Abstract
In this study, Xiangsu hybrid pig growth traits were evaluated via PRKAA2 and MSMB as candidate genes. Sanger sequencing revealed three mutation sites in PRKAA2, namely, g.42101G>T, g.60146A>T, and g.61455G>A, and all these sites were intronic mutations. Moreover, six mutation sites were identified in MSMB: intronic g.4374G>T, exonic g.4564T>C, exonic g.6378G>A, exonic g.6386C>T, intronic g.8643G>A, and intronic g.8857A>G. Association analysis revealed that g.42101G>T, g.60146A>T, g.61455G>A, g.4374G>T, g.4564T>C, g.6378G>A, g.6386C>T, g.8643G>A, and g.8857A>G showed different relationship patterns among body weight, body length, body height, chest circumference, abdominal circumference, tube circumference, and chest depth. Real-time polymerase chain reaction results revealed that the expression of PRKAA2 was highest in the longissimus dorsi muscle, followed by that in the heart, kidney, liver, lung, and spleen. The expression of MSMB was highest in the spleen, followed by that in the liver, kidney, lung, heart, and longissimus dorsi muscle. These results suggest that PRKAA2 and MSMB can be used in marker-assisted selection to improve growth related traits in Xiangsu hybrid pigs, providing new candidate genes for Pig molecular breeding.
Keywords: PRKAA2, MSMB, SNPs, real-time PCR, Xiangsu hybrid pigs, growth traits
1. Introduction
The Xiangsu hybrid pig is a cross between the Guizhou Congjiang Xiang pig and Jiangsu Sutai pig of China. The Xiang Pig, a small pig breed unique to the Guizhou Province in China, is characterized by early sexual maturity, better disease resistance, and strong adaptability than other local breeds [1,2,3]. It is highly homozygous in its genome composition and highly similar to the human genome. It is considered an ideal animal model for toxicology and pharmacology research [4,5,6,7,8]. The Congjiang Xiang pigs have many advantages, but they grow slowly. Therefore, in the early stages of research, Congjiang Xiang pigs and Sutai pigs were crossed, and their offspring were backcrossed. The blood relationship of Congjiang Xiang pigs accounted for 87.5% in the offspring. In this sudy, PRKAA2 and MSMB genes were used as candidate genes for growth traits, and the relationships among different genotypes in these two candidate genes and growth traits at six months of age were analyzed by Sanger sequencing to screen for superior genes or superior growth trait genotypes.
PRKAA2 (adenosine monophosphate-activated protein kinase (AMPK)) is a member of the AMPK family. They are heterotrimeric proteins that mainly detect the state of mammalian cells, regulate the new biosynthesis of fatty acids and cholesterol, and play a key role in cell energy metabolism [9,10,11]. A previous study found that PRKAA2 is primarily expressed and distributed in the heart, skeletal muscle, liver, and neuronal tissue cells of animals [12,13,14]. In clinical research, PRKAA2 has been shown to be associated with susceptibility to type 2 diabetes [15,16], In addition, the high expression of PRKAA2 may predict the poor prognosis of head and neck squamous cell carcinoma [17], colorectal cancer [18], and endometrial cancer [19]. In animal research, the bovine PRKAA2 was located on chromosome 3 using somatic cell hybrid cell panel [20]. The study on PRKAA2 in Pakistani Nili-Ravi and Kundi buffaloes revealed 17 single nucleotide polymorphism (SNP) loci. These SNPs may be related to energy metabolism and production traits [21]. PRKAA2 was also studied in Qinchuan cattle, Nanyang cattle, Jiaxian cattle, and yak cattle. The phylogenetic analysis revealed that Qinchuan cattle and Jiaxian cattle are closely related, followed by Nanyang cattle, and yak cattle has the largest relationship difference, which provided evolutionary information for the genetic background of different cattle breeds in China [22]. However, few studies have evaluated PRKAA2 in pigs.
MSMB (microseminoproteinp-β) is a disulfide rich low molecular weight protein synthesized by prostaglandin epithelial cells. This protein inhibits the growth of prostate tissue via the autocrine and paracrine modes. MSMB has been reported to have a relatively low sequence identity in vertebrates but exhibit strong conservation [23,24,25]. In clinical studies, MSMB was mainly associated with prostate cancer treatment and other aspects [26,27]. In animals, it has been mainly associated with reproduction [28,29,30] and not linked to animal growth, particularly in pigs. MSMB was selected as a candidate gene for evaluating growth traits in this study. Thus, PRKAA2 and MSMB were selected as candidate genes for evaluating growth traits to identify dominant genes or dominant genotypes in Xiangsu hybrid pigs.
2. Materials and Methods
2.1. Ethical Approval
All experiments in this study were performed in accordance with the guidelines of the Animal Welfare Committee of Guizhou University (EAE-GZU-2021-P003, 9 May 2021).
2.2. Bioinformatics Analyses
The amino acid sequences of PRKAA2 and MSMB were obtained from NCBI (https://www.ncbi.nlm.nih.gov/protein accessed on 6 November 2021) for Sus scrofa (NP_999431.1; NP_999017.1), Bos taurus (NP_001192534.1; XP_024842536.1), Bubalus bubalis (XP_045022084.1; XP_006065751.3), Capra hircus (XP_017900141.1; XP_013831148.2), Ovis arie (NP_001106287.1; XP_042096743.1), Equus caballus (NP_001075410.1; XP_014589601.1), Equus asinus (XP_044626814.1; XP_044615142.1), Gallus gallus (NP_001034694.1; XP_004942175.3), Anser cygnoides (XP_047918049.1; XP_013033273.1), and Oryctolagus cuniculus (XP_008263418.1; XP_008268062.2). MEGA 7 (https://www.megasoftware.net/ accessed on 12 October 2021) was used for sequence alignment and phylogenetic tree construction. The structural features and functions of PRKAA2 and MSMB proteins in these 10 species were revealed using MEME (https://meme.nbcr.net/ accessed on 12 October 2021) suite software.
2.3. Animal Weight and Body Size Data and Tissue Collection
A total of 164 Xiangsu hybrid pigs for this study were provided by the Xiang pig breeding farm of Guizhou University. Their blood samples were collected, and their growth traits (body weight, body length, body height, chest circumference, abdominal circumference, tube circumference, chest depth, chest width, and leg and hip circumference) at the age of 6 months were measured for correlation analysis. Three 6-month-old Xiangsu hybrid pigs were sacrificed, and six tissue samples of the heart, liver, spleen, lung, kidney, and longissimus dorsi muscle were collected for subsequent experiments.
2.4. DNA and RNA Extraction and cDNA Synthesis
DNA was extracted from the 164 pig blood samples according to the instructions of Omega blood genomic DNA extraction kit (D3392-01, GA, USA). Total RNA was extracted from six tissue samples using Tiangen RNA extraction kit (DP424, Beijing, China). cDNA was then synthesized using Genstar reverse transcription kit (A212-02, Beijing, China). The concentration and purity of DNA, RNA, and cDNA were measured via Thermo Nanodrop 2000 ultramicro spectrophotometer (NanoDrop 2000, CA, USA) and then stored at −20 °C.
2.5. Primer Design
Using the NCBI data of porcine PRKAA2 (NC_010448.4) and MSMB (NC_010456.5) (https://www.ncbi.nlm.nih.gov/ accessed on 11 September 2020 ) as a reference, Premier 5.0 software was used to design relevant primers (Table 1 and Table 2). The primers were manufactured by Tsingke Biotechnology Co., Ltd. (Beijing, China).
Table 1.
Primers for porcine PRKAA2.
| Primer Name | Primer Sequence (5′-3′) | Amplified Fragment Size (bp) | Annealing Temperature (°C) | Application |
|---|---|---|---|---|
| PRKAA2-1 | F: TTAGACTGACCTTCGAGCAAGGTCG | 973 | 61 | Exon 1 |
| R: TCACAGAGCAGGGCACTGAAGTC | ||||
| PRKAA2-2 | F: ATTTGTTCTTCAATAATGTATGACT | 859 | 61 | Exon 2 |
| R: AAAAATCCTGATATGCTAACTTGAA | ||||
| PRKAA2-3 | F: GCTGGTTGTCTTCATCTTGGTATCA | 581 | 55 | Exon 3 |
| R: ATTACTGCAGAAGCAACCCCAACTT | ||||
| PRKAA2-4 | F: CCAGGGTTTGAATTGGATCTATAGC | 421 | 63 | Exon 4 |
| R: GCAGCTAGTATCCTTCTAAACACCA | ||||
| PRKAA2-5 | F: CAGCATATGGAAGTTCCCAGGCT | 777 | 65 | Exon 5 |
| R: TTTGATCACTGGCCTGAGGCAGT | ||||
| PRKAA2-6 | F: TCAGGTATTGCCGTAGGGCTAGTTA | 812 | 65 | Exon 6 |
| R: GCCTAACATAGATCAGGCATTCAG | ||||
| PRKAA2-7 | F: GCCACATACCCAAGTCTGAATAATC | 823 | 59 | Exon 7 |
| R: GCCAGAAGCATCTAGACCACTAAAT | ||||
| PRKAA2-8 | F: ACAGTACCTGTTACTGTGCCAGGTT | 533 | 63 | Exon 8 |
| R: CTTCTCAGAGTTCATGCCTGCGT | ||||
| PRKAA2-9 | F: TAGTGATGTCTGTTACGATTGAGGG | 667 | 63 | Exon 9 |
| R: CACCTAGTAAAGACACCGCCTATGT | ||||
| PRKAA2 | F: GCCCAGTTACTTATTTCCT | 189 | 60 | Real-time PCR |
| R: TTCATTATTCTCCGATTGTC | ||||
| GAPDH | F: TTGTGATGGGCGTGAACC | 169 | 58 | Reference gene |
| R: GTCTTCTGGGTGGCAGTGAT |
Note: F stands for upstream primer and R stands for downstream primer.
Table 2.
Primers for porcine MSMB.
| Primer Name | Primer Sequence (5′-3′) | Amplified Fragment Size (bp) | Annealing Temperature (°C) | Application |
|---|---|---|---|---|
| MSMB-1 | F: GATGCACATGCCTGAAAGGACTC | 668 | 55 | Exon 1 |
| R: GAGTGATGGTTCCCAATTTGCTGA | ||||
| MSMB-2 | F: GTCCAACAGATCCATATCAGCCTA | 858 | 59 | Exon 2 |
| R: CTGTCCCAACCCTTTCTCTCATATA | ||||
| MSMB-3 | F: ATCACCCTAAATGCCCCTGACTCA | 778 | 57 | Exon 3 |
| R: TGAAAGAGGCATAGCTGTCCTTAG | ||||
| MSMB-4 | F: CCTACCTGGAGTGACTGACACATA | 676 | 61 | Exon 4 |
| R: AGTTAGAGGCCTAGGGAATGAGG | ||||
| MSMB | F: AAAGAAGGACCCAGGAAAG | 145 | 60 | Real-time PCR |
| R: CAATCATAGACAGTTAGAGGC | ||||
| GAPDH | F: TTGTGATGGGCGTGAACC | 169 | 58 | Reference gene |
| R: GTCTTCTGGGTGGCAGTGAT |
Note: F stands for upstream primer and R stands for downstream primer.
2.6. Polymerase Chain Reaction (PCR)
The PCR reaction mixture (30 μL) contained the following components: 15 μL 2 × Taq PCR Starmix (GeneStar, Beijing, China), 10.5 μL ddH2O, 1.5 μL DNA template (40 ng/μL), and 1.5 μL each of the forward and reverse primers. The PCR amplification procedure was as follows: 94 °C for 5 min; 35 cycles of denaturation at 94 °C for 10 s, gradient annealing of 55–65 °C (55 °C, 57 °C, 59 °C, 61 °C and 63 °C) for 20 s, and extension temperature for 30 s; and finally holding at 72 °C for 5 min. The PCR products were subjected to agarose gel electrophoresis for 35 min. A gel imaging system (Universal Hood II, Bio–Rad, USA) was used to identify the bright bands and determine whether the length of the target fragment was consistent.
2.7. Real-Time PCR
The Real-time PCR reaction mixture (10 μL) included 5 μL SsoFastTM EvaGreen® Supermix (Bio-Rad, CA, USA), 0.5 μL cDNA template, 0.5 μL each of the primers, and 3.5 μL ddH2O. The quantitative real-time PCR amplification procedure was as follows: predenaturation at 95 °C for 30 s, 39 cycles of denaturation at 95 °C for 5 s, gradient annealing from 55 °C to 65 °C for 5 s, and extension for 30 s, and final extended at 95 °C for 5 s.
2.8. Data Analysis
Microsoft Excel was used to calculate the gene frequency, genotype frequency, genetic homogeneity, genetic heterogeneity, effective allele number, and polymorphic information content (PIC). The χ2 test and p values were used to determine whether the allele frequency of the mutant group complied with the Hardy–Weinberg equilibrium. SPSS 25 software was used to perform correlation analyses for the body weight, body length, body height, chest circumference, abdominal circumference, tube circumference, chest depth, chest width, and leg hip circumference of the different genotypes, and the results are expressed as the mean ± standard deviation.
The 2−∆∆Ct method was used to calculate the results of real-time PCR. The test data were analyzed using one-way analysis of variance in SPSS 25 and visualized using GraphPad Prism 8 (version 8, Harvey Motulsky, CA, USA).
3. Results
3.1. Bioinformatic Analysis of PRKAA2 and MSMB
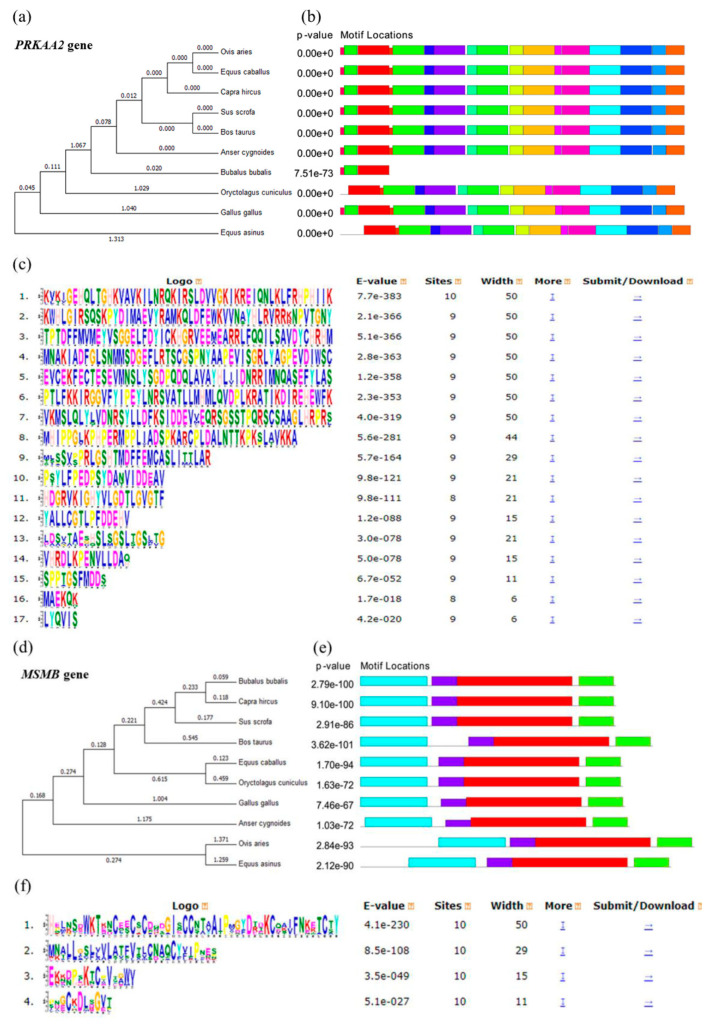
The multiple sequence alignments of PRKAA2 and MSMB proteins were performed with ten mammalian or domestic animals viz: pig (S. scrofa), cattle (B. taurus), buffalo (B. bubalis), goat (C. hircus), sheep (O. aries), horse (E. caballus), donkey (E. asinus), chicken (G. gallus), goose (A. cygnoides), and rabbit (O. cuniculus). As per the PRKAA2 evolutionary tree, sheep and horses are closely related and pigs and cattle are closely related (Figure 1a). A total of 17 significant motifs were found in the 10 species (Figure 1b,c). According to the MSMB evolutionary tree, buffalo and goat are closely related and horse and rabbit are closely related (Figure 1d). Four significant motifs were found in the 10 species (Figure 1e,f).
Figure 1.
Bioinformatics analysis results of PRKAA2 and MSMB. (a–c) are PRKAA2; (d–f) are MSMB. (a,d) Phylogenetic tree for the 10 species. (b,e) Motif structural analysis for the 10 species. (c,f) Significant motifs of PRKAA2 and MSMB across the 10 species. The different colored letters signify the abbreviation of different amino acids.
3.2. Detection of SNP Mutation Sites in PRKAA2 and MSMB
3.2.1. PRKAA2 SNP Mutation Site Detection
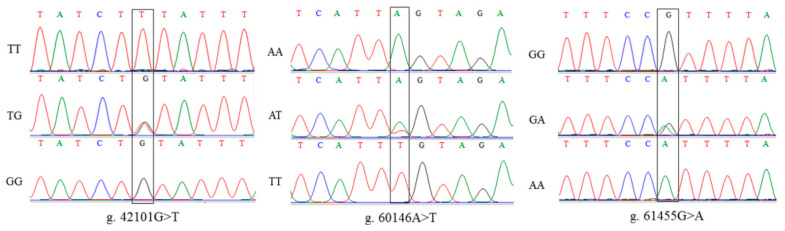
The porcine PRKAA2 is located on chromosome 6 and contains nine exon regions. Sanger sequencing showed three mutation sites in PRKAA2, including intronic g.42101G>T, intronic g.60146A>T, and intronic g.61455G>A (Figure 2).
Figure 2.
Sequencing results of three SNPs in PRKAA2.
3.2.2. MSMB SNP Mutation Site Detection
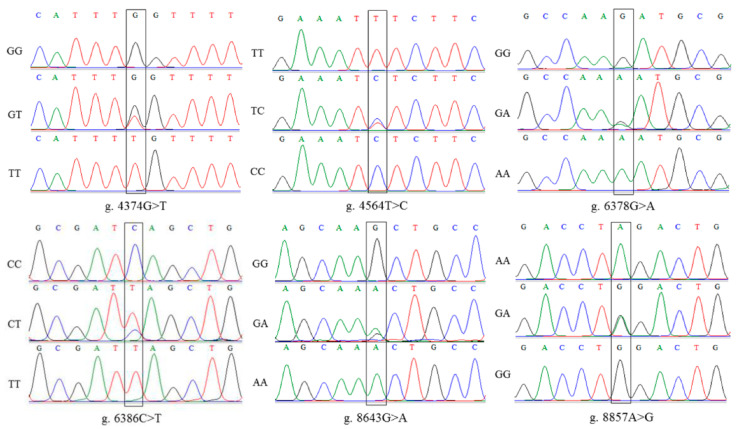
The porcine MSMB is located on chromosome 14 and include four exon regions. Sanger sequencing revealed nine mutation sites in MSMB, including intronic g.4374G>T, exonic g.4564T>C, exonic g.6378G>A, exonic g.6386C>T, intronic g.8643G>A, and intronic g.8857A>G (Figure 3).
Figure 3.
Sequencing results of six SNPs in MSMB.
3.3. Genetic Diversity Analysis of PRKAA2 and MSMB Mutation Groups
3.3.1. Genetic Diversity Analysis of PRKAA2 Mutant Groups
Table 3 shows the genotype frequency and genetic parameters of the three mutant positions of the porcine PRKAA2. Their PIC was 0.374, 0.365, and 0.356 (all 0.25 < PIC < 0.5), indicating moderate polymorphism. The χ2 results showed that the three sites were in Hardy–Weinberg equilibrium.
Table 3.
Genetic diversity analysis of PRKAA2 mutant groups.
| SNPs | Genotypic Frequencies | Allelic Frequency |
He | Ho | Ne | PIC | χ2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| g.42101G>T | GG | GT | TT | G | T | 0.498 | 0.502 | 1.991 | 0.374 | 1.833 |
| 0.244 (40) | 0.445 (73) | 0.311 (51) | 0.466 | 0.534 | ||||||
| g.60146A>T | AA | AT | TT | A | T | 0.480 | 0.520 | 1.922 | 0.365 | 2.482 |
| 0.390 (64) | 0.421 (69) | 0.189 (31) | 0.601 | 0.399 | ||||||
| g.61455G>A | GG | GA | AA | G | A | 0.499 | 0.501 | 1.996 | 0.375 | 0.649 |
| 0.213 (35) | 0.530 (87) | 0.256 (42) | 0.479 | 0.521 | ||||||
Note: HE, heterogeneity; HO, pureness; NE, effective allele; PIC, polymorphic information content; PIC < 0.25 implies low polymorphism; 0.5 > PIC > 0.25 denotes moderate polymorphism; PIC > 0.5 suggests high polymorphism; χ2 < 5.99 denotes Hardy-Weinberg equilibrium; χ2 > 5.99 signifies Hardy-Weinberg disequilibrium.
3.3.2. Genetic Diversity Analysis of MSMB Mutant Groups
Table 4 shows the genotype frequency and genetic parameters of the nine mutant positions of the porcine MSMB. According to the sequencing results, in the g.6378G>A site and g.6386C>T site, the mutant individuals and number of mutations were the same. PIC for all sites were in the range of 0.25 < PIC < 0.5, suggesting moderate polymorphism. The χ2 results showed that the six sites were in Hardy–Weinberg equilibrium.
Table 4.
Genetic diversity analysis of MSMB mutant groups.
| SNPs | Genotypic Frequencies | Allelic Frequency |
He | Ho | Ne | PIC | χ2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| g.4374G>T | GG | GT | TT | G | T | 0.485 | 0.515 | 1.943 | 0.368 | 3.488 |
| 0.378 (62) | 0.415 (68) | 0.207 (34) | 0.585 | 0.415 | ||||||
| g.4564T>C | TT | TC | CC | T | C | 0.477 | 0.523 | 1.913 | 0.363 | 0.285 |
| 0.378 (62) | 0.457 (75) | 0.165 (27) | 0.607 | 0.393 | ||||||
| g.6378G>A | GG | GA | AA | G | A | 0.499 | 0.502 | 1.994 | 0.374 | 0.014 |
| 0.226 (37) | 0.494 (81) | 0.280 (46) | 0.473 | 0.527 | ||||||
| g.6386C>T | CC | CT | TT | C | T | 0.499 | 0.502 | 1.994 | 0.374 | 0.014 |
| 0.226 (37) | 0.494 (81) | 0.280 (46) | 0.473 | 0.527 | ||||||
| g.8643G>A | GG | GA | AA | G | A | 0.491 | 0.509 | 1.965 | 0.371 | 0.518 |
| 0.201 (33) | 0.463 (76) | 0.335 (55) | 0.433 | 0.567 | ||||||
| g.8857A>G | AA | AG | GG | A | G | 0.483 | 0.517 | 1.935 | 0.337 | 0.588 |
| 0.152 (25) | 0.512 (84) | 0.335 (55) | 0.409 | 0.591 | ||||||
Note: HE, heterogeneity; HO, pureness; NE, effective allele; PIC, polymorphic information content; PIC < 0.25 implies low polymorphism; 0.5 > PIC > 0.25 denotes moderate polymorphism; PIC > 0.5 suggests high polymorphism; χ2 < 5.99 denotes Hardy-Weinberg equilibrium; χ2 > 5.99 signifies Hardy-Weinberg disequilibrium.
3.4. Correlation Analysis of Genotypes and Growth Traits
To screen the relationship between the SNPs and growth traits of Xiangsu hybrid pigs, SPSS software was used to analyze the association between PRKAA2 and MSMB mutation sites and nine traits (Table 5 and Table 6). In PRKAA2, the g.42101G>T locus was associated with body weight, body length, chest circumference, abdominal circumference, and tube circumference. the g.60146A>T locus was associated with chest depth; and the g.61455G>A locus was associated with body weight and chest depth. In MSMB, the g.4374G>T locus was associated with body weight, body height, and chest depth; the g.4564T>C locus was associated with body height and chest depth; the g.6378G>A and g.6386C>T loci were associated with chest depth; and the g.8643G>A and g.8857A>G loci were associated with body height and abdominal circumference.
Table 5.
Association analysis of three SNPs in PRKAA2 and growth trait.
| SNPs | Genotype | W/kg | B S/cm | B H/cm | C C/cm | A C/cm | T C/cm | C D/cm | C W/cm | L H C/cm |
|---|---|---|---|---|---|---|---|---|---|---|
| g.42101G>T | GG | 89.09 ± 3.99A | 108.80 ± 4.91A | 74.78 ± 4.16 | 109.83 ± 3.66A | 119.20 ± 4.09A | 19.78 ± 1.31A | 39.38 ± 2.65 | 30.03 ± 2.13 | 71.40 ± 2.04 |
| GT | 91.42 ± 4.35B | 110.01 ± 4.59 | 75.21 ± 3.98 | 110.37 ± 3.31a | 119.66 ± 3.75a | 20.21 ± 1.18 | 40.14 ± 2.78 | 30.22 ± 1.95 | 71.51 ± 2.01 | |
| TT | 92.03 ± 4.81B | 111.39 ± 4.42B | 76.12 ± 3.81 | 111.96 ± 3.86bB | 121.35 ± 3.92bB | 20.59 ± 1.20B | 40.51 ± 3.09 | 30.00 ± 1.84 | 71.69 ± 2.04 | |
| g.60146A>T | AA | 91.67 ± 4.42 | 110.42 ± 4.38 | 75.88 ± 3.82 | 111.06 ± 3.46 | 119.86 ± 4.09 | 20.36 ± 1.17 | 40.41 ± 2.64a | 29.94 ± 1.75 | 71.95 ± 1.68 |
| AT | 90.37 ± 4.75 | 110.29 ± 4.83 | 75.33 ± 4.06 | 110.45 ± 4.00 | 120.16 ± 3.86 | 20.16 ± 1.39 | 40.20 ± 3.10 | 30.10 ± 2.16 | 71.30 ± 2.16 | |
| TT | 91.24 ± 4.19 | 109.26 ± 5.05 | 74.48 ± 4.10 | 110.68 ± 3.24 | 120.32 ± 4.04 | 20.06 ± 1.06 | 39.06 ± 2.62b | 30.45 ± 1.89 | 71.19 ± 2.24 | |
| g.61455G>A | GG | 90.86 ± 4.21 | 109.97 ± 4.79 | 76.03 ± 3.78 | 111.09 ± 3.25 | 120.71 ± 3.62 | 20.43 ± 1.04 | 41.00 ± 2.77a | 29.91 ± 2.02 | 71.37 ± 1.94 |
| GA | 90.40 ± 4.65a | 109.84 ± 4.78 | 75.33 ± 4.03 | 110.69 ± 4.00 | 120.02 ± 4.09 | 20.05 ± 1.31 | 39.64 ± 3.01b | 30.11 ± 1.92 | 71.59 ± 2.19 | |
| AA | 92.52 ± 4.32b | 110.93 ± 4.44 | 74.95 ± 4.09 | 110.52 ± 3.24 | 119.64 ± 4.01 | 20.40 ± 1.25 | 40.17 ± 2.47 | 30.24 ± 2.01 | 71.57 ± 1.74 |
Note: W: Weight/kg, B S: Body straight/cm, B H: Body height/cm, C C: Chest circumference/cm, A C: Abdominal circumference/cm, T C: Tube circumference/cm, C D: Chest depth/cm, C W: Chest width/cm, L H C: Leg and hip circumference/cm. a, b indicate significant differences between different genotypes (p < 0.05); A, B indicate extremely significant differences between different genotypes (p < 0.01).
Table 6.
Association analysis of six SNPs in MSMB and growth traits.
| SNPs | Genotype | W/kg | B S/cm | B H/cm | C C/cm | A C/cm | T C/cm | C D/cm | C W/cm | L H C/cm |
|---|---|---|---|---|---|---|---|---|---|---|
| g.4374G>T | GG | 91.41 ± 4.76a | 110.05 ± 5.01 | 74.02 ± 3.94aA | 110.48 ± 3.61 | 120.60 ± 3.95 | 20.05 ± 1.26 | 39.19 ± 2.95aA | 30.34 ± 2.13 | 71.21 ± 2.16 |
| GT | 91.59 ± 4.21a | 110.31 ± 4.31 | 76.29 ± 3.80B | 110.71 ± 3.57 | 119.41 ± 4.15 | 20.35 ± 1.26 | 40.62 ± 2.65B | 29.84 ± 1.84 | 71.72 ± 1.91 | |
| TT | 89.27 ± 4.43b | 110.00 ± 4.96 | 76.06 ± 3.82b | 111.24 ± 3.93 | 120.44 ± 3.49 | 20.26 ± 1.21 | 40.56 ± 2.81b | 30.21 ± 1.84 | 71.76 ± 1.95 | |
| g.4564T>C | TT | 91.41 ± 4.76 | 110.05 ± 5.01 | 74.02 ± 3.94aA | 110.48 ± 3.61 | 120.60 ± 3.95 | 20.05 ± 1.26 | 39.19 ± 2.95aA | 30.34 ± 2.13 | 71.21 ± 2.16 |
| TC | 91.31 ± 4.35 | 110.15 ± 4.32 | 76.29 ± 3.80B | 110.72 ± 3.45 | 119.47 ± 4.05 | 20.39 ± 1.24 | 40.57 ± 2.61B | 29.93 ± 1.80 | 71.76 ± 1.90 | |
| CC | 89.44 ± 4.32 | 110.37 ± 5.12 | 76.00 ± 3.82b | 111.33 ± 4.29 | 120.56 ± 3.62 | 20.15 ± 1.23 | 40.67 ± 2.95b | 30.04 ± 1.97 | 71.67 ± 1.98 | |
| g.6378G>A | GG | 90.48 ± 4.61 | 110.38 ± 4.95 | 75.11 ± 4.38 | 110.65 ± 3.80 | 120.11 ± 3.56 | 20.19 ± 1.27 | 39.19 ± 3.15a | 30.16 ± 2.02 | 71.76 ± 2.50 |
| GA | 91.62 ± 4.32 | 110.01 ± 4.58 | 75.48 ± 3.98 | 110.88 ± 3.62 | 120.28 ± 4.40 | 20.28 ± 1.25 | 40.40 ± 2.84b | 30.04 ± 1.93 | 71.54 ± 1.91 | |
| AA | 90.48 ± 4.81 | 110.20 ± 4.78 | 75.43 ± 3.73 | 110.54 ± 3.65 | 119.67 ± 3.49 | 20.13 ± 1.26 | 40.20 ± 2.55 | 30.17 ± 1.99 | 71.35 ± 1.79 | |
| g.6386C>T | CC | 90.48 ± 4.61 | 110.38 ± 4.95 | 75.11 ± 4.38 | 110.65 ± 3.80 | 120.11 ± 3.56 | 20.19 ± 1.27 | 39.19 ± 3.15a | 30.16 ± 2.02 | 71.76 ± 2.50 |
| CT | 91.62 ± 4.32 | 110.01 ± 4.58 | 75.48 ± 3.98 | 110.88 ± 3.62 | 120.28 ± 4.40 | 20.28 ± 1.25 | 40.40 ± 2.84b | 30.04 ± 1.93 | 71.54 ± 1.91 | |
| TT | 90.48 ± 4.81 | 110.20 ± 4.78 | 75.43 ± 3.73 | 110.54 ± 3.65 | 119.67 ± 3.49 | 20.13 ± 1.26 | 40.20 ± 2.55 | 30.17 ± 1.99 | 71.35 ± 1.79 | |
| g.8643G>A | GG | 90.22 ± 4.08 | 109.76 ± 4.66 | 74.97 ± 3.80 | 111.03 ± 4.07 | 121.18 ± 4.19a | 20.00 ± 1.27 | 40.39 ± 2.89 | 30.12 ± 2.07 | 71.48 ± 2.35 |
| GA | 90.75 ± 4.77 | 109.87 ± 4.32 | 74.84 ± 4.14a | 110.20 ± 3.64 | 119.33 ± 4.22b | 20.29 ± 1.27 | 39.89 ± 3.04 | 30.22 ± 2.00 | 71.63 ± 2.05 | |
| AA | 91.94 ± 4.40 | 110.76 ± 5.21 | 76.38 ± 3.73b | 111.29 ± 3.34 | 120.44 ± 3.26 | 20.25 ± 1.21 | 40.11 ± 2.61 | 29.93 ± 1.84 | 71.44 ± 1.79 | |
| g.8857A>G | AA | 90.58 ± 4.21 | 109.68 ± 4.63 | 75.28 ± 3.74 | 111.36 ± 4.20 | 121.52 ± 4.23a | 20.08 ± 1.22 | 40.84 ± 2.88 | 30.16 ± 1.84 | 72.04 ± 1.90 |
| AG | 90.59 ± 4.68 | 109.88 ± 4.36 | 74.76 ± 4.12a | 110.18 ± 3.63 | 119.40 ± 4.19b | 20.24 ± 1.30 | 39.81 ± 3.00 | 30.20 ± 2.10 | 71.45 ± 2.19 | |
| GG | 91.94 ± 4.40 | 110.76 ± 5.21 | 76.38 ± 3.73b | 111.29 ± 3.34 | 120.44 ± 3.26 | 20.25 ± 1.21 | 40.11 ± 2.61 | 29.93 ± 1.84 | 71.44 ± 1.79 |
Note: W: Weight/kg, B S: Body straight/cm, B H: Body height/cm, C C: Chest circumference/cm, A C: Abdominal circumference/cm, T C: Tube circumference/cm, C D: Chest depth/cm, C W: Chest width/cm, L H C: Leg and hip circumference/cm. a, b indicate significant differences between different genotypes (p < 0.05); A, B indicate extremely significant differences between different genotypes (p < 0.01).
3.5. Expression of PRKAA2 and MSMB in Different Tissues
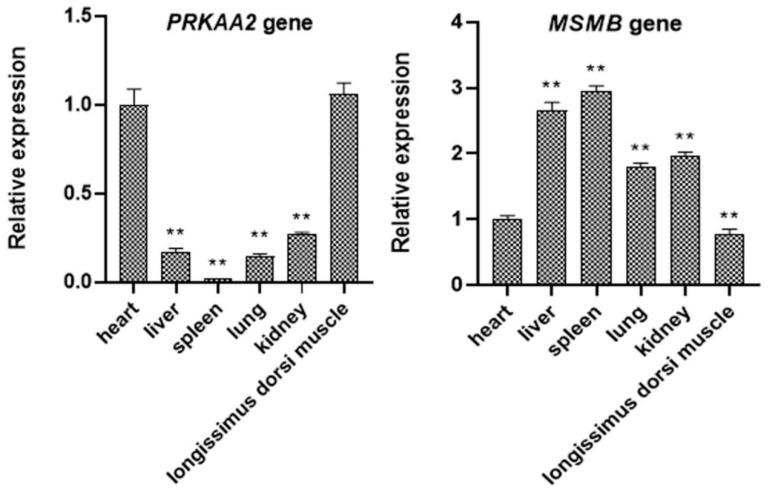
The real-time PCR results are presented using the heart as the reference tissue (Figure 4). In the expression of PRKAA2 in various tissues, there was a significant difference between the heart and liver, spleen, lung, and kidney (p < 0.01) but no significant difference between the heart and longissimus dorsi muscle (p > 0.05). The specific expression level from high to low was as follows: longissimus dorsi muscle > heart > kidney > liver > lung > spleen. In the expression of MSMB in various tissues, there was a significant difference between the heart and liver, spleen, lung, kidney, and longissimus dorsi muscle (p < 0.01). The specific expression level from high to low was as follows: spleen>liver>kidney>lung>heart>longissimus dorsi muscle.
Figure 4.
Expression of PRKAA2 and MSMB in pig tissues. Note: The heart was used as a reference for all tissues. ** implies very significant difference (p < 0.01), and no * signifies no significant difference (p > 0.05).
4. Discussion
In the present study, intronic mutations were detected in PRKAA2 and MSMB. Although an intron is a protein noncoding sequence, studies have found that intron variation can affect the expression and transcription of eukaryotic genes, RNA stability, processing of transcripts, efficiency of mRNA transport and translation from the nucleus to cytoplasm, and detection and elimination of mRNA errors in the transcription process [31,32,33,34]. Therefore, intronic mutations may also affect animal growth traits and be used as gene markers.
A total of three SNPs were identified in the PRKAA2 of the Xiangsu hybrid pig population. Correlation analysis showed that the g.42101G>T locus was correlated with body weight, body length, chest circumference, abdominal circumference, and tube circumference (p < 0.05, p < 0.01); the g.60146A>T locus was correlated with chest depth (p < 0.01); and the g.61455G>A locus was associated with body weight and chest depth (p < 0.01). Therefore, g.42101G>T and g.61455G>A may be used as candidate sites for body weight and g.60146A>T and g.61455G>A may be used as candidate sites for chest depth. However, correlation between PRKAA2 and Hu sheep and Dorper sheep growth revealed a mutation site at 32832382 (namely 32832382G>A locus), and association analysis with growth traits showed that individuals with the AA genotype had significantly higher body weight, body length, chest circumference, and gun circumference than those with the GG and GA genotypes (p < 0.05) [35]. In another study on the correlation between PRKAA2 and the meat quality traits of commercial pigs, it was found that the statistical score of the physical location of the PRKAA2 locus was not significant, thereby not necessitating any follow-up analysis [36]. Real-time PCR results showed that the expression of PRKAA2 was highest in the longissimus dorsi muscle, followed by the heart, kidney, liver, lung, and spleen. The results of PRKAA2 expression in sheep tissues showed that PRKAA2 was expressed in the heart, liver, spleen, lung, kidney, rumen, duodenum, muscle, lymph, and tail fat, with the highest expression in the spleen, followed by the kidney, duodenum, and muscle [35]. These results suggest that the expression pattern of PRKAA2 is different in different species and is species-specific.
Regarding MSMB, six SNPs were detected in the Xiangsu hybrid pig population. The analysis results showed that the g.4374G>T locus was correlated with body weight, body height, and chest depth (p < 0.05, p < 0.01); the g.4564T>C locus was correlated with body height and chest depth (p < 0.05, p < 0.01), the g.6378G>A and g.6386C>T loci were correlated with chest depth (p < 0.01), and the g.8643G>A and g.8857A>G loci were correlated with body height and abdominal circumference (p < 0.01). Therefore, g.4374G>T, g.4564T>C, g.8643G>A, and g.8857A>G may be used as candidate sites for body height, whereas g.4374G>T, g.4564T>C, g.6378G>A, and g.6386C>T may be used as candidate sites for body height. In addition, g.8643G>A and g.8857A>G may be used as candidate sites for abdominal circumference. The T allele of rs10993994 is a potential pathogenic variant of 10q11 that increases the risk of prostate cancer [37,38] and is an important marker for prostate cancer therapeutic targets [39]. Real-time PCR results revealed that the expression of MSMB in the spleen was the highest, followed by the liver, kidney, lung, heart, and longissimus dorsi muscle. The MSMB gene contains one pseudogene and four functional genes in Callithrix jacchus. Real-time RT-PCR analysis of RNA samples from the prostate, seminal vesicles, and testes of marmosets showed transcripts for MSMB1, MSMB2, MSMB3, and MSMB4, four functional genes, present in the testes and parotoid gonads, and levels in the prostate were approximately 100-fold higher than those in the seminal vesicles and 10,000-fold higher than those in the testes [25]. At present, there is no report on the expression of MSMB in animal tissues.
5. Conclusions
In the present study, Sanger sequencing was used to identify three mutation sites in PRKAA2 and six mutation sites in MSMB. The late correlation analysis with growth traits revealed that PRKAA2 was closely related to body weight, body length, chest circumference, abdominal circumference, tube circumference, and chest depth and MSMB was closely related to body weight, body height, abdominal circumference, and chest depth. These results suggest that PRKAA2 and MSMB SNPs may be used as genetic markers for breeding Xiangsu hybrid pigs with desirable growth-related traits, which will provide new candidate genes for the molecular breeding of pigs in the future. The real-time PCR results of PRKAA2 expression from high to low were: longissimus dorsi>heart>kidney>liver>lung>spleen, whereas those of MSMB expression from high to low were: spleen>liver>kidney>lung>heart>longissimus dorsi muscle.
Author Contributions
Funding acquisition, supervision, H.X.; writing—original draft, J.X.; data curation, methodology, Y.R.; writing—review and editing, J.S. and P.S.; investigation, J.H., L.D., and M.X. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The research protocols and animals used in this study strictly comply with the guidelines of the Animal Welfare Committee of Guizhou University (EAE-GZU-2021-P003, 9 May 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Guizhou Provincial Science and Technology Project (QKHFQ-2018,4007, (002)), and the Guizhou Provincial Agricultural Major Industrial Scientific Research Project (QKHKYZ-2019,011).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tang L.T., Ran X.Q., Mao N., Zhang F.P., Niu X., Ruan Y.Q., Yi F.L., Li S., Wang J.F. Analysis of alternative splicing events by RNA sequencing in the ovaries of Xiang pig at estrous and diestrous. Theriogenology. 2018;119:60–68. doi: 10.1016/j.theriogenology.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Liu C., Ran X., Wang J., Li S., Liu J. Detection of genomic structural variations in Guizhou indigenous pigs and the comparison with other breeds. PLoS ONE. 2018;13:194282. doi: 10.1371/journal.pone.0194282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie J., Li R., Li S., Ran X., Wang J., Jiang J., Zhao P. Identification of Copy Number Variations in Xiang and Kele Pigs. PLoS ONE. 2016;11:148565. doi: 10.1371/journal.pone.0148565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bode G., Clausing P., Gervais F., Loegsted J., Luft J., Nogues V., Sims J. The utility of the minipig as an animal model in regulatory toxicology. J. Pharmacol. Toxicol. Methods. 2010;62:196–220. doi: 10.1016/j.vascn.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Luo Z.Y., Dai X.L., Ran X.Q., Cen Y.X., Niu X., Huang S.H., Wang J.F. Identification and profile of microRNAs in Xiang pig testes in four different ages detected by Solexa sequencing. Theriogenology. 2017;117:61–71. doi: 10.1016/j.theriogenology.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Xiao L., Estelle J., Kiilerich P., Ramayo-Caldas Y., Xia Z., Feng Q., Liang S., Pedersen A.O., Kjeldsen N.J., Liu C., et al. A reference gene catalogue of the pig gut microbiome. Nat. Microbiol. 2016;1:16161. doi: 10.1038/nmicrobiol.2016.161. [DOI] [PubMed] [Google Scholar]
- 7.Walters E.M., Wolf E., Whyte J.J., Mao J., Renner S., Nagashima H., Kobayashi E., Zhao J., Wells K.D., Critser J.K. Completion of the swine genome will simplify the production of swine as a large animal biomedical model. BMC Med. Genom. 2012;5:55. doi: 10.1186/1755-8794-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin-Qin J., Biao W., Zhi-Qiang D., Yong R., Yan H., Meng-Qiu H., Shan-Shan D., Yu-Jie T. Expression of IFN-lambda1 from Congjiang pigs and its effect on anti-PRRSV proliferation. Pol. J. Vet. Sci. 2020;23:423–430. doi: 10.24425/pjvs.2020.134687. [DOI] [PubMed] [Google Scholar]
- 9.Hardie D.G., Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur. J. Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.H., Koh H., Kim M., Kim Y., Lee S.Y., Karess R.E., Lee S.H., Shong M., Kim J.M., Kim J., et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 11.Hwang S.L., Chang H.W. Natural vanadium-containing Jeju ground water stimulates glucose uptake through the activation of AMP-activated protein kinase in L6 myotubes. Mol. Cell. Biochem. 2012;360:401–409. doi: 10.1007/s11010-011-1062-4. [DOI] [PubMed] [Google Scholar]
- 12.Stapleton D., Mitchelhill K.I., Gao G., Widmer J., Michell B.J., Teh T., House C.M., Fernandez C.S., Cox T., Witters L.A., et al. Mammalian AMP-activated protein kinase subfamily. J. Biol. Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 13.Turnley A.M., Stapleton D., Mann R.J., Witters L.A., Kemp B.E., Bartlett P.F. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J. Neurochem. 1999;72:1707–1716. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramamurthy S., Chang E., Cao Y., Zhu J., Ronnett G.V. AMPK activation regulates neuronal structure in developing hippocampal neurons. Neuroscience. 2014;259:13–24. doi: 10.1016/j.neuroscience.2013.11.048. [DOI] [PubMed] [Google Scholar]
- 15.Horikoshi M., Hara K., Ohashi J., Miyake K., Tokunaga K., Ito C., Kasuga M., Nagai R., Kadowaki T. A polymorphism in the AMPKalpha2 subunit gene is associated with insulin resistance and type 2 diabetes in the Japanese population. Diabetes. 2006;55:919–923. doi: 10.2337/diabetes.55.04.06.db05-0727. [DOI] [PubMed] [Google Scholar]
- 16.Shen J.Z., Ge W.H., Fang Y., Liu H. A novel polymorphism in protein kinase AMP-activated catalytic subunit alpha 2 (PRKAA2) is associated with type 2 diabetes in the Han Chinese population. J. Diabetes. 2017;9:606–612. doi: 10.1111/1753-0407.12449. [DOI] [PubMed] [Google Scholar]
- 17.Zhou H., He Y., Li L., Wu C., Hu G. Identification novel prognostic signatures for Head and Neck Squamous Cell Carcinoma based on ceRNA network construction and immune infiltration analysis. Int. J. Med. Sci. 2021;18:1297–1311. doi: 10.7150/ijms.53531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q., Hong Z., Zhu J., Zeng C., Huang H. miR-4999-5p Predicts Colorectal Cancer Survival Outcome and Reprograms Glucose Metabolism by Targeting PRKAA2. OncoTargets Ther. 2020;13:1199–1210. doi: 10.2147/OTT.S234666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weijiao Y., Fuchun L., Mengjie C., Xiaoqing Q., Hao L., Yuan L., Desheng Y. Immune infiltration and a ferroptosis-associated gene signature for predicting the prognosis of patients with endometrial cancer. Aging. 2021;13:16713–16732. doi: 10.18632/aging.203190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mckay S.D., White S.N., Kata S.R., Loan R., Womack J.E. The bovine 5’ AMPK gene family: Mapping and single nucleotide polymorphism detection. Mamm. Genome. 2003;14:853–858. doi: 10.1007/s00335-003-2276-x. [DOI] [PubMed] [Google Scholar]
- 21.Khan W.A., Hussain T., Babar M.E., Nadeem A., Saif R. Polymorphic Status of PRKAA2 Gene in Pakistani Buffaloes. Int. J. Agric. Biol. 2015;18:903–905. [Google Scholar]
- 22.Zhang Q., Zhao S., Chen H., Zhang L., Zhang L., Li F., Wang X. SNP discovery and haplotype analysis in the bovine PRKAA2 gene. Mol. Biol. Rep. 2011;38:1551–1556. doi: 10.1007/s11033-010-0263-3. [DOI] [PubMed] [Google Scholar]
- 23.Valtonen-Andre C., Lundwall A. The cotton-top tamarin (Saguinus oedipus) has five beta-microseminoprotein genes, two of which are pseudogenes. DNA Cell Biol. 2008;27:45–54. doi: 10.1089/dna.2007.0641. [DOI] [PubMed] [Google Scholar]
- 24.Makinen M., Valtonen-Andre C., Lundwall A. New world, but not Old World, monkeys carry several genes encoding beta-microseminoprotein. Eur. J. Biochem. 1999;264:407–414. doi: 10.1046/j.1432-1327.1999.00614.x. [DOI] [PubMed] [Google Scholar]
- 25.Lundwall A., Larne O., Nayudu P.L., Ceder Y., Valtonen-Andre C. Rapidly evolving marmoset MSMB genes are differently expressed in the male genital tract. Reprod. Biol. Endocrinol. 2009;7:96. doi: 10.1186/1477-7827-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darayee M., Geramizadeh B., Tabei S., Rezvani A., Soleimanian S., Rahimi A. Suggesting Tissue-Specific MSMB Gene Promoter as a Novel Approach for Prostate Targeted Gene Therapy. Asian Pac. J. Cancer Prev. 2022;23:1993–2000. doi: 10.31557/APJCP.2022.23.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu B., Wang J., Tong N., Mi Y., Min Z., Tao J., Li P., Cheng G., Li J., Wang M., et al. A functional polymorphism in MSMB gene promoter is associated with prostate cancer risk and serum MSMB expression. Prostate. 2010;70:1146–1152. doi: 10.1002/pros.21149. [DOI] [PubMed] [Google Scholar]
- 28.Frankenberg S., Fenelon J., Dopheide B., Shaw G., Renfree M.B. A novel MSMB-related microprotein in the postovulatory egg coats of marsupials. BMC Evol. Biol. 2011;11:373. doi: 10.1186/1471-2148-11-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terenina E., Fabre S., Bonnet A., Monniaux D., Robert-Granie C., Sancristobal M., Sarry J., Vignoles F., Gondret F., Monget P., et al. Differentially expressed genes and gene networks involved in pig ovarian follicular atresia. Physiol. Genom. 2017;49:67–80. doi: 10.1152/physiolgenomics.00069.2016. [DOI] [PubMed] [Google Scholar]
- 30.Zi X.D., Lu J.Y., Zhou H., Ma L., Xia W., Xiong X.R., Lan D.L., Wu X.H. Comparative analysis of ovarian transcriptomes between prolific and non-prolific goat breeds via high-throughput sequencing. Reprod. Domest. Anim. 2018;53:344–351. doi: 10.1111/rda.13111. [DOI] [PubMed] [Google Scholar]
- 31.Chave K.J., Ryan T.J., Chmura S.E., Galivan J. Identification of single nucleotide polymorphisms in the human gamma-glutamyl hydrolase gene and characterization of promoter polymorphisms. Gene. 2003;319:167–175. doi: 10.1016/S0378-1119(03)00807-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhao C., Hamilton T. Introns regulate the rate of unstable mRNA decay. J. Biol. Chem. 2007;282:20230–20237. doi: 10.1074/jbc.M700180200. [DOI] [PubMed] [Google Scholar]
- 33.Liu M., Li M., Wang S., Xu Y., Lan X., Li Z., Lei C., Yang D., Jia Y., Chen H. Association analysis of bovine Foxa2 gene single sequence variant and haplotype combinations with growth traits in Chinese cattle. Gene. 2014;536:385–392. doi: 10.1016/j.gene.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 34.Wang G., Li M., Zhou J., An X., Bai F., Gao Y., Yu J., Li H., Lei C., Dang R. A novel A > G polymorphism in the intron 2 of TBX3 gene is significantly associated with body size in donkeys. Gene. 2021;785:145602. doi: 10.1016/j.gene.2021.145602. [DOI] [PubMed] [Google Scholar]
- 35.Li W., Wang X., Zhang X., Li F., Zhang D., Li X., Zhang Y., Zhao Y., Zhao L., Xu D., et al. Polymorphism of sheep PRKAA2 gene and its association with growth traits. Anim. Biotechnol. 2021;31:1–7. doi: 10.1080/10495398.2021.2021215. [DOI] [PubMed] [Google Scholar]
- 36.Fontanesi L., Davoli R., Nanni C.L., Scotti E., Russo V. Study of candidate genes for glycolytic potential of porcine skeletal muscle: Identification and analysis of mutations, linkage and physical mapping and association with meat quality traits in pigs. Cytogenet. Genome Res. 2003;102:145–151. doi: 10.1159/000075740. [DOI] [PubMed] [Google Scholar]
- 37.Chang B.L., Cramer S.D., Wiklund F., Isaacs S.D., Stevens V.L., Sun J., Smith S., Pruett K., Romero L.M., Wiley K.E., et al. Fine mapping association study and functional analysis implicate a SNP in MSMB at 10q11 as a causal variant for prostate cancer risk. Hum. Mol. Genet. 2009;18:1368–1375. doi: 10.1093/hmg/ddp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeager M., Deng Z., Boland J., Matthews C., Bacior J., Lonsberry V., Hutchinson A., Burdett L.A., Qi L., Jacobs K.B., et al. Comprehensive resequence analysis of a 97 kb region of chromosome 10q11.2 containing the MSMB gene associated with prostate cancer. Hum. Genet. 2009;126:743–750. doi: 10.1007/s00439-009-0723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitaker H.C., Warren A.Y., Eeles R., Kote-Jarai Z., Neal D.E. The potential value of microseminoprotein-beta as a prostate cancer biomarker and therapeutic target. Prostate. 2010;70:333–340. doi: 10.1002/pros.21059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.