Abstract
The stress-induced protease ClpP is required for virulence of the facultative intracellular pathogen Listeria monocytogenes. We previously found that in the absence of ClpP, the virulence of this pathogen was strongly reduced, mainly due to the decreased production of functional listeriolysin O (LLO), a major immunodominant virulence factor promoting intracellular growth. In this work, a clpP deletion mutant of L. monocytogenes was used to study the generation of anti-Listeria protective immunity. We found that ClpP is required for the intracellular growth of L. monocytogenes in resident macrophages in vivo. Mice infected with doses as high as 106 clpP mutant bacteria were not protected against a lethal challenge of wild-type bacteria and did not develop any detectable LLO-specific cytolytic T cells or antibodies, suggesting that the amount of LLO produced in infected mice under these conditions was too low to induce a specific immune response. However, in contrast to the results obtained with a mutant with a disrupted hly gene, this lack of protection was overcome by inoculation of very high infecting doses of clpP mutant bacteria (5 × 108), thus producing sufficient amounts of LLO to stimulate anti-Listeria immunity. The role of ClpP was confirmed by showing that anti-Listeria immunity was restored in mice infected with a clpP-complemented mutant. These results indicate that the stress-induced serine protease ClpP is a potential target for modulating the presentation of protective antigens such as LLO and thereby the immune response against L. monocytogenes.
Listeria monocytogenes is a facultative intracellular gram-positive bacterium responsible for severe infections in humans and animals, including meningoencephalitis and abortion (19). It has been extensively used as a model to study host resistance against intracellular bacterial pathogens. The virulence of this ubiquitous microorganism is due to its capacity to invade and multiply within host cells, including macrophages (29). During its intracellular life cycle, L. monocytogenes produces several virulence factors involved at each step of the invasive process, including listeriolysin O (LLO), internalin, phospholipases, and ActA, all controlled by the pleiotropic transcriptional activator PrfA (11). LLO is a 58-kDa exotoxin allowing bacteria to escape phagosomes of macrophages and to multiply in the cytoplasm (1, 10, 12, 13, 40). LLO is presumably processed by the cytosolic pathway and plays a crucial role in the effective presentation of Listeria antigens to immune T cells (2, 3, 4, 21, 26, 33).
It was recently shown that stress proteins also play a role in the virulence of L. monocytogenes, especially during the early stages of intracellular growth (31, 36, 37). Uptake of L. monocytogenes by macrophages induces a set of bacterial proteins, including stress proteins (20). Direct evidence for the role of these proteins in virulence of L. monocytogenes includes the finding that a ClpC ATPase belonging to the Hsp-100 family promotes early escape of bacteria from the phagosomal compartment of macrophages (36, 37). ClpC acts synergistically with ClpE, another Hsp-100 family member also involved in the expression of virulence (31). Recently, a stress-induced 21.6-kDa protein, designated ClpP, required for L. monocytogenes growth under hostile conditions was identified (15). It was found that ClpP belongs to the large family of ClpP serine proteases highly conserved in prokaryotes and eukaryotes. ClpP of L. monocytogenes acts as a serine protease and prevents the accumulation of altered proteins that might be toxic for the bacteria under stress conditions (15). In the absence of ClpP, the secretion of functional LLO is reduced, thus explaining why a clpP deletion mutant demonstrated poor growth in cultured bone marrow macrophages in vitro (15). Since ClpP promotes the intracellular survival of L. monocytogenes and presumably the antigenic presentation of LLO and other protective antigens, ClpP may represent a potential vaccine target for L. monocytogenes.
In this work, we studied the role of the stress-induced serine protease ClpP in the generation of anti-Listeria protective immunity in vivo. We found that ClpP is essential for intracellular survival in macrophages and modulates LLO-dependent anti-Listeria protection. Following high infecting doses of a clpP deletion mutant, sufficient amounts of LLO were produced to induce specific immunity against L. monocytogenes.
MATERIALS AND METHODS
Bacterial strains and culture media.
We used L. monocytogenes reference strain LO28 (37), a clpP deletion mutant of LO28, and the same mutant trans-complemented with clpP (15). As a control, we used BOF 415, a previously described Tn917 insertion hly mutant of LO28 (10). Bacteria were grown in brain heart infusion (BHI) media. For virulence assays, bacteria were harvested while still in log phase (5 × 108/ml), dispensed into vials (1-ml lots), and stored at −80°C until required. For each experiment, the contents of a vial were thawed and diluted appropriately in saline (0.15 M NaCl) before inoculation.
Infection of mice.
Adult, 6- to 8-week-old pathogen-free female BALB/c mice were supplied by Janvier, Le Geneset St. Isle, France. Mice were maintained in a protected environment under filtered airflow in negative-pressure isolators (ESI, Cachan, France). Animals were fed with sterilized, vitamin-supplemented diet and sterile water (pH 3). Mice were inoculated intravenously (i.v.) with appropriate dilutions of L. monocytogenes in a volume of 0.5 ml via a lateral tail vein. Growth of bacteria in the spleen and the liver was monitored. At intervals, groups of five mice were killed; the organs were removed aseptically and homogenized separately in sterile saline. Then, 0.1-ml volumes of serial 10-fold dilutions were surface plated on BHI agar with a minimal detectable limit of 100 bacteria per organ. Protection was estimated after 30 days by inoculating i.v. a lethal dose of L. monocytogenes LO28 (106 bacteria). The 50% lethal dose of strain LO28 was estimated at 5 × 104 bacteria/mouse. Student's t test was used for statistical analysis.
Titration of hemolytic activity.
The hemolytic activity of supernatants of mid-log-phase cultures incubated at 30 or 40°C was titrated with horse erythrocytes and expressed in arbitrary hemolytic units per 108 bacteria as previously described (15).
Western blot analysis.
Proteins in culture supernatants were precipitated with 10% (vol/vol) trichloroacetic acid. Bacterial whole extracts were prepared by boiling the cells for 5 min in 100 mM Tris (pH 6.8)–200 mM dithiothreitol–4% (wt/vol) sodium dodecyl sulfate–0.2% (wt/vol) bromophenol blue–20% (vol/vol) glycerol. Proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were stained with Coomassie blue or subjected to immunoblot analysis with a rabbit LLO-specific polyclonal antibody (18). Sheep anti-mouse immunoglobulin–horseradish peroxidase conjugate and an ECL kit (Amersham) were used for immunodetection. To determine the anti-LLO response, mice were bled 4 weeks after infection and the presence of anti-LLO antibodies (immunoglobulin G) was determined by Western blot analysis with purified LLO as previously described (39). Coating antibodies were detected with peroxidase-conjugated goat anti-mouse immunoglobulin G diluted 1/1,000 (Organon Teknika, West Chester, United Kingdom).
Anti-LLO cytolytic assay.
Mice were sacrificed by day 6 postinfection. Their spleens were removed, and single-cell suspensions were prepared. Erythrocytes were lysed using ammonium chloride, and splenocytes were washed, counted, and adjusted to the desired cell numbers. LLO-transfected P815 cells, pHEM3.3 cells (33), were incubated with 50 mg of mitomycin C (Boehringer GmbH, Mannheim, Germany)/liter at 37°C. Prior to use in cultures, pHEM3.3 cells were washed extensively in culture media. Cultures containing 2.5 × 105 splenocytes and 2.5 × 104 mitomycin C-treated pHEM3.3 stimulator cells in a volume of 200 μl were established in round-bottom 96-well tissue culture plates (Falcon, Mountain View, Calif.) and incubated at 37°C for 6 days. Recombinant human interleukin-2 (5 U/ml; Peprotech, London, United Kingdom) was present throughout the culture period. Splenocytes were then harvested, washed, counted, and adjusted to the desired cell numbers. The anti-LLO cytotoxicity of the effector cells was determined by use of a 4-h release assay with 3,000 chromium (51Cr)-labeled target cells, either pHEM 3.3 cells or nontransfected P815 cells, in cultures of 200 μl in round-bottom 96-well tissue culture plates. Effector cells were added to target cells at various ratios. Spontaneous release was measured by use of cultures with target cells incubated alone. Total release was measured by use of cultures containing target cells and 2% acetic acid. Spontaneous release was always less than 15% total release. The methods used for harvesting supernatants and the subsequent counting of radioactivity have been described previously (8). The specific cytotoxicity was calculated as follows: percent specific lysis = [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100.
IFN-γ production.
Spleen cells (106/ml) from infected mice and control mice were cultured in triplicate for 48 h in a volume of 200 μl in round-bottom 96-well tissue culture plates in the presence or absence of 107 heat-killed L. monocytogenes LO28. Subsequently, the supernatants were harvested, and the concentration of gamma interferon (IFN-γ) was measured using a sandwich enzyme-linked immunosorbent assay kit (Geneset, Cambridge, Mass.) according to the manufacturer's instructions.
Histologic analysis.
Mice were challenged i.v. with 108 bacteria (in 0.5 ml) and killed by cervical dislocation 1 or 8 h after inoculation. Small pieces of liver were removed and processed for Gram staining and electron microscopy. For light microscopy, samples were fixed in 10% formalin and embedded in paraffin. Semithin sections were cut and stained with 1% toluidine blue. Samples to be processed for electron microscopy were fixed in 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 2 h at room temperature, postfixed in 2% aqueous osmium tetroxide (E. Merck AG, Darmstadt, Germany) for 1 h, dehydrated in graded ethanol solutions, and embedded in Epon 812 (TAA-Jamming). Ultrathin sections of the appropriate blocks were cut, stained with uranyl acetate and lead citrate, and examined with an electron microscope (model CX200; Jeol, Croissy-sur-Seine, France).
RESULTS
ClpP is required for the intracellular growth of L. monocytogenes in infected mice.
It was previously demonstrated that ClpP of L. monocytogenes is required for intracellular growth in cultured bone marrow macrophages, as evidenced by a loss of virulence in a clpP deletion mutant (15). The relevance of this finding in vivo was studied by monitoring the fate of L. monocytogenes during the early phase of infection. Mice were inoculated i.v. with a high dose of either LO28 or its isogenic clpP deletion mutant (108 bacteria). After 1 and 8 h, mice were sacrificed and bacterial survival in the liver was monitored by light and electron microscopy. As illustrated in Fig. 1, bacteria from both strains were exclusively confined within Küpffer cells 1 h after infection. The electron microscopic study shows that most wild-type bacteria were intact inside vacuoles, whereas many of the mutant bacteria were already damaged (Fig. 1). After 8 h, wild-type bacteria were densely packed within Küpffer cells as a result of rapid intracellular growth. This dramatic multiplication of wild-type bacteria was associated with early invasion of adjacent hepatocytes (Fig. 1). As shown by electron microscopy, wild-type bacteria remained apparently undamaged inside the cytoplasm of infected cells. In contrast, rare clpP mutant bacteria were visible at 8 h in liver tissue, most of the bacteria being destroyed in Küpffer cells without any invasion of adjacent hepatocytes (Fig. 1). These results indicate that the ClpP serine protease is required for the intracellular growth of L. monocytogenes in vivo.
FIG. 1.
In vivo survival and replication of L. monocytogenes in the livers of infected mice. Livers of mice infected with L. monocytogenes LO28 (A to D) and an isogenic clpP mutant (E to H) were examined by light microscopy of semithin sections stained with toluidine blue (left panel) and by electronic microscopy of ultrathin sections (right panel). After 1 h, wild-type bacteria (A and B) and mutant bacteria (E and F) were confined inside Küpffer cells of sinusoid capillaries and appeared intact. After 8 h, dense clusters of wild-type bacteria were visible throughout the parenchyma, with invasion of adjacent hepatocytes (C). Electron microscopy showed replicating bacteria in the cytoplasm of hepatocytes with actin polymerization (D). Conversely, clpP mutant bacteria were hardly visible after 8 h, suggesting that they had been cleared by resident phagocytes (G); ghosts of mutant bacteria were seen inside vacuoles of rare Küpffer cells (H).
The ClpP protease favors the induction of anti-Listeria protective immunity.
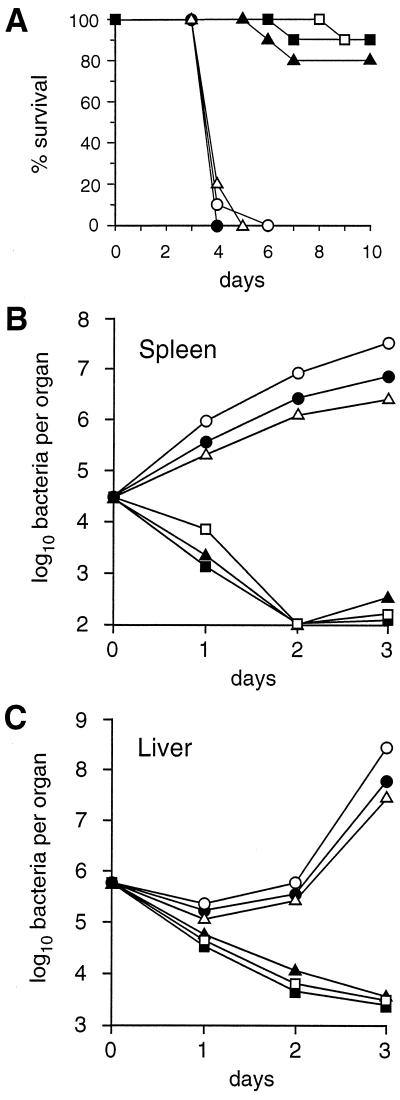
Mice were immunized i.v. with sublethal doses of wild-type bacteria (5 × 103) or clpP mutant bacteria (1 × 106 or 5 × 108). Controls included three groups of mice: (i) mice receiving phosphate-buffered saline (PBS), (ii) mice infected with a clpP-complemented mutant (5 × 103) expressing the same level of virulence as wild-type bacteria (15), and (iii) mice infected with an avirulent hly insertion mutant of LO28 (5 × 108) which does not produce LLO. All mice were challenged i.v. 42 days later with a lethal dose of wild-type LO28 (5 × 105). Mortality was then evaluated over a 10-day period, while bacterial survival in the spleen and the liver during the 3 days following the challenge was studied. As expected, most mice infected with wild-type bacteria or clpP-complemented mutant bacteria survived the lethal challenge (Fig. 2A). In contrast, mice infected with the lower dose of the clpP mutant (1 × 106) were not protected, with rapid death of all mice within 3 to 4 days (P < 0.001). As shown in Fig. 2B and C, bacterial growth was almost unrestricted in these mice, similar to that in uninfected control mice or mice inoculated with the hly mutant (5 × 108). However, mice inoculated with a very high dose of the clpP mutant (5 × 108) were fully protected against a lethal challenge, as demonstrated by their survival (Fig. 2A) and the rapid bacterial elimination in organs (P < 0.01) (Fig. 2B and C). These findings suggest that the lack of protection by the lower dose of the clpP mutant presumably results from insufficient stimulation of anti-Listeria protective T cells.
FIG. 2.
Anti-Listeria protection in mice infected by isogenic mutants of L. monocytogenes. Groups of mice received i.v. PBS (○), 5 × 108 LO28Δhly (●), 1 × 106 LO28ΔclpP (▵), 5 × 108 LO28ΔclpP (▴), 5 × 103 wild-type (▪), or 5 × 103 trans-complemented LO28ΔclpP (□) bacteria. All mice were challenged i.v. 42 days later with a lethal dose of the wild-type strain (5 × 105 bacteria). (A) Mortality was monitored for 10 days with groups of 10 mice. Values are the percent survival after challenge. (B and C) Bacterial growth in the spleen (B) and liver (C) was monitored with groups of 16 mice for 4 days (means of bacterial counts; four organs per time point; standard deviations, ≤0.25). Mice immunized with the wild-type strain, the trans-complemented clpP mutant, or high doses of the clpP mutant (5 × 108 bacteria) survived the lethal challenge, with rapid bacterial elimination from the spleen and liver. In contrast, mice infected with 1 × 106 clpP mutant bacteria or LLO-deficient bacteria were not protected and died rapidly.
The ClpP protease promotes the anti-LLO immune response.
It was previously demonstrated (15) that under stress conditions, the hemolytic activity of LLO produced by a ΔclpP mutant was much lower than that of its wild-type parental strain or of a clpP trans-complemented mutant, although the amounts of secreted LLO were similar in the three strains, as shown by Western blot analysis with an anti-LLO antibody (Fig. 3). Since ClpP favors the production of functional LLO, we studied the production of anti-LLO antibodies in the sera of day 42 infected mice. Anti-LLO antibodies were detected in mice having received low doses of wild-type bacteria (5 × 103) and in mice infected with a high challenge dose of clpP mutant bacteria (5 × 108), at titers of 1/400 and 1/20, respectively. In contrast, anti-LLO antibodies (titer of >1/10) were not found in mice inoculated with 1 × 106 clpP mutant bacteria or 5 × 108 hly mutant bacteria.
FIG. 3.
ClpP modulates the expression of functional LLO under stress conditions. (A) Anti-LLO Western blot analysis of supernatants of bacteria grown to mid-log phase in BHI broth at 30 or 40°C. (B) Hemolytic activity was titrated with the same culture supernatants and is expressed in arbitrary units for 108 bacteria. LLO was overexpressed in all strains at 40°C, but hemolytic activity was strongly reduced in the absence of ClpP.
The generation of protective immunity against L. monocytogenes is dependent upon the activation of LLO-specific major histocompatibility complex (MHC) class I-restricted CD8+ cytotoxic T lymphocytes (CTLs), which recognize the immunodominant H2-Kd-restricted epitope, 91-99, of LLO (21, 33). Therefore, we investigated the induction of LLO-specific CD8+ T cells in mice infected i.v. with LO28 (5 × 103), the clpP mutant (1 × 106 or 5 × 108), or the hly mutant (5 × 108). Spleen cells from infected mice and noninfected mice were collected by day 6 and restimulated with LLO-transfected P815 cells in vitro. The cytotoxic activity of activated splenocytes was subsequently tested against pHEM3.3 cells and nontransfected P815 cells. As expected, mice infected with 5 × 108 clpP mutant bacteria raised an anti-LLO CTL response at a level similar to that of mice infected with wild-type bacteria (5 × 103) (Fig. 4). In contrast, CTLs were not detected in uninfected mice, in mice infected with 1 × 106 clpP mutant bacteria, or in mice receiving the hly mutant (5 × 108), consistent with the absence of protective immunity.
FIG. 4.
In vitro induction of LLO-specific CTL responses by the ΔclpP mutant. Mice were immunized i.v. with 5 × 103 wild-type strain LO28 (▪), 5 × 108 LO28Δhly (●), 1 × 106 LO28ΔclpP (▵), or 5 × 108 LO28ΔclpP (▴) bacteria. Six days after infection, splenocytes were recovered, restimulated, and subsequently tested for cytotoxic activity against LLO-expressing target cells at different effector/target (E:T) ratios. Results are expressed as the mean percent specific lysis of duplicate cultures; error bars show standard deviations. An LLO-specific CTL response was generated from mice infected with the wild-type strain. No CTL activity was detected with LLO-deficient bacteria or with 1 × 106 clpP mutant bacteria. In mice infected with 5 × 108 clpP mutant bacteria, the CTL response was similar to that in mice infected with wild-type bacteria. Lysis produced by effector cells from infected mice sensitized with PBS was <2% and is not represented.
The absence of a CTL response in mice infected with 1 × 106 clpP mutant bacteria could have been due to the rapid elimination of bacteria and therefore to the lack of in vivo T-cell activation. This idea was tested by titrating IFN-γ production by spleen cells collected from mice and incubated in the presence or absence of heat-killed bacteria (LO28) for 48 h. When restimulated with heat-killed Listeria bacteria in vitro, splenocytes from mice infected with 1 × 106 clpP mutant bacteria produced an amount of IFN-γ similar to that produced by splenocytes from mice infected with wild-type LO28 (Fig. 5). Moreover, no difference was observed between mice infected with 1 × 106 and 5 × 108 clpP mutant bacteria, suggesting that T cells can be stimulated by both regimens.
FIG. 5.
IFN-γ response to total listerial antigens. Mice were immunized as described in the legend to Fig. 4. After 6 days, splenocytes were recovered and stimulated in vitro with heat-killed L. monocytogenes; IFN-γ production was measured by an enzyme-linked immunosorbent assay. Data represent the mean and standard deviation for groups of six to eight mice. Splenocytes from mice infected with 106 clpP mutant bacteria produced IFN-γ at levels comparable to those produced by splenocytes from mice infected with wild-type bacteria.
DISCUSSION
We show in this work that the ClpP serine protease is essential for the in vivo intracellular growth of L. monocytogenes and plays a major role in the induction of anti-Listeria protective immunity. By using a clpP deletion mutant of L. monocytogenes, we first demonstrated that mutant bacteria failed to grow in macrophages during the early phase of infection (8 h) in vivo. After i.v. inoculation, most mutant bacteria were destroyed by Küpffer cells of the liver without invasion of hepatocytes (Fig. 1). In contrast, wild-type bacteria multiplied rapidly and invaded adjacent hepatocytes, as previously reported (14, 32). Indeed, the kinetics of elimination of the mutant in host tissues closely resemble those for LLO-deficient mutants (10, 12, 27, 30, 35). The role of ClpP was further confirmed by showing that intracellular growth in Küpffer cells was restored in a clpP-complemented mutant.
Our results are in agreement with the previous finding that the capacity of the clpP mutant to grow in cultured bone marrow-derived macrophages was strongly restricted in vitro (15). Under such conditions, only rare clpP mutant bacteria access the cytoplasm, where they multiply poorly and have very few actin comets (15). This behavior may be due to the reduction of functional LLO in the absence of ClpP, explaining the inability of the bacteria to escape the phagosomal compartment in macrophages. In addition, the reduction of intracytoplasmic multiplication and actin polymerization suggests that ClpP may be required for the expression of other virulence factors, such as ActA (15).
LLO is an immunodominant antigen playing a crucial role in the development of anti-Listeria immunity (2, 4, 5, 9, 22, 26, 41). It has been demonstrated that the expression of LLO in heterologous bacterial hosts, such as Bacillus anthracis and Salmonella enterica serovar Typhimurium, is sufficient to specifically protect against L. monocytogenes (17, 39). Here, we demonstrate that low doses of viable clpP mutant bacteria failed to protect mice against a lethal challenge with wild-type LO28. Neither antibodies nor CTLs against LLO were detectable in these mice. This absence was not due to an inability to stimulate T cells in vivo, since splenocytes from these mice produced the same level of IFN-γ after in vitro exposure to Listeria antigens, thus confirming the major role of LLO in anti-Listeria protection. However, this lack of protection was overcome by use of high infecting doses of mutant bacteria (5 × 108). Under these conditions, a significant level of protection was induced in infected mice, as confirmed by the presence of anti-LLO CTLs and antibodies. This result indicates that the antigenic threshold necessary to stimulate significant anti-Listeria protective immunity is probably very low. Indeed, even with a high challenge dose, the very rapid destruction of bacteria in host tissues visualized in Fig. 1 suggests that minute amounts of LLO are sufficient to protect mice against listeriosis. In contrast, we found that an hly-disrupted mutant producing a truncated LLO completely failed to protect against listeriosis, even with a high infecting dose of 1 × 108, as previously reported for other LLO-deficient mutants (3, 30). These observations demonstrate that LLO must be intact to adequately induce protective immunity.
Escape from the phagosome and intracytosolic multiplication of L. monocytogenes are important steps for the effective presentation of Listeria antigens to T cells, including CD4+ or CD8+ αβ T cells and γδ T cells, a step which is necessary for the development of specific immunity against listeriosis (24, 25). Although an alternative presentation pathway may also play a role (28, 34), Listeria antigens are mainly processed by the cytosolic pathway. Antigens are degraded to shorter peptides by the cytosolic proteasome machinery, transported through the endoplasmic reticulum, and presented as MHC class I-associated Listeria peptides to CD8+ cells (25, 42). The complexes presented by Listeria-infected cells are recognized and lysed by specific CD8+ cytolytic T cells. Among L. monocytogenes-specific antigens, LLO plays a major role through the activation of protective CD8+ MHC class I-restricted LLO-specific CTLs (22, 23, 33).
The LLO 91-99 peptide has been previously identified as the immunodominant epitope of L. monocytogenes (21, 33). However, CD8+ T cells specific for other Listeria-derived peptides (e.g., p60 227-225 and p60 449-457) have been shown to develop simultaneously with LLO 91-99-specific CD8+ T cells (6, 7, 16, 38, 42). Therefore, the lack of protection in the absence of ClpP may be due to a restriction of the availability of intracytoplasmic LLO-derived immunogenic peptides processed and presented to CD8+ T cells. This situation would result in a low cell surface density of MHC class I-presented epitopes, which would reduce the magnitude of the CTL response (6). In addition, the limited access to the cytoplasm for the clpP mutant may also alter the pathway of presentation of peptides from other protective antigens, thus restricting the immune response against L. monocytogenes. In conclusion, our results show that the stress-induced serine protease ClpP modulates the production of LLO during the intracellular survival of L. monocytogenes in host tissues and thus represents a major determinant for immunity to L. monocytogenes by regulating the presentation of protective Listeria peptides by host macrophages.
ACKNOWLEDGMENTS
We thank Michael J. Bevan for the kind gift of pHEM3.3 cells and Pascale Cossart (Institut Pasteur, Paris, France) for providing the LO28 hly insertion mutant. We also thank M. Monnet (CHU Necker-Enfants Malades) for technical assistance in the histologic study and Jean-Luc Beretti for LLO blot assays.
This work was supported by INSERM, Institut Pasteur, University of Paris V, and a grant from the EEC (BMH-4CT 960659). S.B. is sponsored by the Danish Research Agency.
REFERENCES
- 1.Beauregard K E, Lee K D, Collier R J, Swanson J A. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med. 1997;186:1159–1163. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berche P, Gaillard J L, Geoffroy C, Alouf J E. T cell recognition of listeriolysin O is induced during infection with Listeria monocytogenes. J Immunol. 1987;139:3813–3821. [PubMed] [Google Scholar]
- 3.Berche P, Gaillard J L, Sansonetti P J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987;138:2266–2271. [PubMed] [Google Scholar]
- 4.Bouwer H G, Nelson C S, Gibbins B L, Portnoy D A, Hinrichs D J. Listeriolysin O is a target of the immune response to Listeria monocytogenes. J Exp Med. 1992;175:1467–1471. doi: 10.1084/jem.175.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouwer H G, Gibbins B L, Jones S, Hinrichs D J. Antilisterial immunity includes specificity to listeriolysin O (LLO) and non-LLO-derived determinants. Infect Immun. 1994;62:1039–1045. doi: 10.1128/iai.62.3.1039-1045.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouwer H G, Hinrichs D J. Cytotoxic T-lymphocyte responses to epitopes of listeriolysin O and p60 following infection with Listeria monocytogenes. Infect Immun. 1996;64:2515–2522. doi: 10.1128/iai.64.7.2515-2522.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouwer H G, Shen H, Fan X, Miller J F, Barry R A, Hinrichs D J. Existing antilisterial immunity does not inhibit the development of a Listeria monocytogenes-specific primary cytotoxic T-lymphocyte response. Infect Immun. 1999;67:253–258. doi: 10.1128/iai.67.1.253-258.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bregenholt S, Ropke M, Skov S, Claesson M H. Ligation of MHC class I molecules on peripheral blood T lymphocytes induces new phenotypes and functions. J Immunol. 1996;157:993–999. [PubMed] [Google Scholar]
- 9.Brunt L M, Portnoy D A, Unanue E R. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990;145:3540–3546. [PubMed] [Google Scholar]
- 10.Cossart P, Vicente M F, Mengaud J, Baquero F, Perez-Diaz J C, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cossart P, Lecuit M. Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. EMBO J. 1998;17:3797–3806. doi: 10.1093/emboj/17.14.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard J L, Berche P, Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986;52:50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard J L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillard J L, Jaubert F, Berche P. The inlAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J Exp Med. 1996;183:359–369. doi: 10.1084/jem.183.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaillot O, Pellegrini E, Bregenholt S, Nair S, Berche P. The ClpP serine protease is essential for the intracellular parasitism and virulence of Listeria monocytogenes. Mol Microbiol. 2000;35:1286–1294. doi: 10.1046/j.1365-2958.2000.01773.x. [DOI] [PubMed] [Google Scholar]
- 16.Geginat G, Nichterlein T, Kretschmar M, Schenk S, Hof H, Lalic-Multhaler M, Goebel W, Bubert A. Enhancement of the Listeria monocytogenes p60-specific CD4 and CD8 T cell memory by nonpathogenic Listeria innocua. J Immunol. 1999;162:4781–4789. [PubMed] [Google Scholar]
- 17.Gentschev I, Sokolovic Z, Mollenkopf H J, Hess J, Kaufmann S H, Kuhn M, Krohne G F, Goebel W. Salmonella strain secreting active listeriolysin changes its intracellular localization. Infect Immun. 1995;63:4202–4205. doi: 10.1128/iai.63.10.4202-4205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geoffroy C, Gaillard J L, Alouf J E, Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987;55:1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray M L, Killinger A H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966;30:309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanawa T, Yamamoto T, Kamiya S. Listeria monocytogenes can grow in macrophages without the aid of proteins induced by environmental stresses. Infect Immun. 1995;63:4595–4599. doi: 10.1128/iai.63.12.4595-4599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harty J T, Bevan M J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harty J T, Schreiber R D, Bevan M J. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc Natl Acad Sci USA. 1992;89:11612–11616. doi: 10.1073/pnas.89.23.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harty J T, Pamer E G. CD8 T lymphocytes specific for the secreted p60 antigen protect against Listeria monocytogenes infection. J Immunol. 1995;154:4642–4650. [PubMed] [Google Scholar]
- 24.Harty J T, Lenz L L, Bevan M J. Primary and secondary immune responses to Listeria monocytogenes. Curr Opin Immunol. 1996;8:526–530. doi: 10.1016/s0952-7915(96)80041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiltbold E M, Ziegler H K. Mechanisms of processing and presentation of the antigens of Listeria monocytogenes. Infect Agents Dis. 1993;2:314–323. [PubMed] [Google Scholar]
- 26.Hiltbold E M, Safley S A, Ziegler H K. The presentation of class I and class II epitopes of listeriolysin O is regulated by intracellular localization and by intercellular spread of Listeria monocytogenes. J Immunol. 1996;157:1163–1175. [PubMed] [Google Scholar]
- 27.Kathariou S, Metz P, Hof H, Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987;169:1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacsovics-Bankowski M, Rock K L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 29.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;116:381–387. [PubMed] [Google Scholar]
- 30.Michel E, Reich K A, Favier R, Berche P, Cossart P. Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol. 1990;4:2167–2178. doi: 10.1111/j.1365-2958.1990.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 31.Nair S, Frehel C, Nguyen L, Escuyer V, Berche P. ClpE, a novel member of the HSP100 family, is involved in cell division and virulence of Listeria monocytogenes. Mol Microbiol. 1999;31:185–196. doi: 10.1046/j.1365-2958.1999.01159.x. [DOI] [PubMed] [Google Scholar]
- 32.North R J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970;132:521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pamer E G, Harty J T, Bevan M J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991;353:852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeifer J D, Wick M J, Roberts R L, Findlay K, Normark S J, Harding C V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 35.Portnoy D A, Schreiber R D, Connelly P, Tilney L G. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J Exp Med. 1989;170:2141–2146. doi: 10.1084/jem.170.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rouquette C, Ripio M T, Pellegrini E, Bolla J M, Tascon R I, Vazquez-Boland J A, Berche P. Identification of a ClpC ATPase required for stress tolerance and in vivo survival of Listeria monocytogenes. Mol Microbiol. 1996;21:977–987. doi: 10.1046/j.1365-2958.1996.641432.x. [DOI] [PubMed] [Google Scholar]
- 37.Rouquette C, de Chastellier C, Nair S, Berche P. The ClpC ATPase of Listeria monocytogenes is a general stress protein required for virulence and promoting early bacterial escape from the phagosome of macrophages. Mol Microbiol. 1998;27:1235–1245. doi: 10.1046/j.1365-2958.1998.00775.x. [DOI] [PubMed] [Google Scholar]
- 38.Sijts A J, Pilip I, Pamer E G. The Listeria monocytogenes-secreted p60 protein is an N-end rule substrate in the cytosol of infected cells. Implications for major histocompatibility complex class I antigen processing of bacterial proteins. J Biol Chem. 1997;272:19261–19268. doi: 10.1074/jbc.272.31.19261. [DOI] [PubMed] [Google Scholar]
- 39.Sirard J C, Fayolle C, de Chastellier C, Mock M, Leclerc C, Berche P. Intracytoplasmic delivery of listeriolysin O by a vaccinal strain of Bacillus anthracis induces CD8-mediated protection against Listeria monocytogenes. J Immunol. 1997;159:4435–4443. [PubMed] [Google Scholar]
- 40.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villanueva M S, Sijts A J, Pamer E G. Listeriolysin is processed efficiently into an MHC class I-associated epitope in Listeria monocytogenes-infected cells. J Immunol. 1995;155:5227–5233. [PubMed] [Google Scholar]
- 42.Zwickey H L, Potter T A. Antigen secreted from noncytosolic Listeria monocytogenes is processed by the classical MHC class I processing pathway. J Immunol. 1999;162:6341–6350. [PubMed] [Google Scholar]