Abstract
Enterotoxigenic Escherichia coli (ETEC) is a leading cause of diarrhea in travelers to countries where the disease is endemic and causes a major disease burden in the indigenous population, particularly children. We describe here the generation and preclinical characterization of candidate strains of ETEC which are intended to provide the basis of a live attenuated oral vaccine to prevent this disease. It has been shown previously that a spontaneously arising toxin-negative variant ETEC strain, E1392/75-2A, could confer 75% protection against challenge when administered to volunteers. Unfortunately this strain induced mild diarrhea in 15% of recipients. To eliminate the unacceptable reactogenicity of strain E1392/75-2A, it was further attenuated by introducing three different combinations of defined deletion mutations into the chromosome. A mouse intranasal model of immunization was developed and used to show that all of the strains were immunogenic. Immune responses against colonization factor antigens (CFAs) were particularly strong when the bacterial inocula were grown on “CFA agar,” which induces strong expression of these antigens. Two of the strains were selected for a phase I dose escalation safety study with healthy adult volunteers. Freshly grown organisms were harvested from CFA agar plates and administered to volunteers as a suspension containing from 5 × 107 to 5 × 109 CFU. The vaccine was well tolerated at all doses and induced significant immune responses in all recipients at the highest dose of either strain. The results provide the basis for further clinical evaluation of these vaccine candidates.
Enterotoxigenic Escherichia coli (ETEC) is a common cause of dehydrating diarrhea in developing countries and may be life threatening, particularly in weanling infants. In addition, ETEC is the predominant cause of travelers' diarrhea in adults from the developed world visiting regions where ETEC infection is endemic (29). In developing countries, the incidence of ETEC infections leading to clinical disease decreases with age, indicating that immunity to ETEC infection can be acquired and suggesting that an approach to ETEC vaccination involving a live attenuated vaccine may prove successful. In contrast, adults from industrialized countries who visit areas of endemicity are highly susceptible to ETEC infections.
ETEC diarrhea is caused by colonization of the small intestine by enterotoxigenic strains of E. coli and subsequent elaboration of enterotoxins. Two types of enterotoxins have been identified in ETEC strains. The heat-labile toxin (LT) is highly homologous in structure to the cholera toxin, a multisubunit protein of the form AB5. The A subunit is the active component of the toxin and functions to increase the activity of adenylate cyclase. This is delivered into host cells by the B subunits, which bind to gangliosides on the cell surface. The heat-stable toxin (ST) is a small (19-amino-acid) nonimmunogenic polypeptide that has guanylate cyclase-stimulating activity. In addition, it has been demonstrated recently that a large proportion of ETEC strains also produce EAST1, a heat-stable toxin similar to ST, which was originally identified in enteroaggregative E. coli strains (45). Colonization of the ileum requires fimbrial colonization factor antigens (CFAs), which promote adhesion to the intestinal epithelium. Several CFAs have been identified, the most prevalent being CFA/I, CFA/II, and CFA/IV. CFA/II and CFA/IV consist of more than one fimbrial type, CFA/II being composed of E. coli surface antigens (CS) CS3 and CS1 or CS2, while CFA/IV is composed of CS6 and CS4 or CS5. Evidence indicates that anti-CFA immune responses are important for protection against ETEC disease (8, 32, 37, 39, 40).
It has been proposed that derivatives of ETEC strains which have lost the ability to produce toxins may be effective live vaccines against virulent isolates. A derivative of wild-type ETEC strain E1392/75 that has spontaneously lost the ST and LT activities but that continues to express CFA/II was identified and designated E1392/75-2A (7). In human volunteer studies, oral vaccination with 2 × 1010 CFU of E1392/75-2A gave 75% protection against challenge with a toxin-expressing ETEC that belonged to a different serotype but that expressed the same CFAs (reviewed in reference 39). However, approximately 15% of vaccinees experienced mild diarrhea as a side effect of the vaccine. It was concluded that further attenuation of this strain was required before it could be considered for use as a live vaccine against ETEC infections.
To our knowledge, no studies to date have defined suitable attenuating mutations for reducing the virulence of pathogenic E. coli strains. In contrast, a large number of characterized mutations have been shown to attenuate the virulence of Salmonella strains (4, 9, 13, 16, 18, 20, 22, 27, 44, 47). Most of these have been identified in Salmonella enterica serovar Typhimurium, but mutations in the aro or htrA genes have been demonstrated also to attenuate S. enterica serovar Typhi in human volunteers, enabling such strains to be used as live oral vaccines (17, 38, 41, 42). The aro genes are required in the biosynthesis of aromatic amino acids and the metabolic intermediate chorismate. It is thought that mutations in aro genes are attenuating because the mutants cannot synthesize chorismate, which is required in turn to synthesize folate, the major methyl donor in many biosynthetic reactions. Chorismate is also required for the synthesis of enterobactin, a protein involved in the in vivo acquisition of iron.
HtrA is a heat shock protein involved in the degradation of denatured proteins (36) and is required for growth of E. coli at elevated temperatures (25) and for survival of Salmonella in macrophages (1). Salmonella htrA mutants exhibit decreased survival in macrophage cell lines and are more attenuated for mouse virulence than aro mutants (5). Therefore, deletion of htrA could be attenuating for ETEC.
The ompC and ompF genes code for outer membrane proteins, whose expression is regulated by ompR. It has been shown that S. enterica serovar Typhimurium strains with mutations in ompR are attenuated (13), as are strains with combined mutations in ompC and ompF (4).
This paper describes the partial characterization of toxin loss by ETEC strain E1392/75-2A and the derivation of variants which have, in addition, lost expression of one or both of the CS1 and CS3 genes. It further describes the generation of three derivative strains that have been mutated by specifically deleting from the chromosome the gene combinations aroC and htrA, aroC and ompR, or aroC, ompC, and ompF in order to confer an attenuated phenotype in vivo. Two of these strains have been evaluated in a phase I clinical trial as potential live attenuated vaccines against ETEC diarrhea.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains and plasmids used in this work are listed in Table 1. ETEC strains E1392/75 and E1392/75-2A were first isolated by Cravioto (7) and were obtained from the National Collection of Type Cultures (Public Health Laboratory Service, Central Public Health Laboratory, London, United Kingdom). Strain E1392/75 has an O6:H16 serotype and expresses the CFA/II (CS1 and CS3) colonization factors and ST and LT toxins. Strain E1392/75-2A is an ST and LT toxin-negative derivative of strain E1392/75 that arose spontaneously. Both these ETEC strains carry a plasmid-borne streptomycin resistance marker. Bacteria were cultured routinely in L broth or on L agar and incubated at 37°C. When maximal expression of CFA/II was required ETEC strains were grown on, and harvested from, CFA agar (1% Casamino Acids, 2% Noble agar, 0.15% yeast extract, 0.005% MgSO4, 0.0005% MnCl2). Auxotrophy for aromatic amino acids was confirmed by inoculating duplicate plates of M9 minimal salts (Sigma) agar supplemented with 0.4% glucose. One of each duplicate was further supplemented with Aro mixture (40 μg of phenylalanine/ml, 40 μg of tryptophan/ml, 40 μg of tyrosine/ml, 10 μg of ρ-aminobenzoic acid/ml, and 10 μg of 2,3-dihydroxybenzoic acid/ml). Strain EC012 is TG1 (Stratagene) expressing the CS1 pilin from the cooA gene cloned into the expression vector pKK223-3 (Amersham Pharmacia Biotech). This plasmid, called pKCS1, was constructed by amplifying by PCR the cooA gene using oligonucleotides TT26 and TT27 as primers (Table 2) and cloning the fragment into the EcoRI and HindIII sites of pKK223-3.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Description | Source and/or reference |
|---|---|---|
| Strains | ||
| E1392/75 | ETEC strain expressing CS1, CS3, ST, LT, and streptomycin resistance | NCTCa |
| E1392/75-2A | E1392/75 derivative with LT and ST spontaneously deleted | NCTC |
| TG1 | Laboratory E. coli strain for general DNA manipulations | Stratagene |
| ET011 | E1392/75-2A cured of the plasmid that codes for CS1 | This work |
| EC012 | TG1 expressing CS1 from pKCS1 | This work |
| SY327λpir | Nalrpir; host for maintenance of pCVD442 and derivatives | 28 |
| SM10λpir | Kanrpir mobRP4; host for maintenance and mobilization of pCVD442 and derivatives | 35 |
| PTL001 | E1392/75-2A aroC htrA derivative | This work |
| PTL002 | E1392/75-2A aroC ompR derivative | This work |
| PTL003 | E1392/75-2A aroC ompC ompF derivative | This work |
| Plasmids | ||
| pKK223-3 | Expression vector | Amersham Pharmacia Biotech |
| pKCS1 | CS1 expressed from cooB gene cloned in pKK223-3 | This work |
| pStrep | 6-kb plamid from E1392/75-2A coding for streptomycin resistance | 7; this work |
| pCVD442 | sacB suicide vector that codes for ampicillin resistance and requires pir for replication | 12 |
| pCVDΔaroC | pCVD442 derivative carrying ΔaroC fragment | This work |
| pCVDΔhtrA | pCVD442 derivative carrying ΔhtrA fragment | This work |
| pCVDΔompR | pCVD442 derivative carrying ΔompR fragment | This work |
| pCVDΔompC | pCVD442 derivative carrying ΔompC fragment | This work |
| pCVDΔompF | pCVD442 derivative carrying ΔompF fragment | This work |
NCTC, National Collection of Type Cultures.
TABLE 2.
Oligonucleotides used in this study
| Name | Nucleotide sequencea (5′-) | Comments |
|---|---|---|
| CS3-01 | ATACTTATTAATAGGTCTTT | Detection of CS3; hybridizes to CS3 pilin gene |
| CS3-02 | TTGTCGAAGTAATTGTTATA | Detection of CS3; hybridizes to CS3 pilin gene |
| CSA-01 | TGACTTAGTCAGGATAATTG | Detection of CS1; hybridizes to cooA gene |
| CSA-02 | TGGAGTTTATATGAAACTAA | Detection of CS1; hybridizes to cooA gene |
| EST-01 | CATGTTCCGGAGGTAATATGAA | Detection of STI |
| EST-02 | AGTTCCCTTTATATTATTAATA | Detection of STI |
| LT-04 | CATCGCCATTATATGCAAATGGCG | Detection of LT; hybridizes to eltA gene |
| LT-05 | ACTGATTGCCGCAATTGAATT GGG | Detection of LT; hybridizes to eltB gene |
| RNS-01 | TTTGTAGGTATAAGATGGAC | Detection of rns |
| RNS-02 | AATTGCTTTGGTGTAACACC | Detection of rns |
| TT1 | ATCTGTTTGTTGAGCTCAGCAATCTATTTGCAACC | Construction of ΔompF mutation; includes SacI site |
| TT2 | TTTTTTGCCAGCATGCCGGCAGCCACGCGTAGTG | Construction of ΔompF mutation; includes SphI site |
| TT3 | CTCGAGGCTTAGCTCTATTTATTACCCTCATGG | Construction of ΔompF mutation |
| TT4 | GAGCTAAGCCTCGAGTAATAGCACACCTCTTTG | Construction of ΔompF mutation |
| TT7 | TTGCTGGAAAGTCGACGGATGTTAATTATTTGTG | Construction of ΔompC mutation; includes SalI site |
| TT8 | GGCCAAAGCCGAGCTCATTCACCAGCGGCCCGACG | Construction of ΔompC mutation; includes SacI site |
| TT9 | GCTAAGCCTCGAGTAATCTCGATTGATATCCG | Construction of ΔompC mutation |
| TT10 | CTCGAGGCTTAGCGTTATTAACCCTCTGTTA | Construction of ΔompC mutation |
| TT11 | AACTGGTACCGTCGACGGCACAACGATTTATTG | Construction of ΔhtrA mutation; includes SalI site |
| TT12 | CCCATTCAGTGCATGCGGGAGTATTCTCCT | Construction of ΔhtrA mutation; includes SphI site |
| TT13 | CTCGAGTCTAGACTCCCTCAACCCCTTCCT | Construction of ΔhtrA mutation |
| TT14 | TCTAGACTCGAGAGTATTTCAGTCTCGATTA | Construction of ΔhtrA mutation |
| TT15 | GAGTCACGCCGTCGACGTTTTCAATCTCCACCCA | Construction of ΔompR mutation; includes SalI site |
| TT16 | GCCTATCGCCGAGCTCATAATCGCCAGCGTATAG | Construction of ΔompR mutation; includes SacI site |
| TT17 | GCTAAGCGCATGCGAGGCGATTGCGCTTCTC | Construction of ΔompR mutation |
| TT18 | GCATGCGCTTAGCTGTTTGTACTCCCAAAGGT | Construction of ΔompR mutation |
| TT19 | CCGCGCTCGCTCTAGAGTGAACTGATCAACAATA | Construction of ΔaroC mutation; includes XbaI site |
| TT20 | ATGCGCGCGAGAGCTCAACCAGGGTCGCACTTTG | Construction of ΔaroC mutation; includes SacI site |
| TT21 | CTCGAGGCATGCTGAATAAAACCGCGATTG | Construction of ΔaroC mutation |
| TT22 | GCATGCCCTCGAGGGCTCCGTTATTGTTGTG | Construction of ΔaroC mutation |
| TT26 | AATGGAGAATTCATGAAACTAAAGAAAACA | Construction of pKCS1; hybridizes to cooA of CS1 operon; includes EcoRI site |
| TT27 | TGGCTTAAGCTTTTAGTGATGATGATGATGATGCGT TGACTTAGTCAGGATAATTG | Construction of pKCS1; hybridizes to cooA of CS1 operon; includes HindIII site |
Restriction endonuclease sites incorporated into the oligonucleotides for cloning purposes are in boldface. Regions of the oligonucleotides that do not hybridize to the target DNA are underlined.
DNA manipulations.
DNA manipulations were performed using standard procedures (34). Plasmid DNA was prepared using plasmid purification kits from Qiagen (Crawley, United Kingdom), and DNA fragments were isolated from agarose gels using the QIAquick gel extraction kit from Qiagen.
Southern hybridizations were performed using Hybond N+ membranes and an ECL direct nucleic acid labeling and detection kit or a random prime labeling and detection kit (Amersham Pharmacia Biotech). Primer pairs used to generate probes for the analysis of virulence factor genes were as follows: for LT, oligonucleotides LT-04 and LT-05; for ST, EST-01 and EST-02; for CS1, CSA-01 and CSA-02; for CS3, CS3–01 and CS3–02; for rns, RNS-01 and RNS-02 (Table 2).
Conventional agarose gel electrophoresis was performed as described previously (34). Pulsed-field agarose gel electrophoresis (PFGE) was performed using a Bio-Rad CHEF-DRII system set to 6 V cm−1 for 14 h with an initial switch every 10 s and a final switch every 20 s.
Construction of mutant genes.
Deletion mutations were constructed in such a way as to remove most or all of the open reading frame of the relevant gene. Oligonucleotide primers were designed to amplify fragments of 500 to 600 bp flanking the target open reading frame (Table 1). These two flanking DNA fragments were spliced by overlap extension PCR using oligonucleotide primers incorporating appropriate restriction enzyme sites (Table 2). PCR products were digested with appropriate restriction endonucleases and ligated into suicide vector pCVD442, which requires the product of the pir gene for replication, confers ampicillin resistance, and carries the sacB gene, which allows selection of revertants by growth on sucrose (12). Plasmids carrying these mutant constructs were maintained in E. coli strain SY327λpir (28) or SM10λpir (35).
Introduction of mutations into ETEC strains.
Plasmid constructs were introduced into bacterial cells by electroporation (14) or, for some of the pCVD442 mutant gene constructs, by conjugation from SM10λpir. Transformants were selected on L agar supplemented with 50 μg of ampicillin/ml, and transconjugants were selected on MacConkey agar supplemented with streptomycin at 20 μg/ml and ampicillin at 200 μg/ml. Ampicillin enabled selection of recombinants in which the suicide vector-mutant construct had inserted at the relevant locus by homologous recombination. Selection for loss of the pCVD442 suicide vector backbone from ETEC derivatives was achieved on L agar supplemented with 5% (wt/vol) sucrose, with incubation overnight at 30°C. To distinguish mutants from revertants, these recombinants were screened by PCR amplification using primers TT1 and TT2 for ompF, TT7 and TT8 for ompC, TT15 and TT16 for ompR, TT19 and TT20 for aroC, and TT11 and TT12 for htrA (Table 2). Mutants all gave a 1-kbp product, whereas revertants gave a product of 1.6 kbp and 1 kbp.
Extraction of chromosomal DNA from mutants.
Extraction was performed using a Qiagen genomic DNA extraction kit according to the supplier's instructions but with the following modifications. Mutant cells were harvested from a 5-ml culture grown at 37°C for 16 to 24 h. After addition of buffer B1-proteinase K-lysozyme, the mixture was incubated for 10 min at 37°C before the addition of 10% sodium dodecyl sulfate (SDS) to a concentration of 0.5%. The mixture was then incubated at 55°C for 0.5 to 1.5 h, after which buffer B2 was added and the mixture was incubated for an additional 0.5 h at 55°C. The mixture was centrifuged using a Sorvall RC5C Plus centrifuge and SS34 rotor at 14,000 rpm for 5 min, and the supernatant was applied to a Qiagen genomic DNA extraction column. Eluted DNA was precipitated, rinsed in 70% ethanol, partially dried, and then resuspended in 500 μl of 10 mM Tris-HCl (pH 8)–1 mM Na2-EDTA. Chromosomal DNA was digested to completion with restriction endonuclease EcoRV, separated by PFGE in 1% gels, and hybridized with probes specific for the aroC, htrA, ompC, ompF, and ompR genes (Table 2).
Targeted plasmid curing of ETEC strains.
ETEC strain E1392/75-2A was cured of the CS1-encoding plasmid by cloning a fragment of the CS1 operon into suicide vector pCVD442. The cloned fragment was generated by PCR amplification using CS1-specific oligonucleotides (Table 2) as primers and was cloned initially into the pGEM-T Easy PCR cloning vector (Stratagene). The fragment was subcloned into the pCVD442 suicide vector on a SalI-SphI fragment. This construct was introduced into strain E1392/75-2A by conjugation, and ampicillin- and streptomycin-resistant derivatives were selected. The recombinant plasmid was cured by growing the derivatives in the presence of sucrose as described for construction of the mutants. Ampicillin-sensitive colonies were isolated, and the absence of the CS1 operon was confirmed by PCR using CS1-specific oligonucleotides (Table 2). The resulting strain was designated ET011.
Analysis of LPS using SDS-PAGE.
The lipopolysaccharide (LPS) profiles of the mutant strains were compared to that of the parent strain, E1392/75-2A. Cells were grown overnight on L agar, harvested into water, adjusted to an A600 of 20/cm, mixed with an equal volume of 50 mM Tris-HCl (pH 6.8)–2% (wt/vol) SDS–10% (vol/vol) glycerol–0.25% (wt/vol) bromophenol blue–2% (vol/vol) 2-mercaptoethanol, and boiled for 5 min. Proteinase K was then added to a final concentration of 0.2 mg/ml, and the samples were incubated at 60°C for 1 h prior to loading onto a 12% Tris-glycine SDS-polyacrylamide gel electrophoresis (PAGE) gel. LPS was visualized using a Novex SilverXpress silver stain kit.
Preparation of CFA/II.
Bacterial growth on CFA agar was harvested into phosphate-buffered saline (PBS) and incubated at 65°C for 25 min to release the pili. The suspension was centrifuged for 15 min at 11,500 rpm in a Sorvall SS34 rotor at 4°C. The supernatant was then centrifuged at 18,500 rpm in a Sorvall SS34 rotor at 4°C for 2 h to remove membrane debris. The pili were harvested by centrifugation for 2 h at 43,000 rpm in a Beckman 70.1 Ti rotor at 4°C. The pellet was resuspended in PBS, CsCl was added to 0.5 g/ml, and the resulting solution was centrifuged at 55,000 rpm for 16 to 20 h in a Beckman 70.1 Ti rotor. The CFA/II band was removed using a hypodermic needle and syringe and dialyzed against PBS.
Western blot analysis of CFA expression.
Bacteria grown on CFA agar overnight at 37°C were harvested into PBS and adjusted to an A600 of 20/cm. An equal volume of SDS-PAGE sample buffer supplemented with 2% 2-mercoptoethanol was added, and the samples were incubated for 5 min in a boiling-water bath. Volumes of 3 to 6 μl were electrophoresed through 12% polyacrylamide gels (Novex), and the proteins were then electrotransferred to nitrocellulose membranes. Subsequent treatments were performed at room temperature. Membranes were blocked with 5% (wt/vol) skim milk in PBS–0.05% (vol/vol) Tween 20 (PBST) for 1 h and then transferred to primary rabbit antisera (specific for CS1 or CS3; kind gift from M. Levine, Centre for Vaccine Development, Baltimore, Md.) diluted 1:2,000 in 1% (wt/vol) skim milk–PBST for 1 h. Membranes were washed four times for 10 min in 1% (wt/vol) skim milk–PBST and then incubated in goat anti-rabbit immunoglobulin G (IgG)–horseradish peroxidase (HRP) conjugate (Sigma) diluted 1:2,000 in 1% (wt/vol) skim milk–PBST for 1 h. Membranes were washed four times as described previously and then added to enhanced chemiluminescence (ECL) detection reagents (Amersham) before being exposed to ECL film.
Evaluation of immunogenicity of ETEC strains by intranasal inoculation of mice.
Stocks of live ETEC strains for vaccination studies were prepared from cultures grown overnight at 37°C in L broth. Aliquots (1 ml) of stationary-phase cultures were snap-frozen and stored in liquid N2. On the day of vaccination, 200-μl aliquots of thawed stocks were spread onto CFA agar plates. After 4 h of incubation at 37°C, the bacterial lawn on each plate was harvested into 5 ml of PBS, centrifuged at 2,000 × g for 15 min at 4°C, and resuspended in PBS at a density of 5 × 1010 CFU/ml (as estimated from the optical density of the suspension at 600 nm). To confirm inoculation dose, aliquots of serial dilutions of bacterial suspensions were plated on L agar. CFU were enumerated after 24 h of incubation at 37°C.
BALB/c mice for immunization studies were purchased from Harlan United Kingdom. Experiments were carried out according to the United Kingdom Animals (Scientific Procedures) Act of 1986. For intranasal immunization, mice were anesthetized with halothane and 20-μl aliquots of live ETEC bacterial suspension, prepared as described above (i.e., 109 CFU), were slowly dropped onto the nares using a Gilson Pipetman.
Evaluation of serum and mucosal anti-CFA/II antibody responses in mice.
Groups of BALB/c mice were immunized intranasally, and serum samples taken thereafter were individually titrated by enzyme-linked immunosorbent assay (ELISA) using plates coated with purified CFA/II (2.5 μg/ml in PBS; isolated from strain E1392/75-2A). Specific antibodies were detected with biotinylated anti-mouse IgG or IgA (Sigma) and a streptavidin-HRP conjugate (Dako), used in accordance with the manufacturer's recommendations. Sera from mice immunized with PBS were used to define a cutoff value for determining antibody titers. The highest dilution of a test serum which gave an A492 equal to the mean plus twice the standard deviation of the A492 readings of these sera at a dilution of 1:100 was defined as the end point titer. Mucosal antibody responses in lung lavages were determined as ELISA units (EU) per microgram of total IgA as follows. Specific anti-CFA/II IgA in lung lavages was measured using the same ELISA as that used for serum antibodies. The total content of IgA present in individual lavages was assessed in a sandwich ELISA using plates coated with anti-mouse IgA antibody (Sigma) as described previously (15). A reference mouse serum containing a high titer of anti-CFA/II IgA antibody was used as a standard to determine the number of EU of specific anti-CFA present in each lavage.
Summary protocol for phase I trial.
Strains PTL002 and PTL003 were tested in a phase I, open-label safety and immunogenicity study with healthy adult volunteers in the inpatient unit of the General Clinical Research Center at Johns Hopkins Hospital under Investigational New Drug no. BBIND 7922. According to the protocol, a total of 30 volunteers (15 for each vaccine strain) were to be allocated to sequential groups that received oral doses of 5 × 107 (n = 3), 5 × 108 (n = 6), and 5 × 109 (n = 6) CFU along with a bicarbonate buffer. Volunteers were monitored for side effects, fecal excretion of the vaccine strain, and serological responses to CFA/II. Three volunteers were excluded shortly before the study began. There were two resulting omissions from the 5 × 108-CFU group for PTL-ETEC003 and one from the 5 × 109-CFU group for PTL-ETEC002.
The vaccine was given as a suspension, freshly prepared from a lawn of bacteria that were grown overnight at 37°C on CFA agar. The lawn was harvested with PBS and, based on optical density, the concentrations of bacteria were adjusted to prepare the appropriate dose of vaccine. The volunteers drank 120 ml of sodium bicarbonate solution (1.33% in water) first and then drank the vaccine bacteria in 30 ml of the same buffer, which had been filter sterilized. The actual dose ingested was determined by colony counts of an extra dose of vaccine retained in the laboratory and plated after the dosing of the volunteers was complete.
Excretion of the vaccine strains.
Up to two stool specimens were collected each day after the immunization and were cultured on MacConkey agar and on MacConkey agar with streptomycin. Colonies that grew on the MacConkey agar with streptomycin were presumed to be vaccine strains, and five colonies were spotted onto Luria agar and onto minimal media. Control (wild-type) strains of E. coli grow on both of these agars, but the vaccine strains do not grow on the minimal media. At least one colony of the vaccine strains was saved on nutrient agar slants.
ALS assays.
Heparinized blood specimens were obtained immediately prior to vaccination and on days 7, 10, and 14 after vaccination. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque gradient centrifugation, and the cell concentration was adjusted to 107 per ml in complete RPMI medium. One-milliliter aliquots were cultured in 24-well tissue culture plates for 48 h, and the supernatants were harvested by centrifugation and stored frozen at −20°C until ready for the assay for IgG and IgA antibodies to purified CFA/II antigen. ELISA plates were precoated with CFA/II antigen at 1 μg/ml in PBS, blocked with 1% bovine serum albumin in PBS, and washed. Threefold dilutions of antibody lymphocyte supernatant (ALS) samples were prepared, starting with undiluted supernatant in the first cup. The plates were incubated for 1 h, washed with PBS-Tween 20, and subsequently incubated with HRP-labeled anti-human IgG, washed as described above, and developed with o-phenylenediamine dihydrochloride (Sigma). Titers are expressed as the interpolated dilution that corresponds to an optical density at 492 nm of 0.4 above background.
RESULTS
Association between virulence determinants and plasmids of E1392/75 and E1392/75-2A.
The toxins and virulence factors of ETEC strains are commonly encoded by plasmids. Southern hybridization was used to confirm the absence of the toxin genes from ETEC strain E1392/75-2A and to define the association between the virulence genes and plasmids. Plasmid DNA extracted from the wild-type E1392/75 and the toxin-negative E1392/75-2A ETEC strains was separated by pulsed-field and conventional agarose gel electrophoresis and transferred to Hybond N+ membranes. These were probed with labeled PCR products amplified with oligonucleotide pairs specific for the toxin and CFA/II genes and rns (the transcriptional activator for CFA/II). The results indicated that toxin-expressing strain E1392/75 harbors plasmids of approximately 6, 8, 12, and 15 kb and at least three of 40 to 120 kb. Toxin-negative variant E1392/75-2A harbors plasmids of approximately 6 and 12 kb and two or more of 40 to 120 kb (data not shown). In both strains the 6- to 15-kb plasmids were of higher copy number than the others. The ST-specific probe hybridized with the 8-kb plasmid of E1392/75 that is absent in E1392/75-2A. The CS3-, rns-, and CS1-specific probes hybridized to plasmids of 40 to 120 kb in both strains. The hybridization data also indicated that, relative to that of E1392/75, the CS1 plasmid of E1392/75-2A had undergone a deletion. In each strain the CS3 probe gave the same hybridization pattern as the rns probe, but in the toxin-negative strain this plasmid had undergone a significant deletion. When E1392/75-2A was specifically cured of the CS3 plasmid, the rns locus was lost also, suggesting that these two loci are carried by the same large plasmid. The LT-specific probe also hybridized to a plasmid of 40 to 120 kb in E1392/75 but did not hybridize to any of the plasmids from the toxin-negative strain. The plasmid to which the LT probe hybridized had a mobility that was indistinguishable from that of the CS3-rns plasmid in E1392/75, suggesting that all three of these loci may be linked. A DNA probe for the EAST1 toxin gene hybridized to at least one plasmid of between 40 and 120 kb in E1392/75 but not in the toxin-negative derivative. Both ETEC strains are resistant to streptomycin and sulfathiazole. Transformation of E. coli TG1 cells with plasmid DNA from E1392/75-2A followed by selection on streptomycin indicated that this resistance gene was carried by a plasmid. Examination of plasmid DNA from the streptomycin-resistant TG1 derivatives indicated that the streptomycin resistance was encoded by the 6-kb plasmid. These derivatives were resistant to sulfathiazole, indicating that this resistance determinant is encoded by the 6-kb plasmid also.
Introduction of the deletion mutations into the chromosome of ETEC strain E1392/75-2A.
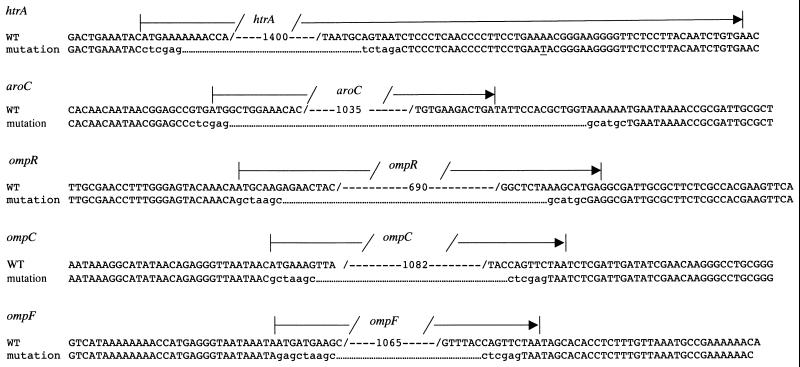
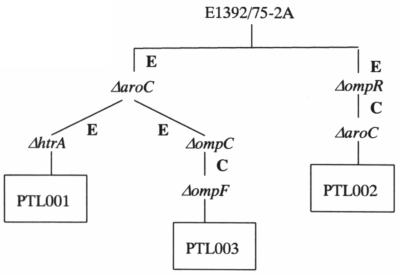
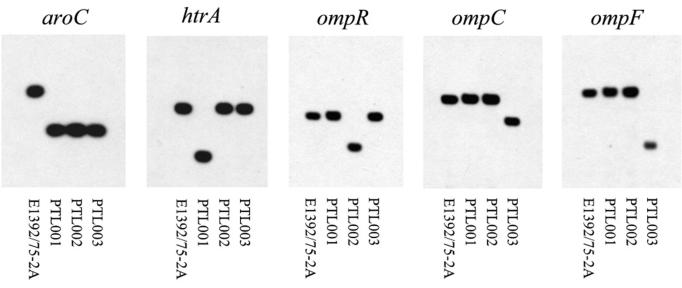
Three ETEC mutants carrying different combinations of chromosomal deletion mutations were constructed. The mutations were ΔaroC ΔhtrA, ΔaroC ΔompR, and ΔaroC ΔompC ΔompF, which were introduced into ETEC strain E1392/75-2A by sequential allelic exchange. The mutations were constructed by overlap extension PCR such that all or most of the open reading frame of the relevant gene was deleted (Fig. 1). The oligonucleotide primers (Table 2) used to amplify these fragments incorporated restriction endonuclease sites to facilitate cloning into suicide vector pCVD442. Each pCVD442 deletion mutation construct was then introduced sequentially into ETEC strain E1392/75-2A by electroporation or conjugation in the order shown in Fig. 2. After isolation of each Ampr recombinant ETEC, sucrose selection was used to identify colonies that had lost the pCVD442 suicide vector backbone through a second recombination step. These Amps derivatives were screened by PCR to identify those in which the target gene had been deleted before introduction of the next pCVD442 deletion construct. The amplified fragments so generated were sequenced to confirm the structure of the recombinant locus (Fig. 1). When all of the mutations had been incorporated, the deletions were checked by Southern hybridizations. For this, chromosomal DNA was extracted and digested with EcoRV before being subjected to PFGE. DNA fragments were probed with a PCR fragment amplified from the wild-type locus. All three mutant strains carried the expected deletion mutations and wild-type alleles (Fig. 3). Hybridization of the membranes with labeled vector pCVD442 confirmed that no vector sequences remained in the chromosome of the mutants.
FIG. 1.
Primary structure of the defined mutations introduced into ETEC strain E1392/75-2A. The upper nucleotide sequence of each alignment represents the wild-type (WT) sequence from E. coli as obtained from GenBank. Numbers within the sequence, numbers of nucleotides omitted; arrows, limits of the open reading frames. The lower nucleotide sequence in each alignment is that resulting from the mutations as confirmed by sequencing reactions; dots were introduced to allow alignment with the wild-type sequence. Nucleotides shown in lowercase were introduced to generate restriction endonuclease sites.
FIG. 2.
Dendrogram indicating the order in which the different mutations were introduced into ETEC strain E1392/75-2A to give PTL001, PTL002, and PTL003. E, introduction by electroporation; C, introduction by conjugation.
FIG. 3.
Southern hybridization confirming the presence of the deletion mutations in PTL001, PTL002, and PTL003. Chromosomal DNA was extracted and digested with restriction endonuclease EcoRV before being subjected to PFGE. DNA fragments were blotted from the gel onto Hybond N+ nylon membranes (Amersham Pharmacia Biotech) and hybridized with labeled fragments from the relevant genes as indicated.
Growth phenotype of PTL001, PTL002, and PTL003.
The dependence of aroC mutants on aromatic amino acids was confirmed by culturing them on M9 minimal salts agar or M9 minimal salts agar supplemented with Aro mixture. After incubation for 48 h at 37°C aroC mutants PTL001, PTL002, and PTL003 showed no significant growth on the M9 minimal salts agar but did grow on M9 minimal salts supplemented with agar Aro mixture. The E1392/75-2A parental strain grew equally well on both media (data not shown).
The HtrA− phenotype of PTL001 was confirmed by comparing growth at elevated temperatures with that of E1392/75-2A. Two aliquots of fresh L broth were inoculated with overnight cultures of each strain, and these were grown to A600 of 0.1, when one of the cultures of each strain was shifted to 45°C. Viable counts were then determined at different time points. As expected, viable counts for both strains continued to increase in the cultures held at 37°C. For the cultures shifted to 45°C the growth of the parental strain was slowed while that of PTL001 was arrested (data not shown).
PTL001, PTL002, and PTL003 possess wild-type LPS.
The LPS of E. coli and Salmonella is vital for persistence of the bacteria in the alimentary tract (6, 24, 44), a trait that is likely to be important for an effective live vaccine. LPS mutants can arise spontaneously during in vitro growth; therefore, the LPS profiles of the mutants were analyzed by SDS-PAGE. There was no significant difference between the mutants and the parental strain (data not shown).
PTL001, PTL002, and PTL003 continue to express both CS1 and CS3 colonization factors.
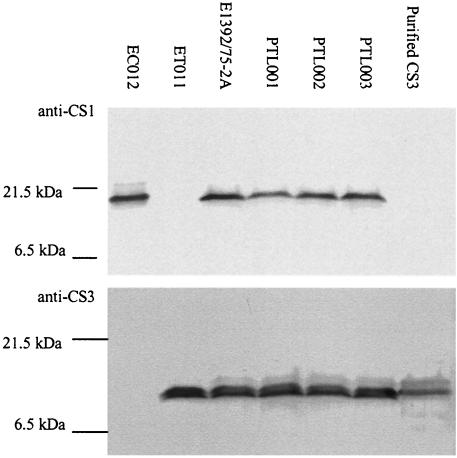
The CFAs are a key component of an ETEC vaccine since these pili mediate adhesion to the epithelial surface and immune responses against them are considered to be protective. It was therefore important to confirm that introduction of the mutations had not affected expression of the CFAs. The three mutants and strains ET011, a CS1− derivative of E1392/75-2A, and EC012, expressing cloned CS1 (Table 1), were grown on CFA agar to optimize CFA/II expression. Western blot analysis of whole-cell lysates with monospecific sera confirmed that the mutants continued to express both CS1 and CS3 (Fig. 4).
FIG. 4.
Western blot to confirm expression of CS1 and CS3 from PTL001, PTL002, and PTL003 following growth on CFA agar. Similar numbers of cells from each strain were processed for SDS-PAGE on a 15% gel. Following electrotransfer, nitrocellulose membranes were probed with anti-CS1- or anti-CS3-specific polyclonal antibodies.
PTL001, PTL002, and PTL003 generate antibody responses against CFA/II in mice when administered via the intranasal route.
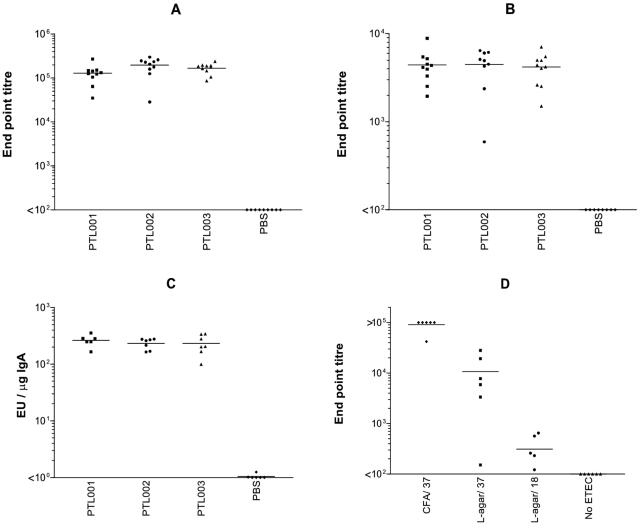
To evaluate the immunogenicity of the attenuated ETEC strains described here, groups of BALB/c mice were immunized intranasally on two occasions with approximately 109 CFU of PTL001, PTL002, or PTL003 or with PBS as a control. Serum samples were obtained following each immunization, and antibody responses were evaluated by ELISA. All three strains induced both IgG and IgA antibody responses against CFA/II (Fig. 5A and B). One intranasal immunization was sufficient to make all animals seroconvert against CFA/II by day 14, and the responses were boosted by the second immunization (data not shown). CFA/II-specific IgA was also detected in lung lavage samples obtained from mice immunized with any of the three mutants (Fig. 5C). No significant difference in the strengths of the anti-CFA/II responses in serum or mucosal secretions elicited by the three different mutants was observed (P > 0.05 in two-way analysis of variance).
FIG. 5.
Serum and mucosal anti-CFA/II antibody responses to PTL001, PTL002, and PTL003 in a mouse intranasal vaccination model. Bars, mean values for each data set. (A to C) Groups of 10 BALB/c mice were vaccinated intranasally on day 0 and day 21 with ca. 109 CFU. Serum samples were taken 21 days later, and lung wash samples were taken on day 35 after the second immunization. Anti-CFA/II antibody levels were determined by ELISA as described in Materials and Methods. (A) Serum anti-CFA/II IgG. (B) Serum anti-CFA/II IgA. (C) Anti-CFA/II IgA from lung lavage samples. (D) Groups of six BALB/c mice were vaccinated intranasally on day 0 with ca. 109 CFU of PTL002 grown on CFA agar or L agar at 37 oC and L agar at 18°C. Serum samples were taken 14 days later, and levels of anti-CFA/II IgG were determined by ELISA.
Bacteria used in the immunization experiments described above were grown on CFA agar formulated to optimize in vitro CFA expression. The effect of the level of CFA/II expression on the titer of the serum anti-CFA/II IgG response was investigated using strain PTL002. Organisms were prepared for vaccination by growth on CFA agar at 37°C, L agar at 37°C, or L agar at 18°C. Serum IgG titers were significantly lower (P < 0.05 by Student's t test) if bacteria were grown in conditions that are suboptimal for CFA/II expression (L agar at 37°C) or those that prevent expression (incubation at 18°C) (Fig. 5D). There was a low but significant anti-CFA/II response in the latter group, which may represent a response to antigen expression induced after administration to the mice. Mucosal anti-CFA/II IgA responses were detected only in the lungs of mice immunized with PTL002 grown on CFA agar (data not shown).
Vaccine strains PTL002 and PTL003 are safe in human volunteers.
The two vaccine candidates were administered orally to healthy volunteers in doses ranging from 5 × 107 to 5 × 109 CFU. The symptoms experienced by each recipient are detailed in Table 3. No serious adverse events were experienced, and no volunteers showed any sign of elevated temperature, though some experienced moderate gastrointestinal symptoms. In the two cases where vomiting occurred, it did not restrict the activities of the volunteers, and they were both eager to eat their next meal. Overall, strain PTL003 was better tolerated than PTL002, with adverse reactions seen only in two of six recipients of the highest dose. There was one case of diarrhea in recipients of the highest dose of both strains.
TABLE 3.
Clinical symptoms in volunteers receiving vaccinea
| Volunteer | Strain | Dose (CFU) | Diarrhea vol (ml) | Vomiting | Cramps |
|---|---|---|---|---|---|
| 1 | PTL002 | 5.7 × 107 | — | — | — |
| 2 | PTL002 | 5.7 × 107 | — | — | — |
| 3 | PTL002 | 5.7 × 107 | — | — | — |
| 4 | PTL002 | 1.4 × 108 | — | — | — |
| 5 | PTL002 | 1.4 × 108 | — | — | — |
| 6 | PTL002 | 1.4 × 108 | — | YESb | MODc |
| 7 | PTL002 | 1.4 × 108 | — | — | — |
| 8 | PTL002 | 1.4 × 108 | — | — | — |
| 9 | PTL002 | 1.4 × 108 | — | — | — |
| 10 | PTL002 | 4.9 × 109 | — | — | MOD |
| 11 | PTL002 | 4.9 × 109 | — | — | MOD |
| 12 | PTL002 | 4.9 × 109 | 545 | YESb | — |
| 13 | PTL002 | 4.9 × 109 | — | — | — |
| 14 | PTL002 | 4.9 × 109 | — | — | — |
| 15 | PTL003 | 6.8 × 107 | — | — | — |
| 16 | PTL003 | 6.8 × 107 | — | — | — |
| 17 | PTL003 | 6.8 × 107 | — | — | — |
| 18 | PTL003 | 3.7 × 108 | — | — | — |
| 19 | PTL003 | 3.7 × 108 | — | — | — |
| 20 | PTL003 | 3.7 × 108 | — | — | — |
| 21 | PTL003 | 3.7 × 108 | — | — | — |
| 22 | PTL003 | 4.7 × 109 | 396 | — | MILD |
| 23 | PTL003 | 4.7 × 109 | — | — | — |
| 24 | PTL003 | 4.7 × 109 | — | — | — |
| 25 | PTL003 | 4.7 × 109 | — | — | — |
| 26 | PTL003 | 4.7 × 109 | — | — | — |
| 27 | PTL003 | 4.7 × 109 | — | — | MILD |
—, no diarrhea or absence of symptom.
Unspecified severity.
MOD, moderate.
The duration of secretion of the vaccine strain in the stools of volunteers was dose related. The vaccine strain was recovered from the stools of all six volunteers receiving a dose of approximately 5 × 107 CFU at some time. It was recovered the same day as vaccination from two volunteers and continued to be excreted for up to 4 days from two volunteers. Eight of nine volunteers who received a dose of approximately 2 × 108 CFU excreted the vaccine strain at some time; two volunteers continued to excrete for up to 4 days. All 11 volunteers who received a dose of approximately 5 × 109 CFU continued to excrete the vaccine strain for 4 days, compared to only 4 of 16 who received lower doses (P < 0.0001; Fisher's exact test). There was no difference in the frequencies or durations of excretion of the two vaccine strains when given at comparable doses.
PTL002 and PTL003 are immunogenic in human volunteers.
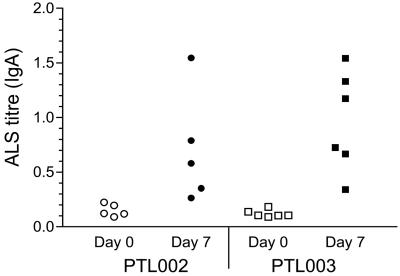
ALS assays were performed with PBMCs isolated from the blood of volunteers immediately prior to and after vaccination. The frequency and magnitude of immune responses were dose dependent although, as the primary outcome of this phase I study was a safety evaluation, the numbers of volunteers involved do not allow for statistically significant conclusions on the immunogenicities of the different dose levels of the two strains to be drawn (data not shown). All volunteers receiving the highest dose of either strain produced a significant increase over baseline in IgA anti-CFA/II reactivity in ALSs (Fig. 6).
FIG. 6.
Anti-CFA/II IgA response of PMNCs from vaccinated human volunteers. Blood samples were taken from human volunteers prior to and 7 days after oral vaccination with ca. 5 × 109 CFU of strain PTL002 or PTL003. PBMCs were isolated and cultured as described in Materials and Methods, and ALSs were assayed for anti-CFA/II IgA using ELISAs.
DISCUSSION
Toxin-negative ETEC strain E1392/75-2A has been shown previously to be significantly less virulent when administered to human volunteers than the parental strain, E1392/75, which expresses both ST and LT. We have confirmed that the toxin genes of spontaneously derived LT- and ST-negative ETEC strain E1392/75-2A have been deleted rather than inactivated by mutation. Analysis of the plasmids using PFGE confirmed that the parent strain, E1392/75, harbors at least seven plasmids. Southern hybridization indicated that in the E1392/75-2A derivative the toxin genes were deleted, the ST gene through complete loss of an 8-kb plasmid. In addition, ETEC strain E1392/75 carries at least one copy of the EAST1 toxin gene on one of the large, low-copy-number plasmids. This toxin gene also is absent from the toxin-negative derivative. The data also indicated that the CS1 and CS3 factors were encoded by separate plasmids, with the transcriptional activator of CS expression, rns, residing on the plasmid encoding CS3. This may explain why CS1 is normally found expressed only in the presence of CS3.
The LT- and ST-negative strain E1392/75-2A induced mild diarrhea in 2 of 12 volunteers, indicating that further attenuation was required before it could be considered for use as a vaccine. The factors responsible for this residual virulence have not been identified. Baumler and coworkers (2) have observed that intragastric injection of S. enterica serovar Typhimurium caused fluid accumulation in infant mice, while mutants lacking expression of pef fimbriae did not. One possibility is that the colonization factors expressed by ETEC allow close and prolonged interaction with epithelial cells and that this alone can cause diarrhea in some individuals. As the CFAs themselves are the target antigens for the induction of anticolonization immunity, they must be retained in the vaccine organisms.
Candidate mutations that have been shown to attenuate other intestinal pathogens such as Salmonella, namely, those of the aro and omp gene families and htrA, were evaluated for their ability to attenuate ETEC strains such as E1319/75-2A. The infections caused by ETEC and Salmonella are generally different. While ETEC is localized to the alimentary tract, Salmonella is often highly invasive and in many cases can produce systemic as well as enteric infections. Mutations in aro, htrA, and omp genes of Salmonella have been shown to be attenuating for systemic disease, but less information regarding the effect on enteric disease is available. However, an aroA mutation did reduce the severity of S. enterica serovar Typhimurium-induced diarrhea in calves (43). Trimethoprim and sulfamethoxazole are effective in the treatment of ETEC infections. These antibiotics interfere with hydrofolate metabolism (46) and in this respect act similarly to aro mutations, which confer folate auxotrophy. We may therefore expect aro mutations to attenuate ETEC in addition to Salmonella infections.
The htrA gene was identified as being required for heat tolerance in E. coli and for Salmonella virulence. HtrA is a stress response protein involved in the degradation of misfolded proteins in the periplasm (21) and is required for survival of Salmonella in macrophages and for full virulence of Salmonella (1, 5). Mutations in clpB, whose product is involved with disaggregation of denatured proteins (11), reduced colonization by S. enterica serovar Typhimurium in the alimentary tracts of chickens (44). Unlike Salmonella, ETEC is primarily noninvasive, and normally infections are localized to the alimentary tract. However, the activity of ClpB and HtrA in removal of denatured proteins and the observation that ClpB is involved in colonization of the chicken alimentary tract suggest that HtrA may be of relevance for ETEC infections also.
Expression of outer membrane proteins OmpC and OmpF is regulated by EnvZ-OmpR two-component signal transduction proteins. High osmolarity represses OmpF, while OmpC, which has a narrower pore, is derepressed (30). It has been shown that S. enterica serovar Typhimurium ompR mutants and ompC ompF double mutants are attenuated (4, 13). OmpR mutants do not express either OmpC or OmpF and so may be expected to function equivalently. However, OmpR is a response regulator affecting the expression of many genes (23) and as such may have been overattenuating; therefore, we constructed the ompC ompF double mutant and evaluated the two in parallel.
Following construction, the mutants were characterized by PCR and by Southern hybridization to confirm that the deletions in the chromosome were as expected. Their phenotypes were confirmed by biochemical and microbiological methods. It has been shown that the LPS of a number of pathogens is required for colonization of their respective hosts (3, 6, 10, 19, 24, 26, 44). A viable live vaccine candidate must therefore express wild-type LPS in addition to the CFAs. Since there is no selective pressure against LPS-defective mutants during growth in vitro, we confirmed that the LPS of PTL001, PTL002, and PTL003 was identical to that of the parent strain, E1392/75-2A, using SDS-PAGE. All three mutants were shown to express CS1 and CS3 by Western blotting of PAGE-separated whole-cell lysates (Fig. 4). All three mutants had deletions in aroC and, as expected, grew on minimal medium only in the presence of Aro mixture (not shown).
Following these genotypic and phenotypic checks we wanted to confirm that the mutants would be immunogenic at the mucosal surface. There is no animal model of infection for human-adapted strains of ETEC, such as E1392/75-2A. The same is true for other human-adapted pathogens, such as S. enterica serovar Typhi. However, immunization of mice via the intranasal route has been used to evaluate the immunogenicity of these bacteria (31). The three candidate ETEC vaccine strains were therefore evaluated in a mouse intranasal vaccination model. This demonstrated that the mutants were all equally immunogenic at the lung mucosal surface, inducing anti-CFA/II serum IgG and IgA responses and sIgA in the lungs. These immune responses to CFA/II were correlated with the level of CFA/II expression by the vaccine strain. They were improved by growth of the inoculum (PTL002) on CFA agar which has been formulated to optimize fimbrial expression. The same growth media were therefore selected to prepare the inocula for the human volunteer study.
Mutant strains PTL002 (ΔaroC ΔompR) and PTL003 (ΔaroC ΔompC ΔompF) were selected for evaluation in human volunteers, since it was hypothesized that aro and omp mutations were probably more likely to attenuate survival of ETEC in the alimentary tract. The results from our first phase I clinical study with human volunteers confirmed that PTL002 (aroC ompR) and PTL003 (aroC ompC ompF) are well tolerated in humans given a single oral dose of up to 5 × 109 CFU. Some mild side effects were noted, but, in the absence of a placebo group, it is not possible to attribute these to the ingestion of vaccine organisms. Indeed, in many similar phase I and II studies these mild side effects were seen to be a result of the ingestion of bicarbonate solution (33). Since evidence indicates that anti-CFA immune responses are important for protection against ETEC (8, 32, 37, 39, 40), secretion of CFA/II-specific IgA from PBMCs isolated from these volunteers was measured. This indicated that significant immune responses were generated in all of the volunteers immunized with the highest dose (5 × 109 CFU) of both PTL002 and PTL003. This assay correlates well with the mucosal antibody response in the intestinal tract (D. Sack, unpublished data) and is readily performed with samples obtained by a minimally invasive method suitable for such pilot studies.
The preliminary clinical data presented here demonstrate that a vaccine based on live attenuated strains of ETEC may prove to be both safe and effective. Additional clinical evaluation of strains PTL002 and PTL003 is under way to determine the ability of these candidate vaccine strains to protect against a challenge by wild-type ETEC. If the results of these studies are positive, then the same mutations will be incorporated into additional strains to create a final multistrain vaccine able to confer protection against a wide range of ETEC strains expressing different CFAs.
ACKNOWLEDGMENTS
We thank M. Levine for the generous gift of CS1- and CS3-specific rabbit sera and S. Chatfield for advice in the early stages of the project.
REFERENCES
- 1.Baumler A J, Kusters J G, Stojiljkovic I, Heffron F. Salmonella typhimurium loci involved in survival within macrophages. Infect Immun. 1994;62:1623–1630. doi: 10.1128/iai.62.5.1623-1630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumler A J, Tsolis R M, Bowe F A, Kusters J G, Hoffmann S, Heffron F. The pef fimbrial operon of Salmonella typhimurium mediates adhesion to murine small intestine and is necessary for fluid accumulation in the infant mouse. Infect Immun. 1996;64:61–68. doi: 10.1128/iai.64.1.61-68.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camprubi S, Merino S, Guillot J F, Tomas J M. The role of the O-antigen lipopolysaccharide on the colonization in vivo of the germfree chicken gut by Klebsiella pneumoniae. Microb Pathog. 1993;14:433–440. doi: 10.1006/mpat.1993.1042. [DOI] [PubMed] [Google Scholar]
- 4.Chatfield S N, Dorman C J, Hayward C, Dougan G. Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both ompC and ompF are attenuated in vivo. Infect Immun. 1991;59:449–452. doi: 10.1128/iai.59.1.449-452.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatfield S N, Strahan K, Pickard D, Charles I G, Hormaeche C E, Dougan G. Evaluation of Salmonella typhimurium strains harbouring defined mutations in htrA and aroA in the murine salmonellosis model. Microb Pathog. 1992;12:145–151. doi: 10.1016/0882-4010(92)90117-7. [DOI] [PubMed] [Google Scholar]
- 6.Cohen P S, Arruda J C, Williams T J, Laux D C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985;48:139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cravioto A. Ph.D thesis. London, United Kingdom: University of London; 1980. [Google Scholar]
- 8.Cravioto A, Reyes R E, Trujillo F, Uribe F, Navarro A, De La Roca J M, Hernandez J M, Perez G, Vazquez V. Risk of diarrhea during the first year of life associated with initial and subsequent colonization by specific enteropathogens. Am J Epidemiol. 1990;131:886–904. doi: 10.1093/oxfordjournals.aje.a115579. [DOI] [PubMed] [Google Scholar]
- 9.Curtiss R, III, Kelly S M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987;55:3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dekkers L C, van der Bij A J, Mulders I H, Phoelich C C, Wentwoord R A, Glandorf D C, Wijffelman C A, Lugtenberg B J. Role of the O-antigen of lipopolysaccharide, and possible roles of growth rate and of NADH:ubiquinone oxidoreductase (nuo) in competitive tomato root-tip colonization by Pseudomonas fluorescens WCS365. Mol Plant-Microbe Interact. 1998;11:763–771. doi: 10.1094/MPMI.1998.11.8.763. [DOI] [PubMed] [Google Scholar]
- 11.Diamant S, Ben-Zvi A P, Bukau B, Goloubinoff P. Size-dependent disaggregation of stable protein aggregates by the DnaK chaperone machinery. J Biol Chem. 2000;275:21107–21113. doi: 10.1074/jbc.M001293200. [DOI] [PubMed] [Google Scholar]
- 12.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorman C J, Chatfield S, Higgins C F, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elson C O, Ealding W, Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J Immunol Methods. 1984;67:101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- 16.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D G. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohmann E L, Oletta C A, Killeen K P, Miller S I. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis. 1996;173:1408–1414. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 18.Hoiseth S K, Stocker B A. Aromatic-dependent Salmonella typhimurium are nonvirulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 19.Jackson A D, Maskell D, Moxon E R, Wilson R. The effect of mutations in genes required for lipopolysaccharide synthesis on Haemophilus influenzae type b colonization of human nasopharyngeal tissue. Microb Pathog. 1996;21:463–470. doi: 10.1006/mpat.1996.0076. [DOI] [PubMed] [Google Scholar]
- 20.Johnson K, Charles I, Dougan G, Pickard D, O'Gaora P, Costa G, Ali T, Miller I, Hormaeche C. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991;5:401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim K I, Park S C, Kang S H, Cheong G W, Chung C H. Selective degradation of unfolded proteins by the self-compartmentalizing HtrA protease, a periplasmic heat shock protein in Escherichia coli. J Mol Biol. 1999;294:1363–1374. doi: 10.1006/jmbi.1999.3320. [DOI] [PubMed] [Google Scholar]
- 22.Klose K E, Mekalanos J J. Simultaneous prevention of glutamine synthesis and high-affinity transport attenuates Salmonella typhimurium virulence. Infect Immun. 1997;65:587–596. doi: 10.1128/iai.65.2.587-596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee A K, Detweiler C S, Falkow S. OmpR regulates the two-component system SsrA-SsrB in Salmonella pathogenicity island 2. J Bacteriol. 2000;182:771–781. doi: 10.1128/jb.182.3.771-781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Licht T R, Krogfelt K A, Cohen P S, Poulsen L K, Urbance J, Molin S. Role of lipopolysaccharide in colonization of the mouse intestine by Salmonella typhimurium studied by in situ hybridization. Infect Immun. 1996;64:3811–3817. doi: 10.1128/iai.64.9.3811-3817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipinska B, Fayet O, Baird L, Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989;171:1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merino S, Rubires X, Aguillar A, Guillot J F, Tomas J M. The role of the O-antigen lipopolysaccharide on the colonization in vivo of the germfree chicken gut by Aeromonas hydrophila serogroup O:34. Microb Pathog. 1996;20:325–333. doi: 10.1006/mpat.1996.0031. [DOI] [PubMed] [Google Scholar]
- 27.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller V L, Mekalanos J J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci USA. 1984;81:3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. . (Erratum, 11:403.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikaido H. Outer membrane. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. [Google Scholar]
- 31.Pickett T E, Pasetti M F, Galen J E, Sztein M B, Levine M M. In vivo characterization of the murine intranasal model for assessing the immunogenicity of attenuated Salmonella enterica serovar Typhi strains as live mucosal vaccines and as live vectors. Infect Immun. 2000;68:205–213. doi: 10.1128/iai.68.1.205-213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rudin A, Olbe L, Svennerholm A M. Monoclonal antibodies against fimbrial subunits of colonization factor antigen I (CFA/I) inhibit binding to human enterocytes and protect against enterotoxigenic Escherichia coli expressing heterologous colonization factors. Microb Pathog. 1996;21:35–45. doi: 10.1006/mpat.1996.0040. [DOI] [PubMed] [Google Scholar]
- 33.Sack D A, Sack R B, Shimko J, Gomes G, O'Sullivan D, Metcalfe K, Spriggs D. Evaluation of Peru-15, a new live oral vaccine for cholera, in volunteers. J Infect Dis. 1997;176:201–205. doi: 10.1086/514025. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Simon R, Priefer U, Puhler A. A broad host range mobilisation system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 36.Spiess C, Beil A, Ehrmann M. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell. 1999;97:339–347. doi: 10.1016/s0092-8674(00)80743-6. [DOI] [PubMed] [Google Scholar]
- 37.Svennerholm A M, Wenneras C, Holmgren J, McConnell M M, Rowe B. Roles of different coli surface antigens of colonization factor antigen II in colonization by and protective immunogenicity of enterotoxigenic Escherichia coli in rabbits. Infect Immun. 1990;58:341–346. doi: 10.1128/iai.58.2.341-346.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tacket C O, Hone D M, Curtiss III R, Kelly S M, Losonsky G, Guers L, Harris A M, Edelman R, Levine M M. Comparison of the safety and immunogenicity of ΔaroC ΔaroD and Δcya Δcrp Salmonella typhi strains in adult volunteers. Infect Immun. 1992;60:536–541. doi: 10.1128/iai.60.2.536-541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tacket C O, Levine M M. Vaccines against enterotoxigenic Escherichia coli infections. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc; 1997. pp. 875–883. [Google Scholar]
- 40.Tacket C O, Reid R H, Boedeker E C, Losonsky G, Nataro J P, Bhagat H, Edelman R. Enteral immunization and challenge of volunteers given enterotoxigenic E. coli CFA/II encapsulated in biodegradable microspheres. Vaccine. 1994;12:1270–1274. doi: 10.1016/s0264-410x(94)80038-2. [DOI] [PubMed] [Google Scholar]
- 41.Tacket C O, Sztein M B, Losonsky G A, Wasserman S S, Nataro J P, Edelman R, Pickard D, Dougan G, Chatfield S N, Levine M M. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tacket C O, Sztein M B, Wasserman S S, Losonsky G, Kotloff K L, Wyant T L, Nataro J P, Edelman R, Perry J, Bedford P, Brown D, Chatfield S, Dougan G, Levine M M. Phase 2 clinical trial of attenuated Salmonella enterica serovar Typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect Immun. 2000;68:1196–1201. doi: 10.1128/iai.68.3.1196-1201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsolis R M, Adams L G, Ficht T A, Baumler A J. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect Immun. 1999;67:4879–4885. doi: 10.1128/iai.67.9.4879-4885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner A K, Lovell M A, Hulme S D, Zhang-Barber L, Barrow P A. Identification of Salmonella typhimurium genes required for colonization of the chicken alimentary tract and for virulence in newly hatched chicks. Infect Immun. 1998;66:2099–2106. doi: 10.1128/iai.66.5.2099-2106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto T, Echeverria P. Detection of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coli strains pathogenic for humans. Infect Immun. 1996;64:1441–1445. doi: 10.1128/iai.64.4.1441-1445.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao J D C, Moellering R C., Jr . Antibacterial agents. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 7th ed. Washington, D.C.: American Society for Microbiology; 1999. pp. 1474–1504. [Google Scholar]
- 47.Zhang-Barber L, Turner A K, Dougan G, Barrow P A. Protection of chickens against experimental fowl typhoid using a nuoG mutant of Salmonella serotype Gallinarum. Vaccine. 1998;16:899–903. doi: 10.1016/s0264-410x(97)00300-9. [DOI] [PubMed] [Google Scholar]