Abstract
Apoptotic regulation of monocytes/macrophages appears to be closely associated with chronic inflammatory reactions. Since it was demonstrated earlier that certain bacterial cell components are involved in apoptotic regulation of these cells, in the present study, we investigated whether the bacterial fimbria, an important cell structure involved in bacterial adherence to host cells, regulates apoptosis of human monocytic THP-1 cells induced under growth factor deprivation. To investigate this point, we used fimbriae of Porphyromonas gingivalis, a pathogen causing periodontal disease, which is a chronic inflammatory disease. The fimbriae inhibited apoptosis of the cells under growth factor deprivation. This inhibitory action of the fimbriae was completely neutralized by anti-fimbrial antibody. The fimbriae stimulated activation of extracellular signal-regulated kinase (ERK) and expression of cyclin-dependent kinase inhibitor p21 Cip/WAF1 (p21) in the cells. The stimulatory effect of the fimbriae on the expression of the p21 protein was inhibited by treatment with PD98059, a specific inhibitor of ERK. The cell apoptosis was inhibited by treatment with Ac-DEVD-CHO, an inhibitor of caspase-3. The fimbriae inhibited the serum withdrawal-induced cleavage of the caspase-3 proform and poly(ADP-ribose) polymerase, one of the caspase-3 substrates. Furthermore, PD98059 and antisense p21 oligonucleotide blocked the fimbrial inhibition of apoptosis and caspase-3 activation of the cells induced by serum withdrawal. These results show that the bacterial fimbriae inhibited apoptosis of THP-1 cells induced under growth factor deprivation via ERK-dependent expression of p21. The present study suggests that bacterial fimbriae act as potent regulators of chronic inflammatory disease, e.g., periodontal disease, through blocking apoptosis of monocytes/macrophages.
It has been well documented that apoptosis plays an important role in the inflammatory response, tumorigenesis, and embryonic development (4). Apoptosis is characterized by distinctive morphological and biochemical changes involving nuclear and chromatin condensation, cell membrane blebbing, and endonuclease activity resulting in DNA fragmentation (37). Therefore, much interest has been generated in demonstrating the signaling mechanisms of specific genes that regulate apoptosis.
Recent studies (30, 32, 48) have shown that several pathogenic bacteria function as promoters or inhibitors of apoptosis of monocytes/macrophages. These observations suggest that several cell components of these bacteria are involved in an important pathogenic mechanism promoting inflammation and concomitant disease via apoptosis of monocytes/macrophages. In fact, lipopolysaccharide of gram-negative bacteria is able to regulate the apoptosis of neutrophils and monocytes/macrophages via direct or indirect action through endogenous cytokines (1, 10, 13, 20, 26–28, 32, 35, 44).
Porphyromonas gingivalis is a pathogen causing periodontal disease, a typical chronic inflammatory disease (14, 23, 24, 41, 47). The bacterial fimbria is an important cell structure that contributes to the adherence to and invasion of host cells. Also, several studies (11, 16–19, 31, 40) have shown that the fimbriae function as a virulence factor in inflammatory reactions because they stimulate production of inflammatory cytokines by macrophages and fibroblasts. These observations suggest the involvement of the fimbriae as regulators of inflammatory reactions caused by bacterial infection. Since apoptosis is an important biological phenomenon regulating the number of monocytes/macrophages at sites of inflammation, it was of interest for us to investigate whether bacterial fimbriae play functional roles as regulators of monocytic-cell apoptosis and to explore a possible intracellular signaling pathway regulating the action of the fimbriae on cell apoptosis.
For this purpose, we investigated the regulatory role of the fimbriae of P. gingivalis in serum withdrawal-induced apoptosis of human monocytic THP-1 cells. We show in this study that P. gingivalis fimbriae inhibited serum withdrawal-induced apoptosis of THP-1 cells and that they did so via extracellular signal-regulated kinase (ERK)- and mitogen-activated protein kinase (MAPK)-dependent expression of p21 Cip/WAF1 (p21), a cyclin-dependent kinase inhibitor.
MATERIALS AND METHODS
Cell culture.
Human monocytic THP-1 cells were maintained in RPMI 1640 medium supplemented with 100 μg of streptomycin sulfate/ml, 100 U of penicillin G potassium/ml, and 5% (vol/vol) heat-inactivated fetal bovine serum (Flow Laboratories, McLean, Va.) in a humidified atmosphere of 5% CO2 and 95% air at 37°C. To induce apoptosis, we washed the cells five times with serum-free medium, cultured them for 24 h in serum-free medium, washed them three times with serum-free medium, and then incubated them with or without the fimbriae for various times under serum withdrawal conditions.
Preparation of P. gingivalis fimbriae and their antibody.
P. gingivalis ATCC 33277 fimbriae were prepared and purified from cell washings by the method of Yoshimura et al. (46) as described previously (16). We had demonstrated earlier that purified fimbriae were able to induce several biological activities that could not be attributed to lipopolysaccharide contamination in the preparation (16–18). The protein content of the fimbriae was measured by the method of Bradford (6). A monoclonal antibody against P. gingivalis fimbriae was used, the preparation of which was described previously (22).
Agarose gel electrophoresis for DNA fragmentation.
To assess DNA fragmentation, we prepared DNA from the THP-1 cells and analyzed it by the electrophoretic method. After incubation, the cells were lysed with lysis buffer (10 mM Tris [pH 8.0], 10 mM EDTA, 0.5% Triton X-100) for 15 min at 4°C, and then the supernatant, including DNA fragments, was harvested from the lysate by centrifugation for 20 min at 13,000 × g. The DNA fragments in the supernatant were precipitated with 0.5 M NaCl and ethanol, electrophoresed on a 2% agarose gel containing ethidium bromide, and then visualized under UV light.
Quantification of DNA fragmentation.
DNA fragmentation was measured by a slight modification of the diphenylamine assay described previously (29). Briefly, after incubation, the cells were scraped off the culture plates and incubated with 200 μl of lysis buffer (10 mM Tris [pH 8.0], 10 mM EDTA, 0.5% Triton X-100) for 15 min at 4°C. Then, the intact chromatin (pellet) was separated from DNA fragments (supernatant) by centrifugation for 20 min at 13,000 × g. The pellets were resuspended in 200 μl of the lysis buffer, and the samples were precipitated with 1 N perchloric acid at 4°C. The DNA was pelleted at 13,000 × g for 20 min, and the supernatant was removed. After addition of 50 μl of 1 N perchloric acid, the samples were boiled for 20 min. The DNA contents were quantified by use of the diphenylamine reagent (7). The percentage of fragmented DNA was calculated as the ratio of the DNA content in the supernatant to the amount in the pellet.
Western blot analysis.
THP-1 cells under serum-free culture conditions were incubated in the presence or absence of test samples. Cell lysis was achieved with lysis buffer (10 mM Tris-HCl [pH 7.9], 1% sodium deoxycholate, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 150 mM NaCl, 5 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride). The samples were electrophoresed on 10.0, 15.0, or 17.5% polyacrylamide gels by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a Tris-glycine buffer system (0.025 M Tris, 0.192 M glycine, 0.1% sodium dodecyl sulfate). The proteins were transferred to a polyvinylidene difluoride membrane (Millipore Co., Bedford, Mass.) by using the semidry transblot system (ATTO Co., Tokyo, Japan). The blots were blocked with 5% skim milk in Tris-buffered saline including 0.1% Tween 20 (TBS-T) overnight at 4°C and then washed with TBS-T. Then, the membrane was incubated with the primary antibody in 5% skim milk in TBS-T overnight at 4°C. Proteins were detected with a Phototope-HRP Western blot detection kit (New England Biolabs, Inc., Beverly, Mass.). The blots were exposed to X-Omat film (Eastman Kodak Co., Rochester, N.Y.).
Antibodies and inhibitors.
The following antibodies were used for Western blot analysis: anti-CPP32 (Santa Cruz Biotechnology, Santa Cruz, Calif.), anti-p21 (Santa Cruz Biotechnology), anti-PARP (Enzyme Systems Products, Livermore, Calif.), anti- MAPK, and anti-phosphorylated p44/42 MAPK (New England Biolabs, Inc). Secondary-antibody–horseradish peroxidase conjugates were purchased from Santa Cruz Biotechnology and Bio-Rad Laboratories (Richmond, Calif.). PD98059 was purchased from Calbiochem-Novabiochem Corporation (La Jolla, Calif.). Ac-YVAD-CHO (acetyl-l-tyrosyl-l-valyl-l-alanyl-l-aspart-1-al), a caspase-1 family inhibitor, and Ac-DEVD-CHO (acetyl-l-aspartyl-l-glutamyl-l-valyl-l-aspart-1-al), a caspase-3 family inhibitor, were purchased from Peptide Institute, Inc. (Osaka, Japan).
Antisense oligonucleotide.
The antisense oligonucleotide used was based on the p21 coding sequence. Antisense p21 oligonucleotide (5′-TCC CCA GCC GGT TCT GAC AT-3′) is complementary to the region around the initiation codon, and the control sense p21 oligonucleotide (5′-ATG TCA GAA CCG GCT GGG GA-3′) is complementary to the antisense p21 oligonucleotide. Each oligonucleotide and Lipofectin (Life Technologies, Inc., Rockville, Md.) were incubated for 15 min at 37°C. The mixture was diluted with medium to a final concentration of 0.5 or 1 μM oligonucleotide and added to the THP-1 cells.
RESULTS
P. gingivalis fimbriae inhibit THP-1 cell apoptosis under growth factor deprivation.
In the absence of an appropriate survival stimulus, human monocytes undergo spontaneous apoptosis (13, 26–28). Therefore, we first looked by DNA fragmentation assay for apoptosis of the untreated or fimbria-treated cells under growth factor deprivation. In preliminary experiments, we observed that the fimbriae inhibited the apoptosis of THP-1 cells under these conditions in a dose-dependent manner (1 to 5 μg of protein/ml), with the maximum inhibitory action at 5 μg of protein/ml of fimbriae. Therefore, we used this fimbrial concentration in the following experiments.
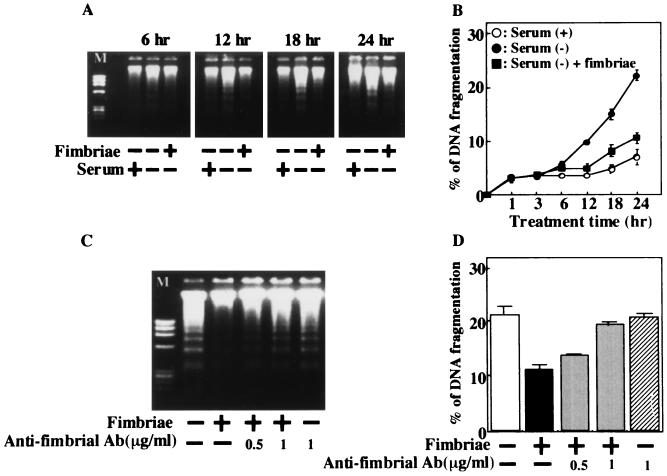
Figure 1A shows that the characteristic DNA laddering of the cells under these conditions increases in a culture time-related manner. However, such significantly increased DNA laddering was not observed when the medium was supplemented with 5% serum. Interestingly, we observed that the fimbriae clearly inhibit DNA laddering of the cells induced under growth factor deprivation. In addition, this inhibitory action of the fimbriae was also supported by the results of the diphenylamine assay (Fig. 1B). Furthermore, to confirm the inhibitory action of the fimbriae, we examined by DNA fragmentation and diphenylamine assays whether the fimbrial inhibition could be neutralized by a monoclonal antibody against the fimbriae. The monoclonal antibody completely neutralized the fimbrial inhibition of cell apoptosis (Fig. 1C and D).
FIG. 1.
Inhibitory effect of P. gingivalis fimbriae on THP-1 cell apoptosis induced by growth factor deprivation. (A) THP-1 cells were incubated with or without serum in the absence or presence of fimbriae at 5 μg of protein/ml. DNA fragments from the supernatant of the lysed cells were isolated at selected times after the initiation of the cultures and then subjected to agarose gel electrophoresis. φX174 RF DNA HaeIII fragments were used as molecular weight markers (M). (B) The cells were treated as described for panel A, and DNA fragmentation was quantified by the diphenylamine assay. (C) Cells in serum-free medium were incubated in the absence or presence of fimbriae at 5 μg of protein/ml that had been pretreated or not with anti-fimbrial antibody (Ab). DNA fragments from the supernatant of the lysed cells were analyzed by agarose gel electrophoresis at 24 h after the initiation of the cultures. (D) Cells were treated as described for panel C, and DNA fragmentation was quantified by the diphenylamine assay at 24 h after the initiation of the cultures. The results of the diphenylamine assays are expressed as the mean ± standard deviation of three cultures. An identical experiment independently performed gave similar results.
P. gingivalis fimbriae stimulate expression of p21 in THP-1 cells.
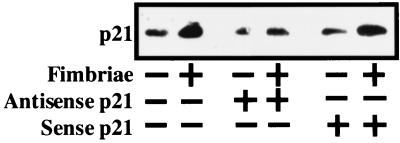
Many studies (2, 3, 15, 34, 42, 43) have shown that expression of p21 is essential for the differentiation and survival of several kinds of cells. Also, there is a study showing that the fimbriae were able to induce monocytic-cell differentiation (21). Therefore, we suspected the involvement of p21 expression in the fimbrial inhibition of THP-1 cell apoptosis under growth factor deprivation. Based on this suspicion, we examined by Western blot analysis whether the fimbriae could stimulate the expression of p21 protein in the cells under these culture conditions. As a result, we observed the clearly stimulated expression of p21 protein in the cells when the expression was examined at 12 or 24 h after the initiation of the fimbrial treatment (data not shown). In addition, using an antisense p21 oligonucleotide, we confirmed the fimbria-stimulated expression of the p21 protein. Figure 2 shows that the fimbria-stimulated expression of the p21 protein was inhibited by antisense p21 oligonucleotide treatment but not by sense p21 oligonucleotide. These results clearly demonstrate that the fimbriae act as potent stimulators of p21 expression in the cells in the absence of growth factors.
FIG. 2.
Inducing effect of P. gingivalis fimbriae on expression of p21 protein in THP-1 cells under growth factor deprivation. THP-1 cells in serum-free medium were pretreated for 24 h in the absence or presence of 1 μM antisense or sense p21 oligonucleotide and were then incubated, also in serum-free medium, in the absence or presence of the fimbriae at 5 μg of protein/ml. The expression of p21 protein in the cells was analyzed by Western blotting with anti-p21 antibody 24 h after the initiation of the fimbrial treatment. An identical experiment independently performed gave similar results.
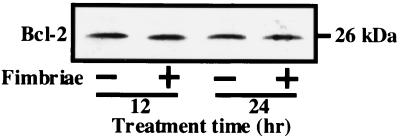
On the other hand, since Bcl-2, an oncoprotein, acts as an inhibitor of cell apoptosis, we also examined by Western blot analysis the effect of the fimbriae on the expression of the Bcl-2 protein in THP-1 cells under growth factor deprivation. Figure 3 shows that the fimbriae had no effect on the expression of the Bcl-2 protein, suggesting to us that the regulation of Bcl-2 expression is probably not involved in the fimbrial inhibition of THP-1 cell apoptosis induced under growth factor deprivation.
FIG. 3.
Effect of P. gingivalis fimbriae on expression of Bcl-2 protein in THP-1 cells under growth factor deprivation. THP-1 cells were treated as described in the legend to Fig. 2, and the expression of Bcl-2 protein in the cells was analyzed by Western blotting with anti-Bcl-2 antibody 12 and 24 h after the initiation of the cultures. An identical experiment independently performed gave similar results.
P. gingivalis fimbriae inhibit THP-1 cell apoptosis via p21 under growth factor deprivation.
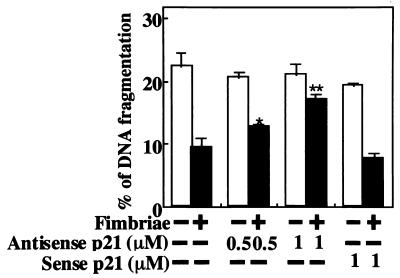
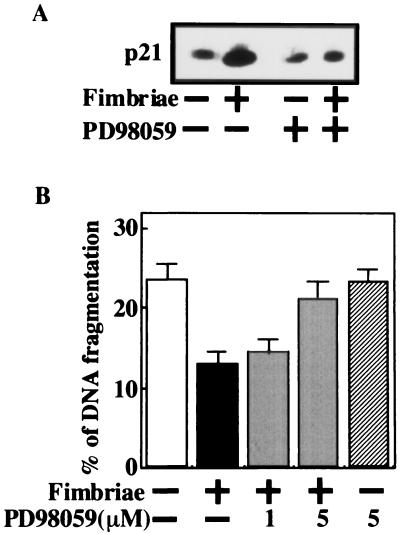
The fimbrial stimulation of the expression of the p21 protein in THP-1 cells suggested to us the possibility that the p21 protein is involved in the fimbrial inhibition of cell apoptosis under growth factor deprivation. To demonstrate the possibility, we examined whether fimbrial inhibition is blocked by antisense p21 oligonucleotide treatment, and we observed that the antisense oligonucleotide at 1 μM, but not the sense oligonucleotide, significantly blocked fimbrial inhibition of apoptosis (Fig. 4). These results indicate an important role for the p21 protein in the inhibitory action of the fimbriae on cell apoptosis under growth factor deprivation.
FIG. 4.
Antisense p21 oligonucleotide blocks fimbrial inhibition of THP-1 cell apoptosis induced under growth factor deprivation. THP-1 cells were treated as described in the legend to Fig. 2, and DNA fragmentation was quantified by the diphenylamine assay 24 h after the initiation of the fimbrial treatment. The results of the assay are expressed as the mean ± standard deviation of three cultures. Variations between values from the fimbria-treated groups with or without antisense p21 oligonucleotide that were found to be statistically significant by Student's t test are indicated by asterisks (∗, P < 0.05; ∗∗, P < 0.01). An identical experiment independently performed gave similar results.
P. gingivalis fimbriae inhibit THP-1 cell apoptosis under growth factor deprivation via ERK- and MAPK-dependent expression of p21.
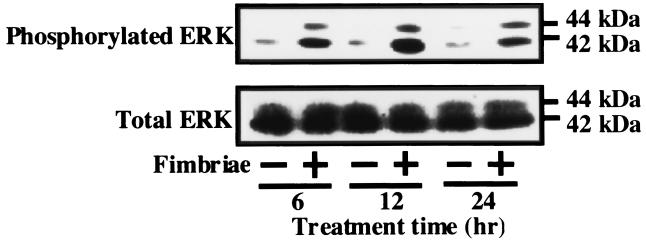
Since we found the p21 protein to be involved in the fimbrial inhibition of THP-1 cell apoptosis under growth factor deprivation, it was of interest to us to explore which protein kinase acts in signaling of the fimbria-stimulated expression of p21 in the cells. Interestingly, several studies (5, 25) have suggested that p21 expression is induced by activation of ERK and MAPK. Therefore, using Western blot analysis with anti-phosphorylated p44/42 MAPK antibody, we investigated whether the fimbriae stimulated expression of p21 in THP-1 cells through activation (i.e., phosphorylation) of ERK and MAPK. As shown in Fig. 5, although they had no effect on the total amount of the ERK and MAPK proteins in THP-1 cells, the fimbriae markedly increased the expression of the phosphorylated forms of ERK and MAPK in the cells. Since a recent study (2) demonstrated that p21 inhibits apoptosis of monocytic cells via inhibition of c-Jun N-terminal kinase–stress-activated protein kinase (JNK-SAPK) activation, we also examined whether JNK-SAPK activation was involved in THP-1 cell apoptosis under growth factor deprivation. Although the data are not shown, the increased expression of phosphorylated JNK-SAPK was not observed in the absence of growth factors. In addition, our Western blot analysis showed that the fimbria-stimulated expression of p21 protein in the cells was inhibited by PD98059, a specific inhibitor of mitogen-activated protein-ERK kinase (MEK) activation (12) (Fig. 6A). These observations caused to us to investigate the effect of PD98059 on the fimbrial inhibition of cell apoptosis. Figure 6B shows that the inhibitor blocked the fimbrial inhibition of cell apoptosis in a dose-dependent manner. Taken together, these results suggest that the fimbriae inhibited THP-1 cell apoptosis under growth factor deprivation via ERK- and MAPK-dependent expression of p21.
FIG. 5.
Effect of P. gingivalis fimbriae on phosphorylation of ERK and MAPK in THP-1 cells under growth factor deprivation. The cells were treated as described in the legend to Fig. 2. The expression of ERK or phosphorylated ERK proteins in the cells was analyzed by Western blotting with anti-p44/42 MAPK antibody or anti-phosphorylated p44/42 MAPK antibody at selected times after the initiation of the cultures. An identical experiment independently performed gave similar results.
FIG. 6.
Involvement of ERK and MAPK activity in fimbria-stimulated expression of p21 protein in and inhibitory effect of the fimbriae on THP-1 cell apoptosis induced under growth factor deprivation. (A) THP-1 cells in serum-free medium were pretreated or not for 1 h with PD98059 at 5 μM and then incubated in the absence or presence of fimbriae at 5 μg of protein/ml. After 24 h, the expression of the p21 protein in the cells was analyzed by Western blotting with anti-p21 antibody. (B) Cells were treated as described for panel A, and DNA fragmentation was quantified by the diphenylamine assay 24 h after the initiation of the fimbrial treatment. The results of the assay are expressed as the mean ± standard deviation of three cultures. An identical experiment independently performed gave similar results.
Caspase-3 inhibitor blocks growth factor deprivation-induced apoptosis of THP-1 cells.
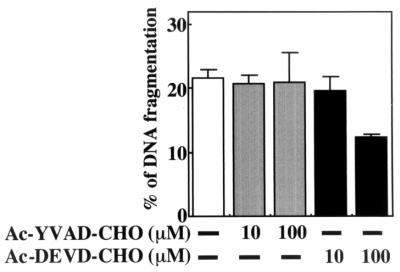
It is well demonstrated that the caspase cascade plays an important role in the signaling of apoptosis of many kinds of cells. Therefore, using Ac-YVAD-CHO and Ac-DEVD-CHO, inhibitors of caspase-1 and -3, respectively, we examined which caspase mediates THP-1 cell apoptosis under growth factor deprivation. As shown in Fig. 7, cell apoptosis was significantly inhibited by the caspase-3 inhibitor but not by the caspase-1 inhibitor, thus suggesting that caspase-3 activation may be involved in cell apoptosis.
FIG. 7.
Effect of caspase inhibitors on THP-1 cell apoptosis induced under growth factor deprivation. THP-1 cells in serum-free medium were treated with the indicated concentration of Ac-YVAD-CHO or Ac-DEVD-CHO or untreated. Then DNA fragmentation was quantified by the diphenylamine assay 24 h after the initiation of the treatment. The results of the assay are expressed as the mean ± standard deviation of three cultures. Similar results were obtained in an identical experiment.
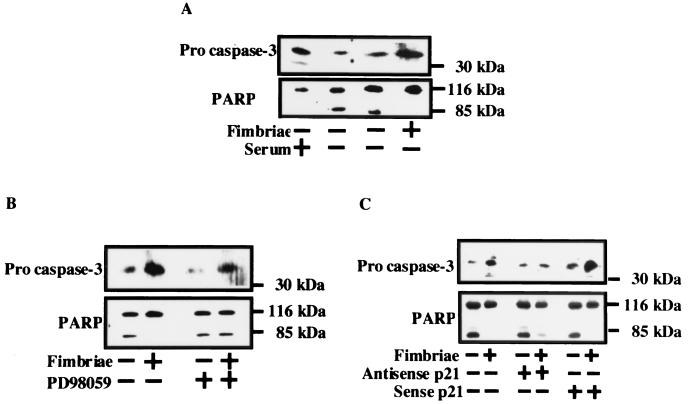
P. gingivalis fimbriae inhibit growth factor deprivation-induced caspase-3 activation in THP-1 cells via activation of ERK and MAPK and expression of p21.
To confirm caspase-3 activation during induction of THP-1 cell apoptosis under growth factor deprivation, we examined by Western blot analysis whether the caspase-3 proform and PARP would be cleaved in the cells under growth factor deprivation. As shown in Fig. 8A, the caspase-3 proform and PARP of the cells in the presence of serum were cleaved when the cells were incubated under growth factor deprivation. However, the fimbriae inhibited the cleavage of the caspase-3 proform and PARP in the cells. Importantly, our interest was to explore whether the fimbrial inhibition of caspase-3 activation would be blocked by antisense p21 oligonucleotide and PD98059 treatment. We observed that the fimbrial inhibition of caspase-3 activation in the cells was blocked by antisense p21 oligonucleotide and PD98059 treatment, because the inhibition of cleavage of the caspase-3 proform and PARP in fimbria-treated cells was reduced by the treatment (Fig. 8B and C).
FIG. 8.
Inhibitory effect of P. gingivalis fimbria-stimulated ERK and MAPK activity and expression of p21 protein on caspase-3 activation in THP-1 cells induced under growth factor deprivation. (A) THP-1 cells were treated as described in the legend to Fig. 1A. (B) Cells were treated as described in the legend to Fig. 6A. (C) Cells were treated as described in the legend to Fig. 2. After 24 h, the cleavage of the caspase-3 proform and PARP was analyzed by Western blotting with anti-CPP32 antibody or anti-PARP antibody. An identical experiment independently performed gave similar results.
DISCUSSION
It is of interest to investigate whether bacterial fimbriae, important structures in the triggering of bacterial infection, modulate apoptosis of monocytes/macrophages, cells that play a central role in inflammatory reactions caused by pathogenic bacteria. Therefore, using fimbriae of P. gingivalis, which is a pathogen causing periodontal disease, we investigated the regulatory role of bacterial fimbriae in the apoptosis of cultured human monocytic THP-1 cells. The present study demonstrates that the bacterial fimbriae inhibited THP-1 cell apoptosis under growth factor deprivation via ERK- and MAPK-dependent expression of p21.
Growth factor deprivation has been shown to provoke apoptosis in a variety of cells, including monocytes/macrophages and endothelial cells (8, 9, 13, 26–28). Although the induction of monocytic apoptosis under growth factor or serum withdrawal conditions is physiologically irrelevant, these experimental conditions appear to provide an effective method for examining the regulation of monocytic apoptosis. Therefore, in this study, we employed this experimental design to explore the regulation of apoptosis in THP-1 cells by P. gingivalis fimbriae. In fact, we observed that THP-1 cells effectively undergo apoptosis upon growth factor deprivation as determined by detection of the characteristic genomic DNA laddering. The bacterial fimbriae inhibited monocytic-cell apoptosis. This inhibitory action of fimbriae was confirmed by their inability to rescue the apoptotic cells when the fimbriae were pretreated with a specific monoclonal antibody against themselves. To our knowledge, this is the first demonstration that bacterial fimbriae play a functional role as inhibitors of monocytic-cell apoptosis.
It is known that p21 is involved in DNA damage repair, differentiation, and apoptosis, and many studies (2, 3, 15, 34, 42, 43) have demonstrated that expression of p21 inhibits apoptosis of several kinds of cells, such as monocytic cells, muscle cells, neuroblastoma cells, and melanoma cells. Therefore, we explored whether p21 was involved in the fimbrial inhibition of THP-1 cell apoptosis induced under growth factor deprivation. Using Western blot analysis, we observed that expression of p21 protein in the cells markedly increased following the fimbrial treatment. The antisense p21 oligonucleotide inhibited the fimbria-stimulated expression of the p21 protein and also blocked the fimbrial inhibition of cell apoptosis. These observations strongly suggest that fimbrial inhibition of cell apoptosis is mediated by the action of the p21 protein.
Since we showed earlier that fimbrial signaling of P. gingivalis occurred via β2 integrin on macrophages (40), our interest was in exploring a possible intracellular signaling pathway responsible for the inhibitory effects of the fimbriae on the apoptotic cells. A recent study (36) demonstrated that lipopolysaccharide inhibited growth factor withdrawal-induced apoptosis of dendritic cells via an ERK- and MAPK-dependent pathway. Therefore, we investigated the possibility that the fimbriae inhibited serum withdrawal-induced apoptosis via the ERK- and MAPK-dependent expression of p21. Our Western blot analysis using phosphospecific antibody against ERK and MAPK showed that the fimbriae increased the phosphorylation of ERK and MAPK in the cells at 6 h after the initiation of the treatment and that this increase was sustained up to 24 h. However, the total amount of the ERK and MAPK proteins was not affected by the fimbriae within the period tested. Using PD98059, a MEK1-specific inhibitor, we addressed whether the increased phosphorylation of ERK and MAPK was MEK dependent. The fimbrial inhibition of the apoptotic cells was blocked by the inhibitor. In addition, the fimbrial stimulation of p21 expression in the cells was inhibited by PD98059 treatment. Since PD98059 has little effect on other kinases, including cyclic-AMP-dependent kinase, protein kinase C, and other serine and threonine kinases, these results suggest that the fimbrial inhibition of cell apoptosis under growth factor deprivation is mediated by the phosphorylated ERK- and MAPK-dependent expression of p21.
On the other hand, Bcl-2 prevents apoptosis induced by a wide range of agents. Our Western blot analysis showed that there was no change in expression of the Bcl-2 protein following fimbrial treatment. Several studies (33, 45) have demonstrated that protection from apoptosis stimuli is not determined simply by Bcl-2 expression per se but by heterodimers of Bcl-2 and its homologues. Although other members of the Bcl-2 family were not examined here, Bcl-2 protein expression probably does not contribute to the fimbrial inhibition of growth factor withdrawal-induced apoptosis of the cells.
In addition, our interest was to identify the target molecule of the p21 protein that contributes to the fimbrial inhibition of THP-1 cell apoptosis under growth factor deprivation. In order to elucidate this point, it was important for us to address the question of which molecule plays a central role in the induction of cell apoptosis. A recent study (13) demonstrated that caspase-3 plays an important role in spontaneous monocytic-cell apoptosis. In this study, we observed that Ac-DEVD-CHO, a potent inhibitor of caspase-3, significantly blocked growth factor withdrawal-induced cell apoptosis. However, such blockage was not observed with Ac-YVAD-CHO, a caspase-1 inhibitor. These observations suggest an important role for caspase-3 in cell apoptosis under growth factor deprivation. Interestingly, recent studies (38, 39) have shown that p21 acts as a caspase-3 inactivator. Therefore, we assumed that caspase-3 might be a target molecule of the p21 protein that contributes to the fimbrial inhibition of THP-1 cell apoptosis. As expected, the fimbriae inhibited caspase-3 activity of the cells induced by the absence of serum. And, interestingly, the fimbrial inhibition of caspase-3 activity was blocked by PD98059 and antisense p21 oligonucleotide treatment. These results strongly suggest that caspase-3 is a target molecule of p21 that mediates the fimbrial inhibition of THP-1 cell apoptosis under growth factor deprivation.
In view of the signaling pathway of the fimbriae described above, although it is of interest to demonstrate whether the fimbria-induced ERK–p21–caspase-3 signaling pathway is mediated via binding of the fimbriae to β2 integrin, as suggested by our previous study (40), this point still remains obscure.
An understanding of the processes regulating apoptosis of monocytes/macrophages is critical to the elucidation of the pathogenic mechanism of chronic inflammation. Although the inflammatory reaction in bacterial infection has been well demonstrated, we suggest here that bacterial fimbriae act as inhibitors of growth factor withdrawal-induced apoptosis of cultured monocytic cells through p21 expression via the ERK- and MAPK-dependent pathway. This action suggests that bacterial fimbriae play a functional role as potent regulators of chronic inflammatory disease, such as periodontal disease caused by periodontal pathogens.
In conclusion, the present study shows that P. gingivalis fimbriae inhibit caspase-3-mediated apoptosis of monocytic THP-1 cells under growth factor deprivation via ERK- and MAPK-dependent expression of p21.
ACKNOWLEDGMENT
This work was supported by a grant-in-aid for scientific research (11470380) from the Ministry of Education, Science, and Culture of Japan.
REFERENCES
- 1.Albina J E, Cui S, Mateo R B, Reichner J S. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- 2.Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S. Apoptosis inhibitory activity of cytoplasmic p21Cip1/WAF1 in monocytic differentiation. EMBO J. 1999;18:1223–1234. doi: 10.1093/emboj/18.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asada M, Yamada T, Fukumuro K, Mizutani S. p21Cip/WAF1 is important for differentiation and survival of U937 cells. Leukemia. 1998;12:1944–1950. doi: 10.1038/sj.leu.2401228. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 5.Auer K L, Spector M S, Tombes R M, Seth P, Fisher P B, Gao B, Dent P, Kunos G. α-Adrenergic inhibiton of proliferation in HepG2 cells stably transfected with the α1B-adrenergic receptor through a p42MAP kinase/p21Cip1/WAF1-dependent pathway. FEBS Lett. 1998;436:131–138. doi: 10.1016/s0014-5793(98)01074-6. [DOI] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the estimation of DNA. Biochem J. 1956;62:315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin B Y, Petrache I, Choi A M K, Choi M E. Transforming growth factor β1 rescues serum deprivation-induced apoptosis via the mitogen-activated protein kinase (MAPK) pathway in macrophages. J Biol Chem. 1999;274:11362–11368. doi: 10.1074/jbc.274.16.11362. [DOI] [PubMed] [Google Scholar]
- 9.Choi M E, Ballermann B J. Inhibition of capillary morphogenesis and associated apoptosis by dominant negative mutant transforming growth factor-β. J Biol Chem. 1995;270:21144–21150. doi: 10.1074/jbc.270.36.21144. [DOI] [PubMed] [Google Scholar]
- 10.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 11.Connell H, Hedlund M, Agace W, Svanborg C. Bacterial attachment to uro-epithelial cells: mechanisms and consequences. Adv Dent Res. 1997;11:50–58. doi: 10.1177/08959374970110011701. [DOI] [PubMed] [Google Scholar]
- 12.Dudly D T, Pang L, Decker S J, Bridges A J, Saltiet A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahy R J, Doseff A I, Wewers M D. Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J Immunol. 1999;163:1755–1762. [PubMed] [Google Scholar]
- 14.Genco C A, Van Dyke T, Amar S. Animal models for Porphyromonas gingivalis-mediated periodontal disease. Trends Microbiol. 1998;6:444–449. doi: 10.1016/s0966-842x(98)01363-8. [DOI] [PubMed] [Google Scholar]
- 15.Gorospe M, Cirielli C, Wang X, Seth P, Capogrossi M C, Holbrook N J. p21 (Waf1/Cip1) protects against p53-mediated apoptosis of human melanoma cells. Oncogene. 1997;14:929–935. doi: 10.1038/sj.onc.1200897. [DOI] [PubMed] [Google Scholar]
- 16.Hanazawa S, Hirose K, Ohmori Y, Amano S, Kitano S. Bacteroides gingivalis fimbriae stimulate production of thymocyte-activating factor by human gingival fibroblasts. Infect Immun. 1988;56:272–274. doi: 10.1128/iai.56.1.272-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanazawa S, Murakami Y, Takeshita A, Kitami H, Ohta K, Amano S, Kitano S. Porphyromonas gingivalis fimbriae induce expression of the neutrophil chemotactic factor KC gene of mouse peritoneal macrophages: role of protein kinase C. Infect Immun. 1992;60:1544–1549. doi: 10.1128/iai.60.4.1544-1549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanazawa S, Murakami Y, Hirose K, Amano S, Ohmori Y, Higuchi H, Kitano S. Bacteroides (Porphyromonas) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect Immun. 1991;59:1972–1977. doi: 10.1128/iai.59.6.1972-1977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedges S, Svensson M, Svanborg C. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect Immun. 1992;60:1295–1301. doi: 10.1128/iai.60.4.1295-1301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiroi M, Shimojima T, Kashimata M, Miyata T, Takano H, Takahama M, Sakagami H. Inhibition by Porphyromonas gingivalis LPS of apoptosis induction in human peripheral blood polymorphonuclear leukocytes. Anticancer Res. 1998;18:3475–3479. [PubMed] [Google Scholar]
- 21.Hirose K, Isogai E, Mizugai H, Ueda I. Inductive effect of Porphyromonas gingivalis fimbriae on differentiation of human monocytic tumor cell line U937. Oral Microbiol Immunol. 1996;11:62–64. doi: 10.1111/j.1399-302x.1996.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 22.Kawata Y, Hanazawa S, Amano S, Murakami Y, Matsumoto T, Nishida K, Kitano S. Porphyromonas gingivalis fimbriae stimulate bone resorption in vitro. Infect Immun. 1994;62:3012–3016. doi: 10.1128/iai.62.7.3012-3016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamont R J, Jenkinson H F. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landi L, Amar S, Polins A S, Van Dyke T E. Host mechanisms in the pathogenesis of periodontal disease. Curr Opin Periodontol. 1997;4:3–10. [PubMed] [Google Scholar]
- 25.Liu Y, Martindale J L, Gorospe M, Holbrook N J. Regulation of p21WAF1/CIP1 expression through mitogen-activated protein kinase signaling pathway. Cancer Res. 1996;56:31–35. [PubMed] [Google Scholar]
- 26.Mangan D F, Welch G R, Wahl S M. Lipopolysaccharide, tumor necrosis factor-α, and IL-1β prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991;146:1541–1546. [PubMed] [Google Scholar]
- 27.Mangan D F, Mergenhagen S E, Wahl S M. Apoptosis in human monocytes: possible role in chronic inflammatory disease. J Periodontol. 1993;64:461–466. [PubMed] [Google Scholar]
- 28.Mangan D F, Wahl S M. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol. 1991;147:3408–3412. [PubMed] [Google Scholar]
- 29.McConkey D J, Nicotera P, Hartzell P, Bellomo G, Wyllie A H, Orrenius S. Glucocorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys. 1989;269:365–370. doi: 10.1016/0003-9861(89)90119-7. [DOI] [PubMed] [Google Scholar]
- 30.Moore K J, Matlashewski G. Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J Immunol. 1994;152:2930–2937. [PubMed] [Google Scholar]
- 31.Murakami Y, Iwahashi H, Yasuda H, Umemoto T, Namikawa I, Kitano S, Hanazawa S. Porphyromonas gingivalis fimbrillin is one of the fibronectin-binding proteins. Infect Immun. 1996;64:2571–2576. doi: 10.1128/iai.64.7.2571-2576.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muro M, Nakashima K, Tomioka J, Kato S, Nonaka K, Yoshida T, Inoue M, Nishihara T, Kowashi Y. Inhibitory effect of lipopolysaccharide on apoptotic cell death in macrophages infected with Actinobacillus actinomycetemcomitans. FEMS Microbiol Lett. 1999;175:211–216. doi: 10.1111/j.1574-6968.1999.tb13622.x. [DOI] [PubMed] [Google Scholar]
- 33.Oltvai Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 34.Poluha W, Poluha D K, Chang B, Crosbie N E, Schonhoff C M, Kilpatrick D L, Ross A H. The cyclin-dependent kinase inhibitor p21WAF1 is required for survival of differentiating neuroblastoma cells. Mol Cell Biol. 1996;16:1335–1341. doi: 10.1128/mcb.16.4.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preshaw P M, Schifferle R E, Walters J D. Porphyromonas gingivalis lipopolysaccharide delays human polymorphonuclear leukocyte apoptosis in vitro. J Periodontal Res. 1999;34:197–202. doi: 10.1111/j.1600-0765.1999.tb02242.x. [DOI] [PubMed] [Google Scholar]
- 36.Rescigno M, Martino M, Sutherland C L, Gold M R, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki A, Tsutomi Y, Akahane K, Araki T, Miura M. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene. 1998;17:931–940. doi: 10.1038/sj.onc.1202021. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki A, Tsutomi Y, Yamamoto N, Shibutani T, Akahane K. Mitochondrial regulation of cell death: mitochondria are essential for procaspase 3-p21 complex formation to resist Fas-mediated cell death. Mol Cell Biol. 1999;19:3842–3847. doi: 10.1128/mcb.19.5.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeshita A, Murakami Y, Yamashita Y, Ishida M, Fujisawa S, Kitano S, Hanazawa S. Porphyromonas gingivalis fimbriae use β2 integrin (CD11/CD18) on mouse peritoneal macrophages as a cellular receptor, and the CD18 β chain plays a functional role in fimbrial signaling. Infect Immun. 1998;66:4056–4060. doi: 10.1128/iai.66.9.4056-4060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Winkelhoff A J, de Graaff J. Microbiology in the management of destructive periodontal disease. J Clin Periodontol. 1991;18:406–410. doi: 10.1111/j.1600-051x.1991.tb02308.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Walsh K. Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science. 1996;273:359–361. doi: 10.1126/science.273.5273.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Van Tuyle G, Conard D, Fisher P B, Dent P, Grant S. Dysregulation of cyclin-dependent kinase inhibitor p21WAF1/CIP1/MDM6 increases the susceptibility of human leukemia cells (U937) to 1-β-D- arabinofuranosylcytosine-mediated mitochondrial dysfunction and apoptosis. Cancer Res. 1999;59:1259–1267. [PubMed] [Google Scholar]
- 44.William R, Watson G, Rotstein O D, Parodo J, Bitar R, Marshall J C. The IL-1β-converting enzyme (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1β. J Immunol. 1998;161:957–962. [PubMed] [Google Scholar]
- 45.Yang E, Zha J, Jockel J, Boise L M, Thompson C B, Korsmeyer S J. Bad, a heterodimeric partner for Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 46.Yoshimura F, Takahashi K, Nodasaka Y, Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984;160:949–954. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zambon J J. Periodontal diseases: microbial factors. Ann Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- 48.Zychlinsky A, Sansonetti P. Perspectives series: host/pathogen interactions. J Clin Investig. 1997;100:493–496. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]