qPCR targeting the N and S genes.
Results
All 185 samples were negative for SARS-CoV-2.
Clinical relevance
This study constitutes the first report on the surveillance of SARS-CoV-2 from wildlife treated in rehabilitation centers in Chile, and supports the biosafety procedures adopted in those centers.
Keywords: Chile, wildlife conservation, wildlife rehabilitation centers, COVID-19, SARS-CoV-2
1. Introduction
The current COVID-19 pandemic, caused by the coronavirus SARS-CoV-2, has infected more than 250 million humans and has caused more than 5 million deaths worldwide (WHO 2022). Early in the pandemic, based on the ability of coronaviruses to infect different vertebrate hosts (Kayode et al. 2021), many species were proposed as the zoonotic origin of SARS-CoV-2 (Gupta et al. 2021; Islam et al. 2022; Shahhosseini et al. 2021; K. Sharun et al. 2021a). Bats were presented as the natural reservoir of SARS-CoV-2, since chiropterans are the natural reservoir hosts of SARS and MERS, among other zoonotic viruses (Letko et al. 2020; Alves et al. 2021; Gupta et al. 2021; Hernandez-Aguilar et al. 2021; Jacob Machado et al. 2021; Kayode et al. 2021; Ruiz-Aravena et al. 2022). Because there were no SARS-like viruses obtained from bats that perfectly matched the sequence of SARS-CoV-2, an unknown intermediate host was proposed as the bridge before it became a human infection (Farrag et al. 2021). Although the spillover model was accurate for MERS (Gupta et al. 2021; Jacob Machado et al. 2021; Weidinger et al. 2021), currently there is no experimental data proving the spillover model for SARS and SARS-CoV-2 infections (Frutos et al. 2021; Jacob Machado et al. 2021). Nonetheless, wildlife can play a critical role in the transmission of SARS-CoV-2, as was the case with minks (Neovison vison). SARS-CoV-2 infection in mink farms showed horizontal transmission of the virus from humans to mink, between minks, and from minks to humans (Rabalski et al. 2021; Shriner et al. 2021). These transmission events caused the emergence of a new variant named “Cluster 5”, which had a higher affinity to the ACE2 receptor (Peacock et al. 2021; K. Sharun et al. 2021a), leading to a massive culling of minks in different countries (Frutos et al. 2021). Likewise, white-tailed deer (Odocoileus virginianus) in North America display a high rate of infection, with hundreds of cases reported (Le Page 2021; Kuchipudi et al. 2022). Therefore, the risk of SARS-CoV-2 infection in wildlife is relevant both for wild animal health and, to an extent, ecosystem health, as well as human health, since wildlife can act as reservoirs of many infectious diseases (Grange et al. 2021).
A suitable host for a virus, has target cells available to become infected and allows efficient replication, so it can then spread to other individuals (Frutos et al. 2021). In the case of SARS-CoV-2, the infection of target cells occurs through recognition of the angiotensin-converting enzyme 2 (ACE2) receptor by the spike protein of the virus (Devaux et al. 2020). Many in silico analyses quickly identified species that were more or less susceptible to SARS-CoV-2 infection based on the receptor binding domain (RBD) of the spike protein and ACE2 receptor coding sequences (Islam et al. 2022). Based on ACE2 receptor coding sequences, several lists of different vertebrate species were proposed, classifying potential host susceptibility based on this data (Mathavarajah and Dellaire 2020). Combining both in silico and experimental data, SARS-CoV-2 susceptible animal hosts are now clearly defined: bats, felids, non-human primates, mustelids, and deer are highly susceptible (Islam et al. 2022; Palmer et al. 2021; Parolin et al. 2021); domestic dogs have low susceptibility, and other vertebrates such as sheep, birds, and reptiles are not susceptible (Villanueva-Saz et al. 2021; Fischhoff et al. 2021a). Besides mink farms, other places that have a high contact of human and wild animals are zoological parks and wildlife rehabilitation centers (WRCs, hereafter). Indeed, zoological parks were the first place where susceptible wild animals such as tigers and cougars were reported both with infection and clinical symptoms of SARS-CoV-2 (Jemeršić et al. 2021). Moreover, WRCs are places of high significance in their potential to spread SARS-CoV-2 infection to susceptible wildlife reservoir hosts; this due to the fact that animals that arrive at WRCs have several instances of interaction with humans: both with the people who find them, with the WRC trained staff that receives them, and later when they are released back to the environment (Hedman et al. 2021). Consequently, there are concerns that WRCs could act as a potential threat in the dissemination of SARS-CoV-2 from humans to wildlife (Islam et al. 2022; Sharun et al. 2021a), especially in places such as Latin America, where most WRCs are funded by visitors and donations, both heavily impacted by COVID-19 restrictions, and also because in Latin America there are many potentially susceptible wildlife species to SARS-CoV-2 infection (Chaves et al. 2021).
In Chile, the first reported human case of COVID-19 was on March 3rd, 2020, with the first wave between May and June 2020 with 231,948 reported cases, and the second wave between February and March 2021 with 470,542 reported cases. Vaccination started on December 24th, 2020 with both inactivated and mRNA vaccines. As of November 2022, the total accumulated number of confirmed cases in Chile is 4,769,638 with 50,063 deceased (MINSAL 2022). Regarding WRCs in Chile, there are currently 26 centers officially registered by the Chilean Agricultural and Livestock Service (SAG), and are distributed in several regions of the country (SAG 2022). Therefore, our aim was to perform molecular surveillance of SARS-CoV-2 at six WRCs and three wildlife associated institutions, located in different geographical areas, for viral detection in potentially susceptible native wild animals. To the best of our knowledge, there are no reports of molecular surveillance of SARS-CoV-2 in wildlife admitted at WRCs in Latin America and the rest of the world.
2. Materials and methods
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. This study was approved by the Universidad Andres Bello Ethics Board, protocol number 041/2020. This study was conducted for a year, between October 2020 and October 2021. The Chilean Agricultural and Livestock Service (SAG) authorized the WRCs to be able to work with wildlife with the following permits: N° 1506/2012, 803/2014, 1355/2015, 3717/2015, 132/2017, 455/2017, 2186/2019, 7490/2021.
2.1. Animal selection and sampling
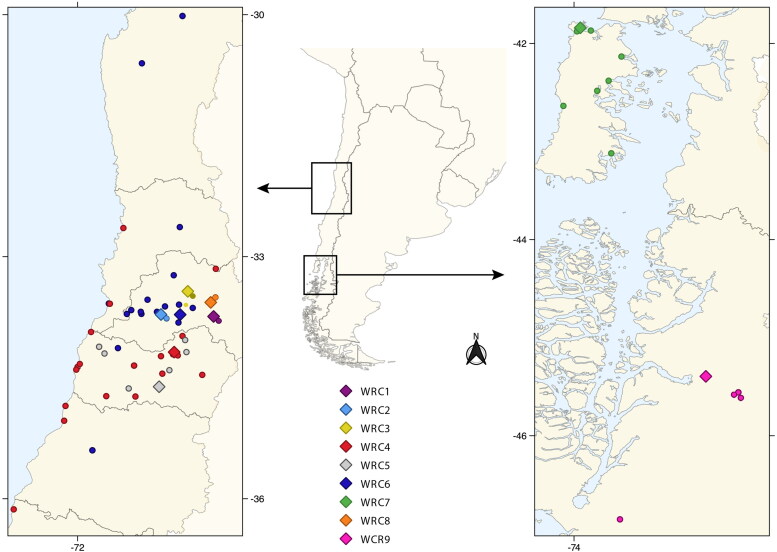
The survey was conducted in six WRCs and three wildlife associated institutions (WRC1-WRC9). WRCs 1,3,4,6-8 received, treated and released/euthanized wildlife. WRC2 operated only as an exhibition center, WRC5 handled exclusively road-accidents, and WRC9 captured, sampled, and then released animals. Sampling kits were sent to each WRC. They contained personal protective equipment (PPE), disinfectants, and swabs with DNA/RNA shield (Zymo Research, Irvine, CA, USA). Each WRC received materials to sample at least 10 different animals. Wild mammals in Chile usually are admitted at WRCs after trauma (Romero et al. 2019), nonetheless, the common admittance causes for animals sampled in this study were mostly trauma, followed by disease and orphaning. Following the OIE Guidelines of Handling wild animals during the COVID-19 pandemic (OIE 2022), no animals were anesthetized solely for the purpose of obtaining a sample, and they were only sampled when other medical procedures had to be performed. Also, local veterinary staff determined that sampling the animals did not put them at risk in the current condition that they were admitted. Because of the aforementioned factors, there was no standardized number of sampled animals or time frame of sampling, as it was performed based on opportunity.
The is no national entity that regulates the measures WRCs should take to handle susceptible individuals during the pandemic, each WRC had their own protocol to try and not inadvertently infect the individuals. In most WRCs, personal protection equipment was used such as, KN95 or surgical masks, nitrile gloves, goggles or face shields, gowns or aprons, surgical caps and disposable shoe covers to handle all susceptible patients. Furthermore, before and after treating or working with the patients, surfaces were thoroughly disinfected with quaternary ammonium, to decrease the probability of having cross species infections between different sampled individuals. WRC staff were not regularly screened; however, if they become PCR positive to COVID-19 they could not come back to work unless 2 weeks had passed due to national legislative protocols that were in place during this study. Furthermore, if the staff are a close contact or confirmed case, they have an immediate medical license to stay at home until 2 weeks have passed.
Potentially infected animal selection criteria were as followed: 1. Confirmed species positivity reported in the literature; 2. Possible infection based on ACE2 receptor aminoacidic sequences; 3. Possible infection based on the taxonomic family of previously reported SARS-CoV-2 positive species.
2.2. Sample collection
In all live animals, one sample was obtained by two nasopharyngeal swabs in animals with large nostrils (one for each nostril) or one oropharyngeal swab in animals with nostrils smaller than the swab diameter. In necropsied animals, one tracheal swab was obtained. In almost all animals (live and necropsied), a second rectal swabbing was performed with one swab. All live sampling was performed in previously anesthetized animals by qualified veterinary staff in charge of each WRC. Each collection tube had 1 mL of DNA/RNA shield, which allows virus inactivation and RNA stabilization until extraction (Dunbar and Tang 2022). Swabs were then frozen at −20 °C until they were transported on dry ice to the laboratory. Samples from WRCs 1-6 and 8 were processed within a week of being obtained. Samples from WRCs 7 and 9 were stored frozen at −20 °C for a month and then shipped to the laboratory.
2.3. RNA isolation
Whole RNA was isolated from the samples using the Rneasy® Mini kit (QIAGEN, Germantown, MD, USA) with a maximum of 600 µL, following the manufacturer’s recommendations, with an elution volume of 50 µL. RNA was quantified by absorbance using a Qubit 4 Fluorometer, and only samples with >1 µg of total RNA were included in the study. An aliquot of 10 µL was separated for One-Step RT-qPCR assays and stored at −20 °C. The remaining 40 µL were immediately retrotranscribed to cDNA.
2.4. cDNA synthesis
RNA was retrotranscribed to cDNA using the Quantitec® reverse transcription kit (QIAGEN, USA). The manufacturer’s recommendations were as follows, with a step to eliminate contamination from genomic DNA (gDNA): 2 µL of gDNA Wipeout Buffer 7X, 2 µL of Rnase-free water and 10 µL of template RNA were incubated for 2 min at 42 °C. Afterwards, the template RNA was added to the reverse-transcription master mix and the incubation was carried out in one step: 30 min at 42 °C and 3 min at 95 °C. cDNA samples were stored at −20 °C until the following molecular analyses.
2.5. RT-PCR assays of Pan-Coronavirus (Pan-CoV)
A first screening was performed to evaluate the overall presence of Coronavirus in the samples, following the protocol described by Hu et al. (Hu et al. 2018), as previously validated for SARS-CoV-2 (Erlichster et al. 2021). The RT-PCR protocol was performed as follows: 10 µM of primers Pan-CoV-18 F2 (5′-AARTTYTAYGGHGGYTGG-3′) and Pan-CoV-18 R1 (5′-GARCARAATTCATGHGGDCC-3′), 5X Green GoTaq® Flexi Buffer (Fitchburg, WI, USA), 10 mM of dNTPs, MgCl2 solution 25 mM, GoTaq® G2 Flexi DNA Polymerase, nuclease free water and 2 µL of DNA template, in a final volume of 20 µL. Cycling conditions were 30 min at 50 °C, 2 min at 95 °C, followed by 35 cycles at 94 °C for 40 s, 52 °C for 40 s and 72 °C for 45 s, finishing with 72 °C for 5 min. A sample was considered positive with an amplicon of 668 bp. As a positive control we used a cDNA extracted from Nobilis IB MA5 vaccine and nuclease-free water as the no-template control in each assay.
2.6. Real-time PCR assays of SARS-CoV-2 (RT-qPCR)
qPCR assays were performed using the SARS-CoV-2 GenomeCoV19 Detection Kit (Applied Biological Materials Inc., Richmond, BC, Canada). The optimized protocol, ABM.G628V2-200M, consisted in COVID-19 Primers/Probes (G628-1.V2), RT-qPCR Enzyme mix (RT-13), Luna® Universal Probe qPCR master mix (Ipswich, MA, USA) and 6 μL of RNA template, in a final volume of 20 μL. Cycling conditions were 15 min at 50 °C, 2 min at 95 °C followed by 3 cycles at 95 °C for 5 s and 60 °C for 15 s, finishing with a 40 cycles at 90 °C for 5 s and 60 °C for 30 s. Detection of SARS-CoV-2 was considered positive when the two fluorophores FAM and HEX were amplified (N and S genes). All the assays were run in a Bioer LineGene k plus FQD-48A Real-time PCR (Hangzhou, Binjiang District, China), using the positive control template and negative extraction control included in the kit, and nuclease-free water as the no-template control in each assay.
3. Results
3.1. Susceptible native wild animals sampled
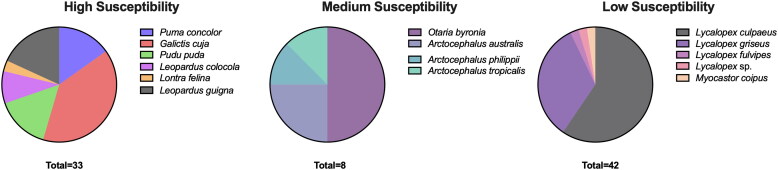
A total of 185 samples were obtained from 83 individuals belonging to 15 different species in 9 WRCs (Figure 1). Species were classified into low, medium, and high susceptibility to SARS-CoV-2, according to available in silico and experimental infection data (Fischhoff et al. 2021a; Figure 2).
Figure 1.
Geographical distribution of sampled animals. Each dot represents where the animal was found, and each diamond represents the location of Wildlife Rehabilitation Centers (WRC) where the animal was admitted for sampling (WRC1-WRC9). The color of each circle indicates the respective WRC where the animals were sampled.
Figure 2.
Susceptibility to SARS-CoV-2 infection of sampled animals based on published data (Islam et al. 2022; Palmer et al. 2021; Parolin et al. 2021; Villanueva-Saz et al. 2021; Fischhoff et al. 2021a; 2021b). Since there are no in silico or experimental susceptibility reports for most species (Galictis cuja, Pudu puda, Lontra felina, Lycalopex culpaeus, Lycalopex griseus, Lycalopex fulvipes, Otaria byronia, Arctocephalus australis, Arctocephalus philippi, Arctocephalus tropicalis, Leopardus guigna, and Leopardus colocola), susceptibility was estimated based on taxonomic family. Each color represents the proportion of sampled individuals in each susceptibility category.
3.2. Pan-CoV RT-PCR results
All 185 samples were negative to the Pan-Cov RT-PCR assay.
3.3. SARS-CoV-2 qPCR assay results
All 185 samples were tested with two different qPCR assays, and all animals were negative to both. The number and type of samples analyzed per species are available in Table 1. Detailed results for each specimen, such as sex, cause of admission and type of sample assayed are available in Table 2.
Table 1.
Total number of wild animals sampled in this study. Each sampled animal is identified with common name, scientific name, number of sampled animals, wildlife rehabilitation center, number and kind of sample analyzed, and qPCR assay results.
| Common Name | Scientific Name | Nº of specimens | WRC | OS | NS | RS | TS | FAM qPCR | HEX qPCR |
|---|---|---|---|---|---|---|---|---|---|
| Mountain Lion | Puma concolor | 5 | WRC1, WRC2, WRC3 | 0 | 5 | 5 | 0 | Negative | Negative |
| Lesser Grison | Galictis cuja | 13 | WRC3, WRC4, WRC5, WRC6 | 12 | 0 | 11 | 1 | Negative | Negative |
| Pudu | Pudu puda | 5 | WRC7 | 0 | 5 | 5 | 0 | Negative | Negative |
| Pampas Cat | Leopardus colocola | 3 | WRC3, WRC6 | 0 | 1 | 1 | 0 | Negative | Negative |
| Kodkod | Leopardus guigna | 6 | WRC5, WRC7, WRC9 | 2 | 2 | 4 | 0 | Negative | Negative |
| Marine Otter | Lontra felina | 1 | WRC4 | 0 | 0 | 1 | 0 | Negative | Negative |
| South American Sea Lion | Otaria byronia | 4 | WRC4 | 4 | 0 | 3 | 0 | Negative | Negative |
| South American Fur Seal | Arctocephalus australis | 2 | WRC4, WRC7 | 0 | 1 | 2 | 0 | Negative | Negative |
| Subantarctic Fur Seal | Arctocephalus tropicalis | 1 | WRC4 | 0 | 1 | 0 | 0 | Negative | Negative |
| Juan Fernández Fur Seal | Arctocephalus philippii | 1 | WRC4 | 1 | 0 | 1 | 0 | Negative | Negative |
| Andean Fox | Lycalopex culpaeus | 25 | WRC1, WRC4, WRC6, WRC8 | 3 | 8 | 19 | 12 | Negative | Negative |
| South American Gray Fox | Lycalopex griseus | 14 | WRC4, WRC6 | 2 | 4 | 12 | 5 | Negative | Negative |
| Darwin’s Fox | Lycalopex fulvipes | 1 | WRC7 | 0 | 1 | 0 | 0 | Negative | Negative |
| Fox | Lycalopex sp. | 1 | WRC4 | 1 | 0 | 1 | 0 | Negative | Negative |
| Coypu | Myocastor coipus | 1 | WRC6 | 1 | 0 | 1 | 0 | Negative | Negative |
WRC = Wildlife rehabilitation center; OS = oropharyngeal swab; NS = nasal swab; RS = rectal swab; TS = tracheal swab; WRC2 operates as a wildlife exhibition center; WRC5 and WRC9 did wildlife sampling but not rehabilitation.
Table 2.
Animal species sampled, sample kind, sex, cause of admission and wildlife rehabilitation center of admittance. Samples are listed in chronological order.
| Species | Sample Kind | Sex | Cause of Admission | Wildlife Rehabilitation Center |
|---|---|---|---|---|
| Puma concolor | NS | Male | Orphaned | WRC1 |
| RS | ||||
| Puma concolor | NS | Male | Orphaned | WRC1 |
| RS | ||||
| Lycalopex culpaeus | NS | Female | Orphaned | WRC4 |
| Puma concolor | NS | Female | Orphaned | WRC2 |
| RS | ||||
| Galictis cuja | OS | Male | Trauma | WRC4 |
| RS | ||||
| Lycalopex culpaeus | OS | Female | Trauma | WRC4 |
| RS | ||||
| Otaria byronia | OS | Male | Trauma | WRC4 |
| RS | ||||
| Arctocephalus philippii | OS | – | Orphaned | WRC4 |
| RS | ||||
| Lycalopex culpaeus | RS | Female | Trauma | WRC4 |
| Lycalopex griseus | NS | Ilegal trapping | WRC6 | |
| RS | ||||
| Lycalopex culpaeus | NS | Male | Collision with vehicle | WRC6 |
| RS | ||||
| Lycalopex culpaeus | NS | Male | Disease (Distemper) | WRC6 |
| RS | ||||
| Lycalopex griseus | TS | Female | Orphaned | WRC6 |
| RS | ||||
| Lycalopex griseus | TS | Female | Collision with vehicle | WRC6 |
| RS | ||||
| Leopardus colocola | NS | Male | Collision with vehicle | WRC6 |
| RS | ||||
| Lycalopex griseus | RS | Female | Ilegal captivity | WRC6 |
| Otaria byronia | NS | Male | Trauma | WRC4 |
| Galictis cuja | OS | Male | Collision with vehicle | WRC5 |
| RS | ||||
| Lycalopex culpaeus | NS | Male | Sarna | WRC4 |
| Lycalopex griseus | NS | Male | Disease (Distemper) | WRC4 |
| Galictis cuja | OS | Collision with vehicle | WRC4 | |
| RS | ||||
| Galictis cuja | OS | Collision with vehicle | WRC4 | |
| RS | ||||
| Lycalopex sp. | OS | Orphaned | WRC4 | |
| RS | ||||
| Galictis cuja | OS | Collision with vehicle | WRC4 | |
| RS | ||||
| Galictis cuja | OS | Female | Collision with vehicle | WRC5 |
| RS | ||||
| Leopardus guigna | OS | Orphaned | WRC5 | |
| RS | ||||
| Otaria byronia | OS | Orphaned | WRC4 | |
| RS | ||||
| Galictis cuja | OS | Male | Collision with vehicle | WRC4 |
| RS | ||||
| Lycalopex culpaeus | TS | Male | Unknown/Not Recorded | WRC8 |
| RS | ||||
| Lycalopex culpaeus | OS | Unknown/Not Recorded | WRC1 | |
| RS | ||||
| Lycalopex culpaeus | TS | Collision with vehicle | WRC6 | |
| Lycalopex culpaeus | TS | Male | Unknown/Not Recorded | WRC8 |
| RS | ||||
| Lycalopex culpaeus | NS | Unknown/Not Recorded | WRC1 | |
| RS | ||||
| Lycalopex culpaeus | NS | Unknown/Not Recorded | WRC1 | |
| RS | ||||
| Lycalopex culpaeus | TS | Female | Unknown/Not Recorded | WRC8 |
| RS | ||||
| Lycalopex culpaeus | TS | Male | Unknown/Not Recorded | WRC8 |
| RS | ||||
| Lycalopex culpaeus | TS | Juvenile with suboptimal condition | WRC6 | |
| RS | ||||
| Lycalopex culpaeus | TS | Attacked by dog | WRC6 | |
| RS | ||||
| Galictis cuja | OS | Female | Trauma | WRC4 |
| RS | ||||
| Galictis cuja | OS | – | Dead | WRC4 |
| RS | ||||
| Lycalopex griseus | RS | Female | Disease (Scabies) | WRC4 |
| Lycalopex griseus | OS | Female | Trauma | WRC4 |
| RS | ||||
| Otaria byronia | OS | Female | Orphaned | WRC4 |
| RS | ||||
| Galictis cuja | OS | Female | Trauma | WRC4 |
| RS | ||||
| Lycalopex griseus | NS | Female | Disease (Scabies) | WRC4 |
| Galictis cuja | OS | Male | Malnutrition | WRC4 |
| Galictis cuja | OS | Unknown/Not Recorded | WRC5 | |
| Puma concolor | NS | Unknown/Not Recorded | WRC3 | |
| RS | ||||
| Puma concolor | NS | Female | Unknown/Not Recorded | WRC3 |
| RS | ||||
| Galictis cuja | TS | Male | Collision with vehicle | WRC6 |
| RS | ||||
| Leopardus colocola | Female | Unknown/Not Recorded | WRC3 | |
| Leopardus colocola | Female | Unknown/Not Recorded | WRC3 | |
| Myocastor coipus | OS | Male | Unknown trauma | WRC6 |
| RS | ||||
| Lontra felina | RS | Male | Trauma | WRC4 |
| Arctocephalus tropicalis | NS | – | Orphaned | WRC4 |
| RS | ||||
| Lycalopex fulvipes | NS | Unknown/Not Recorded | WRC7 | |
| Pudu puda | NS | Unknown/Not Recorded | WRC7 | |
| RS | ||||
| Pudu puda | NS | Female | Unknown/Not Recorded | WRC7 |
| RS | ||||
| Leopardus guigna | NS | Male | Unknown/Not Recorded | WRC7 |
| RS | ||||
| Pudu puda | NS | Female | Unknown/Not Recorded | WRC7 |
| RS | ||||
| Pudu puda | NS | Female | Unknown/Not Recorded | WRC7 |
| RS | ||||
| Arctocephalus australis | NS | Unknown/Not Recorded | WRC7 | |
| RS | ||||
| Arctocephalus australis | NS | Male | Trauma | WRC4 |
| RS | ||||
| Lycalopex culpaeus | TS | Unknown trauma | WRC6 | |
| RS | ||||
| Lycalopex culpaeus | TS | Poisoned | WRC6 | |
| RS | ||||
| Lycalopex griseus | TS | Attacked by dog | WRC6 | |
| RS | ||||
| Lycalopex griseus | RS | Collision with vehicle | WRC6 | |
| TS | ||||
| Lycalopex griseus | TS | Ilegal trapping | WRC6 | |
| RS | ||||
| Lycalopex griseus | OS | Orphaned | WRC6 | |
| RS | ||||
| Lycalopex culpaeus | TS | Juvenile with suboptimal condition | WRC6 | |
| RS | ||||
| Lycalopex culpaeus | OS | Unknown/Not Recorded | WRC4 | |
| RS | ||||
| Lycalopex culpaeus | NS | Female | Disease (Gut Infection) | WRC4 |
| RS | ||||
| Lycalopex culpaeus | OS | Female | Trauma | WRC4 |
| RS | ||||
| Lycalopex griseus | NS | Female | Trauma | WRC4 |
| RS | ||||
| Lycalopex griseus | RS | Unknown/Not Recorded | WRC4 | |
| Otaria byronia | OS | Female | Unknown/Not Recorded | WRC4 |
| RS | ||||
| Lycalopex culpaeus | OS | Male | Trauma | WRC4 |
| RS | ||||
| Lycalopex culpaeus | NS | Female | Unknown/Not Recorded | WRC5 |
| RS | ||||
| Leopardus guigna | NS | Unknown/Not Recorded | WRC9 | |
| Leopardus guigna | RS | Unknown/Not Pecorded | WRC9 | |
| Leopardus guigna | OS | Unknown/Not Recorded | WRC9 | |
| Leopardus guigna | RS | Unknown/Not Recorded | WRC9 |
WRC = Wildlife rehabilitation center; OS = oropharyngeal swab; NS = nasal swab; RS = rectal swab; TS = tracheal swab; WRC2 operates as a wildlife exhibition center; WRC5 and WRC9 did wildlife sampling but not rehabilitation.
4. Discussion
Most of the evaluated animals were sampled within the first days of admission during their initial physical exams, and due to their negative results, this could be indicating that these individuals are not getting infected in their previous natural habitat. This is in accordance with previous studies reporting that the evidence of the maintenance of the virus in the wild is scant (Delahay et al. 2021), although the exposure of wild animals to the virus has been reported (Chandler et al. 2021). Preventive measures adopted at WRCs will continue to be followed when necessary, as they could prevent transmission from asymptomatic staff. However, the cross-sectional design of our study prevented more permanent monitoring of the animals, which were only sampled when they were subjected to other interventions that required their direct manipulation. In addition, serological survey of antibodies against SARS-CoV-2 should be included in future surveillance and monitoring, to obtain information of virus exposure in wildlife. Ideally, future studies should also monitor the WRC personnel SARS-CoV-2 infection status in a periodic manner, to test the animals in case there are human cases of COVID-19 in the compound, which is a scenario that did not happen during this study. Asymptomatic healthcare workers have been identified as critical points in SARS-CoV-2 transmission, as they cannot do physical distancing from patients. In this group, the use of PPE has been paramount in preventing SARS-CoV-2 transmission (Olmos et al. 2021). This also applies to WRCs staff, which is why the use of face masks, face shields, gloves, disposable overalls, and shoe covers is mandatory in most of the WRCs included in this study.
Vaccination for preventing SARS-CoV-2 is not yet performed in animals from these WRCs, so this would not be influencing our results. Also, a large proportion of the samples analyzed in this study were obtained when vaccination was not yet available to neither domestic and wild animals, the general public, animal handlers, nor the veterinarians in all the WRCs included in this study. Currently, in Chile 92.1% of the population is fully vaccinated (either with a single or two shots), and 78.3% has received a booster immunization (MINSAL 2022). This vaccination effort is likely to contribute to a smaller chance of horizontal viral transmission between humans and wildlife. Now, vaccination of both domestic and wild animals is a possibility, since vaccine candidates were first tested in non-human animals prior to clinical trials; domestic cats have shown high levels of neutralizing antibodies, and non-human primates in zoos have also been immunized (Khan Sharun et al. 2021b). However, vaccination at WRCs is not warranted, because these vaccination efforts are unlikely to prevent SARS-CoV-2 infection in wild animals, therefore, vaccinating wild animals is not a part of the animal handling protocols at WRCs, and they usually can only receive one shot. Nonetheless, domestic animals and captive animals at zoos should be vaccinated to minimize symptoms and risk of severe disease.
According to the International Union for Conservation of Nature (IUCN), some animals sampled in this study belong to vulnerable (Pudu puda, Leopardus guigna), near threatened (Leopardus colocola), and endangered (Lontra felina, Lycalopex fulvipes) categories. Since felids, mustelids and cervids have been reported previously naturally infected by SARS-COV-2, the possibility of SARS-CoV-2 infection was of high concern, enhancing the need to assess the infection in those mentioned species. Regarding the other taxonomic groups, negative results were previously reported in wild canids (Jemeršić et al. 2021), and, to the best of our knowledge, this is the first assessment of the infection in Otariidae and Myocastoriidae. We are confident in our results, because the RT-PCR and RT-qPCR assays have high sensitivity and specificity for SARS-CoV-2 infection (Camporesi et al. 2022; Pratelli et al. 2022), the GenomeCoV19 Detection Kit used in this study has been validated previously (Buchta et al. 2021; Wozniak A et al. 2020; Peña et al. 2021; Sarwar et al. 2021); with only one of these publications reporting a 95% sensitivity and 100% specificity (Wozniak A et al. 2020). This commercial kit is designed with two different SARS-CoV-2 genes (N and S), minimizing the possibility of false positives. To further prove our results, each of these animals were evaluated from two different biological samples. However, since the OIE guidelines recommended not to sample animals at zoos and WRCs for the sole purpose of detecting SARS-CoV-2 (OIE 2022), animals were sampled once and only during other procedures, which could lead to false negatives (Sánchez‐Montes et al. 2022).
The main limitation of our study lies in the sampling method; because of the limited manipulation that each animal was subjected to, we could only sample each individual once. Also, due to anatomical differences, the sample kind also fluctuated, as we were unable to obtain nasal swabs from every species, since the nostrils of small carnivores were narrower than the swabs used. Future efforts to monitor infected wildlife that are admitted at WRCs and released back to the environment should include at least 2 samplings, one at admittance and the other before release. In this way we could ensure that released fauna are not carrying SARS-CoV-2 infection back to nature.
5. Conclusion
Our study constitutes the first report on the molecular surveillance of SARS-CoV-2 from wildlife treated in rehabilitation centers of Chile. Efforts must be made to continue molecular surveillance of SARS-CoV-2, especially in cervids, and serological assays must be implemented to assess previous exposure to the virus. Both assays should be performed in released fauna, to ensure ecosystem and planetary health.
Acknowledgments
We would like to thank Dr. Renzo Boccanegra for his help in implementing the Pan-CoV RT-PCR assays, Dr. Susan Christen for her technical help, and SAG and CONAF for their assistance. We would also like to thank all the wildlife rehabilitation center staff that were involved in the process of obtaining the samples from the various animals included in this study.
Funding Statement
This work was supported by the Agencia Nacional de Investigación y Desarrollo (ANID) under Grants ANID/COVID 0728 (GR), Fondecyt Postdoc PD3180707 (GR), Fondecyt Regular 1180940 (EC), and PAI77180009 (AR).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alves RS, do Canto Olegário J, Weber M, da Silva M, Canova R, Sauthier J, Baumbach L, Witt A, Varela A, Mayer F, et al. 2022. Detection of coronavirus in vampire bats (Desmodus rotundus) in southern Brazil. Transboun Emerg Dis. 69(4):2384–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchta C, Görzer I, Chiba P, Camp JV, Holzmann H, Puchhammer-Stöckl E, Mayerhofer M, Müller MM, Aberle SW.. 2021. Variability of cycle threshold values in an external quality assessment scheme for detection of the SARS-CoV-2 virus genome by RT-PCR. Clin Chem Lab Med. 59(5):987–994. [DOI] [PubMed] [Google Scholar]
- Camporesi A, De Silvestri A, Diotto V, Ferrario S, Eccher L, De Ferrari A, Messina F, Pelizzo G, Mileto D, Calcaterra V.. 2022. Very high negative concordance rate of RT-PCR for SARS-CoV-2 in nasopharyngeal swab and tracheo-bronchial aspirate in children. Front Pediatr. 10:866111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler JC, Bevins SN, Ellis JW, Linder TJ, Tell RM, Jenkins-Moore M, Root JJ, Lenoch JB, Robbe-Austerman S, DeLiberto TJ.. 2021. SARS-CoV-2 exposure in wild white-tailed deer (Odocoileus virginianus). Proc Natl Acad Sci USA. 118(47):e2114828118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves A, Montecino‐Latorre D, Alcázar P, Suzán G.. 2021. Wildlife rehabilitation centers as a potential source of transmission of SARS‐CoV‐2 into native wildlife of Latin America. Biotropica. 53(4):987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahay RJ, de la Fuente J, Smith GC, Sharun K, Snary EL, Flores Girón L, Nziza J, Fooks AR, Brookes SM, Lean FZX.. 2021. Assessing the risks of SARS-CoV-2 in wildlife. One Health Outlook. 3(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux CA, Pinault L, Osman IO, Raoult D.. 2020. Can ACE2 receptor polymorphism predict species susceptibility to SARS-CoV-2? Front Public Health. 8:608765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar SA, Tang Y-W.. 2022. Diagnostic tests and procedures during the COVID-19 pandemic. In: Pandemics: insurance and social protection. Cham: Springer; p. 191–216. [Google Scholar]
- Erlichster M, Chana G, Zantomio D, Goudey B, Skafidas E.. 2021. Pan-family assays for rapid viral screening: reducing delays in public health responses during pandemics. Clin Infect Dis. 73(9):e3047–e3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrag MA, Amer HM, Bhat R, Hamed ME, Aziz IM, Mubarak A, Dawoud TM, Almalki SG, Alghofaili F, Alnemare AK, et al. 2021. SARS-CoV-2: an overview of virus genetics, transmission, and immunopathogenesis. Int J Environ Res Public Health. 18(12):6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff IR, Castellanos AA, Rodrigues J, Varsani A, Han BA.. 2021a. Predicting the zoonotic capacity of mammals to transmit SARS-CoV-2. Proc Biol Sci. 288(1963):20211651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischhoff IR, Castellanos AA, Rodrigues JPGLM, Varsani A, Han BA. 2021b. Predicting the zoonotic capacity of mammals to transmit SARS-CoV-2. Proc Biol Sci. 288(1963):20211651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R, Gavotte L, Devaux CA.. 2021. Understanding the origin of COVID-19 requires to change the paradigm on zoonotic emergence from the spillover to the circulation model. Infect Genet Evol. 95:104812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange ZL, Goldstein T, Johnson CK, Anthony S, Gilardi K, Daszak P, Olival KJ, O'Rourke T, et al. ; Expert Panel; PREDICT Consortium; Mazet JAK; University of Edinburgh Epigroup members those who wish to remain anonymous. 2021. Ranking the risk of animal-to-human spillover for newly discovered viruses. Proc Natl Acad Sci USA. 118(39):e2115409118 [Google Scholar]
- Gupta P, Singh MP, Goyal K, Tripti P, Ansari MI, Obli Rajendran V, Dhama K, Malik YS.. 2021. Bats and viruses: a death-defying friendship. VirusDis. 32(3):467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman HD, Krawczyk E, Helmy YA, Zhang L, Varga C.. 2021. Host diversity and potential transmission pathways of SARS-CoV-2 at the human-animal interface. Pathogens. 10(2):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Aguilar I, Lorenzo C, Santos-Moreno A, Naranjo EJ, Navarrete-Gutierrez D.. 2021. Coronaviruses in bats: a review for the Americas. Viruses. 13(7):1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Jung K, Wang Q, Saif LJ, Vlasova AN.. 2018. Development of a one-step RT-PCR assay for detection of pancoronaviruses (α-, β-, γ-, and δ-coronaviruses) using newly designed degenerate primers for porcine and avian ′fecal samples. J Virol Methods. 256:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam A, Ferdous J, Islam S, Sayeed MA, Rahman MK, Saha O, Hassan MM, Shirin T.. 2022. Transmission dynamics and susceptibility patterns of SARS-CoV-2 in domestic, farmed and wild animals: Sustainable One Health surveillance for conservation and public health to prevent future epidemics and pandemics. Transbound Emerg Dis. 69(5):2523–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob Machado D, Scott R, Guirales S, Janies DA.. 2021. Fundamental evolution of all Orthocoronavirinae including three deadly lineages descendent from Chiroptera-hosted coronaviruses: SARS-CoV, MERS-CoV and SARS-CoV-2. Cladistics. 37(5):461–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemeršić L, Lojkić I, Krešić N, Keros T, Zelenika TA, Jurinović L, Skok D, Bata I, Boras J, Habrun B.. 2021. Investigating the Presence of SARS CoV-2 in free-living and captive animals. Pathogens. 10(6):635. https://www.mdpi.com/2076-0817/10/6/635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayode AJ, Banji-Onisile FO, Olaniran AO, Okoh AI.. 2021. An overview of the pathogenesis, transmission, diagnosis, and management of endemic human coronaviruses: a reflection on the past and present episodes and possible future outbreaks. Pathogens. 10(9):1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchipudi SV, Surendran-Nair M, Ruden RM, Yon M, Nissly RH, Vandegrift KJ, Nelli RK, Li L, Jayarao BM, Maranas CD, et al.. 2022. Multiple spillovers from humans and onward transmission of SARS-CoV-2 in white-tailed deer. Proc Natl Acad Sci U S A. 119(6):e2121644119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Page M. 2021. Should we be worried by wild animals with covid-19? New Sci. 252(3361):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M, Seifert SN, Olival KJ, Plowright RK, Munster VJ.. 2020. Bat-borne virus diversity, spillover and emergence. Nat Rev Microbiol. 18(8):461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathavarajah S, Dellaire G.. 2020. Lions, tigers and kittens too: ACE2 and susceptibility to COVID-19. Evol Med Public Health. 2020(1):109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINSAL . 2022. Epidemiological Report and Diary Report of the Health Minister of Chile. https://www.gob.cl/coronavirus/cifrasoficiales/.
- OIE . 2022. OIE Wildlife Health Framework: ‘Protecting Wildlife Health to Achieve One Health’. https://www.oie.int/fileadmin/Home/eng/Internationa_Standard_Setting/docs/pdf/WGWildlife/A_Wildlifehealth_conceptnote.pdf.
- Olmos C, Campaña G, Monreal V, Pidal P, Sanchez N, Airola C, Sanhueza D, Tapia P, Muñoz AM, Corvalan F.. 2021. SARS-CoV-2 infection in asymptomatic healthcare workers at a clinic in Chile. PLoS One. 16(1):e0245913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MV, Martins M, Falkenberg S, Buckley A, Caserta LC, Mitchell PK, Cassmann ED, Rollins A, Zylich NC, Renshaw RW.. 2021. Susceptibility of white-tailed deer (Odocoileus virginianus) to SARS-CoV-2. J Virol. 95(11):e00083–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin C, Virtuoso S, Giovanetti M, Angeletti S, Ciccozzi M, Borsetti A.. 2021. Animal hosts and experimental models of SARS-CoV-2 infection. Chemotherapy. 66(1–2):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock TP, Penrice-Randal R, Hiscox JA, Barclay WS.. 2021. SARS-CoV-2 one year on: evidence for ongoing viral adaptation. J General Virol. 102(4):001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña M, Ampuero M, Garcés C, Gaggero A, García P, Velasquez MS, Luza R, Alvarez P, Paredes F, Acevedo J.. 2021. Performance of SARS-CoV-2 rapid antigen test compared with real-time RT-PCR in asymptomatic individuals. Int J Infect Dis. 107:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratelli A, Pellegrini F, Ceci L, Tatò D, Lucente MS, Capozzi L, Camero M, Buonavoglia A.. 2022. Severe acute respiratory syndrome coronavirus 2 detection by real time polymerase chain reaction using pooling strategy of nasal samples. Front Microbiol. 13:957957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabalski L, Kosinski M, Smura T, Aaltonen K, Kant R, Sironen T, Szewczyk B, Grzybek M.. 2021. Severe acute respiratory syndrome coronavirus 2 in farmed mink (neovison vison), Poland. Emerg Infect Dis. 27(9):2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero F, Espinoza A, Sallaberry-Pincheira N, Napolitano C.. 2019. A five-year retrospective study on patterns of casuistry and insights on the current status of wildlife rescue and rehabilitation centers in Chile. Rev Chil de Hist Nat. 92(1):1–10. [Google Scholar]
- Ruiz-Aravena M, McKee C, Gamble A, Lunn T, Morris A, Snedden CE, Yinda CK, Port JR, Buchholz DW, Yeo YY.. 2022. Ecology, evolution and spillover of coronaviruses from bats. Nat Rev Microbiol. 20(5):299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAG . 2022. National Registry of Wildlife Tenence. https://www.sag.gob.cl/ambitos-de-accion/registro-nacional-de-tenedores-de-fauna-silvestre-rntfs/1374/registros.
- Sánchez‐Montes S, Ballados‐González GG, Gamboa‐Prieto J, Cruz‐Romero A, Romero‐Salas D, Pérez‐Brígido CD, Austria‐Ruíz MJ, Guerrero‐Reyes A, Lammoglia‐Villagómez MA, Camacho‐Peralta IP.. 2022. No molecular evidence of SARS‐CoV‐2 infection in companion animals from Veracruz, Mexico. Transbounding Emerging Dis. 69(4):2398–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar MB, Yasir M, Alikhan N-F, Afzal N, de Oliveira Martins L, Le Viet T, Trotter AJ, Prosolek SJ, Kay GL, Foster-Nyarko E.. 2021. SARS-CoV-2 variants of concern dominate in Lahore, Pakistan in April 2021. Microbial Genomics. 7(11):000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahhosseini N, Wong G, Kobinger GP, Chinikar S.. 2021. SARS-CoV-2 spillover transmission due to recombination event. Gene Rep. 23:101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharun K, Dhama K, Pawde AM, Gortázar C, Tiwari R, Bonilla-Aldana DK, Rodriguez-Morales AJ, de la Fuente J, Michalak I, Attia YA.. 2021a. SARS-CoV-2 in animals: potential for unknown reservoir hosts and public health implications. Vet Q. 41(1):181–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharun K, Tiwari R, Saied AA, Dhama K.. 2021b. SARS-CoV-2 vaccine for domestic and captive animals: An effort to counter COVID-19 pandemic at the human-animal interface. Vaccine. 39(49):7119–7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriner SA, Ellis JW, Root JJ, Roug A, Stopak SR, Wiscomb GW, Zierenberg JR, Ip HS, Torchetti MK, DeLiberto TJ.. 2021. SARS-CoV-2 exposure in Escaped Mink, Utah, USA. Emerg Infect Dis. 27(3):988–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva-Saz S, Giner J, Fernández A, Lacasta D, Ortín A, Ramos JJ, Ferrer LM, Ruiz de Arcaute M, Tobajas AP, Pérez MD.. 2021. Absence of SARS-CoV-2 antibodies in natural environment exposure in sheep in close contact with humans. Animals (Basel). 11(7):1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger P, Kolodziejek J, Camp JV, Loney T, Kannan DO, Ramaswamy S, Tayoun AA, Corman VM, Nowotny N.. 2022. MERS-CoV in sheep, goats, and cattle, United Arab Emirates, 2019: Virological and serological investigations reveal an accidental spillover from dromedaries. Transbound Emerg Dis. 69(5):3066–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak A, Cerda A, Ibarra-Henríquez C, Sebastian V, Armijo G, Lamig L, Miranda C, Lagos M, Solari S, Guzmán AM, et al. 2020. A simple RNA preparation method for SARS-CoV-2 detection by RT-qPCR. Sci Rep. 10(1):16608. [DOI] [PMC free article] [PubMed]
- WHO . 2022. World Health Organization (WHO) Coronavirus (COVID-19) Dashboard. https://covid19.who.int/.