Abstract
Lipopolysaccharide (LPS) preparations from gram-negative black-pigmented bacteria such as Porphyromonas gingivalis and Prevotella intermedia activate cells from non-LPS-responsive C3H/HeJ mice, but it is still unclear whether this activity is due to the unique structure of LPS or to a minor component(s) responsible for the activity in the preparation. A nonendotoxic glycoprotein with bioactivity against cells from C3H/HeJ mice was purified from a hot phenol-water extract of P. intermedia ATCC 25611 and designated Prevotella glycoprotein (PGP). Treatment of human monocytic THP-1 cells with 22-oxyacalcitriol (OCT) induced maturation and marked expression of CD14 on the cells, but the cells constitutively expressed Toll-like receptor 2 (TLR2) and TLR4 on the cells irrespective of the treatment. PGP induced a high level of interleukin-8 production at doses of 100 ng/ml and higher in OCT-treated THP-1 cells compared with Salmonella LPS, and the production was significantly inhibited by anti-CD14 and anti-TLR2 but not anti-TLR4 antibodies. Consistent with this, TLR2-deficient murine macrophages did not respond to PGP. It was also shown that PGP activity on the THP-1 cells was LPS-binding protein dependent and was inhibited by a synthetic lipid A precursor IVA. These results indicate that PGP activates monocytic cells in a CD14- and TLR2-dependent manner.
CD14 is expressed mainly on monocytes and neutrophils and has recently been shown to act as a pattern recognition receptor for various bacterial cell surface components in addition to lipopolysaccharide (LPS) (29, 45). LPS-binding protein (LBP) in serum accelerates the binding of low concentrations of LPS to CD14 (6, 32, 46). Since CD14 is a 55-kDa glycosylphosphatidylinositol-anchored membrane protein that lacks transmembrane and cytoplasmic domains, CD14 itself does not elicit intracellular signaling events (41). Members of the vertebrate Toll-like receptor (TLR) family, homologues of Drosophila Toll, have been implicated as important to innate immune responses in vertebrates (22). Recently, the gene responsible for the LPS nonresponsiveness of C3H/HeJ mice was mapped (28, 30). In this mouse, proline at cytoplasmic position 712 of the TLR4 polypeptide chain was replaced with histidine, a substitution which prevented LPS signaling in the mouse. Supporting this evidence, overexpression of wild-type TLR4 but not mutant TLR4 from C3H/HeJ mice activates nuclear factor κB (11). Several recent studies showed that TLR4 mediates signals of LPS and that TLR2 mediates signals of other bacterial cell surface components, such as peptidoglycan, lipoprotein, and lipoarabinomannan (11, 21, 28, 30, 33, 37, 38, 42, 48).
Gram-negative anaerobic black-pigmented bacteria (BPB) such as Porphyromonas gingivalis and Prevotella intermedia have been suggested to be the principal bacteria associated with periodontal diseases (10, 34). LPS specimens prepared from BPB and related bacteria (formerly called Bacteroides species) have been reported to possess chemical and biological properties different from those of LPSs from the family Enterobacteriaceae (7, 20, 44). LPS specimens extracted from BPB and related bacteria with hot phenol-water activate macrophages and lymphocytes from both LPS-responsive C3H/HeN and non-LPS-responsive C3H/HeJ (TLR4 mutant) mice (8, 13, 16), and the purified lipid A moiety of P. gingivalis LPS induced production of proinflammatory cytokines from the cells of C3H/HeJ mice (26, 40). It has recently been shown that purified P. gingivalis LPS activated macrophages from TLR4-deficient mice (39), suggesting that this type of LPS preparation interacts with molecules other than TLR4.
We have recently isolated an immunobiologically active nonendotoxic glycoprotein from a hot phenol-water extract of P. intermedia ATCC 25611 and have designated it Prevotella glycoprotein (PGP) (12). PGP consists mainly of carbohydrate and protein and is devoid of fatty acid. PGP showed strong mitogenicity to splenocytes and showed cytokine-inducing activity in macrophages from C3H/HeJ as well as C3H/HeN mice. In contrast, LPS extracted from the same bacterium with a phenol-chloroform-petroleum ether (PCP) mixture exhibited strong Limulus activity and activated only the cells from C3H/HeN mice, and its activity was completely inhibited by polymixin B. The LPS fraction extracted with hot phenol-water exhibited the properties of both PGP and PCP-extracted LPS preparations. These findings strongly suggested that the unique bioactivities of LPS preparations extracted from BPB and related bacteria with hot phenol-water reported to date were due to this type of material and not to LPS itself. To further examine this possibility, we investigated whether PGP and LPS preparations from P. intermedia can stimulate a human monocytic cell line, THP-1 cells, and macrophages of TLR2- and TLR4-deficient mice by interacting with CD14, TLR2, and TLR4.
MATERIALS AND METHODS
Reagents. (i) PGP preparation.
PGP was prepared from P. intermedia ATCC 25611 as described previously (12). Briefly, lyophilized cells of P. intermedia were extracted with phenol-water at 67°C, and the extract in the water phase was ultracentrifuged at 140,000 × g. The LPS sediment was designated LPS-phenol-water (LPS-PW). The supernatant was then applied to a column of Sephadex G-100 (Pharmacia, Uppsala, Sweden), and the fractions free of Limulus activity and mitogenic to C3H/HeJ splenocytes were collected, treated with NP1 nuclease (Yamasa, Choshi, Japan), and rechromatographed on Sephadex G-100 to obtain the final PGP fraction. Another LPS preparation was extracted from the same bacteria with the PCP mixture and designated LPS-PCP. The PGP fraction was scarcely active in the Limulus test (22 ng/mg) (Endospecy test; Seikagaku Co., Tokyo, Japan) (23) and contained rhamnose, galactose, and glucose as neutral sugars and small amounts of glucosamine as described previously (12). Chemical analytical data for LPS-PW and LPS-PCP were also described in a previous paper (12).
(ii) Other reagents.
An ultrapurified LPS preparation from Salmonella enterica serovar Abortus-equi (Novo-Pyrexal) (4) was a gift from C. Galanos (Max Plank Institut für Immunbiologie, Freiburg, Germany) and was used as a reference LPS of Enterobacteriaceae. A synthetic lipid A precursor IVA, LA-14-PP (compound 406), was obtained from Daiichi Chemical Co. (Tokyo, Japan). Anti-human TLR2 monoclonal antibody (MAb) TL2.1 (mouse immunoglobulin G2a [IgG2a]) and TLR4 MAb HTA125 (mouse IgG2a) were raised as described previously (1, 35). Purified anti-human CD14 MAb MY4 (mouse IgG2b) and isotype control IgG were purchased from Coulter (Miami, Fla.) and dialyzed against phosphate-buffered saline. Recombinant human LBP (rLBP) was purchased from Biometec (Greifswald, Germany). All other reagents were obtained from Sigma (St. Louis, Mo.) unless otherwise indicated.
Cell line and mice.
THP-1, a human leukemia cell line of monocyte/macrophage lineage, was obtained from Human Science Research Resource Bank (Osaka, Japan) and grown in RPMI 1640 with 10% fetal bovine serum (FBS) (heat inactivated at 56°C for 30 min) (Life Technologies, Grand Island, N.Y.). To induce maturation, cultures were grown in 24-well plates (Falcon; Becton Dickinson Labware, Lincoln Park, N.J.) in the presence of 0.1 μM 22-oxyacalcitriol (OCT) (Chugai Pharmaceutical Co., Tokyo, Japan) (17), a potent analogue of 1,25-dihydroxyvitamin D3, for 3 days.
Mutant mice deficient in TLR2 and TLR4 were generated by gene targeting as described previously (11, 37). Age-matched groups of wild-type, TLR2-deficient, and TLR4-deficient mice on the same genetic background were used for the experiments.
Flow cytometry.
Flow cytometric analyses were performed with a fluorescence-activated cell sorter (FACScan; Becton Dickinson, Mountain View, Calif.) (36). Briefly, cells were stained with fluorescein isothiocyanate (FITC)-conjugated MY4 or FITC-conjugated isotype control IgG2b (Coulter) at 4°C for 30 min. For TLR2 and TLR4 staining, cells were treated with TL2.1, HTA125, or isotype control IgG2a at 4°C for 30 min and then incubated with FITC-conjugated goat anti-mouse IgG (BioSource International, Camarillo, Calif.) at 4°C for a further 30 min.
Detection of cytokines by ELISA.
OCT-treated THP-1 cells were seeded in a 96-well flat-bottomed plate (Falcon) at 5 × 104 cells/well and were incubated with or without test stimulants in 200 μl of RPMI 1640 with 1% FBS for 24 h (unless otherwise indicated) for interleukin-8 (IL-8) production. For the inhibition experiments, the cells were preincubated with given concentrations of MAbs or LA-14-PP for 30 min at 37°C and then stimulated with test stimulants at 37°C in a CO2 incubator. For the LBP dependency experiment, the cells were stimulated in the presence or absence of 0.1 μg of rLBP/ml in medium containing 0.1% bovine serum albumin (BSA) (Roche Diagnostics, Mannheim, Germany). After the incubation, the supernatants were collected and the level of IL-8 in the supernatants was determined with an OptEIA human IL-8 enzyme-linked immunosorbent assay (ELISA) set (PharMingen, San Diego, Calif.). The concentrations of IL-8 in the supernatants were determined using the Softmax data analysis program (Molecular Devices Corp., Menlo Park, Calif.).
Measurement of tumor necrosis factor alpha (TNF-α) production from murine macrophages was performed as described previously (37). Briefly, mice were intraperitoneally injected with 2 ml of 4% thioglycolate medium (Difco, Detroit, Mich.). Three days later, peritoneal exudate cells were isolated from the peritoneal cavity by washing with ice-cold Hanks' balanced salt solution (Life Technologies). Cells were cultured for 2 h and washed with warmed Hanks' balanced salt solution to remove nonadherent cells. Adherent monolayer cells were used as peritoneal macrophages and were cultured in RPMI 1640 medium with 10% FBS. Peritoneal macrophages were cultured with 10 μg of stimulants per ml for 24 h. Concentrations of TNF-α in culture supernatants were measured by ELISA according to the instructions of the manufacturer (Genzyme/Techne, Minneapolis, Minn.)
Data analysis.
All of the experiments in this study were conducted at least three times. The data shown are representative results. Experimental values are given as means ± standard deviations (SD) from triplicate cultures. The statistical significance of differences between two means was evaluated by Student's unpaired t test, and P values of less than 0.05 were considered significant.
RESULTS
Effect of OCT on expression of CD14, TLR2, and TLR4 by THP-1 cells.
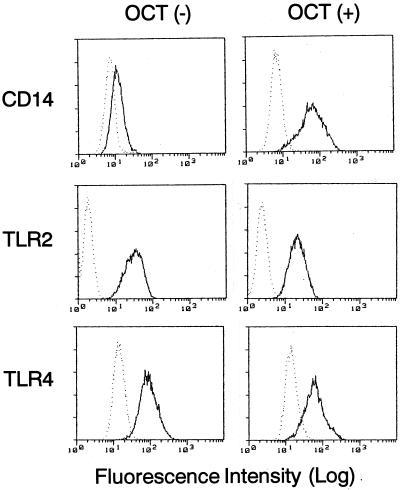
It has been shown that an active form of vitamin D3 (1,25-dihydroxyvitamin D3) and its potent analogue, OCT, induce maturation of THP-1 cells and consequently expression of CD14 on the cells (15, 47). Therefore, we first examined whether OCT induces not only CD14 but also TLR2 and TLR4 by flow cytometry. When THP-1 cells were incubated with 0.1 μM OCT for 3 days, a strong induction of CD14 was observed on the cell surface (Fig. 1). By contrast, TLR2 and TLR4 were already expressed on the untreated cell surface of THP-1 cells, and the expression was almost unaffected after exposure to OCT. This observation was confirmed by reverse transcription-PCR (data not shown). Based on this observation, OCT-treated THP-1 cells were utilized in further experiments.
FIG. 1.
Effect of OCT on the expression of CD14, TLR2, and TLR4 by THP-1 cells. THP-1 cells were incubated with or without 0.1 μM OCT for 3 days. Cells were stained with anti-CD14 MY4, anti-TLR2 TL2.1, or anti-TLR4 HTA125 MAbs (solid lines) or with control IgG (dotted lines) and were analyzed by flow cytometry. Similar results were obtained from three distinct experiments.
Stimulation of THP-1 cells with PGP and P. intermedia LPS preparations to produce IL-8.
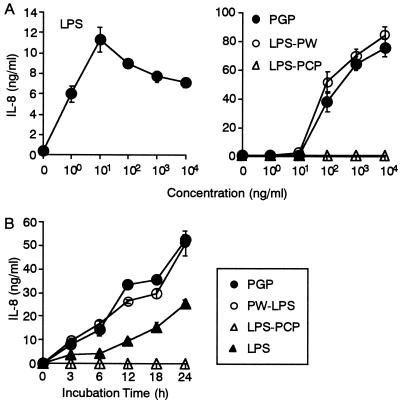
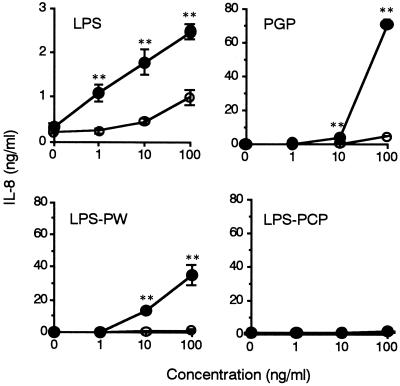
We next examined whether PGP and P. intermedia LPS preparations stimulate THP-1 cells by measuring production of IL-8 from the cells. The reference Salmonella serovar Abortus-equi LPS at 1 ng/ml stimulated THP-1 cells to produce IL-8, and the production reached a maximum at 10 ng/ml (Fig. 2A). PGP and P. intermedia LPS-PW at 100 ng/ml started to induce production of IL-8 from the THP-1 cells, and the production was increased at higher concentrations. By contrast, P. intermedia LPS-PCP showed no effect on the cells. A time kinetic study showed that IL-8 production from the THP-1 cells in response to PGP and P. intermedia LPS-PW was time dependent, and the production was highest at 24 h, showing a pattern similar to that for the reference Salmonella serovar Abortus-equi LPS (Fig. 2B). P. intermedia LPS-PCP had no effect on the production of IL-8 at any time point. It is notable that PGP and P. intermedia LPS-PW induced high levels of IL-8 at 100 ng/ml and induced higher concentrations than the reference Salmonella serovar Abortus-equi LPS.
FIG. 2.
IL-8 production from THP-1 cells in response to PGP and P. intermedia LPS preparations. OCT-treated THP-1 cells were stimulated with the indicated concentrations of Salmonella serovar Abortus-equi LPS, PGP, P. intermedia LPS-PW, and P. intermedia LPS-PCP for 24 h (A) or were stimulated with a 100-ng/ml concentration of Salmonella serovar Abortus-equi LPS, PGP, P. intermedia LPS-PW, and P. intermedia LPS-PCP for the times indicated (B). The culture supernatants were collected and analyzed for the presence of IL-8 by ELISA. Error bars indicate SD. Results are representative of those from four distinct experiments with similar results.
Inhibition of PGP activity by anti-CD14 and anti-TLR2 MAbs but not by anti-TLR4 MAb.
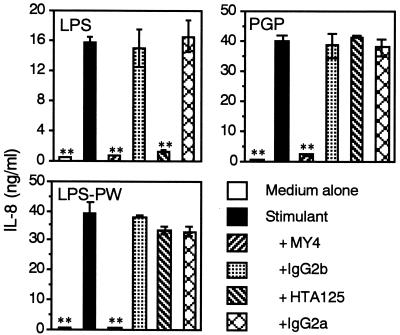
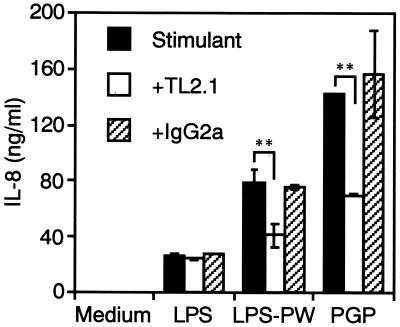
It has recently been shown that LPS activity was CD14 and TLR4 dependent (11, 28, 30, 37, 42), and therefore, we next examined the CD14 and TLR dependency of THP-1 cell activation by PGP using neutralizing MAbs. The reference Salmonella serovar Abortus-equi LPS (100 ng/ml)-induced production of IL-8 from the THP-1 cells was significantly inhibited not only by anti-CD14 but also by anti-TLR4 MAbs (Fig. 3), as recently reported for human monocytes (36). By contrast, the production induced by PGP (100 ng/ml) and P. intermedia LPS-PW (100 ng/ml) was significantly inhibited by anti-CD14 MAb but not by anti-TLR4 MAb. In addition, anti-TLR2 MAb significantly inhibited PGP- and P. intermedia LPS-PW-induced but not the reference Salmonella serovar Abortus-equi LPS-induced production of IL-8 from the cells (Fig. 4). These results suggest that PGP activates human monocytic cells via a CD14- and TLR2-dependent but TLR4-independent pathway.
FIG. 3.
Effect of anti-CD14 and anti-TLR4 MAbs on PGP-induced IL-8 production from THP-1 cells. OCT-treated THP-1 cells were stimulated with a 100-ng/ml concentration of Salmonella serovar Abortus-equi LPS, PGP, and P. intermedia LPS-PW in the presence or absence of anti-CD14 MAb MY4 (10 μg/ml), its isotype control mouse IgG2b (10 μg/ml), anti-TLR4 MAb HTA125 (5 μg/ml), or its isotype control mouse IgG2a (5 μg/ml) for 24 h. The culture supernatants were collected and analyzed for the presence of IL-8 by ELISA. Error bars indicate SD. ∗∗, P < 0.01 versus stimulants alone. Results are representative of those from three distinct experiments with similar results.
FIG. 4.
Effect of anti-TLR2 MAb on PGP-induced IL-8 production from THP-1 cells. OCT-treated THP-1 cells were stimulated with a 100-ng/ml concentration of Salmonella serovar Abortus-equi LPS, PGP, or P. intermedia LPS-PW in the presence or absence of anti-TLR2 MAb TL2.1 (10 μg/ml) or its isotype control mouse IgG2a (10 μg/ml) for 24 h. The culture supernatants were collected and analyzed for the presence of IL-8 by ELISA. Error bars indicate SD. ∗∗, P < 0.01 versus stimulants alone. Results are representative of those from three distinct experiments with similar results.
Responsiveness of TLR2- and TLR4-deficient murine peritoneal macrophages to PGP.
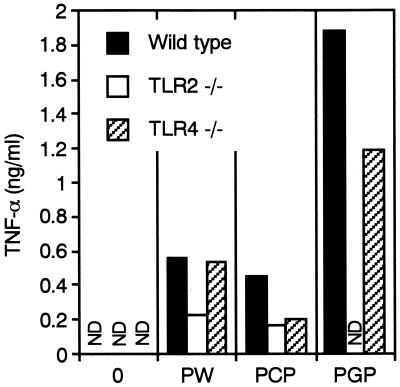
In order to confirm the above-described observations obtained with human monocytic cells in culture and the previous observation that PGP activates cells from non-LPS-responsive C3H/HeJ mice (12), the responsiveness of peritoneal macrophages of TLR2- and TLR4-deficient mice to PGP was next examined by measuring TNF-α production. P. intermedia LPS-PW induced TNF-α production from the cells of both wild-type and TLR4-deficient mice and lesser amounts from the cells of TLR2-deficient mice (Fig. 5). Although P. intermedia LPS-PCP had no effect on human monocytic THP-1 cells, as shown in Fig. 2A, it induced TNF-α production in macrophages from wild-type mice, and the responses of both TLR2- and TLR4-deficient mice were reduced by approximately 50% compared with those of wild-type mice. The residual TLR2- and TLR4-mediated responses to LPS-PCP may be attributable to an endotoxin protein(s) and LPS itself, respectively, because the LPS-PCP contained marked amounts of protein (12) and lipoprotein activates cells through TLR2 (9, 38). By contrast, PGP induced TNF-α production from both wild-type and TLR4-deficient macrophages but had no effect on the production of TNF-α from the cells of TLR2-deficient mice, indicating clearly that PGP activity is TLR2 dependent.
FIG. 5.
Responsiveness of murine peritoneal macrophages to PGP and P. intermedia LPS preparations. Peritoneal macrophages from wild-type, TLR2-deficient, and TLR4-deficient mice were cultured with a 10-μg/ml concentration of PGP, P. intermedia LPS-PW (PW), or P. intermedia LPS-PCP (PCP) for 24 h. Concentrations of TNF-α in the culture supernatants were measured by ELISA. ND, Not detected. The results are representative of those from three different experiments with similar results.
PGP activity is LBP dependent.
Since LBP in serum accelerated the binding of low concentrations of LPS to CD14 (6, 32, 46), we next examined the possible involvement of LBP in PGP-induced cell activation. The reference Salmonella serovar Abortus-equi LPS-induced IL-8 production from the THP-1 cells was significantly augmented in the presence of 100 ng of rLBP/ml in the medium with 0.1% BSA (Fig. 6). IL-8 production from the THP-1 cells induced by PGP and P. intermedia LPS-PW was also significantly augmented at 10 and 100 ng/ml. By contrast, P. intermedia LPS-PCP did not induce production of IL-8 even in the presence of rLBP. These results indicated that even though PGP is different from LPS in chemical structure, PGP activity was enhanced in the presence of LBP.
FIG. 6.
PGP activity is LBP dependent. OCT-treated THP-1 cells were incubated with the indicated concentrations of Salmonella serovar Abortus-equi LPS, PGP, P. intermedia LPS-PW, and P. intermedia LPS-PCP in medium supplemented with 0.1% BSA in the presence (closed circles) or absence (open circles) of 100 ng of human rLBP/ml for 24 h. The amounts of IL-8 in the supernatants were analyzed by ELISA. Error bars indicate SD. ∗, P < 0.05; ∗∗, P < 0.01 (versus without LBP). The results are representative of those from three different experiments with similar results.
Lipid IVA acts as an antagonist of PGP.
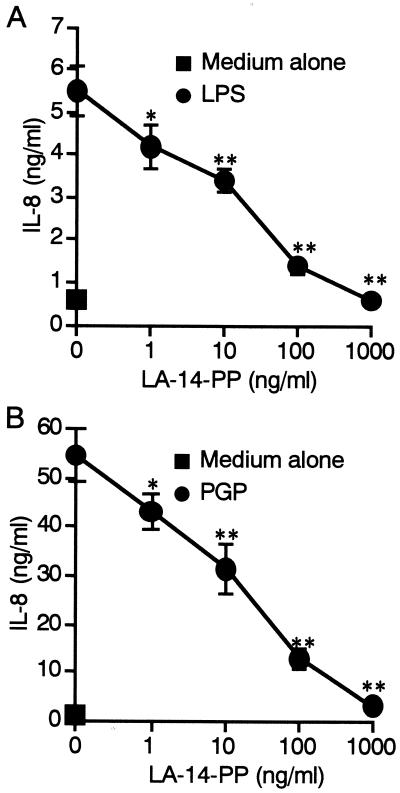
We next analyzed whether synthetic lipid IVA (LA-14-PP or compound 406), a well-known LPS antagonist in human cells, inhibits the activity of PGP. The THP-1 cells were stimulated with the reference Salmonella serovar Abortus-equi LPS (100 ng/ml) or PGP (100 ng/ml) with or without different concentrations of LA-14-PP for 24 h. As shown in Fig. 7, IL-8 production induced by PGP as well as LPS was inhibited by LA-14-PP in a dose-dependent manner, and almost complete inhibitions of LPS and PGP activities were observed at a 10-fold excess amount of LA-14-PP, indicating that LA-14-PP acts as an antagonist not only of LPS but also of PGP.
FIG. 7.
LA-14-PP acts as an antagonist against LPS and PGP. OCT-treated THP-1 cells were stimulated with Salmonella serovar Abortus-equi LPS (100 ng/ml) (A) or PGP (100 ng/ml) (B) in the presence or absence of LA-14-PP at the indicated concentrations for 24 h. The amounts of IL-8 in the supernatants were analyzed by ELISA. Error bars indicate SD. ∗, P < 0.05; ∗∗, P < 0.01 (versus stimulant alone). The results are representative of those from three different experiments with similar results.
DISCUSSION
It has been reported that LPS specimens extracted from BPB and related bacteria with hot phenol-water exert unique biological activities on human and murine cells. In particular, the BPB-derived LPS preparations activated cells from non-LPS-responsive C3H/HeJ mice as well as LPS-responsive C3H/HeN mice (8, 13, 16, 26, 40). However, it is still unclear whether the unique biological activity of the BPB and related bacterial LPS is due to differences in the chemical structure of the LPS, especially the lipid A portion, compared to that of Enterobacteriaceae LPS or to a minor component(s) in the LPS preparations specific to BPB and related bacteria, which are responsible for the apparent activities of these LPS preparations (see the discussion in reference 12). The PGP used in this study consisted mainly of rhamnose, galactose, and glucose as neutral sugars, with small amounts of glucosamine (12). PGP contains only trace amounts of fatty acids and shows very little activity in the Limulus test (22 ng/mg) (12), indicating that PGP was not contaminated by LPS. The LPS-PW and LPS-PCP used in this study showed Limulus activity comparable to that of reference Escherichia coli LPS (12), but LPS-PCP showed no IL-8-inducing activity in human monocytic cells (Fig. 2). In contrast, LPS-PW exhibited the same activity as PGP on the cells (Fig. 2), and LPS-PW exhibited the properties of both PGP and LPS-PCP in murine cells (12), suggesting that the unique bioactivities of LPS preparations of BPB and related bacteria reported to date are derived from some PGP-like molecule(s). In support of this possibility, a chemically synthesized P. gingivalis lipid A activated macrophages to produce IL-6 and TNF-α from C3H/HeN mice but not from C3H/HeJ mice (25).
It has been documented that established protocols for isolating LPS with hot phenol-water result in the coextraction of capsular polysaccharide complex (CPC) of Bacteroides fragilis (27) and that the purified CPC induced IL-1, IL-8, and TNF-α production from human and murine monocytes/macrophages (5). Although the chemical structure of PGP has not been defined, PGP consists mainly of carbohydrate and protein and is devoid of fatty acid, and the activity was resistant to heat (100°C, 1 h) and protease (pronase E) treatment but sensitive to periodate treatment (12), suggesting that (i) PGP is not a endotoxin protein, (ii) the carbohydrate but not the protein moiety of PGP was important for the activity, and (iii) PGP might be a member of the CPC, a unique constituent of BPB and related bacteria (formerly called Bacteroides species). Friedman et al. previously reported a bioactive carbohydrate-rich fraction of Serratia marcescens that was devoid of endotoxic activity (2, 3), suggesting that there is some similarity between these fractions and that a carbohydrate-rich fraction with bioactivity may be distributed among other bacterial species.
P. intermedia LPS-PCP did not induce IL-8 production in human monocytic THP-1 cells (Fig. 2) but activated murine macrophages to induce TNF-α and IL-6 production (Fig. 5) (12). The chemical structure of lipid A of P. gingivalis was proposed by two groups (18, 24). These groups noted considerable variations concerning the lipid A structure, but the P. gingivalis lipid A possesses fewer and longer fatty acids than the E. coli-type lipid A. The chemical analysis of P. intermedia LPS (LPS-PCP) indicated structural similarity to P. gingivalis LPS (12). It is conceivable that the biological activity of P. intermedia LPS-PCP may have similarity to that of lipid IVA in human and murine monocytic cells. Our preliminary study showed that P. intermedia LPS-PCP acts as an antagonist of LPS in human monocytic cells, like lipid IVA (S. Yang, S. Sugawara, and H. Takada, unpublished data).
Several recent studies showed that TLR4 mediates signals of LPS and that TLR2 mediates signals of other bacterial cell surface components, such as peptidoglycan, lipoprotein, and lipoarabinomannan (11, 21, 28, 30, 33, 37, 38, 42, 48). We have previously shown that PGP activates cells from C3H/HeJ mice (TLR4 mutant) (12). We showed in the present study that PGP strongly induced production of IL-8 at a higher level than that induced by LPS from OCT-treated THP-1 cells, which express both TLR2 and TLR4, and that anti-CD14 and anti-TLR2 but not anti-TLR4 MAbs inhibited PGP-induced production of IL-8 from the cells (Fig. 3 and 4). This observation was supported by the evidence in the murine system that TLR2-deficient macrophages did not produce TNF-α in response to PGP (Fig. 5), indicating that PGP activates human and murine monocytic cells via a CD14- and TLR2-dependent pathway.
Kitchens et al. (14, 15) suggested that lipid IVA can antagonize LPS not only by competing to bind CD14 and/or LBP but also by interacting at a site distal to CD14, and recently Lien et al. (19) demonstrated that lipid IVA antagonized LPS-induced cell activation in Chinese hamster ovary fibroblasts overexpressing human CD14 and TLR4 but not TLR2, indicating that lipid IVA competes with LPS to bind CD14 and TLR4. However, gram-positive bacterial cell wall components such as peptidoglycan and lipoarabinomannan activate cells via the CD14-TLR2 pathway (21, 33, 37), and both activities were also blocked by lipid IVA (31, 43), probably competing with CD14. Therefore, it is possible that complete inhibition of PGP activity on THP-1 by lipid IVA (Fig. 7) resulted from competition to bind CD14, probably because of the low affinity of PGP for CD14. Even though the chemical structure of PGP is different from that of LPS, PGP activity was enhanced in the presence of LBP (Fig. 6), suggesting that PGP activity is LBP dependent. In support of this observation, it has been shown that LBP also enhanced the sensitivity of THP-1 cells to a TLR2 ligand, lipoarabinomannan (31).
In conclusion, we have proposed that BPB and related bacteria possess unique PGP-like molecules, and in the course of purification of LPS by established protocols, these molecules may be coisolated in the LPS preparation and are probably responsible for the unique bioactivity of BPB and the related bacterial LPS fraction. In the present study, we showed that nonendotoxic glycoprotein from periodontopathic P. intermedia activated human monocytic cells with different usage of TLRs from LPS.
ACKNOWLEDGMENTS
We thank C. Galanos for providing an LPS preparation and Ø. Halaas and R. Ryan, Norwegian University of Science and Technology, for helpful discussion on the TL2.1 MAb.
This work was supported in part by Grants-in Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture, Japan (10470378, 11671796, and 12470380).
REFERENCES
- 1.Flo T H, Halaas Ø, Lien E, Ryan L, Teti G, Golenbock D T, Sundan A, Espevik T. Human Toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 2.Friedman H, Blanchard D K, Newton C, Klein. Stewart T W, 2nd, Keler T, Nowotny A. Distinctive immunomodulatory effects of endotoxin and nontoxic lipopolysaccharide derivatives in lymphoid cell cultures. J Biol Response Mod. 1987;6:664–677. [PubMed] [Google Scholar]
- 3.Friedman H, Butler R C, Nowotny A. Enhanced antibody response in retrovirus-infected mice treated with endotoxin or nontoxic polysaccharide derivative. Proc Soc Exp Biol Med. 1987;186:275–279. doi: 10.3181/00379727-186-42613. [DOI] [PubMed] [Google Scholar]
- 4.Galanos C, Lüderitz D, Westphal O. Preparation and properties of a standardized lipopolysaccharide from Salmonella abortus equi (Novo-Pyrexal) Zentbl Bakteriol Mikrobiol Hyg Abt 1 Orig Reihe A. 1979;243:226–244. [PubMed] [Google Scholar]
- 5.Gibson I I I, C. F, Tzianabos A O, Onderdonk A B. The capsular polysaccharide complex of Bacteroides fragilis induces cytokine production from human and murine phagocytic cells. Infect Immun. 1996;64:1065–1069. doi: 10.1128/iai.64.3.1065-1069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamada S, Takada H, Ogawa T, Fujiwara T, Mihara J. Lipopolysaccharides of oral anaerobes associated with chronic inflammation: chemical and immunomodulating properties. Int Rev Immunol. 1990;6:247–261. doi: 10.3109/08830189009056635. [DOI] [PubMed] [Google Scholar]
- 8.Hanazawa S, Nakada K, Ohmori Y, Miyoshi T, Amano S, Kitano S. Functional role of interleukin 1 in periodontal disease: induction of interleukin 1 production by Bacteroides gingivalis lipopolysaccharide in peritoneal macrophages from C3H/HeN and C3H/HeJ mice. Infect Immun. 1985;50:262–270. doi: 10.1128/iai.50.1.262-270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirschfeld M, Ma Y, Weis J H, Vogel S N, Weis J J. Repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 10.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 12.Iki K, Kawahara K, Sawamura S, Arakaki R, Sakuta T, Sugiyama A, Tamura H, Sueda T, Hamada S, Takada H. A novel component different from endotoxin extracted from Prevotella intermedia ATCC 25611 activates lymphoid cells from C3H/HeJ mice and gingival fibroblasts from humans. Infect Immun. 1997;65:4531–4538. doi: 10.1128/iai.65.11.4531-4538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirikae T, Nitta T, Kirikae F, Suda Y, Kusumoto S, Qureshi N, Nakano M. Lipopolysaccharides (LPS) of oral black-pigmented bacteria induce tumor necrosis factor production by LPS-refractory C3H/HeJ macrophages in a way different from that of Salmonella LPS. Infect Immun. 1999;67:1736–1742. doi: 10.1128/iai.67.4.1736-1742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitchens R L, Munford R S. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J Biol Chem. 1995;270:9904–9910. doi: 10.1074/jbc.270.17.9904. [DOI] [PubMed] [Google Scholar]
- 15.Kitchens R L, Ulevitch R J, Munford R S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992;176:485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga T, Nishihara T, Fujiwara T, Nishizawa T, Okahashi N, Noguchi T, Hamada S. Biochemical and immunobiological properties of lipopolysaccharide (LPS) from Bacteroides gingivalis and comparison with LPS from Escherichia coli. Infect Immun. 1985;47:638–647. doi: 10.1128/iai.47.3.638-647.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubodera N, Sato K, Nishii Y. Characteristics of 22-oxacalcitriol (OCT) and 2b-(3-hydroxypropoxy)-calcitriol (ED-71) In: Feldman D, Glorieux F H, Pike J W, editors. Vitamin D. San Diego, Calif: Academic Press; 1997. pp. 1071–1086. [Google Scholar]
- 18.Kumada H, Haishima Y, Umemoto T, Tanamoto K. Structural study on the free lipid A isolated from lipopolysaccharide of Porphyromonas gingivalis. J Bacteriol. 1995;177:2098–2106. doi: 10.1128/jb.177.8.2098-2106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lien E, Means T K, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton M J, Oikawa M, Qureshi N, Monks B, Finberg R W, Ingalls R R, Golenbock D T. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Investig. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindberg A A, Weintraub A, Zähringer U, Rietschel E T. Structure-activity relationships in lipopolysaccharides of Bacteroides fragilis. Rev Infect Dis. 1990;12:S133–S141. doi: 10.1093/clinids/12.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 21.Means T K, Lien E, Yoshimura A, Wang S, Golenbock D T, Fenton M J. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748–6755. [PubMed] [Google Scholar]
- 22.Medzhitov R, Preston-Hurlburt P, Janeway C A., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 23.Obayashi T, Tamura H, Tanaka S, Ohki M, Takahashi S, Arai M, Masuda M, Kawai T. A new chromogenic endotoxin-specific assay using recombined limulus coagulation enzymes and its clinical applications. Clin Chim Acta. 1985;149:55–65. doi: 10.1016/0009-8981(85)90273-6. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa T. Chemical structure of lipid A from Porphyromonas (Bacteroides) gingivalis lipopolysaccharide. FEBS Lett. 1993;332:197–201. doi: 10.1016/0014-5793(93)80512-s. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa T, Asai Y, Yamamoto H, Taiji Y, Jinno T, Kodama T, Niwata S, Shimauchi H, Ochiai K. Immunobiological activities of a chemically synthesized lipid A of Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2000;28:273–281. doi: 10.1111/j.1574-695X.2000.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa T, Shimauchi H, Uchida H, Mori Y. Stimulation of splenocytes in C3H/HeJ mice with Porphyromonas gingivalis lipid A in comparison with enterobacterial lipid A. Immunobiology. 1996;196:399–414. doi: 10.1016/S0171-2985(96)80062-3. [DOI] [PubMed] [Google Scholar]
- 27.Pantosti A, Tzianabos A O, Onderdonk A B, Kasper D L. Immunochemical characterization of two surface polysaccharides of Bacteroides fragilis. Infect Immun. 1991;59:2075–2082. doi: 10.1128/iai.59.6.2075-2082.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poltorak A, He X, Smirnova I, Liu M-Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 29.Pugin J, Heumann D, Tomasz A, Kravchenko V V, Akamatsu Y, Nishijima M, Glauser M P, Tobias P S, Ulevitch R J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi S T, Larivi L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savedra R, Jr, Delude R L, Ingalls R R, Fenton M J, Golenbock D T. Mycobacterial lipoarabinomannan recognition requires a receptor that shares components of the signaling system. J Immunol. 1996;157:2549–2554. [PubMed] [Google Scholar]
- 32.Schumann R R, Leong S R, Flaggs G W, Gray P W, Wright S D, Mathison J C, Tobias P S, Ulevitch R J. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 33.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 34.Shah H N, Mayrand D, Genco R, editors. Biology of the species Porphyromonas gingivalis. Boca Raton, Fla: CRC Press, Inc.; 1993. [Google Scholar]
- 35.Shimazu R, Akashi S, Ogata H, Nakagi Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugawara S, Nemoto E, Tada H, Miyake K, Imamura T, Takada H. Proteolysis of human monocyte CD14 by cysteine proteinases (gingipains) from Porphyromonas gingivalis leading to lipopolysaccharide hyporesponsiveness. J Immunol. 2000;165:411–418. doi: 10.4049/jimmunol.165.1.411. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Mühlradt P F, Akira S. Preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a Toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi O, Takeda K, Hoshino K, Adachi O, Ogawa T, Akira S. Cellular responses to bacterial cell wall components are mediated through MyD88-dependent signaling cascades. Int Immunol. 2000;12:113–117. doi: 10.1093/intimm/12.1.113. [DOI] [PubMed] [Google Scholar]
- 40.Tanamoto K, Azumi S, Haishima Y, Kumada H, Umemoto T. The lipid A moiety of Porphyromonas gingivalis lipopolysaccharide specifically mediates the activation of C3H/HeJ mice. J Immunol. 1997;158:4430–4436. [PubMed] [Google Scholar]
- 41.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 42.Underhill D M, Ozinsky A, Hajjar A M, Stevens A, Wilson C B, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 43.Weidemann B, Brade H, Rietschel E T, Dziarski R, Bazil V, Kusumoto S, Flad H-D, Ulmer A J. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun. 1994;62:4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson M. Biological activities of lipopolysaccharide and endotoxin. In: Shah H N, Mayrand D, Genco R J, editors. Biology of the species Porphyromonas gingivalis. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 171–197. [Google Scholar]
- 45.Wright S D. CD14 and innate recognition of bacteria. J Immunol. 1995;155:6–9. [PubMed] [Google Scholar]
- 46.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 47.Yang S, Tamai R, Akashi S, Takeuchi O, Akira S, Sugawara S, Takada H. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect Immun. 2001;69:2045–2053. doi: 10.1128/IAI.69.4.2045-2053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshimura A, Lien E, Ingalls R R, Tuomanen E, Dziarski R, Golenbock D. Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]