Abstract
Egg production is a vital biological and economic trait for poultry breeding. The ‘hypothalamic–pituitary–ovarian (HPO) axis’ determines the egg production, which affects the layer hens industry income. At the organism level, the HPO axis is influenced by the factors related to metabolic and nutritional status, environment, and genetics, whereas at the cellular and molecular levels, the HPO axis is influenced by the factors related to endocrine and metabolic regulation, cytokines, key genes, signaling pathways, post-transcriptional processing, and epigenetic modifications. MiRNAs and lncRNAs play a critical role in follicle selection and development, atresia, and ovulation in layer hens; in particular, miRNA is known to affect the development and atresia of follicles by regulating apoptosis and autophagy of granulosa cells. The current review elaborates on the regulation of the HPO axis and its role in the laying performance of hens at the organism, cellular, and molecular levels. In addition, this review provides an overview of the interactive network regulation mechanism of the HPO axis in layer hens, as well as comprehensive knowledge for successfully utilizing their genetic resources.
Keywords: hypothalamic–pituitary–ovarian axis, laying performance, molecular mechanism, interactive network regulation mechanism
1. Introduction
Egg production is one of the vital biological and economic traits for poultry breeding, which has profound economic impacts on the laying hen industry. The concept, “700 eggs produced in 500 days”, proposed by the French company Institute Selection Animal in 2011 [1], provides a clear goal for the China layer hens breeding industry. This concept has also been included in the future goals of the China Layer Hens Genetic Improvement Plan (2021–2035) [2]. The current production level of layer hens in China is 14–18 kg egg mass per hen, and a few domestic laying farms have achieved 20 kg per hen. However, internationally, the production level of layer hens has reached 20–21 kg per hen, indicating the gap in layer hens performance in China.
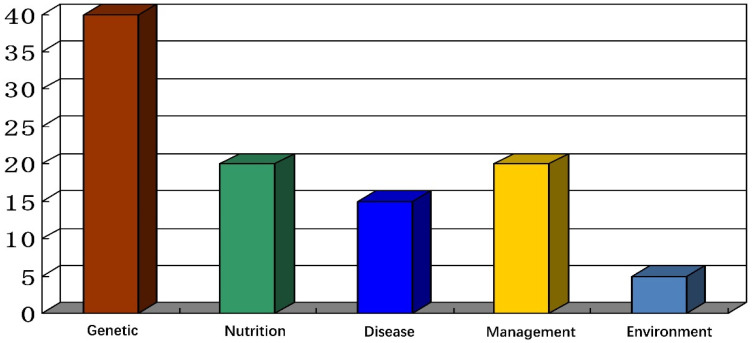
Over the last 50 years, layer hens’ egg production has been influenced by different factors such as genetics (40%), nutrition (20%), disease control (15%), feeding management (20%), and environment (5%) (Figure 1) [3], and these factors directly or indirectly affect the egg production of layer hens. It is commonly believed that egg production is directly regulated by the hypothalamus–pituitary–ovarian (HPO) axis, which comprises a comprehensive network of regulatory molecules, such as endocrine hormones and metabolic cytokines, and these molecules activate or inhibit the HPO axis signaling pathways. Furthermore, the influence of methylation or epigenetic modifications is a biological characteristic that has an economic impact on the layer hen breeding industry [4,5,6,7,8,9]. Noncoding RNAs also regulate the target genes related to endocrine and metabolism signaling pathways. A study reported that microRNAs present in avian ovarian tissue, such as gga-miR-34b, gga-miR-34c, and gga-miR-216b, were differently expressed in high-yield laying hens [10]. By binding to the target genes, noncoding RNAs regulate the synthesis, transport, and secretion of genes related to reproductive hormones downstream of signaling pathways, namely STAR, CYP11A1, and CYP19A1 [11,12].
Figure 1.
Factors influencing layer hens egg production over the last 50 years.
The present review focuses on interacting networks of the HPO axis regulating layer hen performance through endocrine and metabolic regulations, providing an overview of the interactive network regulation mechanism of the HPO axis in layer hens and comprehensive knowledge for successfully utilizing their genetic resources.
2. Organism-Level Regulation Mechanism of the HPO Axis
2.1. Development Characteristics of the HPO Axis
The hypothalamus, the central regulator of physiological homeostasis, regulates reproductive activities in hens [13]. The hypothalamus, pituitary gland, and ovary process signals through positive and negative feedback precision regulation. The hypothalamus secretes gonadotropin-releasing hormone (GnRH) or gonadotropin inhibitory hormone (GnIH) through physiological stimulation, controlling the secretion of anterior pituitary prolactin (PRL), follicle-stimulating hormone (FSH) [14], luteinizing hormone (LH) [15], and oxytocin (OXT), which are associated with egg production performance. FSH, LH, and progesterone (P4) secretion levels progressively increase at the peak stage of laying, whereas P4, estradiol (E2), and PRL secretion concentrations gradually increase at the time of the first egg. The cascade response triggered by GnRH and GnRHR regulates the HPO axis. Research has shown that distinct GnRH might have different receptor types. GnRH is classified into two major classes in most vertebrates, namely GnRH-I and GnRH-II. GnRH-I operates primarily on the pituitary gland, whereas avian GnRH, which is evolutionarily highly conserved, is related to GnRH-II [16,17]. GnRH-I and GnRH-II signals produced by the hypothalamus are significantly correlated with egg production and body weight of White Leghorn laying hens. However, unlike GnRH gene expression, GnIHR mRNA expression in the hypothalamus is significantly higher in the pituitary of sexually immature chicken than in the pituitary of mature chicken. When avians achieve sexual maturity and begin to produce gonadotropins, a surge in circulating E2 and progesterone concentrations may downregulate GnIHR gene expression. Furthermore, GnIHR protein expressed in FSHβ mRNA- or LHβ mRNA-containing cells is likely to promote GnIH-mediated inhibition of LH and FSH secretion. BMP15 and FSHR genes are also linked to reproduction and hence to the laying performance [18]. Therefore, GnRH may play an important role in the initiation signal of the physiological process of egg production. Furthermore, vasoactive intestinal peptides (VIPs) secreted by the hypothalamus inhibit GnRH secretion through its receptor (YIR) and regulate ovulation [19]. The positive and negative feedback regulation of these hormones and neuroendocrine pathways are crucial for avian reproductive performance. Hence, the primary challenge in improving the reproductive performance of laying hens is to elucidate the key mechanisms controlling the HPO axis and identifying the differently expressed genes involved.
The development of chicken ovaries is regulated by hormones secreted in the hypothalamus and the pituitary gland, Ovarian development is the key element determining egg production of hens [20]. The ovary of a chicken begins to grow at approximately 14 weeks and attains sexual maturity between the age of 18 and 20 weeks [21,22]. During hens’ reproductive life, most ovarian follicles undergo atresia, with only approximately 5% progressing to the final hierarchical stages of maturation and ovulation. Growth of the avian ovarian follicle occurs in two stages: the pre-hierarchical follicle stage and the hierarchical follicle stage [23,24]. Pre-hierarchical follicles have been classified as small white follicles (SWF, 2–4 mm), large white follicles (LWF, 4–6 mm), and small yellow follicles (SYF, 6–8 mm), whereas hierarchical follicles are the pre-ovulation follicles (9–12 mm) that have been classified using a rigorous grading system from grade F6 to grade F1, with F1 representing mature follicles and F6 representing hierarchical follicles destined to become fully mature follicles. This is a highly hierarchical process in which one follicle is picked from the small yellow follicles pool and placed in the hierarchical follicle pool sequential order, which then grows into ovulatory follicles [25,26,27,28].
In addition to analyzing the level of egg-laying performance from the perspective of hormones, the challenges related to the bird’s phenotype [29] involve commonly used qualities, such as age at first egg (AFE) [30], egg weight, egg number at the age of 300 days (EN300), and other external indicators used for artificial selection to improve egg production efficiency [31].
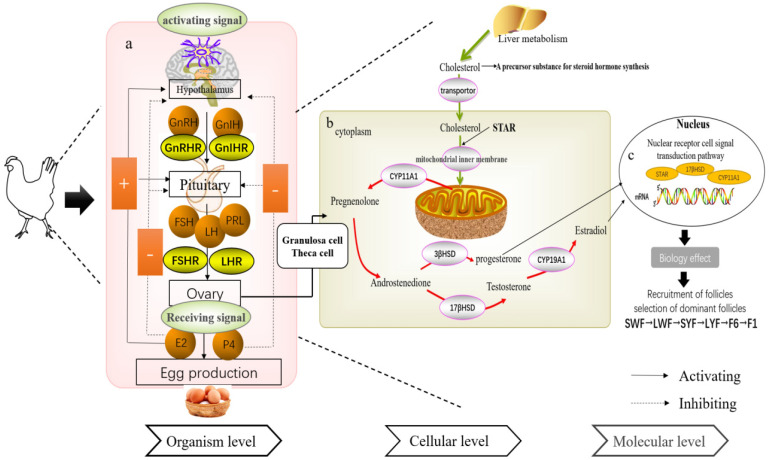
Figure 2a shows that egg production of layer hens is regulated by the HPO axis. The hypothalamus receives activating signals and secretes the gonadotropin-releasing hormone (GnRH) or gonadotropin inhibitory hormone (GnIH). The pituitary gland receives signals from the hypothalamus, which binds to GnRHR receptors on the pituitary gland and secretes anterior pituitary prolactin (PRL), FSH, and LH. The ovary receives signals from the pituitary gland and secretes progesterone (P4) and estradiol (E2), both of which play a vital role in regulation by initiating follicle selection. Figure 2b shows the mechanisms of steroid hormone production by granulosa and theca cells. Steroid hormones are synthesized via two main pathways; granulosa cells (GCs) regulate the synthesis and secretion of P4 through the transcription of STAR and CYP11A1 genes, whereas theca cells regulate the synthesis and secretion of E2 through 17βHSD and CYP19A1 gene transcription (Figure 2c). These genes involved in reproductive hormone synthesis are transported to the nucleus and exert the biological effect of follicle selection by activating the nuclear receptor signaling pathway.
Figure 2.
(a–c) Interaction mechanism of the avian HPO axis network.
2.2. Factors Affecting the Organism-Level Regulation Mechanism
The layer hens performance is not only regulated by HPO axis, but also affected by genetic, environmental, and nutritional metabolic status.
2.2.1. Factors Related to the Metabolic and Nutritional Status
Nutrition affects the secretion of reproductive hormones and the concentration of metabolic substrates in blood. Research showed that food restriction promoted release of P4, reduced out of the T, but did not affect E release, suggesting that metabolic status can control the release of these hormones [32]. The estrogen estradiol-17ß is known as one of the major gonadal steroid hormones with different functions in reproduction [33]. Modern layer hens have consistently high plasma estradiol-17ß levels [34], estrogen divided into two types of receptors, ER receptor α and ER receptor β. The mRNA expression of Erα was significantly higher compared to ERβ in layer hens granulosa cells [35], suggesting that estrogen plays a role in regulating follicular development through ERα receptors. Previous research reported that follicle selection is indispensable in the reproductive process in female chickens, the symbol of which is the proliferation and differentiation of granulosa cells (GCs), In chickens follicular GCs, the expression of steroidogenic acute regulatory protein (StAR) and cytochrome P450 family 11 subfamily A member 1 (CYP11A1) is a prerequisite for progesterone synthesis and is related to follicle selection [36,37,38]. It suggests that estrogen and progesterone work together to complete the physiological process of follicle selection in layer hens. Many studies have shown that estrogen is critical for lipid metabolism in the liver. In vivo and in vitro experiments showed that estrogen decreased the mRNA expression of PPARGC1B, which had been reported to be linked with lipid metabolism, by directly increasing the expression of miR-144-3p [39,40]. These findings show that the liver ERα controls the regulation of liver lipid metabolism, thus connecting liver lipid metabolism to the reproduction cycle.
2.2.2. Environmental Factors
Environmental factors exert a substantial impact on the HPO axis and thus can be utilized as exogenous factors to directly regulate the laying performance. Light and temperature are the key environmental factors. Firstly, light affects the hypothalamus, stimulating GnRH neurons to produce GnRH-II in response to light cues and regulating the reproduction cycle by modulating melatonin production [41]. Secondly, the temperature affects the laying performance of hens in two ways. Research has shown that cold stress can increase free radicals in the body, which affects the antioxidant capacity and causes an imbalance in the stable state of free radicals, thus representing the main three-way linkage reaction of oxidative stress, inflammatory response, and immunological stress [42]. A change in temperature of the environment triggers a series of neuroendocrine responses, causing a systemic control reaction to adjust the endocrine system. To maintain a constant body temperature, the HPO axis is activated, which accelerates metabolism, enhances protein breakdown, and increases insulin secretion [43].
2.2.3. Genetic Factors
Heredity factors also play a crucial role in the hens’ egg-laying performance. Differentially expressed genes (DEGs) were identify in the hypothalamus and pituitary tissues of high and low-yield hens by transcriptomics. Laying performance is linked to the signaling pathways, such as extracellular matrix, glycosaminoglycan biosynthesis, protein extracellular matrix, and extracellular space. GWAS was used to mine SNP loci in commercial egg-type chickens and indigenous chickens. Some genes, such as NELL2 (neural EGFL like 2), KITLG (KIT ligand), GHRHR (Growth hormone releasing hormone receptor), NCOA1 (Nuclear receptor coactivator 1), ITPR1 (inositol 1, 4, 5-trisphosphate receptor type 1), GAMT (guanidinoacetate N-methyltransferase), and CAMK4 (calcium/calmodulin-dependent protein kinase IV), deserve our attention and further study since they have been reported to be closely related to egg production, egg number, and reproductive traits. In addition, the most significant genomic region obtained in this study was located at 48.61–48.84 Mb on GGA5. In this region, we have repeatedly identified four genes, in which YY1 (YY1 transcription factor) and WDR25 (WD repeat domain 25) have been shown to be related to oocytes and reproductive tissues, respectively, which implies that this region may be a candidate region underlying egg number traits [44]. Hence, GWAS and transcriptome analyses and other modern technologies need to be applied to investigate the genes related to laying traits. Such investigation might accelerate the improvement of existing genotypes and the breeding of new breeds.
3. Cellular-Level Regulation of the HPO Axis in Avians
3.1. Insulin-like Growth Factor
Because of the complicated physiological effects of insulin-like growth factors and growth hormone, the roles of growth hormone and IGF-I are often concurrently investigated. Growth hormone (GH) and IGF-I exert biological effects by attaching to their receptors and engaging in downstream signaling cascades. Autocrine production of insulin-like growth factor-I mediates growth hormone-mediated DNA synthesis and proliferation in primary cultured hepatocytes of adult rats. GH is expressed in the GCs of avian ovarian follicles, suggesting that GH is involved in the development of the avian reproductive system via autocrine/paracrine secretion [45]. In vitro tests on primary cultured cells have revealed that GH enhanced the autocrine capacity of IGF-I via the JAK2/PLC signaling pathway of its receptor [46]. Subsequently, its receptor activated the tyrosine kinase signaling pathway of the IGF-I receptor, resulting in the biological impact of IGF-I [46]. This result indicates that growth hormone (GH) and insulin-like growth factor (IGF-1) are fundamental in poultry follicle development.
3.2. Epidermal Growth Factor
Pituitary gonadotropins (FSH and LH) play a vital role in ovarian development. However, pieces of evidence suggest that the local growth-promoting factors may influence gonadotropin secretion and enhance oocyte maturation through paracrine and autocrine mechanisms. Epidermal growth factor (EGF) interacts with the KIT receptor of oocytes via its ligand, thereby triggering the recruitment of primordial follicles and boosting oocyte development. EGF, transforming growth factor α (TGF-α), heparin-binding EGF-like growth factor (HB-EGF), amphiregulin (AR), epiregulin (ER), betacellulin (BTC), and β cytokine, are members of the EGFR family [47]. AR, ER, and BTC are expressed in the granulosa and thecal layers of ovarian follicles [48]. Exogenous EGF increases GC proliferation, inhibits granulosa cell apoptosis, and regulates steroid hormone production by GCs [49]. These findings suggest that heparin-binding EGF-like growth factor (HB-EGF) in the avian ovary can be utilized as an oocyte signal source to regulate granulosa cell proliferation.
3.3. Transforming Growth Factor β
TGF-β is a versatile cytokine of the transforming growth factor β superfamily that is primarily involved in the formation of extracellular matrix (ECM) in poultry. TGF-β is involved in the primary follicular development signaling pathway that controls follicular development [50]. The TGF-β signaling pathway is crucial in selecting hierarchical follicles from the small yellow follicle pool. The physiological process of follicular selection is affected by many endocrine, autocrine, and paracrine pathways. The major component of ECM is collagen, which is mostly dispersed in the follicular membrane layer and generated with the development of follicular GCs and follicular expansion [51]. These results indicate that ECM can bind to TGFβ, activate the TGFβ signaling pathway and regulate the process of follicle selection.
3.4. Immune Regulatory Factors
The occurrence of reproductive disorders in poultry is strongly correlated with the HPO axis and neuro–endocrine–immune network molecules, such as tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ), represented by inflammatory cytokines such as interleukin-10 (IL-10) and interleukin-6 (IL-6). The hosts’ natural immunity maintains follicular development, ovulation, fertilization, and egg formation in poultry, which may be related to several neurotransmitter receptors and endocrine hormone receptors on the surface of proliferating immune cells. Cell surface receptors and their ligands promote or inhibit immune cells. The nervous, endocrine, and immune systems interact to produce neurotransmitters, hormones, and cytokines, thereby forming a nerve–endocrine–immune network control system to maintain homeostasis in the body [52]. These results show that immune factors may play an important role in layer hens reproductive performance.
4. Molecular Regulation of the HPO Axis in Avians
4.1. The Role of Reproduction-Related Genes in HPO Axis Development
Transcriptome technology has been utilized to identify differentially expressed genes of the HPO axis, and the KEGG enrichment pathway analysis has been employed to establish the HPO signaling pathways. A study identified 414 differentially expressed genes in the pituitary gland, enriched in 108 GO terms in the high-laying and low-laying groups, which were connected to 4 enrichment signaling pathways. The ovary was found to have 356 differentially expressed genes enriched in 4 signaling networks, whereas the hypothalamus exhibited no KEGG signaling pathway [53]. Research has shown that GEGs were identified and analyzed in the HPO axis by transcriptome, and these genes are associated with layer hens reproductive performance. Rho-associated coiled-coil containing protein kinase 2 (ROCK2) is involved in the regulation of GTP synthesis in the hypothalamus, which may affect GnRH secretion after being activated by signals and directly interact with the pituitary gland to secrete gonadotropin, thereby acting on target organs and participating in biological activities [22]. Insulin-like growth factor binding protein 7 (IGFBP7) is an inducer of ovarian follicular development that is involved in the ovarian response to FSH [54]. The GTPase activating the Rap/Ran GAP domain-like 1 gene (GARNL1) is linked to EN300 and AFE [19].The POU class 1 homeobox 1 gene (POU1F1) is primarily responsible for activating promoters of the genes producing GH, prolactin, and the gonadotropin chain [55].
Most of the genes found are involved in follicles development, such as bone morphogenetic protein 15 (BMP15), which is a growth factor produced by oocytes involved in mammalian ovarian development and ovulation [56]. Steroidogenic acute regulatory protein (StAR) is a fast steroid synthesis regulatory protein that acts along with the cytochrome P450 family 11 subfamilies A member 1 (CYP11A1) gene to complete follicular selection [57,58]. CYP11A1, CYP17A1, and 17β-hydroxysteroid dehydrogenase type 2 (17β-HSD2) control the production of steroid hormones, including estrogen and progesterone, and among these genes, the expression of CYP11A1 is the most significant [59]. CYP11A1 expression in avian follicular GCs is required for progesterone secretion [60]. The major components of ECM, namely collagen type-VI α 2 chain (COL6A2), collagen type-IV α 1 chain (COL4A1), collagen type-IV α 2 chain (COL4A2), and collagen type-VI α 1 chain (COL6A1) are involved in the production of steroid hormones. ECM is primarily composed of collagen [61], laminin, proteoglycan, fibronectin, and glycoprotein, which influence biological processes, such as cell differentiation, proliferation, adhesion, morphogenesis, and phenotypic expression [62,63,64]. In several species, IGF-I enhances oocyte maturation. Bone morphogenetic protein 6 (BMP6) can increase FSH and anti-Mullerian hormone (AMH) expression in GCs, and AMH can promote the differentiation of GCs [65]. Inhibin subunit β B (INHBB) genes can induce early differentiation of GCs and enhance their capacity to stimulate steroid production. The function of these genes also suggests that they are involved in follicle selection in chickens.
4.2. The Role of Signaling Pathways in Granulosa Cell Development
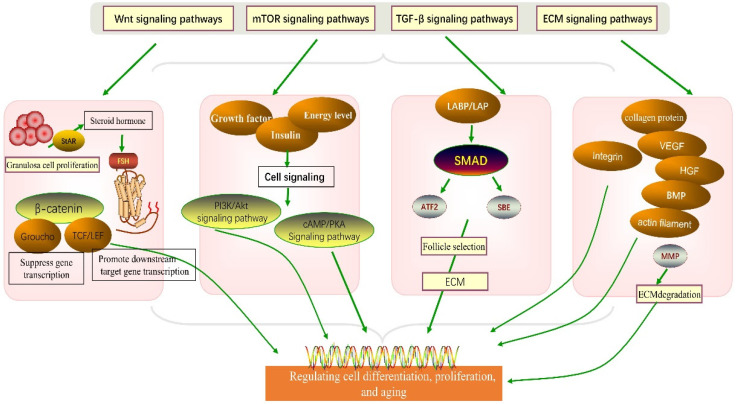
The HPO axis molecular signaling pathways play a crucial role in the laying performance of hens [66]. The whole-genome and transcriptome analysis techniques have been extensively applied in studying avian skeletal muscle development [67], examining sperm movement regulating factors [68], diagnosing poultry disease [69], and understanding genetic diversity [70]. However, the genes regulating egg-laying performance on the HPO axis are still poorly studied. Research has shown that the HPO axis is abundant in several signaling pathways associated with follicle selection. The pathway plays an important role in granulosa cell proliferation, differentiation, and apoptosis, including the mTOR signaling pathway, Wnt signaling pathway, TGF-β signaling pathway, ECM receptor signaling pathway, and steroid biosynthesis signaling pathway (Figure 3).
Figure 3.
The role of four signaling pathways in granulosa cell development.
4.2.1. Wnt Signaling Pathway
The Wnt signaling pathway plays a crucial role in the follicle selection of hens [71]. This pathway can regulate granulosa cell proliferation in follicles to increase steroid hormone production, and the biological mechanism of follicle selection in hens depends on the proliferation and differentiation of GCs [71]. The ovulation process in hens is hierarchical and begins from F6 to F1 follicles [25]. It is estimated that the ovary of sexually mature hens contains approximately 12,000 oocytes [28]; however, only a few of these oocytes reach the ovulation stage, and most small yellow follicles are not selected and undergo apoptosis [25]. Research has shown that BMPs, AHM, FSH, OCLN, STAR, CPY11A1, and other genes are expressed in ovarian GCs. OCLN and STAR have been linked to follicle selection; these genes may mediate the activation of a β-catenin-dependent pathway in the Wnt signaling pathway, which regulates GC proliferation, differentiation, and apoptosis. In the Wnt4 signaling pathway, wnt4 mRNA expression peaks at the small yellow follicles stage that promotes the proliferation of GCs [71]. The mRNA expression levels of STAR and CYP11A1 were enhanced in the pre-hierarchical follicle and hierarchical follicle stages. The Wnt signaling pathway promotes follicle selection and collagen synthesis in the ECM. Hence, the Wnt signaling pathway is a key biological signaling route in the follicular selection process. Further research is required to explore the molecular mechanism of the Wnt signaling pathway in improving the degree of follicle development and decreasing the number of follicles atretic. This could lay the groundwork for identifying molecular genetic markers of laying performance in hens and breeding new breeds.
4.2.2. mTOR Signaling Pathway
The mTOR signaling pathway is a conserved serine/threonine-protein kinase pathway that integrates extracellular signals and affects gene transcription and protein translation by phosphorylating the downstream target protein ribosomal P70S6 kinase, thereby regulating the biological processes, such as cell growth, proliferation, and differentiation [72,73]. Abnormal mTOR signaling pathways can cause reproductive disorders, such as premature ovarian failure and polycystic ovarian syndrome (PCOS) [74], cancer [75], diabetes [76], neurological disorders [77], and aging [78]. According to research, the mTOR signaling pathway regulates follicle development, meiosis and oocyte maturation, ovarian cell differentiation and steroid hormone release, sexual maturity, ovarian aging, and embryo development. During ovary development, the mTOR signaling pathways play a vital role in the development of the avian ovary and function in two ways. First, the pathway might be involved in the primordial follicle activation, promote sexual maturity, determine the ovarian reserves, and influence the length of the reproductive cycle, thus playing a vital role in avian ovary development. Second, the pathway mediates granulosa cell sensitivity to FSH and promotes granulosa cell growth. It primarily mediates the cyclic adenosine monophosphate/protein kinase A system signaling pathway (cAMP/PKA), which subsequently activates the mTOR signaling pathway and promotes follicle growth [79]. Research has shown that treatment of the 75-week and 35-week chicken granulosa cells with mTOR agonist MHY1485 enhances GCs proliferation and inhibits GCs apoptosis in 75-week layer hens, suggesting that the mTOR signaling pathway can regulate ovarian follicular development in aged laying hens [80].
4.2.3. TGF-β Signaling Pathway
TGF-β functions in tissue growth, homeostasis regulation, and repair [81] and is produced by immune cells [82] including T and B cells, dendritic cells, and macrophages. TGF-β is activated by a complex composed of LABP and LAP, which phosphorylates the TGF-βR-I receptor, activating SAMD and interacting with transcription factors at DNA sequence-specific sites ATF2 and SBE in the promoter region to mediate the TGF-β biological function. This regulates gene expression [83]. Additionally, TGF-β promotes other SAMD signal transduction pathways, including numerous mitogen-activated protein kinase (MAPK) and MAPK/ERK kinase (MEK) pathways, which are involved in RhoA and Ras upstream activation [84]. Depending on the cell type, TGF-β produces a range of complicated physiological responses, including late G1 growth arrest, differentiation programmed modifications, and apoptosis involving the phosphatidylinositol-3-kinase (PI3K) and protein phosphatase-2A. The differentiation, proliferation, and aging processes of follicles are all crucial for the selection of avian follicles, and the extracellular matrix signaling pathway is also implicated in the selection of avian follicles, resulting in the rapid growth and development of follicles. Research has shown that during follicle growth, collagen was secreted by GCs under TGF-β1 stimulation and was subsequently collaboratively transferred to neighboring TCs to increase cell proliferation and thus to promote follicle development via an intercellular cooperative pattern during development of chicken growing follicles [85]. These result show that TGF-β signaling pathway plays a critical role in follicular selection.
4.2.4. ECM Receptor Signaling Pathway
The ECM signaling pathway, with collagen as the major component, is a key pathway that influences follicle growth and development [86]. Cells modify the ECM through degradation and recombination mechanisms and interact with integrins to govern the cell differentiation and proliferation processes [87]. In addition, ECM can combine with growth factors, such as vascular endothelial growth factor, hepatocyte growth factor, and BMP, to regulate germ cell formation [88]. Actin recombination also regulates cell proliferation via the ECM. In this process, aberrant ECM recombination can result in various changes, among which pathological changes disrupt the ECM via the regulatory impact, thus altering cell differentiation, proliferation, and apoptosis. Matrix metalloproteinases (MMPs) are involved in several biological processes, such as follicle development, ovulation, atresia, and degeneration, all of which require ECM remodeling. PRL regulates the transcription and translation of certain components of the MMP metalloenzyme protein system, thereby regulating the proliferation of GCs [89].
4.3. The Role of Epigenetic Modification in Granulosa Cell Development
Noncoding RNA, DNA methylation, and RNA methylation is an important information carrier in epigenetic modification. With the continuous optimization and improvement of transcriptome technology, increasing noncoding sequences, which were originally considered as “transcriptional noise”, have been discovered [90]. Noncoding RNAs represented by microRNAs, long noncoding RNAs, circRNAs, and PiWi protein-interacting RNAs (piRNAs) play a vital role in granulosa cell differentiation, proliferation, growth, apoptosis, and other important physiological processes [91,92,93]. Therefore, examining the role of noncoding RNAs in GCs is essential.
4.3.1. The Role of Noncoding RNAs in Granulosa Cell Development
MicroRNAs (miRNA) are endogenous noncoding RNAs that exhibit a range of biological activities, including cell proliferation, differentiation, apoptosis, gonad development, and lipid metabolism [94,95]. Under the action of RNA polymerase II, miRNA primarily develops a lengthy stem–loop structure, referred to as primary miRNA (pri-miRNA). Pri-miRNA is processed into a pre-miRNA with a stem–loop structure of approximately 70 nucleotides under the action of the ribozyme Drosha III and other cofactors [96]. Pri-miRNA is subsequently carried to the nucleus by the RNA-GTP enzyme and exportin 5, where it is converted by nuclease into mature miRNAs with a length of approximately 22 nucleotides. Research has revealed that the mature miRNAs are expressed in various tissues, including embryos [97], ovaries, adipose tissues [98], lungs, and immunological organs. The role of miRNAs in avian ovarian development has been extensively studied. Transcriptomic studies of avian ovaries have revealed that the quantity of miR-26a-5p differs significantly between sexually mature and immature chickens [99]. AFE was linked to 10 single nucleotide polymorphisms, and miR-26a-5p reduced the expression of the target gene TRNCA [100]. The antiapoptotic gene B-cell lymphoma 2 (BCL-2) is upregulated, which regulates granulosa cell growth [100]. Simultaneously, miR-1a and miR-21 are differentially expressed in sexually mature and immature hens and follicles at various developmental stages, suggesting that miRNAs either inhibit mRNA translation or promote mRNA degradation, eventually affecting follicle development in the ovary [101]. The miRNAs are stably present in human and animal serum and plasma [102]. Therefore, it is a novel class of biomarkers for diagnosis of cancer and other diseases. However, a few studies have been conducted on the role of miRNAs in chicken GCs. As shown in Table 1.
Table 1.
Species and role of miRNA in granulosa cell development.
| miRNA | Target Genes | Signaling Pathways | Function | Tissue | Reference |
|---|---|---|---|---|---|
| miR-29c-3p | FOXL2 | cAMP/focal adhesion | Transcription factor Promotes differentiation of pre-hierarchical GCs (PhGCs) and preovulatory GCs (PoGCs) | Chicken (PhGCs/PoGCs) | [71] |
| / | WNT6/LRP1/FOXO1 | Wnt/β-catenin dependent pathway | WNT6 cooperates with FSH to promote follicle development | Hyline-brown (SYF) | [103] |
| gga-miR-30a-5p | BCL1 | miR-30a-5p regulatory pathway | Activates (proliferation) | Chicken | [104] |
| gga-miR-146b-3p | AKT1 | PI3K/ATK | Inhibits (proliferation) | Chicken | [105] |
| miR-458b-5p | CTNNB1 (3′UTR) | Wnt/β-catenin | Inhibits (proliferation) | Chicken (Hyline Brown ) | [106] |
| miR-26a-5p | TNRC6A | / | facilitates chicken ovarian cell proliferation | Chicken (ovarian) | [100] |
| miR-23b-3p | GDF9 | TGF-β signaling pathway | Inhibits (proliferation) steroid hormone synthesis |
Chicken (SYF) | [107] |
| miR-200a | ZEB1/SIP1/SIRT1 | reproduction regulation pathways | Inhibit granulosa cells proliferation | Lu hua chicken | [10] |
| miR-138-1-3p | COL4A5 | matrix metalloproteinase (MMP) | Remodeling of the extracellular matrix during ovarian follicle development in chickens | Chicken (largest preovulatory follicles) | [108] |
| miR-449b-5p | IGF2BP3 | Steroid hormone synthesis signaling pathway | Regulate the expression of key steroidogenesis-related genes (StAR and CYP19A1) | Chicken (SYF) | [109] |
| miR-135a-5p | KLF4/ATP8A1/CPLX1 | p53 Signaling pathway | Involve in proliferation and differentiation in chicken ovarian follicular | Chicken | [110] |
| miR-138-5p | SIRT1 | Apoptosis signaling pathway | Promotes apoptosis and follicular atresia | Chicken | [111] |
| miR-10a-5p | MAPRE1 | / | Inhibits proliferation and progesterone synthesis | Tian fu chicken | [112] |
| miR-122-5p | MAPK3 | / | Promotes apoptosis through the post-transcriptional downregulation of MAPK3. | Chicken (hierarchal follicles) | [113] |
| miR-302a-3p | DRD1 | / | Inhibits GCs proliferation | Chicken (Small yellow follicle) | [114] |
Abbreviations: Forkheadbox L2 (FOXL2); WNT family member 6 (Wnt6); forkhead box transcription factor O1 (FOXO1); B-cell lymphoma 1 (BCL1); trinucleotide repeat-containing gene 6a (TNRC6a); growth and differentiation factor 9 (GDF9); collagen type IV α 5 chain (COL4A5); insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3); dopamine receptors 1 (DRD1); Krüppel-like factor 4 (KLF4); ATPase phospholipid transporting 8A1 (ATP8A1); complexin-1 (CPLX1); silencing information regulator 2 related enzyme 1 (SIRT1); mitogen-activated protein kinase 3 (MAPK3); microtubule associated protein RP/EB family member 1 (MAPRE1); catenin β 1 (CTNNB1); zine finger E-box binding homeobox (ZEB1); Smad interacting protein 1 (SIP1).
Long noncoding RNAs (LncRNA) are longer than 200 nucleotides and lack protein coding functions and are pivotal epigenomic factors [115]. Additionally, they are involved in many biological functions, such as mRNA splicing, gene regulation, and protein stabilization [116,117]. LncRNAs are also essential molecular components that regulate the laying performance and play a role in post-translational mRNA processing [118,119]. Long noncoding RNAs control the formation of small yellow follicles in the ovary [120]. Many distinct mRNAs may play a role in follicle growth by regulating steroid hormone production, oocyte meiosis, and the p13K-Akt signaling pathway [121]. A transcriptomic-based analysis identified 160 mRNAs and 550 lncRNAs that regulate follicular development in various methods, many of which are involved in oocyte meiosis, progesterone-mediated oocyte maturation, and cell cycle [122]. As shown in Table 2.
Table 2.
Species and role of lncRNAs in granulosa cell development.
| LncRNA | Target Gene | Function | Species | Reference |
|---|---|---|---|---|
| LncRNAXLOC_110025 | MOS BMP15 WNT6 CDC25A | Promote follicle development | Hy-line Brown | [122] |
| LncRNA_138134 | PSMD6 | Affect oocyte maturation | Human (GCs) | [123] |
| LncRNA_210520.2 | SOWAHA | Inhibited the proliferation of granulosa cells | Chicken (SYF) | [124] |
| Lnc RNAGLM | ERα | Regulated by estrogen through ERα | Laying hens | [125] |
| LncRNALTR | ERβ | Involved in lipid metabolism | Chicken | [10] |
| LncRAN MTSRG.17017.1 MTSRG.6475.20 |
CACNA1C
TGFB1 |
Affect gonad development and GnRH signaling pathway | White Leghorns and Beijing You chickens | [126] |
| LncRNA XLOC_001347 XLOC_016063 XLOC_02660 XLOC_03201 XLOC_005141 |
CASP6/MMP2/SMAD2 | Involved cell growth, proliferation, and development | Broody chickens (BC) and normal ovaries (NO) | [52] |
| LncRNA RP4-545C24.1 | RAD51 WT1 | Inhibition of DNA damage repair capacity | Human | [127] |
Abbreviations: MOS (MOS proto-oncogene, serine/threonine kinase); PSMD6 (proteasome 26S subunit, non-ATPase 6); sosondowah ankyrin repeat domain family member A (SOWAHA); calcium volt aggregated subunit alphal C (CACNA1C); transforming growth factor beta1 (TGFB1); caspase 6 (CASP6); RAD51 (RAD51 recombinase); WT1 transcription factor (WT1).
CircRNAs are a novel type of noncoding RNA that form a covalently closed continuous loop, and they lack the 5′ terminal cap structure and 3’ terminal ploy (A) tail; these noncoding RNAs are ubiquitous in living organisms [128]. With the development of sequencing technology, some important circRNAs that participate in various biological functions have been discovered [20]; circRNA can be used as a biomarker of diseases to guide production practices [129]. Recent studies have revealed that circRNA molecules have numerous miRNA-binding sites; thus, they can function as miRNA sponges, as well as the regulators of splicing and transcription, and then indirectly regulate the expression of downstream target genes of miRNAs [130]. Studies on circRNA have focused on its role in only human and mouse granulosa cell development, and only a few studies have investigated the role of circRNAs in layer hen GCs, necessitating further studies. As shown in Table 3.
Table 3.
Species and role of circRNAs in granulosa cell development.
| CircRNA | miRNA | Target Gene | Function | Species | Reference |
|---|---|---|---|---|---|
| circRNA-aplacirc_13267 | apla-miR-1-13 | THBS1 | Promotes granulosa cell apoptosis | Duck | [131] |
| circRNA_RANBP9 | miR-136-5p | XIAP | Inhibits granulosa cell apoptosis | Human (Polycystic ovary syndrome) | [132] |
| circRNA_EML1 | miR-449a | IGF2BP3 | Promotes granulosa cell steroid hormone synthesis and estrogen and progesterone secretion, down regulate miR-449a | Hyline Brown | [133] |
| circRNA_0320 circRNA_0185 |
miR-143-3p | FSHR | Promotes GC differentiation and follicle development | Chicken (SYF) |
[134] |
| sss-circINHA-001 | miR-24-5p miR-7144-3p miR-9830-5p |
INHA | Resisting GC apoptosis and follicular atresia | Chicken | [135] |
| circRNA_8:6369673|6402248 circRNA_8:6369673|642209 circRNA_8:6384248|6402248 |
miR-1625-3p miR-1552-3p miR-16-2-3p miR-18b-3p miR-200a-3p |
RalGPS2 | Regulate GC development | Chicken (SYF/F6/F1) | [72] |
| novel_circ0004730 | / | ESR | Cell growth, proliferation, differentiation, and apoptosis | Chicken (SYF/F6/F1) | [136] |
Abbreviations: throbospondin1 (THBS1); X-linked inhibitor of apoptosis (XIAP); RalGEF with PH domain and SH3 binding motif 2 (RalGPS2).
4.3.2. The Role of DNA Methylation in Granulosa Cell Development
Environmental factors can impact epigenetic DNA modification by modifying the gene activity or regulating the gene expression. Epigenetic modification is a type of DNA variation that influences gene expression without altering the DNA coding sequence [137,138,139]. The DNA methylation level is mainly determined by the presence of CpG island in the promoter region. CpG differential methylated regions (DMRs) are significant epigenetic modification markers and functional areas involved in gene transcription [140,141,142]. To begin, 5 progesterone-mediated oocyte maturation genes, namely cell division cycle 27 (CDC27), adenylate cyclase 8 (ADCY8), AKT serine/threonine kinase 3 (AKT3), and microtubule-associated serine/threonine kinase 2 (MAST2), were discovered by the KEGG pathway enrichment analysis of the hypothalamus and ovary genes [143]. CDC27 is a protein required for encoding cell cycle progression, and it is believed to be involved in cell proliferation and division [144,145]. The CpG island methylation level of this gene was reported to be high in Langshan chickens with a high degree of inbreeding, indicating that methylation of this gene affected transcription expression, thereby affecting oocyte maturation, which was one of the main reasons for the decline in reproductive performance of the inbred Langshan hens [146].
Furthermore, laying hens serve as a model for human ovarian cancer research [147]. In laying hens, DNA methyltransferases 1, 3A, 3B (DNMT1, DNMT3A, and DNMT3B) genes are strongly expressed in GCs of cancerous ovaries, and miR-1741, miR-16c, miR-222, or miR-1632 directly bind to the DNMT3A or DNMT3B transcripts. Following transcription, the expression of DNMT3A and DNMT3B genes is regulated. The expression level of DNMTs was determined in normal and cancerous ovaries, and the miRNA target validation assays indicated that the DNMTs are regulated by the post-transcriptional effects of particular miRNAs [148]. The DNMT genes exhibit cell-specific expression patterns, and the present review elaborates on the methylation state of the CpG island and the promoter region of a tumor suppressor gene in normal and cancerous laying hen ovaries.
DNMT3A and DNMT3B may methylate semi-methylated or unmethylated CpG islands at the same rate. Although the overall structure of DNMT3 is comparable to that of DNMT1, its total length is shorter, and it carries a distinct proline–tryptophan–tryptophan–proline (PWWP) tetrapeptide. Furthermore, depending on the type of tumor, overexpression of DNMT3A or DNMT3B is linked to tumorigenesis in humans [149]. These findings imply that DNMT3A and DNMT3B function as DNA methyltransferases and play vital roles in normal development and diseases [150].As shown in Figure 4
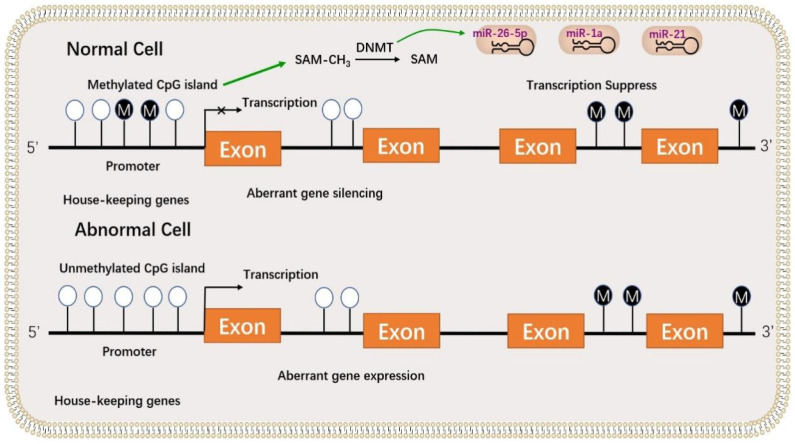
Figure 4.
The role of DNA methylation in granulosa cell development. There are two main patterns of DNA methylation, including methylation of CpG islands (normal cells) and unmethylation of CpG islands (abnormal cells). These CpG islands are located in the promoter regions of housekeeping genes. If the CpG islands are methylated in the promoter region, the expression of downstream genes is inhibited, and cell development is normal. If the CpG islands are unmethylated in the promoter region, downstream genes are activated, and cell development is abnormal. The CpG island for methylation donors complete the process of demethylation under the action of the DNMT enzyme, which combines with miRNAs (miR-26-5p, miR-1a, and miR-21) to suppress transcription.
5. Interaction Network Regulation Mechanism of the HPO Axis
5.1. Organism-Level Regulation
The HPO axis is crucial for improving egg production performance. From a systematic standpoint, clarifying the molecular mechanism of the HPO axis control of laying performance is crucial. The laying performance is directly regulated at the organism level and indirectly regulated by the ‘Gut–Brain Axis’. Organism-level regulation induces the release of signal molecules directly to the hypothalamus, triggering GnRH production, acting on the pituitary gland to secrete FSH and LH and regulating the target ovary tissues to release E2 and P4. These hormones regulate the proliferation, differentiation, and development of ovarian follicles at the organism level, thereby improving egg production performance. Simultaneously, dietary status and environmental factors directly or indirectly influence the HPO axis.
5.2. Cellular-Level Regulation
At the cellular level, the HPO axis is controlled by several processes. First, the liver cells produce and release IGF-I in response to GHs secreted by the pituicytes. Therefore, IGF-I is sometimes referred to as somatomedins (SM). Second, transforming growth factor and EGF indirectly regulate the synthesis and development of ECM and primary follicles. Third, certain reproductive functions are directly linked to the immune regulatory factors that regulate ovarian follicle formation through the ‘neuro–endocrine–immune’ pathway comprising T cells, TNF-α, and TNF-β produced by macrophages.
5.3. Molecular-Level Regulation
Many genes and signaling pathways jointly regulate the HPO axis and perform biological functions at the molecular level. First, egg production is determined by follicular selection, which facilitates the regulation of steroid-related genes StAR and CYP11A1 that mediates the TGF-β signaling pathway. Second, FSHR, OCLN, AMH, and other genes in the Wnt signaling pathway, mTOR signaling pathway, and ECM signaling pathway influence follicular regeneration or delay follicular atresia by enhancing gene expression to accomplish follicular renewal and improve hens’ laying performance. Third, miRNA and lncRNA are also involved in the proliferation, differentiation, and atrestic regulation of the follicular GCs of hens. MiRNAs and lncRNAs may be involved in forming follicular cells, potentially providing a novel means and methodology for the molecular breeding of chickens. Fourth, DNA methylation can directly affect the methylation level of each tissue along with the HPO axis and significantly affect the methylation level of the CpG island in associated tissues. Furthermore, the liver connects hepatic lipid metabolism to the reproductive cycle through ERα, and the primary mechanism is related to yolk production through VLDL.
The laying performance is directly controlled by the HPO axis, thereby providing key genes and molecular signaling pathways involved in egg production, which can be identified through the genome, transcriptome, and other emerging technologies. Moreover, selecting key gene SNP markers can improve the efficiency of the choice of livestock and poultry breeds, laying a foundation for breeding new breeds.
6. Recommendations for Future Research
The economic benefits of large-scale chicken egg farms are determined by laying performance, which is one of the most important economic aspects of laying hen production. The HPO axis, which regulates egg production, is connected to the adrenal gland, thyroid gland, gut–brain axis, and liver lipid metabolism. Many researchers are investigating the differential expression genes and signaling pathway networks across the hypothalamus, pituitary, and ovarian tissues; however, from the perspective of system biology, studies investigating the key HPO axis controlling genes and signaling pathways of these function are limited. Therefore, future studies should focus on the following aspects:
-
(1)
Compared with the common transcriptome, single-cell RNA sequencing is a high-precision technique that can provide accurate resolution from the multicellular level to a single-cell level. This separates specific characteristics of the reaction cells or a group of cells based on RNA abundance to evaluate the condition of a single cell. Additionally, the single-cell RNA sequencing can be valuable for studying different cell types and developmental track. Therefore, single-cell RNA sequencing is a valuable tool for studying the molecular mechanism of GCs involved in the regulation of the egg production performance, which can effectively improve the efficiency of molecular breeding and accelerate breed selection.
-
(2)
Metabolomics is often referred to as the bridge between genomics and phenotype, and metabolic components can be used as biomarkers for determining complex biological traits. Using the transcriptome data to obtain a large number of differentially expressed genes and differential metabolites obtained through correlation analysis, and from two levels, causes and consequences of organism are analyzed, identifying key gene targets, metabolites, and metabolic pathways to build the key control networks. Efficient seed selection based on biomarkers is also the development direction in the breeding of layer hens.
-
(3)
Proliferation, differentiation, and apoptosis of GCs are the key to prolonging the laying cycle, and GC gene expression is temporally and spatially regulated. Future studies should utilize single-cell RNA sequencing and spatial transcriptomics to analyze the gene expression from both temporal and spatial dimensions in GCs. Moreover, the chromatin accessibility analysis technique can be used to predict the dynamic changes of chromatin conformation during follicle development, which may provide ideas for the breeding of high-reproductive performance hens.
Acknowledgments
We would like to thank the Major Science and Technology Project of Joint Funds of the National Natural Science Foundation of China, Yunnan Xichou black bone chicken Industry science and technology mission, Yunnan Su Zhengchang Expert Workstation, and National System for Layer Production Technology.
Author Contributions
Conceptualization, J.Z. and H.P.; methodology, J.Z.; software, J.Z..; validation, Y.L.; writing—original draft preparation, J.Z. and H.P.; writing—review and editing, H.S.; visualization, J.Z.; supervision, Y.H.; project administration, C.G.; funding acquisition, C.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Major Science and Technology Project of Joint Funds of the National Natural Science Foundation of China (U2002205), Yunnan Xichou black bone chicken Industry science and technology mission (202104BI090020), Yunnan Su Zhengchang Expert Workstation (20149IC008), National System for Layer Production Technology (CARS-40-S25).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.van Smabeek F. Breeding for 500 Eggs in 100 Weeks [EB/OL] Mar 2, 2011. [(accessed on 2 March 2011)]. Available online: https://www.poultryworld.net/poultry/breeding-for-500-eggs-in-100-weeks/
- 2.Ministry of Agriculture and Rural Affairs of the People’s Republic of China Announcemengt No.2 of the Ministry of Agricukture of the Peopie’s Republic of China. [(accessed on 29 April 2021)]; Available online: http://www.gov.cn/xinwen/202104/29/content_5603727.htm.
- 3.Yang S., Chen J., Cheng Y. Characteristics of Livestock Production System in USA and Enlightenment for China. Food Nutr. China. 2017;23:20–24. [Google Scholar]
- 4.Onagbesan O.M., Mast J., Goddeeris B., Decuypere E. Effect of TNF-alpha on LH and IGF-I modulated chicken granulosa cell proliferation and progesterone production during follicular development. Reproduction. 2000;120:433–442. doi: 10.1530/jrf.0.1200433. [DOI] [PubMed] [Google Scholar]
- 5.Lovell T.M., Gladwell R.T., Groome N.P., Knight P.G. Ovarian follicle development in the laying hen is accompanied by divergent changes in inhibin A, inhibin B, activin A and follistatin production in granulosa and theca layers. J. Endocrinol. 2003;177:45–55. doi: 10.1677/joe.0.1770045. [DOI] [PubMed] [Google Scholar]
- 6.Rivas R.E.C., Nieto M.P.C., Kamiyoshi M. Effects of Steroid Hormone in Avian Follicles. Asian-Australas. J. Anim. Sci. 2016;29:487–499. doi: 10.5713/ajas.15.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komatsu K., Masubuchi S. Observation of the dynamics of follicular development in the ovary. Reprod. Med. Biol. 2017;16:21–27. doi: 10.1002/rmb2.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowak M., Grzesiak M., Saito N., Kwaśniewska M., Sechman A., Hrabia A. Expression of aquaporin 4 in the chicken ovary in relation to follicle development. Reprod. Domest. Anim. 2017;52:857–864. doi: 10.1111/rda.12990. [DOI] [PubMed] [Google Scholar]
- 9.Brady K., Porter T.E., Liu H.C., Long J.A. Characterization of the hypothalamo–pituitary–gonadal axis in low and high egg producing turkey hens. Poult. Sci. 2020;99:1163–1173. doi: 10.1016/j.psj.2019.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu N., Gaur U., Zhu Q., Chen B., Xu Z., Zhao X., Yang M., Li D. Expressed microRNA associated with high rate of egg production in chicken ovarian follicles. Anim. Genet. 2017;48:205–216. doi: 10.1111/age.12516. [DOI] [PubMed] [Google Scholar]
- 11.Shi S., Zhou X., Li J., Zhang L., Hu Y., Li Y., Yang G., Chu G. MiR-214-3p promotes proliferation and inhibits estradiol synthesis in porcine granulosa cells. J. Anim. Sci. Biotechnol. 2020;11:94. doi: 10.1186/s40104-020-00500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M., Wang Y., Yao W., Du X., Li Q. Lnc2300 is a cis-acting long noncoding RNA of CYP11A1 in ovarian granulosa cells. J. Cell. Physiol. 2022;237:4238–4250. doi: 10.1002/jcp.30872. [DOI] [PubMed] [Google Scholar]
- 13.Kuo Y.M., Shiue Y.L., Chen C.F., Tang P.C., Lee Y.P. Proteomic analysis of hypothalamic proteins of high and low egg production strains of chickens. Theriogenology. 2005;64:1490–1502. doi: 10.1016/j.theriogenology.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Padmanabhan V., Karsch F.J., Lee J.S. Hypothalamic, pituitary and gonadal regulation of FSH. Reprod. Suppl. 2002;59:67–82. [PubMed] [Google Scholar]
- 15.Cao C., Ding Y., Kong X., Feng G., Xiang W., Chen L., Yang F., Zhang K., Chu M., Wang P., et al. Reproductive role of miRNA in the hypothalamic-pituitary axis. Mol. Cell. Neurosci. 2018;88:130–137. doi: 10.1016/j.mcn.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Millar R.P., Lu Z.L., Pawson A.J., Flanagan C.A., Morgan K., Maudsley S.R. Gonadotropin-Releasing Hormone Receptors. Endocr. Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- 17.Metallinou C., Asimakopoulos B., Schröer A., Nikolettos N. Gonadotropin-releasing hormone in the ovary. Reprod. Sci. 2007;14:737–749. doi: 10.1177/1933719107310707. [DOI] [PubMed] [Google Scholar]
- 18.Maddineni S., Ocón-Grove O.M., Krzysik-Walker S.M., Hendricks G.L., 3rd, Proudman J.A., Ramachandran R. Gonadotrophin-inhibitory hormone receptor expression in the chicken pituitary gland: Potential influence of sexual maturation and ovarian steroids. J. Neuroendocrinol. 2008;20:1078–1088. doi: 10.1111/j.1365-2826.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 19.Shen X., Zeng H., Xie L., He J., Li J., Xie X., Luo C., Xu H., Zhou M., Nie Q., et al. The GTPase Activating Rap/RanGAP Domain-Like 1 Gene Is Associated with Chicken Reproductive Traits. PLoS ONE. 2012;7:e33851. doi: 10.1371/journal.pone.0033851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X., Sun X., Chimbaka I.M., Qin N., Xu X., Liswaniso S., Xu R., Gonzalez J.M. Transcriptome Analysis of Ovarian Follicles Reveals Potential Pivotal Genes Associated with Increased and Decreased Rates of Chicken Egg Production. Front. Genet. 2021;12:622751. doi: 10.3389/fgene.2021.622751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson F.E., Renema R.A., Oosterhoff H.H., Zuidhof M.J., Wilson J.L. Carcass Traits, Ovarian Morphology and Egg Laying Characteristics in Early Versus Late Maturing Strains of Commercial Egg-Type Hens. Poult. Sci. 2001;80:37–46. doi: 10.1093/ps/80.1.37. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Li C., Li Q., Li G., Li W., Li H., Kang X., Tian Y. Novel Regulatory Factors in the Hypothalamic-Pituitary-Ovarian Axis of Hens at Four Developmental Stages. Front. Genet. 2020;11:591672. doi: 10.3389/fgene.2020.591672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu G., Mao Y., Zhou W., Jiang Y. Dynamic Changes in the Follicular Transcriptome and Promoter DNA Methylation Pattern of Steroidogenic Genes in Chicken Follicles throughout the Ovulation Cycle. PLoS ONE. 2015;10:e0146028. doi: 10.1371/journal.pone.0146028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson P.A. Follicle Selection in the Avian Ovary. Reprod. Domest. Anim. 2012;47:283–287. doi: 10.1111/j.1439-0531.2012.02087.x. [DOI] [PubMed] [Google Scholar]
- 25.Johnson A.L., Woods D.C. Dynamics of avian ovarian follicle development: Cellular mechanisms of granulosa cell differentiation. Gen. Comp. Endocrinol. 2009;163:12–17. doi: 10.1016/j.ygcen.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Lyu Z., Qin N., Tyasi T.L., Zhu H., Liu D., Yuan S., Xu R. The Hippo/MST Pathway Member SAV1 Plays a Suppressive Role in Development of the Prehierarchical Follicles in Hen Ovary. PLoS ONE. 2016;11:e0160896. doi: 10.1371/journal.pone.0160896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson F.E., Fasenko G.M., Renema R.A. Optimizing Chick Production in Broiler Breeders. Volume 1 Spotted Cow Press; Edmonton, AB, Canada: 2003. [Google Scholar]
- 28.Onagbesan O., Bruggeman V., Decuypere E. Intra-ovarian growth factors regulating ovarian function in avian species: A review. Anim. Reprod. Sci. 2009;111:121–140. doi: 10.1016/j.anireprosci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Sharp P.J. Photoperiodic Control of Reproduction in the Domestic Hen1. Poult. Sci. 1993;72:897–905. doi: 10.3382/ps.0720897. [DOI] [PubMed] [Google Scholar]
- 30.Liu G., Dunnington E.A., Siegel P.B. Correlated Responses to Long-Term Divergent Selection for Eight-Week Body Weight in Chickens: Growth, Sexual Maturity, and Egg Production. Poult. Sci. 1995;74:1259–1268. doi: 10.3382/ps.0741259. [DOI] [PubMed] [Google Scholar]
- 31.Yang N., Jiang R.S. Recent advances in breeding for quality chickens. Proc. Nutr. Soc. 2005;61:373–381. doi: 10.1079/WPS200563. [DOI] [Google Scholar]
- 32.Sirotkin A.V., Harrath A.H., Grossmann R. The role of metabolic state and obestatin in control of chicken ovarian hormone release. Poult. Sci. 2016;95:1939–1942. doi: 10.3382/ps/pew108. [DOI] [PubMed] [Google Scholar]
- 33.Mehlhorn J., Höhne A., Baulain U., Schrader L., Weigend S., Petow S. Estradiol-17ß Is Influenced by Age, Housing System, and Laying Performance in Genetically Divergent Laying Hens (Gallus gallus f.d.) Front. Physiol. 2022;13:954399. doi: 10.3389/fphys.2022.954399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eusemann B.K., Baulain U., Schrader L., Thöne-Reineke C., Patt A., Petow S. Radiographic examination of keel bone damage in living laying hens of different strains kept in two housing systems. PLoS ONE. 2018;13:e0194974. doi: 10.1371/journal.pone.0194974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu L., Li D., Gilbert E.R., Xiao Q., Zhao X., Wang Y., Yin H., Zhu Q. Effect of Monochromatic Light on Expression of Estrogen Receptor (ER) and Progesterone Receptor (PR) in Ovarian Follicles of Chicken. PLoS ONE. 2015;10:e0144102. doi: 10.1371/journal.pone.0144102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson A.L., Solovieva E.V., Bridgham J.T. Relationship between steroidogenic acute regulatory protein expression and progesterone production in hen granulosa cells during follicle development. J. Biol. Reprod. 2002;67:1313. doi: 10.1095/biolreprod67.4.1313. [DOI] [PubMed] [Google Scholar]
- 37.Zhou S., Ma Y., Zhao D., Mi Y., Zhang C. Transcriptome profiling analysis of underlying regulation of growing follicle development in the chicken. J. Poult. Sci. 2020;99:2861–2872. doi: 10.1016/j.psj.2019.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang T., Chen L., Han K., Zhang X., Zhang G., Dai G., Wang J., Xie K. Transcriptome analysis of ovary in relatively greater and lesser egg producing Jinghai Yellow Chicken. J. Anim. Reprod. Sci. 2019;208:106114. doi: 10.1016/j.anireprosci.2019.106114. [DOI] [PubMed] [Google Scholar]
- 39.Ren J., Tian W., Jiang K., Wang Z., Wang D., Li Z., Yan F., Wang Y., Tian Y., Ou K., et al. Global investigation of estrogen-responsive genes regulating lipid metabolism in the liver of laying hens. BMC Genom. 2021;22:428. doi: 10.1186/s12864-021-07679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H., Wang T., Xu C., Wang D., Ren J., Li Y., Tian Y., Wang Y., Jiao Y., Kang X., et al. Transcriptome profile of liver at different physiological stages reveals potential mode for lipid metabolism in laying hens. BMC Genom. 2015;16:763. doi: 10.1186/s12864-015-1943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L., Chen F., Cao J., Dong Y., Wang Z., Hu M., Chen Y. Green light inhibits GnRH-I expression by stimulating the melatonin-GnIH pathway in the chick brain. J. Neuroendocr. 2017;29 doi: 10.1111/jne.12468. [DOI] [PubMed] [Google Scholar]
- 42.Zhao F.Q., Zhang Z.W., Qu J.P., Yao H.D., Li M., Li S., Xu S.W. Cold stress induces antioxidants and Hsps in chicken immune organs. Cell Stress Chaperones. 2014;19:635–648. doi: 10.1007/s12192-013-0489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farag M.R., Alagawany M. Physiological alterations of poultry to the high environmental temperature. J. Therm. Biol. 2018;76:101–106. doi: 10.1016/j.jtherbio.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Zhao X., Nie C., Zhang J., Li X., Zhu T., Guan Z., Chen Y., Wang L., Lv X.Z., Yang W., et al. Identification of candidate genomic regions for chicken egg number traits based on genome-wide association study. BMC Genom. 2021;22:610. doi: 10.1186/s12864-021-07755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahumada S.S.M., Martínez M.C.G., Carranza M., Ávila M.J., Luna A.J.L., Harvey S., Luna M., Arámburo C. Autocrine/paracrine proliferative effect of ovarian GH and IGF-I in chicken granulosa cell cultures. Gen. Comp. Endocrinol. 2016;234:47–56. doi: 10.1016/j.ygcen.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Kurihara K., Moteki H., Kimura M., Ogihara M. Autocrine secretion of insulin-like growth factor-I mediates growth hormone-stimulated DNA synthesis and proliferation in primary cultures of adult rat hepatocytes. Eur. J. Pharmacol. 2021;891:173753. doi: 10.1016/j.ejphar.2020.173753. [DOI] [PubMed] [Google Scholar]
- 47.Richani D., Gilchrist R.B. The epidermal growth factor network: Role in oocyte growth, maturation and developmental competence. Hum. Reprod. Update. 2018;24:1–14. doi: 10.1093/humupd/dmx029. [DOI] [PubMed] [Google Scholar]
- 48.Luo J., Ye H., Hao L., Sun Y., Li R., Li Y., Yang Z. SRSFs mediate the function of AR in the ovarian granulosa cells of patients with PCOS. Genes Dis. 2019;8:94–109. doi: 10.1016/j.gendis.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiara Perego M., Bellitto N., Maylem E.R.S., Caloni F., Spicer L.J. Effects of selected hormones and their combination on progesterone and estradiol production and proliferation of feline granulosa cells cultured in vitro. Theriogenology. 2021;168:1–12. doi: 10.1016/j.theriogenology.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Liu H., Zhang W., Li Q., Liu J., Zhang T., Zhou T., Li L., Wang J., Xu H., He H. The comprehensive mechanisms underlying nonhierarchical follicular development in geese (Anser cygnoides) Anim. Reprod. Sci. 2015;159:131–140. doi: 10.1016/j.anireprosci.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Li H., Chang H.M., Shi Z., Leung P.C.K. The p38 signaling pathway mediates the TGF-β1-induced increase in type I collagen deposition in human granulosa cells. FASEB J. 2020;34:15591–15604. doi: 10.1096/fj.202001377R. [DOI] [PubMed] [Google Scholar]
- 52.Liu L., Xiao Q., Gilbert E.R., Cui Z., Zhao X., Wang Y., Yin H., Li D., Zhang H., Zhu Q. Whole-transcriptome analysis of atrophic ovaries in broody chickens reveals regulatory pathways associated with proliferation and apoptosis. Sci. Rep. 2018;8:7231. doi: 10.1038/s41598-018-25103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mishra S.K., Chen B., Zhu Q., Xu Z., Ning C., Yin H., Wang Y., Zhao X., Fan X., Yang M., et al. Transcriptome analysis reveals differentially expressed genes associated with high rates of egg production in chicken hypothalamic-pituitary-ovarian axis. Sci. Rep. 2020;10:5976. doi: 10.1038/s41598-020-62886-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamura K., Matsushita M., Endo A., Kutsukake M., Kogo H. Effect of Insulin-Like Growth Factor-Binding Protein 7 on Steroidogenesis in Granulosa Cells Derived from Equine Chorionic Gonadotropin-Primed Immature Rat Ovaries. Biol. Reprod. 2007;77:485–491. doi: 10.1095/biolreprod.106.058867. [DOI] [PubMed] [Google Scholar]
- 55.Jiang R., Li J., Qu L., Li H., Yang N. A new single nucleotide polymorphism in the chicken pituitary-specific transcription factor (POU1F1) gene associated with growth rate. Anim. Genet. 2004;35:344–346. doi: 10.1111/j.1365-2052.2004.01164.x. [DOI] [PubMed] [Google Scholar]
- 56.Han H., Lei Q., Zhou Y., Gao J., Liu W., Li F., Zhang Q., Lu Y., Cao D. Association between BMP15 Gene Polymorphism and Reproduction Traits and Its Tissues Expression Characteristics in Chicken. PLoS ONE. 2015;10:e0143298. doi: 10.1371/journal.pone.0143298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou R., Miao Y., Li Y., Li X., Xi J., Zhang Z. MicroRNA-150 promote apoptosis of ovine ovarian granulosa cells by targeting STAR gene. Theriogenology. 2019;127:66–71. doi: 10.1016/j.theriogenology.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Rytelewska E., Kisielewska K., Gudelska M., Kiezun M., Dobrzyn K., Bors K., Wyrebek J., Kaminska B., Kaminski T., Smolinska N. The effect of orexin a on the StAR, CYP11A1 and HSD3B1 gene expression, as well as progesterone and androstenedione secretion in the porcine uterus during early pregnancy and the oestrous cycle. Theriogenology. 2020;143:179–190. doi: 10.1016/j.theriogenology.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Shih M.C.M., Chiu Y.N., Hu M.C., Guo I.C., Chung B. Regulation of steroid production: Analysis of Cyp11a1 promoter. Mol. Cell. Endocrinol. 2011;336:80–84. doi: 10.1016/j.mce.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 60.Chien Y., Cheng W.C., Wu M.R., Jiang S.T., Shen C.K.J., Chung B. Misregulated Progesterone Secretion and Impaired Pregnancy in Cyp11a1 Transgenic Mice1. Biol. Reprod. 2013;89:91. doi: 10.1095/biolreprod.113.110833. [DOI] [PubMed] [Google Scholar]
- 61.Chen P., Cescon M., Bonaldo P. Collagen VI in cancer and its biological mechanisms. Trends Mol. Med. 2013;19:410–417. doi: 10.1016/j.molmed.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Theocharis A.D., Skandalis S.S., Gialeli C., Karamanos N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Akbari D.N., Shoorei H., Sharifi G., Mohaqiq M., Majidpoor J., Dinger M.E., Taheri M., Ghafouri F.S. Non-coding RNAs modulate function of extracellular matrix proteins. Biomed. Pharmacother. 2021;136:111240. doi: 10.1016/j.biopha.2021.111240. [DOI] [PubMed] [Google Scholar]
- 65.Umer S., Sammad A., Zou H., Khan A., Weldegebriall S.B., Hao H., Zhao X., Wang Y., Zhao S., Zhu H. Regulation of AMH, AMHR-II, and BMPs (2,6) Genes of Bovine Granulosa Cells Treated with Exogenous FSH and Their Association with Protein Hormones. Genes. 2019;10:1038. doi: 10.3390/genes10121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costa V., Angelini C., De Feis I., Ciccodicola A. Uncovering the Complexity of Transcriptomes with RNA-Seq. J. Biomed. Biotechnol. 2010;2010:853916. doi: 10.1155/2010/853916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li T., Wang S., Wu R., Zhou X., Zhu D., Zhang Y. Identification of long non-protein coding RNAs in chicken skeletal muscle using next generation sequencing. Genomics. 2012;99:292–298. doi: 10.1016/j.ygeno.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Sun Y., Fu L., Xue F., Li Y., Xu H., Chen J. Digital gene expression profiling and validation study highlight Cyclin F as an important regulator for sperm motility of chickens. Poult. Sci. 2019;98:5118–5126. doi: 10.3382/ps/pez212. [DOI] [PubMed] [Google Scholar]
- 69.Matulova M., Rajova J., Vlasatikova L., Volf J., Stepanova H., Havlickova H., Sisak F., Rychlik I. Characterization of Chicken Spleen Transcriptome after Infection with Salmonella enterica Serovar Enteritidis. PLoS ONE. 2012;7:e48101. doi: 10.1371/journal.pone.0048101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Q., Wang N., Du Z., Hu X., Chen L., Fei J., Wang Y., Li N. Gastrocnemius transcriptome analysis reveals domestication induced gene expression changes between wild and domestic chickens. Genomics. 2012;100:314–319. doi: 10.1016/j.ygeno.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y., Chen Q., Liu Z., Guo X., Du Y., Yuan Z., Guo M., Kang L., Sun Y., Jiang Y. Transcriptome Analysis on Single Small Yellow Follicles Reveals That Wnt4 Is Involved in Chicken Follicle Selection. Front. Endocrinol. 2017;8:317. doi: 10.3389/fendo.2017.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen M., Li T., Zhang G., Wu P., Chen F., Lou Q., Chen L., Yin X., Zhang T., Wang J. Dynamic expression and functional analysis of circRNA in granulosa cells during follicular development in chicken. BMC Genom. 2019;20:96. doi: 10.1186/s12864-019-5462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magaway C., Kim E., Jacinto E. Targeting mTOR and Metabolism in Cancer: Lessons and Innovations. Cells. 2019;8:1584. doi: 10.3390/cells8121584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu J., Wu D.C., Qu L.H., Liao H.Q., Li M.X. The role of mTOR in ovarian Neoplasms, polycystic ovary syndrome, and ovarian aging. Clin. Anat. 2018;31:891–898. doi: 10.1002/ca.23211. [DOI] [PubMed] [Google Scholar]
- 75.Moschetta M., Reale A., Marasco C., Vacca A., Carratù M.R. Therapeutic targeting of the mTOR-signalling pathway in cancer: Benefits and limitations. Br. J. Pharmacol. 2014;171:3801–3813. doi: 10.1111/bph.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ardestani A., Lupse B., Kido Y., Leibowitz G., Maedler K. mTORC1 Signaling: A Double-Edged Sword in Diabetic β Cells. Cell Metab. 2018;27:314–331. doi: 10.1016/j.cmet.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Perluigi M., Di Domenico F., Butterfield D.A. mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol. Dis. 2015;84:39–49. doi: 10.1016/j.nbd.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 78.Zhu J., Thompson C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019;20:436–450. doi: 10.1038/s41580-019-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ocón-Grove O.M., Poole D.H., Johnson A.L. Bone morphogenetic protein 6 promotes FSH receptor and anti-Müllerian hormone mRNA expression in granulosa cells from hen prehierarchal follicles. Reproduction. 2012;143:825–833. doi: 10.1530/REP-11-0271. [DOI] [PubMed] [Google Scholar]
- 80.Hao E.Y., Wang D.H., Chen Y.F., Zhou R.Y., Chen H., Huang R.L. The relationship between the mTOR signaling pathway and ovarian aging in peak-phase and late-phase laying hens. Poult. Sci. 2021;100:334–347. doi: 10.1016/j.psj.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fabregat I., Moreno C.J., Sánchez A., Dooley S., Dewidar B., Giannelli G., Dijke P. TGF-β signalling and liver disease. FEBS J. 2016;283:2219–2232. doi: 10.1111/febs.13665. [DOI] [PubMed] [Google Scholar]
- 82.Batlle E., Massagué J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roberts A.B., Derynck R. Meeting Report: Signaling Schemes for TGF-β. Sci. STKE. 2001;2001:pe43. doi: 10.1126/stke.2001.113.pe43. [DOI] [PubMed] [Google Scholar]
- 84.Moustakas A., Pardali K., Gaal A., Heldin C.H. Mechanisms of TGF-β signaling in regulation of cell growth and differentiation. Immunol. Lett. 2002;82:85–91. doi: 10.1016/S0165-2478(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 85.Zhou S., Ma Y., Yao J., Zhao A., Xie C., Mi Y., Zhang C. TGF-β1-induced collagen promotes chicken ovarian follicle development via an intercellular cooperative pattern. Cell Biol. Int. 2021;45:1336–1348. doi: 10.1002/cbin.11580. [DOI] [PubMed] [Google Scholar]
- 86.Irving R.H.F., Rodgers R.J. Extracellular matrix in ovarian follicular development and disease. Cell Tissue Res. 2005;322:89–98. doi: 10.1007/s00441-005-0042-y. [DOI] [PubMed] [Google Scholar]
- 87.Hynes R.O., Naba A. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chun S.Y., Lim J.O., Lee E.H., Han M.H., Ha Y.S., Lee J.N., Kim B.S., Park M.J., Yeo M., Jung B., et al. Preparation and Characterization of Human Adipose Tissue-Derived Extracellular Matrix, Growth Factors, and Stem Cells: A Concise Review. Tissue Eng. Regen. Med. 2019;16:385–393. doi: 10.1007/s13770-019-00199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hrabia A., Wolak D., Sechman A. Response of the matrix metalloproteinase system of the chicken ovary to prolactin treatment. Theriogenology. 2021;169:21–28. doi: 10.1016/j.theriogenology.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Jathar S., Kumar V., Srivastava J., Tripathi V. Technological Developments in lncRNA Biology. Adv. Exp. Med. Biol. 2017;1008:283–323. doi: 10.1007/978-981-10-5203-3_10. [DOI] [PubMed] [Google Scholar]
- 91.Gebremedhn S., Salilew-Wondim D., Ahmad I., Sahadevan S., Hossain M.M., Hoelker M., Rings F., Neuhoff C., Tholen E., Looft C., et al. MicroRNA Expression Profile in Bovine Granulosa Cells of Preovulatory Dominant and Subordinate Follicles during the Late Follicular Phase of the Estrous Cycle. PLoS One. 2015;10:e0125912. doi: 10.1371/journal.pone.0125912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zielak-Steciwko A.E., Browne J.A., McGettigan P.A., Gajewska M., Dzięcioł M., Szulc T., Evans A.C. Expression of microRNAs and their target genes and pathways associated with ovarian follicle development in cattle. Physiol. Genom. 2014;46:735–745. doi: 10.1152/physiolgenomics.00036.2014. [DOI] [PubMed] [Google Scholar]
- 93.Yin H., Lin H. An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster. Nature. 2007;450:304–308. doi: 10.1038/nature06263. [DOI] [PubMed] [Google Scholar]
- 94.Zhang P., Wang L., Li Y., Jiang P., Wang Y., Wang P., Kang L., Wang Y., Sun Y., Jiang Y. Identification and characterization of microRNA in the lung tissue of pigs with different susceptibilities to PCV2 infection. Vet. Res. 2018;49:18. doi: 10.1186/s13567-018-0512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang W., Li X., Ding N., Teng J., Zhang S., Zhang Q., Tang H. miR-34a regulates adipogenesis in porcine intramuscular adipocytes by targeting ACSL4. BMC Genet. 2020;21:33. doi: 10.1186/s12863-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Rådmark O., Kim S., et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 97.Darnell D.K., Kaur S., Stanislaw S., Konieczka J.K., Yatskievych T.A., Antin P.B. MicroRNA expression during chick embryo development. Dev. Dyn. 2006;235:3156–3165. doi: 10.1002/dvdy.20956. [DOI] [PubMed] [Google Scholar]
- 98.Hicks J.A., Trakooljul N., Liu H.C. Discovery of chicken microRNAs associated with lipogenesis and cell proliferation. Physiol. Genom. 2010;41:185–193. doi: 10.1152/physiolgenomics.00156.2009. [DOI] [PubMed] [Google Scholar]
- 99.Wu H., Fan F., Liang C., Zhou Y., Qiao X., Sun Y., Jiang Y., Kang L. Variants of pri-miR-26a-5p polymorphisms are associated with values for chicken egg production variables and affects abundance of mature miRNA. Anim. Reprod. Sci. 2019;201:93–101. doi: 10.1016/j.anireprosci.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 100.Kang L., Yang C., Wu H., Chen Q., Huang L., Li X., Tang H., Jiang Y. miR-26a-5p Regulates TNRC6A Expression and Facilitates Theca Cell Proliferation in Chicken Ovarian Follicles. DNA Cell Biol. 2017;36:922–929. doi: 10.1089/dna.2017.3863. [DOI] [PubMed] [Google Scholar]
- 101.Pasquinelli A.E., Reinhart B.J., Slack F., Martindale M.Q., Kuroda M.I., Maller B., Hayward D.C., Ball E.E., Degnan B., Müller P., et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–89. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 102.Chen X., Ba Y., Ma L., Cai X., Yin Y., Wang K., Guo J., Zhang Y., Chen J., Guo X., et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 103.Zhu Q., Xie X., Yang H., Wang J., Pian H., Yu D. Study on the Effect of WNT6 Gene on Granulosa Cells of Laying Hens and Its Mechanism. J. Nanjing Agric. Univ. 2021:44. [Google Scholar]
- 104.He H., Li D., Tian Y., Wei Q., Amevor F.K., Sun C., Yu C., Yang C., Du H., Jiang X., et al. miRNA sequencing analysis of healthy and atretic follicles of chickens revealed that miR-30a-5p inhibits granulosa cell death via targeting Beclin1. J. Anim. Sci. Biotechnol. 2022;13:55. doi: 10.1186/s40104-022-00697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei Q., Xue H., Sun C., Li J., He H., Amevor F.K., Tan B., Ma M., Tian K., Zhang Z., et al. Gga-miR-146b-3p promotes apoptosis and attenuate autophagy by targeting AKT1 in chicken granulosa cells. Theriogenology. 2022;190:52–64. doi: 10.1016/j.theriogenology.2022.07.019. [DOI] [PubMed] [Google Scholar]
- 106.Wang W., Teng J., Han X., Zhang S., Zhang Q., Tang H. miR-458b-5p regulates ovarian granulosa cells proliferation through Wnt/β-catenin signaling pathway by targeting catenin beta-1. Anim. Biosci. 2021;34:957–966. doi: 10.5713/ajas.20.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei Q., Li J., He H., Cao Y., Li D., Amevor F.K., Zhang Y., Wang J., Yu C., Yang C., et al. miR-23b-3p inhibits chicken granulosa cell proliferation and steroid hormone synthesis via targeting GDF9. Theriogenology. 2022;177:84–93. doi: 10.1016/j.theriogenology.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 108.Ocłoń E, Hrabia A. miRNA expression profile in chicken ovarian follicles throughout development and miRNA-mediated MMP expression. Theriogenology. 2021;160:116–127. doi: 10.1016/j.theriogenology.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 109.Wu X., Zhang N., Li J., Zhang Z., Guo Y., Li D., Zhang Y., Gong Y., Jiang R., Li H., et al. gga-miR-449b-5p Regulates Steroid Hormone Synthesis in Laying Hen Ovarian Granulosa Cells by Targeting the IGF2BP3 Gene. Animals. 2022;12:2710. doi: 10.3390/ani12192710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou Y., Liu J., Lei Q., Han H., Liu W., Cunwei T., Li F., Cao D. Transcriptome Analysis of the Chicken Follicular Theca Cells with miR-135a-5p Suppressed. G3. 2020;10:4071–4081. doi: 10.1534/g3.120.401701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yu C., Qiu M., Yin H., Zhang Z., Hu C., Jiang X., Du H., Li Q., Li J., Xiong X., et al. miR-138-5p promotes chicken granulosa cell apoptosis viatargeting SIRT1. Anim. Biotechnol. 2022;6:1–10. doi: 10.1080/10495398.2022.2095642. [DOI] [PubMed] [Google Scholar]
- 112.Li D., Li X., He H., Zhang Y., He H., Sun C., Zhang X., Wang X., Kan Z., Su Y., et al. miR-10a-5p inhibits chicken granulosa cells proliferation and Progesterone(P4) synthesis by targeting MAPRE1 to suppress CDK2. Theriogenology. 2022;192:97–108. doi: 10.1016/j.theriogenology.2022.08.019. [DOI] [PubMed] [Google Scholar]
- 113.Zhang G., Cui Z., Li J., Zhang D., Li Z., Lin Z., Yin H., Ran J., Wang Y., Liu Y. miR-122-5p regulates proliferation and apoptosis of chicken granulosa cells of hierarchal follicles by targeting MAPK3. Gene. 2022;824:146397. doi: 10.1016/j.gene.2022.146397. [DOI] [PubMed] [Google Scholar]
- 114.Liu Y., Zhou Z., Zhang H., Han H., Yang J., Li W., Wang K. Transcriptome Analysis Reveals miR-302a-3p Affects Granulosa Cell Proliferation by Targeting DRD1 in Chickens. Front. Genet. 2022;13:832762. doi: 10.3389/fgene.2022.832762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Batista P.J., Chang H.Y. Long noncoding RNAs: Cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rinn J.L., Chang H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kopp F., Mendell J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kretz M., Webster D.E., Flockhart R.J., Lee C.S., Zehnder A., Lopez P.V., Qu K., Zheng G.X., Chow J., Kim G.E. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev. 2012;26:338–343. doi: 10.1101/gad.182121.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheetham S.W., Gruhl F., Mattick J.S., Dinger M.E. Long noncoding RNAs and the genetics of cancer. Br. J. Cancer. 2013;108:2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y., Shi H., Zhang G., Wu P., Chen L., Shen M., Li T., Lv X., Gu Y., Wang J. Transcriptome analysis of long noncoding RNAs and mRNAs in granulosa cells of Jinghai Yellow chickens illuminated with red light. Front. Genet. 2021;12:104. doi: 10.3389/fgene.2021.563623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y., Yang H., Zi C., Wang Z. Transcriptomic analysis of the red and green light responses in Columba livia domestica. 3 Biotech. 2019;9:20. doi: 10.1007/s13205-018-1551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Peng Y., Chang L., Wang Y., Wang R., Hu L., Zhao Z., Geng L., Liu Z., Gong Y., Li J., et al. Genome-wide differential expression of long noncoding RNAs and mRNAs in ovarian follicles of two different chicken breeds. Genomics. 2019;111:1395–1403. doi: 10.1016/j.ygeno.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 123.Xu X.F., Li J., Cao Y.X., Chen D.W., Zhang Z.G., He X.J., Ji D.M., Chen B.L. Differential Expression of Long Noncoding RNAs in Human Cumulus Cells Related to Embryo Developmental Potential: A Microarray Analysis. Reprod. Sci. 2015;22:672–678. doi: 10.1177/1933719114561562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhong C., Liu Z., Qiao X., Kang L., Sun Y., Jiang Y. Integrated transcriptomic analysis on small yellow follicles reveals that sosondowah ankyrin repeat domain family member A inhibits chicken follicle selection. Anim. Biosci. 2021;34:1290–1302. doi: 10.5713/ajas.20.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang D., Xia T., Li H., Li Z., Sun G., Li G., Tian Y., Liu X., Xu D., Kang X. Estrogen enhances the expression of a growth-associated long noncoding RNA in chicken liver via ERα. Br. Poult. Sci. 2021;62:336–345. doi: 10.1080/00071668.2020.1868405. [DOI] [PubMed] [Google Scholar]
- 126.Wang Y., Yuan J., Sun Y., Li Y., Wang P., Shi L., Ni A., Zong Y., Zhao J., Bian S., et al. Genetic Basis of Sexual Maturation Heterosis: Insights from Ovary lncRNA and mRNA Repertoire in Chicken. Front. Endocrinol. 2022;13:951534. doi: 10.3389/fendo.2022.951534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li D., Xu W., Wang X., Dang Y., Xu L., Lu G., Chan W.Y., Leung P.C.K., Zhao S., Qin Y. lncRNA DDGC participates in premature ovarian insufficiency through regulating RAD51 and WT1. Mol. Ther. Nucleic Acids. 2021;26:1092–1106. doi: 10.1016/j.omtn.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qu S., Yang X., Li X., Wang J., Gao Y., Shang R., Sun W., Dou K., Li H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015;365:141–148. doi: 10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 129.Li Y., Zheng Q., Bao C., Li S., Guo W., Zhao J., Chen D., Gu J., He X., Huang S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 131.Wu Y., Xiao H., Pi J., Zhang H., Pan A., Pu Y., Liang Z., Shen J., Du J. The circular RNA aplacirc_13267 upregulates duck granulosa cell apoptosis by the apla-miR-1-13/THBS1 signaling pathway. J. Cell. Physiol. 2020;235:5750–5763. doi: 10.1002/jcp.29509. [DOI] [PubMed] [Google Scholar]
- 132.Lu X., Gao H., Zhu B., Lin G. Circular RNA circ_RANBP9 exacerbates polycystic ovary syndrome via microRNA-136-5p/XIAP axis. Bioengineered. 2021;12:6748–6758. doi: 10.1080/21655979.2021.1964157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li J. Ph.D. Thesis. Henan Agricultural University; Henan, China: 2022. circ EML1 Modulates Steroid Synthesis and Secretion of Hen’s Follicular Granulosa Cells via Targeting the gga-mi R-449a/IGF2BP3 axis. [Google Scholar]
- 134.Wang Y., Guo Z., Zi C., Wu P., Lv X., Chen L., Chen F., Zhang G., Wang J. CircRNA expression in chicken granulosa cells illuminated with red light. Poult. Sci. 2022;101:101734. doi: 10.1016/j.psj.2022.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma M., Wang H., Zhang Y., Zhang J., Liu J., Pan Z. circRNA-Mediated Inhibin-Activin Balance Regulation in Ovarian Granulosa Cell Apoptosis and Follicular Atresia. Int. J. Mol. Sci. 2021;22:9113. doi: 10.3390/ijms22179113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shen M., Li T., Chen F., Wu P., Wang Y., Chen L., Xie K., Wang J., Zhang G. Transcriptomic Analysis of circRNAs and mRNAs Reveals a Complex Regulatory Network That Participate in Follicular Development in Chickens. Front. Genet. 2020;11:503. doi: 10.3389/fgene.2020.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mehta A., Dobersch S., Romero O.A.J., Barreto G. Epigenetics in lung cancer diagnosis and therapy. Cancer Metastasis Rev. 2015;34:229–241. doi: 10.1007/s10555-015-9563-3. [DOI] [PubMed] [Google Scholar]
- 138.Elhamamsy A.R. DNA methylation dynamics in plants and mammals: Overview of regulation and dysregulation. Cell Biochem. Funct. 2016;34:289–298. doi: 10.1002/cbf.3183. [DOI] [PubMed] [Google Scholar]
- 139.Law P.P., Holland M.L. DNA methylation at the crossroads of gene and environment interactions. Essays Biochem. 2019;63:717–726. doi: 10.1042/EBC20190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chapman A.G., Cotton A.M., Kelsey A.D., Brown C.J. Differentially methylated CpG island within human XIST mediates alternative P2 transcription and YY1 binding. BMC Genet. 2014;15:89. doi: 10.1186/s12863-014-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moore L.D., Le T., Fan G. DNA Methylation and Its Basic Function. Neuropsychopharmacology. 2013;38:23–38. doi: 10.1038/npp.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Meng H., Cao Y., Qin J., Song X., Zhang Q., Shi Y., Cao L. DNA Methylation, Its Mediators and Genome Integrity. Int. J. Biol. Sci. 2015;11:604–617. doi: 10.7150/ijbs.11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chambers S., Tarasov K., Aon M.A., Lammons J., Chakir K., Tarasova Y., Lukyanenko Y., Lyashkov A., Lakatta E. Metabolic Enzyme Acetylation is Elicited in Response to Stress Induced by Cardiac Specific Overexpression of Human ADCY8. FASEB J. 2020;34:1. doi: 10.1096/fasebj.2020.34.s1.09716. [DOI] [Google Scholar]
- 144.Xin Y., Ning S., Zhang L., Cui M. CDC27 Facilitates Gastric Cancer Cell Proliferation, Invasion and Metastasis via Twist-Induced Epithelial-Mesenchymal Transition. Cell. Physiol. Biochem. 2018;50:501–511. doi: 10.1159/000494164. [DOI] [PubMed] [Google Scholar]
- 145.Abbaspourkharyeki M., Anvekar N.J., Ramachandra N.B. The Possible Role of Point Mutations and Activation of the CDC27 Gene in Progression of Multiple Myeloma. Meta Gene. 2020;26:100761. doi: 10.1016/j.mgene.2020.100761. [DOI] [Google Scholar]
- 146.Qian U., Li G., Yin J., Zhang H., Zhou C., Zhu Y., Xing W.J. Screening of Genes with Differential Methylated CpG Island Related to Inbreeding Depression of Chicken Reproduction. Acta Vet. Zootech. Sin. 2021;52:943–953. [Google Scholar]
- 147.Barua A., Bitterman P., Abramowicz J.S., Dirks A.L., Bahr J.M., Hales D.B., Bradaric M.J., Edassery S.L., Rotmensch J., Luborsky J.L. Histopathology of Ovarian Tumors in Laying Hens: A Preclinical Model of Human Ovarian Cancer. Int. J. Gynecol. Cancer. 2009;19:531–539. doi: 10.1111/IGC.0b013e3181a41613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee J.Y., Jeong W., Lim W., Lim C.H., Bae S.M., Kim J., Bazer F.W., Song G. Hypermethylation and Post-Transcriptional Regulation of DNA Methyltransferases in the Ovarian Carcinomas of the Laying Hen. PLoS ONE. 2013;8:e61658. doi: 10.1371/journal.pone.0061658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dvorská D., Braný D., Nagy B., Grendár M., Poka R., Soltész B., Jagelková M., Zelinová K., Lasabová Z., Zubor P., et al. Aberrant Methylation Status of Tumour Suppressor Genes in Ovarian Cancer Tissue and Paired Plasma Samples. Int. J. Mol. Sci. 2019;20:4119. doi: 10.3390/ijms20174119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sendžikaitė G., Hanna C.W., Stewart M.K.R., Ivanova E., Kelsey G. A DNMT3A PWWP mutation leads to methylation of bivalent chromatin and growth retardation in mice. Nat. Commun. 2019;10:1884. doi: 10.1038/s41467-019-09713-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support this study are available in the article.