Abstract
The streptococcal pyrogenic exotoxins (Spes) play a central role in the pathogenesis of invasive group A streptococcal (GAS) infections. The majority of recent invasive GAS infections have been caused by an M1T1 strain that harbors the genes for several streptococcal superantigens, including speA, speB, speF, speG, and smeZ. However, considerable variation in the expression of Spe proteins among clonal M1 isolates has been found, and many of the speA-positive M1 strains do not produce detectable amounts of SpeA in vitro. This study was designed to test the hypothesis that speA gene expression can be induced in vivo. A mouse infection chamber model that allows sequential sampling of GAS isolates at various time points postinfection was developed and used to monitor the kinetics of Spe production in vivo. Micropore Teflon diffusion chambers were implanted subcutaneously in BALB/c mice, and after 3 weeks the pores became sealed with connective tissue and sterile fluid containing a white blood cell infiltrate accumulated inside the infection chambers. Representative clonal M1T1 isolates expressing no detectable SpeA were inoculated into the implanted chambers, and the expression of SpeA in the aspirated aliquots of the chamber fluid was analyzed on successive days postinfection. Expression of SpeA was detected in the chamber fluid as early as days 3 to 5 postinfection in most animals, with a significant increase in expression by day 7 in all infected mice. Isolates recovered from the chamber and grown in vitro continued to produce SpeA even after 21 passages in vitro, suggesting stable switch on of the speA gene. A temporal relation between the upregulation of SpeA expression and the downregulation of SpeB expression was observed in vivo. These data suggest that in vivo host and/or environmental signals induced speA gene expression and suppressed speB gene expression. This underscores the role of the host-pathogen interaction in regulating the expression of streptococcal virulence factors in vivo. The model described here should facilitate such studies.
Group A streptococci (GAS) are important human pathogens capable of causing a wide variety of infections ranging from simple nasopharyngitis to highly invasive and fatal infections such as necrotizing fasciitis and streptococcal toxic shock syndrome (STSS). GAS have a vast repertoire of virulence factors that participate in pathogenesis (reviewed in references 13, 15, and 38). The streptococcal pyrogenic exotoxins (Spes) are superantigens that induce potent inflammatory responses and cause tissue damage (19, 48) and play a major role in the pathogenesis of STSS (19). Expression of many of the GAS virulence factors can vary considerably among the different GAS strains as well as within clinical isolates of the same clonal strain (7). The mechanism by which expression of these virulence factors is regulated and the impact of variable expression of GAS virulence factors on pathogenesis are currently intense areas of investigation in many laboratories (1, 2, 4, 14, 16, 22, 23, 25, 30, 32, 40–42). However, despite the central role of the other Spe superantigens such as SpeA in the pathogenesis of invasive GAS infections (19, 20, 38), little is known about the regulation of its expression in vitro or in vivo.
The majority of recent invasive GAS infections have been caused by a clonal M1T1 strain that has the speA, speB, speF, speG, and smeZ genes (7, 11, 28; A. McGeer, K. Green, D. Cann, B. Schwartz, R. Kaul, A. Fletcher, S. Matsumura, the Ontario Group A Streptococcal Study Group, and D. E. Low, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. K135, 1995). However, a recent study by Chatellier et al. (7) noted highly variable expression of Spes among clonal M1T1 isolates from invasive infection cases. In particular, the expression of SpeA was either very low or undetectable in 40% of the isolates (7), and an inverse relation between SpeB expression and disease severity was found (18). Variable expression of SpeA among speA-positive isolates has also been noted by several investigators, who observed that only 20 to 50% of the speA-positive M1 isolates they studied expressed SpeA (9, 17, 34, 47).
The failure of some M1 speA-positive isolates to express detectable amounts of SpeA protein while other M1 isolates derived from the same clone express constitutive high levels of SpeA remains unexplained. Further, the biological relevance of this phenomenon is unclear. In an attempt to address these issues we investigated whether speA gene expression can be induced in vitro under various growth conditions or in vivo in a mouse model that we have developed. Here we report that SpeA expression was stably turned on in vivo, and a reciprocal temporal relation between SpeA and SpeB expression was observed. The data support the hypothesis that host factors may play an important role in regulating the expression of GAS virulence genes and underscore the role of host-pathogen interactions in GAS pathogenesis.
MATERIALS AND METHODS
Bacterial strains.
Seven representative M1T1 speA-positive strains of Streptococcus pyogenes were isolated from patients with STSS cases that were recruited from December 1994 to July 1996 through an ongoing population-based surveillance for invasive GAS infections in Ontario, Canada. All strains were determined to be derived from the same clone as detailed elsewhere (7). Original isolates recovered from patients were grown once to avoid in vitro passage, aliquoted, and stored frozen. The serotype of the M1 strains used here was confirmed by sequencing the emm gene, and the presence of Spe genes was determined by PCR as previously described (7). All M1T1 strains had the emm1.1, speA.2, speB, speF, speG, and smeZ genes. With the exception of isolate 8004, none of the isolates selected for this study expressed the SpeA protein. Therefore, isolate 8004 served as positive control, and M49 isolate NZ131, which lacks the speA gene, served as a negative control for speA expression. Isolates 5459, 5628, and 8004 did not express SpeB.
Animals.
Female 6- to 8-week-old BALB/c mice weighing 22 to 25 g were obtained from Jackson Laboratories (Bar Harbor, Maine). The mice were housed on hardwood chip bedding in microisolator cages in a room kept at 23°C with 50 to 60% relative humidity and a 12-h light-dark cycle and were given tap water and sterile irradiated rodent chow (Rodent Chow 2001; Ralston Purina, St. Louis, Mo.) ad libitum. The mice were housed five per cage, were randomly assigned to treatment or control groups, and were allowed to acclimate to the laboratory environment for a minimum of 10 days before being prepared for surgery. All protocols involving animals were approved by the Institutional Animal Care and use Committee of The University of Tennessee, Memphis.
Implantation of tissue chambers.
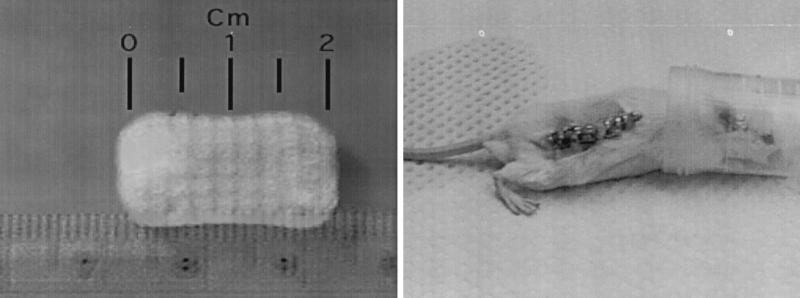
Autoclaved sterile Teflon-FEP (Fisher, Suwanee, Ga.) tissue chambers, which were 20 by 10 mm and perforated by 110 equally spaced 1-mm-diameter holes with two larger holes at both ends to allow penetrance of a 27-gauge needle, were manufactured in our Biomedical Instrumentation Department (University of Tennessee, Memphis) (Fig. 1). The sterile tissue chambers were implanted under aseptic conditions through a small incision in the subcutaneous connective tissue in the backsides of 6- to 8-week-old BALB/c mice. The incisions were closed with 9-mm wound clips, and a Betadine-iodine solution was sprayed over the wound to avoid infection. After inoculation of the bacteria into the implanted chambers, each mouse was housed in a separate cage and all were fed sterilized chow. The chambers were checked for sterility 3 weeks after implantation by culturing 100-μl aliquots of chamber fluid on sheep blood agar plates (Difco, Detroit, Mich.) for 24 h at 37°C.
FIG. 1.
Tissue chamber used in localized infection model. Teflon-FEP tissue chambers (20 by 10 mm) were perforated by 110 equally spaced 1-mm-diameter holes with a larger hole at one end to allow penetration of a 25-gauge needle. The chambers were implanted through a small incision in the subcutaneous connective tissue of the backsides of mice. After 3 weeks, the pores were sealed with vascularized connective tissue and the chamber was filled with a straw-colored sterile fluid.
Effect of environmental conditions on the expression of SpeA in vitro.
GAS isolates recovered from sterile sites of patients with invasive disease were streaked on blood agar plates. After overnight incubation, three pure colonies were picked and cultured overnight in 10 ml of standard medium (i.e., Todd-Hewitt broth plus 1.5% yeast extract). In some experiments, GAS isolates were cultured under different environmental conditions that included (i) incubation at different temperatures (25, 30, 37, or 42°C); (ii) culturing in broth supplemented with riboflavin (2 mg/ml), ferric citrate (13 mM), or the iron-chelating agents ethylenediaminediacetic acid (18 mM) and/or nitrilotriacetic acid (18 mM) alone or in combination with ferric citrate; and (iii) incubation under static or shaking conditions. In addition, GAS cultures were incubated in ambient air or in the presence of 5 and 10% CO2. Variation in CO2 content was made possible by using a commercial gas generator (Campy GasPac; BBL).
Partially purified bacterial culture supernatants were prepared by ethanol precipitation as detailed elsewhere (18). In some experiments the bacteria were grown in the presence of cysteine protease inhibitor E-64 (28 μM) to block SpeB proteolytic activity. Complete inhibition of cysteine protease activity by E-64 was confirmed using the EnzChek protease assay kit (Molecular Probes Inc., Eugene, Oreg.) as previously described (18).
Preparation of bacterial inoculum.
To prepare the bacteria for in vivo inoculation into mice, pure colonies were isolated and cultured overnight at 37°C in standard media under static conditions. The number of CFU per milliliter was determined, and, depending on the experiment, 1 × 103 to 5 × 105 CFU were inoculated into each tissue chamber. Isolate supernatants prepared immediately prior to infection were designated day 0 supernatants, and these were analyzed for Spe expression by Western blotting as described below.
Animal infection.
Infection of the sealed, implanted tissue chambers was done 3 weeks postimplantation. On day 0 of infection, 100 μl of the chamber fluid was aspirated from each chamber and cultured for a sterility check. Each chamber in the test group was inoculated with 100 μl containing 1 × 105 to 5 × 105 CFU of the GAS strain tested diluted in phosphate-buffered saline (PBS). Control animals received the same volume of endotoxin-free PBS (Gibco). Sequential aspiration of tissue chamber fluid was done on specified days after inoculation; the fluid was from the same mouse or in some cases from different mice designated for sampling on different days. The progress of infection inside the chamber was monitored by determining the number of CFU in 100 μl of chamber fluid aspirated from the chambers on different days.
The chamber fluid recovered on various days was centrifuged to remove cells, bacteria, and debris prior to Western blot analysis for SpeA, SpeB, and SpeF expression, as described below. Bacteria recovered from the chambers on different days were grown in vitro for up to 21 passages, and the partially purified supernatants from the bacterial cultures of each passage were also analyzed in Western blots for Spe production as described below.
In some experiments mice were sacrificed on different days postinfection to observe gross morphological changes in liver, heart, spleen, and kidney. Blood samples, peritoneal fluid, and several organs, including spleen, lung, liver, and kidney, were processed for determination of CFU per milliliter to investigate the systemic spread of infection.
Detection of SpeA, SpeB, and SpeF by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting.
Expression of Spe proteins in bacterial culture supernatants or in the fluid recovered from the chambers was detected by Western blotting as described previously (7, 18) using rabbit polyclonal antibodies raised against recombinant SpeA (rSpeA) or rSpeF, both of which were generated as previously reported (3), or an anti-SpeB mouse monoclonal antiserum, which was a gift from James Musser (National Institute of Allergy and Infectious Diseases, Hamilton, Mont.). Spe expression was detected by the luminol-chemiluminescence reagents (ECL; Amersham Life Sciences Ltd., Buckinghamshire, England). The processed blots were exposed to X-ray films, and the autoradiograms were analyzed.
Colony lift immunoblot assay.
Original isolates or isolates recovered from the chambers on the various days postinfection were diluted (103- to 106-fold) and plated on blood agar plates. Colonies were overlaid with a nitrocellulose membrane disk and incubated to allow the binding of secreted proteins. SpeA was detected by probing the membranes with anti-rSpeA rabbit antisera (at 1:2,000 dilution) followed by goat anti-rabbit peroxidase-conjugated immunoglobulin (IgG), which was diluted 1:10,000 in Tris-buffered saline (TBS). SpeB was detected using an anti-SpeB mouse monoclonal antibody, diluted 1:25,000 in TBS, followed by goat anti-mouse peroxidase-conjugated IgG (at 1:10,000 dilution). The blots were developed with the ECL reagent as described above.
SpeA- or SpeB-negative or -positive individual colonies derived from the original isolate or from isolates that were recovered from the infection chambers on various days postinfection were identified and picked for further growth and analysis or for reinoculation into new tissue chambers.
RESULTS
Effect of environmental factors on the induction of SpeA expression in vivo.
In many pathogenic bacteria, environmental conditions, including a change in incubation temperature, iron starvation, and the relative levels of atmospheric O2 and CO2, and other growth factors serve as signals that potentiate the expression of virulence factors. In this study we have attempted to induce the expression of SpeA by speA-positive, SpeA-negative isolates in vitro by growing the bacteria under different conditions that have been previously reported to affect the expression of other virulence genes of GAS (8, 10, 12, 31, 32, 49). Isolates were grown at temperatures ranging from ambient temperature to 42°C or in different media with and without various supplements including riboflavin, as well as under iron deprivation conditions by adding ethylenediaminediacetic acid or nitrilotriacetic acid with or without ferric citrate. GAS isolates were also subjected to growth in the presence of different levels of CO2 (5 to 10%) with shaking or static incubation. Isolates were also grown and recovered at different phases of growth, including early, mid-, and post-log phases. Many of these conditions affected the growth of the bacteria; however, none induced SpeA expression (data not shown). Accordingly, we tested whether expression of SpeA could be turned on in vivo.
Teflon tissue chamber infection model.
Implantation of the micropore Teflon-FEP tissue chambers (Fig. 1) subcutaneously in mice for 3 weeks resulted in the sealing of the chamber pores and the accumulation of sterile straw-colored chamber fluid, which could be sampled using a 25-gauge needle. Vascularized connective tissue surrounded the chamber, and a white blood cell infiltrate consisting primarily of neutrophils was detected in the recovered fluid. Seven M1T1 GAS isolates and one M49 isolate were individually inoculated into the connective tissue-sealed chambers (a minimum of five mice per isolate per experiment). All M1T1 isolates harbored the speA gene, and except for isolate 8004 none expressed the SpeA protein. M49 isolate NZ131 lacked the speA gene and served as a negative control (Table 1). Isolate 8004 expressed constitutive high levels of SpeA (Fig. 2)
TABLE 1.
spe genotypes and Spe phenotypes for GAS isolates included in this study
| GAS isolate |
spe genotype
|
Spe phenotype
|
||||
|---|---|---|---|---|---|---|
| speA | speB | speF | SpeA | SpeB | SpeF | |
| 5448 | + | + | + | − | + | + |
| 5449 | + | + | + | − | + | + |
| 5459 | + | + | + | − | − | + |
| 5628 | + | + | + | − | − | + |
| 5836 | + | + | + | − | + | + |
| 6050 | + | + | + | − | + | + |
| 8004 | + | + | + | + | − | − |
| NZ131 | − | − | − | − | + | + |
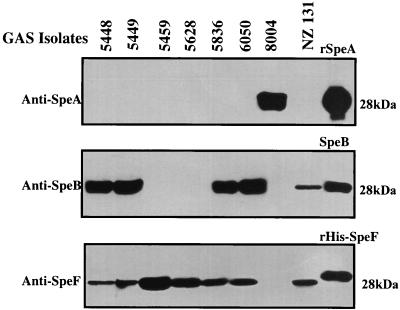
FIG. 2.
Expression of SpeA, SpeB, and SpeF in partially purified culture supernatants of GAS isolates prior to inoculation into tissue chambers. The expression of SpeA, SpeB, and SpeF in culture supernatants of the various isolates prior to in vivo inoculation into the tissue chambers was determined by Western blotting using antibodies to SpeA, SpeB, and SpeF as described in Materials and Methods.
The chambers were inoculated with 100 μl containing 1 × 105 to 5 × 105 CFU of the various isolates, and growth rate was monitored by in vitro culture of chamber fluid recovered on successive days postinfection. The growth curve profiles for the various isolates were similar, and neither the presence of the speA gene nor the expression of the SpeA protein by the isolates prior to the infection had a significant effect on the in vivo growth of the isolates (data not shown). To investigate whether the inoculum size affects the growth of the bacteria in the chambers, isolate 6050 was inoculated at levels ranging from 1 × 103 to 5 × 105 CFU and growth kinetics in vivo were monitored. Regardless of the initial inoculum size, a progressive and significant increase in the number of CFU per milliliter was evident between day 1 and day 5, after which a plateau was reached, i.e., between days 5 to 7, when a value of ∼109 CFU/ml was reached (data not shown). When a low inoculum was used (i.e., 103 CFU/chamber), several mice cleared the infection; however, in those mice where infection was established, the growth of the bacteria reached the same level (CFU per milliliter) in the chamber on day 5 as in mice inoculated with 104 or 105 CFU. Infection was more readily established in the majority of mice receiving 105 CFU. Accordingly, for the remainder of the study, chambers were inoculated with 100 μl containing 105 CFU of the GAS isolates.
Although neutrophils infiltrated the tissue-sealed chamber by diapedesis, the bacteria, which cannot perform this function, remained confined to the chambers. It is possible that a few bacteria were able to penetrate the chambers; however, there was no sign of bacterial dissemination because blood agar cultures of tissue homogenate from liver, spleen, lung, and kidney or cultures of blood and peritoneal fluid from infected animals failed to grow bacteria. Therefore, even if a few bacteria escaped the chamber they appear to have been cleared by the mouse innate immunity. In the few cases when the outside of the chamber became clearly infected due to technical problems, the bacteria disseminated and the mice died within 24 h. Despite evidence that the bacteria remained confined in the tissue chambers and despite the lack of detectable bacterial dissemination in the infected mice, there was clear evidence for a strong systemic inflammation in all mice examined. A mucoid pus surrounded the tissue chambers of infected animals, and significant increases in the sizes and weights of spleens (threefold increases) were noted in infected animals by day 14 postinfection (data not shown). These changes were not seen in control animals whose chambers were inoculated with PBS. Furthermore, injection of 100 μl of sterile PBS into the tissue chambers caused no change in the number of white blood cell infiltrates over a 21-day infection course. By contrast, inoculation with 105 CFU of GAS strains induced an inflammatory response and caused an approximately 100-fold increase in the number of white blood cell infiltrates inside the chamber by day 7 (data not shown). Together the data indicate that the sealing of the chamber with vascularized connective tissue allowed the diffusion of bacterial soluble products and infiltration of immune cells in and out of the chamber, while the bacterium itself remained confined inside the chamber. This allowed sequential sampling of GAS isolates from the chamber on different days following infection in order to investigate possible changes in Spe expression in vivo.
Induction of SpeA expression in vivo.
The expression of SpeA, SpeB, and SpeF was analyzed by Western blotting in the partially purified culture supernatants prepared from the isolates on the day of infection (day 0) as well as in chamber fluid aliquots, aspirated from the chambers on various days postinfection. The production of SpeA, SpeB, and SpeF in the culture supernatants of the GAS isolates prior to inoculation into chambers varied for the different isolates. Prior to infection, isolate 8004 expressed high levels of SpeA but did not express SpeB or SpeF, whereas isolates 5459 and 5628 expressed neither SpeA nor SpeB but both expressed SpeF. M1T1 isolates 5448, 5449, 5836, and 6050 expressed SpeB but not SpeA. Isolate NZ131 (M49), which lacks the speA gene, expressed SpeB. Therefore, prior to in vivo inoculation, all M1T1 isolates except isolate 8004 expressed no detectable amounts of SpeA protein even in the 20-fold-concentrated supernatants (Fig. 2).
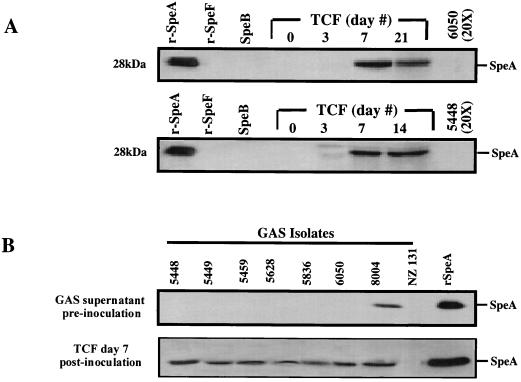
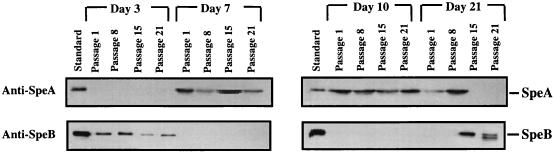
By contrast, after in vivo inoculation of the SpeA-negative isolates into the tissue chambers, low levels of expression of the SpeA protein were detected in the aspirated chamber fluid recovered from most infected animals on day 3 postinfection, and the amount of SpeA in the chamber fluid gradually increased with time (Fig. 3A). Variation of SpeA expression and the amount of SpeA produced in the chamber fluid among mice with respect to time was seen, but by day 7 the chamber fluid aspirated from all M1T1-infected animals showed evidence for SpeA expression (Fig. 3B). Expression of SpeA persisted for the duration of the observation period (14 to 21 days postinfection) (Fig. 3A). Expression of SpeA in chamber fluids of animals infected with isolate 8004, which expressed high levels of SpeA in vitro prior to infection, remained essentially unchanged during the course of the infection, and, as expected, no SpeA was detected in the chamber fluid of animals infected with isolate NZ131, which lacks the speA gene (Fig. 3B).
FIG. 3.
Temporal expression of SpeA in chamber fluid following infection with speA+ SpeA− GAS isolates. Isolates that harbor the speA gene but that express no detectable SpeA protein in vitro were inoculated into tissue chambers of mice (105 CFU/100 μl/chamber). (A) On the indicated days, 100 μl of chamber fluid was aspirated and analyzed for SpeA expression by Western blotting as described in Materials and Methods. Lanes 1 to 3 (counting from the left), standard rSpeA, SpeB, and rSpeF (100 ng each); lanes 4 to 8, tissue chamber fluid (TCF) aspirated on days 0 to 21 postinfection with isolate 5448 or isolate 6005. The same pattern of temporal induction of SpeA expression was seen with the other speA+ SpeA− M1T1 GAS isolates. (B) Expression of SpeA on day 7 postinfection with all isolates. A minimum of five mice per isolate per time point were studied, and results shown are from a single representative chamber fluid.
Stable expression of SpeA by GAS isolates recovered from the chamber fluid.
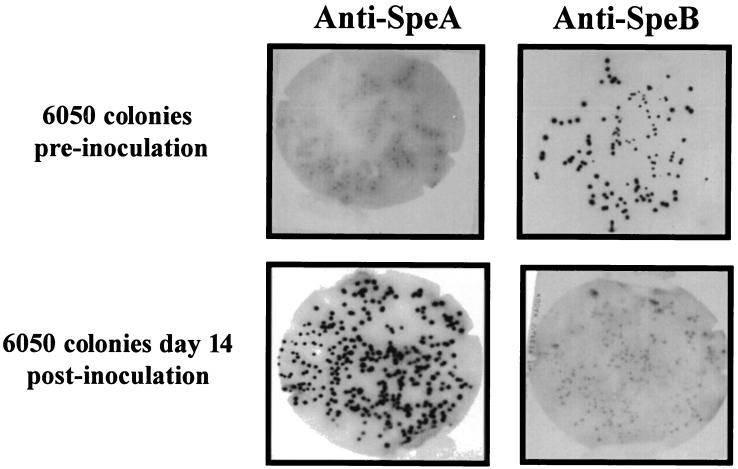
To determine whether the induction of speA gene expression after infection persists in vitro after recovery of isolates from the infection chambers, representative isolate 6050 was inoculated into chambers of mice and bacteria recovered from the infected chambers on days 3, 7, 10, 14, and 21 postinoculation were grown in vitro. The production of SpeA and SpeB in individual colonies as well as in the overnight culture supernatants from the recovered bacteria in vitro was detected by colony lift assays and by Western blotting, respectively. As shown in Fig. 4, the original 6050 colonies were all SpeA negative and SpeB positive, whereas the majority of colonies derived from 6050 bacteria, recovered 14 days postinfection, were all SpeA positive and SpeB negative.
FIG. 4.
Colony lift immunoblot of isolate 6050 colonies before and after recovery from an infected-tissue chamber. Isolates were recovered from chamber fluid aspirates obtained on day 7 or 14 postinfection, diluted, and plated on solid media to obtain individual colonies. Colonies from the original and recovered isolates were overlaid with nitrocellulose membranes, which were probed for SpeA expression using anti-SpeA antibodies as described in Materials and Methods. The blots shown are representative of a minimum of five different experiments with blots performed on bacteria recovered on different days.
In addition to the colony lift assay, bacteria recovered on various days postinfection were grown in vitro for 21 passages and the production of SpeA and SpeB was analyzed. Induction of SpeA expression in vivo was temporally paralleled by decreased SpeB expression in bacteria recovered on days 7 to 14 postinfection (Fig. 5). Bacteria recovered 3 days postinfection reverted to the original SpeA-negative, SpeB-positive phenotype. By contrast, bacteria recovered between days 7 and 14, which had switched on the speA gene and which became SpeA positive and SpeB negative, retained this phenotype for up to 21 passages. Isolates recovered 21 days postinoculation were also SpeA positive and SpeB negative and retained this phenotype upon successive passages; however, at passage 15 they reverted back to the original SpeA-negative phenotype. As isolates recovered on day 21 postinfection reverted back to the SpeA-negative phenotype, SpeB expression was regained.
FIG. 5.
Persistence of SpeA expression by isolates recovered from the infection chambers. Mouse tissue chambers were inoculated with isolate 6050 as detailed in the legend to Fig. 4. Bacteria were recovered from chamber fluid aspirates obtained on days 3, 7, 10, and 21 postinfection and passaged in vitro for the indicated passage numbers (passages 1 to 21). Partially purified culture supernatants from the various passages were analyzed for SpeA and SpeB expression by Western blotting as described in Materials and Methods.
The temporal inverse relation between SpeA and SpeB production raised the possibility that SpeB, a cysteine protease, may be causing proteolysis of SpeA. This possibly was unlikely because two other isolates included in this study, isolates 5459 and 5628, expressed neither SpeA nor SpeB prior to in vivo inoculation; however, both isolates turned on speA gene expression following in vivo inoculation into the chambers (Fig. 3B). Nonetheless, to further address the possible effect of SpeB on the SpeA protein, several original SpeA-negative, SpeB-positive isolates were grown in the presence or in the absence of SpeB protease inhibitor E-64. Inhibition of cysteine protease activity had no effect on the original SpeA-negative phenotype of these isolates (data not shown). Together, the data indicate that the lack of expression of SpeA by the original M1T1 isolates and its induction in vivo are not due to downregulation of SpeB expression but rather to a switch off of speA.
Induction of SpeA expression in vivo is not due to selective growth of SpeA-positive colonies.
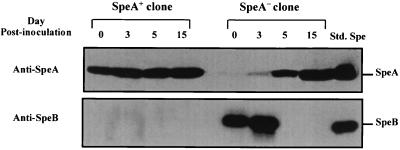
The possibility that the original 6050 inoculum contained rare SpeA-positive colonies that could have had a selective growth advantage in vivo was addressed. Isolate 6050 was inoculated into the chambers, and the bacteria recovered from the chamber fluids on day 5 postinfection, which contained a mixture of SpeA-positive and a few SpeA-negative colonies, were analyzed by the colony lift blot assay for SpeA expression. Two colonies that were clearly either SpeA positive or SpeA negative were selected and grown in vitro separately. The 6050 SpeA-positive and 6050 SpeA-negative colonies were individually reinoculated into mouse chambers, and expression of SpeA and SpeB was monitored on various days postinfection (Fig. 6). The bacteria derived from chambers inoculated with the SpeA-negative colony switched on SpeA expression by day 5 postinfection, and those derived from the SpeA-positive colony continued to express SpeA (Fig. 6). Together the data indicate that the expression of SpeA in vivo was not attributed to a selective growth advantage of rare SpeA-positive isolates in the original inoculum; rather, speA gene expression is indeed turned on in vivo.
FIG. 6.
In vivo expression of SpeA by SpeA-positive and SpeA-negative clones derived from isolate 6050 recovered 5 days postinfection from the tissue chamber. Isolate 6050 was inoculated into a tissue chamber and recovered on day 5 from the chamber fluid. The bacteria were diluted and spread on blood agar plates to obtain individual colonies. The colonies were analyzed for SpeA expression by the colony lift immunoblot assay described in Materials and Methods and illustrated in Fig. 5. SpeA-positive and SpeA-negative colonies were identified and picked for further growth. The bacteria derived from the SpeA-positive and SpeA-negative colonies were grown in vitro, and the supernatants were analyzed for SpeA and SpeB expression. In addition, the bacteria derived from the SpeA-positive and SpeA-negative colonies were reinoculated into tissue chambers of mice and then recovered on days 3, 5, and 15. Production of SpeA and SpeB by the recovered bacteria, which were originally derived from the SpeA-positive and SpeA-negative colonies, was analyzed by Western blotting as detailed in Materials and Methods.
The possibility that the induction of the speA gene in the chamber was due to a quorum-sensing mechanism (i.e., interbacterial communication signal as a result of growth phase regulation) related to the inability of the bacteria to leave the chamber was addressed. The bacteria were grown in vitro under conditions that simulate the “crowded” condition inside the tissue cage (initial inoculum, 105 to 106 CFU/ml). In one set, samples of the growing culture were tested for SpeA and SpeB expression on days 1, 3, 4, 5, and 7 and were subcultured in 10 ml of fresh medium to mimic the sampling protocol used with the mouse chamber model. Another set was established as a continuous culture; the culture was centrifuged daily, the supernatant was removed for analysis, and the pellet was regrown in the same volume of fresh medium according to the above schedule. None of the above conditions induced SpeA expression (data not shown). Growing the bacteria in human plasma also failed to induce SpeA expression.
DISCUSSION
Despite the many elegant studies investigating regulatory mechanisms controlling the transcription and/or posttranslational modification of key streptococcal virulence factors such as SpeB, little is known about the regulation of other Spes, including SpeA. This toxin is considered to be an important virulence component of GAS because of its potent superantigenic properties (19, 21). Much interest has focused on SpeA because the majority of invasive GAS infections since the 1980s have been caused by highly related M1T1 strains that harbor the speA gene (7, 24, 46). In addition, low levels of antibodies to SpeA, as well as to other Spes, in serum have been associated with increased risk of invasive disease (3, 26, 39, 45). However, the role of SpeA in STSS has been questioned in part because in vitro toxin production by clinical isolates is highly variable (7, 10, 33, 47). A number of studies, including our own, have noted that 20 to 50% of speA-positive clinical M1 isolates do not express the SpeA protein (7, 10, 17, 34, 47). The question of whether SpeA is expressed in vivo but not in vitro or vice versa has not been directly answered.
In this study we investigated the in vitro and in vivo expression of SpeA by clonal M1T1 isolates recovered from invasive GAS cases. Attempts to induce superantigen, SpeA, expression in vitro in six representative speA+ SpeA− isolates by varying culture conditions (e.g., temperature, pH, CO2, O2, etc.) or the composition of the growth media failed. Inasmuch as several of our clinical isolates derived from the clonal M1T1 strain were either producing no, low, or high levels of SpeA when originally isolated from the patients (7) and these isolates retained the original Spe phenotype upon continuous passage in culture, we hypothesized that in vivo environmental signals may stably turn on or off expression of specific Spe genes. Here we provided evidence that SpeA expression can be stably turned on in vivo, while SpeB expression is turned off. Thus, the data support the hypothesis and open the door for future investigations into the underlying molecular mechanism responsible for this phenomenon.
To monitor the in vivo expression of Spes over a relatively long period of time, it was necessary to develop a nonlethal model of infection. A mouse chamber model of localized GAS infection was, therefore, developed to investigate the effect of host-pathogen interaction on the expression of bacterial virulence genes. Several murine, rabbit, and monkey models of streptococcus infections have been developed, and these have generated important information regarding the pathogenesis of GAS and the role of various virulence factors in the in vivo infection (5, 29, 35, 43). The micropore Teflon chamber used here is a modification of the steel cage model that was developed by Nordstrand et al. (35). The vascularized connective tissue-sealed Teflon micropore chamber allows sequential sampling of tissue fluid containing the bacteria at different times postinfection from the same mouse. This feature reduces the variability among animals. Thus, our Teflon tissue chamber model combines the favorable properties of the air pouch model (5) and the innovation of the steel tissue chamber model developed by Nordstrand et al. (35). Further, because the bacteria remain confined in the chamber, the mice survive for a prolonged period of time, thereby allowing long-term monitoring of the in vivo environmental effects on the kinetics of expression of bacterial virulence genes.
As the expression of SpeA increased following in vivo inoculation, expression of SpeB was temporally downregulated. To our knowledge, the production of SpeA locally or systemically under in vivo conditions by strains which had failed to produce detectable levels of the protein in vitro has not been previously described. Although the kinetics of speA gene induction varied slightly for different isolates and in different mice, all speA-positive, SpeA-negative isolates expressed high levels of SpeA by day 7 postinfection. A bacterial environmental monitoring system which operates in the in vivo hostile environment may have triggered the maximal expression of the speA gene. Recent studies by Broudy et al. (6) showed that GAS cocultured with a human pharyngeal cell line upregulated SpeC expression within 3 h of coincubation. In our model, induction occurred after 3 to 5 days in the chamber, which contained high numbers of leukocytic infiltrate. The difference in the kinetics of Spe expression between the two systems could be due to differences in speA and speC regulation. Alternatively, the pharyngeal cell line used in the study by Broudy et al. may have acquired a constitutive signal as a result of cellular transformation that induces Spe expression, whereas in our model expression of this signal may require 3 to 5 days and thus represents actual in vivo kinetics. Future studies will explore the difference in kinetics of SpeA expression between the ex vivo and in vivo systems and will aim to identify the nature of the signal derived from the host.
Isolates recovered from the tissue chamber starting on day 7 and up to day 14 continued to express SpeA in vitro after their removal from the in vivo environment, and serial passages in vitro failed to turn off the speA gene. This suggests that an environmental or host signal in vivo has caused the gene to be irreversibly turned on. The nature of this signal is currently under investigation, but it is noteworthy that several isolates in our series of over 100 clonal M1T1 isolates expressed constitutive high levels of SpeA in vitro immediately after recovery from the patients and that this expression was not attenuated or turned off by serial passages in vitro. Isolate 8004 studied here is representative of isolates expressing high levels of SpeA; for this isolate speA gene expression was constitutive both in vitro and in vivo and speB gene expression was turned off. Therefore, it appears that events occurring both in humans and mice can permanently turn on the speA gene. However, this signal may be different in different individuals inasmuch as 40% of clonal M1T1 isolates recovered from our patients had not switched on the SpeA gene.
The advantage of turning the speA gene on or off is not clear. Although production of SpeA in vivo may influence the severity of infection, sustained production of this toxin can lead to the development of antibodies that can neutralize its superantigenic activity and protect the host against subsequent infections. Indeed, vaccination with SpeA has been shown to confer protection in animal models of STSS and necrotizing fasciitis (44), and low levels of anti-SpeA neutralizing antibodies have been reported in sera from invasive GAS infection cases (3, 26, 27, 39, 45) as well as in various intravenous immunoglobulin preparations (36, 37). Interestingly, a recent report by Sriskandan et al. (44) showed that, in a murine model of peritoneal infection, speA-negative mutants were more virulent than the isogenic speA-positive wild-type strain. Increased mortality was seen after intravenous injection of the mutant strain. Furthermore, when the mutant strain was injected intramuscularly, it led to increased bacteremia and a reduction in neutrophil sequestration at the site of primary muscle infection. Therefore, it is possible that, under certain conditions, the bacteria may need to turn off certain virulence genes; while important to the pathogen, the encoded toxins may compromise the ability of the bacteria to continue to survive in the host. Support for this notion is found in a recent study by Kansal et al. (18), which demonstrated that a lack of SpeB expression is associated with increased severity of invasive GAS disease. Future work should reveal the role of the differential regulation of Spe gene expression in pathogenesis.
In summary, the current Teflon tissue chamber model allowed us to establish a streptococcal infection leading to induction of the speA gene in vivo. This model, which allows repeated sampling from the infection focus to determine the temporal expression of Spe genes, will enable us to investigate the in vivo signals required for turning on the speA gene and turning off the speB gene. This information will help us better understand the role of host-pathogen interactions in regulating the expression of streptococcal virulence factors.
ACKNOWLEDGMENTS
This work was supported by grants AI40198 from the National Institutes of Health NIAID (M.K.) and by a Merit Review Award from the U.S. Veterans Affairs (M.K.), as well as by funds from the joint Veterans Affairs/Department of Defense Research Initiative (M.K.).
We thank James Musser for the kind gift of anti-SpeB antibodies and Andy Lundberg for his surgical assistance.
REFERENCES
- 1.Ashbaugh C D, Alberti S, Wessels M R. Molecular analysis of the capsule gene region of group A Streptococcus: the hasAB genes are sufficient for capsule expression. J Bacteriol. 1998;180:4955–4959. doi: 10.1128/jb.180.18.4955-4959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashbaugh C D, Warren H B, Carey V J, Wessels M R. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J Clin Investig. 1998;102:550–560. doi: 10.1172/JCI3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basma H, Norrby-Teglund A, Guedez Y, McGeer A, Low D E, El-Ahmedy O, Schwartz B, Kotb M. Risk factors in the pathogenesis of invasive group A streptococcal infections: role of protective humoral immunity. Infect Immun. 1999;67:1871–1877. doi: 10.1128/iai.67.4.1871-1877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernish B, van de Rijn I. Characterization of a two-component system in Streptococcus pyogenes which is involved in regulation of hyaluronic acid production. J Biol Chem. 1999;274:4786–4793. doi: 10.1074/jbc.274.8.4786. [DOI] [PubMed] [Google Scholar]
- 5.Boyle M D, Raeder R, Flosdorff A, Podbielski A. Role of emm and mrp genes in the virulence of group A streptococcal isolate 64/14 in a mouse model of skin infection. J Infect Dis. 1998;177:991–997. doi: 10.1086/515241. [DOI] [PubMed] [Google Scholar]
- 6.Broudy T B, Pancholi V, Fischetti V A. Induction of lysogenic bacteriophage and phage-associated toxin from group A streptococci during coculture with human pharyngeal cells. Infect Immun. 2001;69:1440–1443. doi: 10.1128/IAI.69.3.1440-1443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatellier S, Ihendyane N, Kansal R G, Khambaty F, Basma H, Norrby-Teglund A, Low D E, McGeer A, Kotb M. Genetic relatedness and superantigen expression of M type 1 group A streptococcal isolates from severe and nonsevere invasive disease. Infect Immun. 2000;68:3523–3534. doi: 10.1128/iai.68.6.3523-3534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaussee M S, Liu J, Stevens D L, Ferretti J J. Effects of environmental factors on streptococcal erythrogenic toxin A (SPE A) production by Streptococcus pyogenes. Adv Exp Med Biol. 1997;418:551–554. doi: 10.1007/978-1-4899-1825-3_127. [DOI] [PubMed] [Google Scholar]
- 9.Chaussee M S, Liu J, Stevens D L, Ferretti J J. Genetic and phenotypic diversity among isolates of Streptococcus pyogenes from invasive infections. J Infect Dis. 1996;173:901–908. doi: 10.1093/infdis/173.4.901. [DOI] [PubMed] [Google Scholar]
- 10.Chaussee M S, Phillips E R, Ferretti J J. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect Immun. 1997;65:1956–1959. doi: 10.1128/iai.65.5.1956-1959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleary P P, Kaplan E L, Handley J P, Wlazlo A, Kim M H, Hauser A R, Schlievert P M. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet. 1992;339:518–521. doi: 10.1016/0140-6736(92)90339-5. [DOI] [PubMed] [Google Scholar]
- 12.Cleary P P, McLandsborough L, Ikeda L, Cue D, Krawczak J, Lam H. High-frequency intracellular infection and erythrogenic toxin A expression undergo phase variation in M1 group A streptococci. Mol Microbiol. 1998;28:157–167. doi: 10.1046/j.1365-2958.1998.00786.x. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham M W. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Federle M J, McIver K S, Scott J R. A response regulator that represses transcription of several virulence operons in the group A streptococcus. J Bacteriol. 1999;181:3649–3657. doi: 10.1128/jb.181.12.3649-3657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrieri P. Microbiological features of current virulent strains of group A streptococci. Pediatr Infect Dis J. 1991;10(Suppl.):S20–S24. doi: 10.1097/00006454-199110001-00005. [DOI] [PubMed] [Google Scholar]
- 16.Heath A, DiRita V J, Barg N L, Engleberg N C. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect Immun. 1999;67:5298–5305. doi: 10.1128/iai.67.10.5298-5305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holm S E, Norrby A, Bergholm A M, Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988 to 1989. J Infect Dis. 1992;166:31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Kansal R G, McGeer A, Low D E, Norrby-Teglund A, Kotb M. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect Immun. 2000;68:6362–6369. doi: 10.1128/iai.68.11.6362-6369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotb M. Bacterial pyrogenic exotoxins as superantigens. Clin Microbiol Rev. 1995;8:411–426. doi: 10.1128/cmr.8.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotb M. Role of superantigens in the pathogenesis of infectious diseases and their sequelae. Curr Opin Infect Dis. 1992;5:364–374. [Google Scholar]
- 21.Kotb M. Superantigens of gram-positive bacteria: structure-function analyses and their implications for biological activity. Curr Opin Microbiol. 1998;1:56–65. doi: 10.1016/s1369-5274(98)80143-4. [DOI] [PubMed] [Google Scholar]
- 22.Levin J C, Wessels M R. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A streptococcus. Mol Microbiol. 1998;30:209–219. doi: 10.1046/j.1365-2958.1998.01057.x. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Sledjeski D D, Kreikemeyer B, Podbielski A, Boyle M D. Identification of pel, a Streptococcus pyogenes locus that affects both surface and secreted proteins. J Bacteriol. 1999;181:6019–6027. doi: 10.1128/jb.181.19.6019-6027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Low D E, Schwartz B, McGeer A. Emerging infections. Vol. 1. Washington, D.C.: ASM Press; 1998. The reemergence of severe group A streptococcal disease: an evolutionary perspective; pp. 93–123. [Google Scholar]
- 25.Lyon W R, Gibson C M, Caparon M G. A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 1998;17:6263–6275. doi: 10.1093/emboj/17.21.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascini E M, Hazenberg M M, van Dalen A, Verhoef-Verhage L A, van Leeuwen N J, Verhoef J, van Dijk H. Invasive group A streptococcal disease. Association with a lack of anti-exotoxin antibodies. Adv Exp Med Biol. 1997;418:921–922. [PubMed] [Google Scholar]
- 27.Mascini E M, Jansze M, Schellekens J F, Musser J M, Faber J A, Verhoef-Verhage L A, Schouls L, van Leeuwen W J, Verhoef J, van Dijk H. Invasive group A streptococcal disease in The Netherlands: evidence for a protective role of anti-exotoxin A antibodies. J Infect Dis. 2000;181:631–638. doi: 10.1086/315222. [DOI] [PubMed] [Google Scholar]
- 28.Mascini E M, Jansze M, Schouls L M, Fluit A C, Verhoef J, van Dijk H. Invasive and noninvasive group A streptococcal isolates with different speA alleles in The Netherlands: genetic relatedness and production of pyrogenic exotoxins A and B. J Clin Microbiol. 1999;37:3469–3474. doi: 10.1128/jcm.37.11.3469-3474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick J K, Tripp T J, Olmsted S B, Matsuka Y V, Gahr P J, Ohlendorf D H, Schlievert P M. Development of streptococcal pyrogenic exotoxin C vaccine toxoids that are protective in the rabbit model of toxic shock syndrome. J Immunol. 2000;165:2306–2312. doi: 10.4049/jimmunol.165.4.2306. [DOI] [PubMed] [Google Scholar]
- 30.McIver K S, Heath A S, Green B D, Scott J R. Specific binding of the activator Mga to promoter sequences of the emm and scpA genes in the group A streptococcus. J Bacteriol. 1995;177:6619–6624. doi: 10.1128/jb.177.22.6619-6624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIver K S, Scott J R. Role of mga in growth phase regulation of virulence genes of the group A streptococcus. J Bacteriol. 1997;179:5178–5187. doi: 10.1128/jb.179.16.5178-5187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McIver K S, Thurman A S, Scott J R. Regulation of mga transcription in the group A streptococcus: specific binding of mga within its own promoter and evidence for a negative regulator. J Bacteriol. 1999;181:5373–5383. doi: 10.1128/jb.181.17.5373-5383.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musser J M, Hauser A R, Kim M H, Schlievert P M, Nelson K, Selander R K. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci USA. 1991;88:2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakashima K, Ichiyama S, Iinuma Y, Hasegawa Y, Ohta M, Ooe K, Shimizu Y, Igarashi H, Murai T, Shimokata K. A clinical and bacteriologic investigation of invasive streptococcal infections in Japan on the basis of serotypes, toxin production, and genomic DNA fingerprints. Clin Infect Dis. 1997;25:260–266. doi: 10.1086/514543. [DOI] [PubMed] [Google Scholar]
- 35.Nordstrand A, Norgren M, Holm S E. An experimental model for acute poststreptococcal glomerulonephritis in mice. APMIS. 1996;104:805–816. doi: 10.1111/j.1699-0463.1996.tb04946.x. [DOI] [PubMed] [Google Scholar]
- 36.Norrby-Teglund A, Basma H, Andersson J, McGeer A, Low D E, Kotb M. Varying titers of neutralizing antibodies to streptococcal superantigens in different preparations of normal polyspecific immunoglobulin G: implications for therapeutic efficacy. Clin Infect Dis. 1998;26:631–638. doi: 10.1086/514588. [DOI] [PubMed] [Google Scholar]
- 37.Norrby-Teglund A, Ihendyane N, Kansal R, Basma H, Kotb M, Andersson J, Hammarstrom L. Relative neutralizing activity in polyspecific IgM, IgA, and IgG preparations against group A streptococcal superantigens. Clin Infect Dis. 2000;31:1175–1182. doi: 10.1086/317423. [DOI] [PubMed] [Google Scholar]
- 38.Norrby-Teglund A, Kotb M. Host-microbe interactions in the pathogenesis of invasive group A streptococcal infections. J Med Microbiol. 2000;49:849–852. doi: 10.1099/0022-1317-49-10-849. [DOI] [PubMed] [Google Scholar]
- 39.Norrby-Teglund A, Pauksens K, Holm S E, Norgren M. Relation between low capacity of human sera to inhibit streptococcal mitogens and serious manifestation of disease. J Infect Dis. 1994;170:585–591. doi: 10.1093/infdis/170.3.585. [DOI] [PubMed] [Google Scholar]
- 40.Okada N, Geist R T, Caparon M G. Positive transcriptional control of mry regulates virulence in the group A streptococcus. Mol Microbiol. 1993;7:893–903. doi: 10.1111/j.1365-2958.1993.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 41.Podbielski A, Flosdorff A, Weber-Heynemann J. The group A streptococcal virR49 gene controls expression of four structural vir regulon genes. Infect Immun. 1995;63:9–20. doi: 10.1128/iai.63.1.9-20.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Podbielski A, Leonard B A. The group A streptococcal dipeptide permease (Dpp) is involved in the uptake of essential amino acids and affects the expression of cysteine protease. Mol Microbiol. 1998;28:1323–1334. doi: 10.1046/j.1365-2958.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- 43.Roggiani M, Stoehr J A, Olmsted S B, Matsuka Y V, Pillai S, Ohlendorf D H, Schlievert P M. Toxoids of streptococcal pyrogenic exotoxin A are protective in rabbit models of streptococcal toxic shock syndrome. Infect Immun. 2000;68:5011–5017. doi: 10.1128/iai.68.9.5011-5017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sriskandan S, Unnikrishnan M, Krausz T, Cohen J. Molecular analysis of the role of streptococcal pyrogenic exotoxin A (SPEA) in invasive soft-tissue infection resulting from Streptococcus pyogenes. Mol Microbiol. 1999;33:778–790. doi: 10.1046/j.1365-2958.1999.01525.x. [DOI] [PubMed] [Google Scholar]
- 45.Stevens D L. Streptococcal toxic-shock syndrome: spectrum of disease, pathogenesis, and new concepts in treatment. Emerg Infect Dis. 1995;1:69–78. doi: 10.3201/eid0103.950301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevens D L. The toxins of group A streptococcus, the flesh eating bacteria. Immunol Investig. 1997;26:129–150. doi: 10.3109/08820139709048921. [DOI] [PubMed] [Google Scholar]
- 47.Talkington D, Schwartz B, Black C, Todd J, Elliott J, Breiman R, Facklam R. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of streptococcal toxic shock syndrome. Infect Immun. 1993;61:3369–3374. doi: 10.1128/iai.61.8.3369-3374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomai M, Schlievert P M, Kotb M. Distinct T-cell receptor Vβ gene usage by human T lymphocytes stimulated with the streptococcal pyrogenic exotoxins and M protein. Infect Immun. 1992;60:701–705. doi: 10.1128/iai.60.2.701-705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu S, Collins C M. Temperature regulation of the streptococcal pyrogenic exotoxin A-encoding gene (speA) Infect Immun. 1996;64:5399–5402. doi: 10.1128/iai.64.12.5399-5402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]