Abstract
Objectives
In this early retrospective cohort study, a total of 26 patients with SARS-CoV-2 were treated with bamlanivimab or casirivimab/imdevimab, and the reduction of the viral load associated with the developed clinical symptoms was analyzed.
Methods
Patients in the intervention groups received bamlanivimab or casirivimab/imdevimab. Patients without treatment served as control. Outcomes were assessed by clinical symptoms and change in log viral load from baseline based on the cycle threshold over a period of 18 days.
Results
Median log viral load decline was higher in both intervention groups after 3 and 6 days compared to control. However, at later time points, the decline of the viral load was more distinct in the control group. Mild symptoms of COVID-19 were observed in 6.3% of the intervention groups and in no patient of the control. No patients treated with bamlanivimab, 18.8% treated with casirivimab/imdevimab, and 14.2% in the control group developed moderate symptoms. Severe symptoms were recorded only in the control group (14.2%), including one related death.
Conclusion
Treatment with monoclonal SARS-CoV-2 antibodies seems to accelerate decline of virus loads, especially in the first 6 days after administration, compared to control. This may be associated with a reduced likeliness of a severe course of COVID-19.
Keywords: COVID-19, Bamlanivimab, Casirivimab, Imdevimab, Antibody therapy, Viral load
Introduction
The first patient with COVID-19 caused by SARS-CoV-2 was officially registered in December 2019. Only 3 months after that, the World Health Organization declared COVID-19 as a pandemic [1].
Usually, after infection, most people have no or only mild symptoms such as cough, fever, malaise, myalgias, gastrointestinal symptoms, or ageusia. A typical symptom seems to be anosmia, which was described in patients all over the world in early stages after infection [2,3]. However, some individuals develop severe respiratory distress syndrome with life-threatening complications. Usually, the severe sickness occurs after 1-2 weeks after the onset of first symptoms and can rapidly progress to acute pneumonia with hypoxemia and the need for oxygen supplementation [4], [5], [6], [7]. Interestingly, in recent studies, it was reported that hospitalized patients show high viral loads, suggesting that high virus titers are associated with the development of hypoxemia and, thus, a severe progression after infection [8]. Other important factors in developing a severe progression are various risk factors such as male sex, age, cardiovascular diseases, lung disease, hypertension, diabetes mellitus, or obesity [9,10].
There are several treatments available in later stages after infection, but especially the administration of steroids and other immunomodulators is recommended as primary treatment by many professional societies and the World Health Organization [11]. Potential therapeutics during the early disease stage are antivirals like remdesivir and molnupiravir as inhibitors of the viral RNA-dependent RNA polymerase or nirmatrelvir as 3CL protease inhibitor [12], [13], [14], [15].
Another promising therapy for the treatment of early COVID-19 is therapy with neutralizing antibodies. In several studies, it could be demonstrated that the use of monoclonal antibodies may reduce the viral load and thus prevent severe progression and hospitalization [16]. There are several studies that analyzed the effect of either a monotherapy with the monoclonal antibodies i.e. bamlanivimab, regdanmivab, or sotrovimab, or a combination of different monoclonal antibodies such as bamlanivimab/etesevimab or casirivimab/imdevimab [16], [17], [18], [19]. Because the emergency authorization for the bamlanivimab monotherapy was revoked in April 2021 due to a lower effect against so-called escape variants of the coronavirus [20], combination therapies were later approved to treat COVID-19 in early stages after infection [21]. In the present early cohort study, monotherapy with bamlanivimab was compared to a combination treatment with casirivimab/imdevimab. The monotherapy with bamlanivimab was administered from January to the beginning of February 2021, when the rate of infection with the more resistant variant B.1.1.7 (Alpha-variant) was at a maximum of 5,6% only in Germany [22]. Combination therapy with casirivimab/imdevimab was used from February to May 2021 when B.1.1.7 (Alpha-variant) became dominant in Germany.
Material and methods
Study design
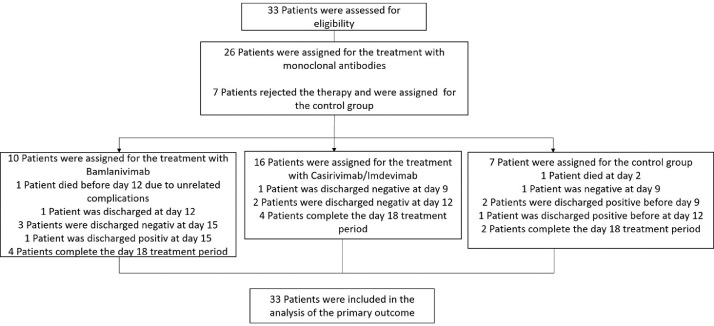
In this study, a total of 33 patients were included that tested positive for SARS-CoV-2 during their hospitalization for other reasons and presented no symptoms. Of the 33 patients, 10 patients were treated with the monotherapy bamlanivimab and 16 were treated with casirivimab/imdevimab, and seven patients were included in the control group. Furthermore, only patients were included that have had at least one possible risk factor for the development of a severe progression, such as age above 65, diabetes mellitus, cardiovascular diseases, lung disease, hypertension, obesity, or a malignant tumor, and that were serum antibody-negative at baseline. As control group patients were included, they would have fit the mentioned criteria, but rejected a treatment with neutralizing antibodies. In Figure 1 a flow chart of the study design is shown.
Figure 1.
Flow chart of the study design.
In the intervention group, patients received a single-dose administration of either bamlanivimab (700 mg) or casirivimab/imdevimab (1200 mg/1200 mg). The neutralizing antibodies were administered as a single intravenous infusion over 1 hour.
Patients in the control group did not receive any treatment as long as they were asymptomatic. As soon as they developed symptoms, a symptomatic treatment was performed, such as oxygen therapy and treatment with dexamethasone following the German S3 guidelines of the AWMF (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften) [23].
The preplanned analysis was conducted from January to May 2021, when the last patient who was included in the study finished the antibody treatment. The therapy with bamlanivimab was administered during January and February 2021, when the number of the more resistant Alpha-variant (B.1.1.7) was at a very low level (5,6 % of all infections) in Germany [22]. The combination therapy with casirivimab/imdevimab started after February and was administered until May 2021, when variant B.1.1.7 became successively dominant in Germany. In total, 10 patients received the bamlanivimab therapy and 16 patients a therapy with casirivimab/imdevimab, respectively.
Outcomes
The primary outcome was the change in SARS-CoV-2 log viral load from baseline [45- cycle threshold [cycle threshold (CT)]/Log(2)10] based on the CT values of a quantitative polymerase chain reaction (qPCR) over an investigation period of 18 days. The baseline was defined as first positive PCR test before the antibody therapy was administered. During the investigation time, additional data regarding severe progression and the development of symptoms were collected. The viral loads were analyzed by collecting nasopharyngeal swabs, followed by qPCR assay of Roche (Basel, Switzerland) and GFE (Frankfurt am Main, Germany) at the laboratory of the DRK-Blutspendedienst-Blutspendezentrum in Frankfurt/Main. In contrast to the qPCR assay of Roche, in which the PCR analyses started directly with the first CT, the qPCR Assay of GFE was performed with five pre-cycles. To compare the results of the different methods, the CT values of GFE were normalized to the values of Roche before statistical analyses.
Secondary outcomes were symptoms observed during the hospitalization time. In total, four main symptoms, such as fever, dyspnea, cough, or myalgias, were defined to determine COVID-19 progress. Patients with one symptom were classified as “mild,” with two symptoms as “moderate,” and more than three symptoms as “severe.” During the whole investigation time, one death was observed of a patient who suffered from severe symptoms.
Statistical analyses of the viral loads
The SARS-CoV-2 viral load data were evaluated using the change in log viral load from baseline based on the CT values and the standard deviations in the intervention groups (bamlanivimab or casirivimab/imdevimab) and the control group (no treatment with monoclonal antibodies). The treatment effects were compared using a two-sided Student's t-test of independent variables with an α level of 0.05.
In general, only CT values of hospitalized patients were included. Depending on the symptoms or severity of the infection, patients with no or only mild symptoms were partly discharged before day 18 to home isolation. These patients were excluded from the statistical analyses. Because of one related death and four earlier discharged patients in the control group, the number of measurements declined over time, and only two patients could be included in the statistical analyses on days 15 and 18. In the bamlanivimab group, one patient died due to unrelated complications of the primary disease.
Results
The administration of bamlanivimab was performed during January and February 2021. In total, 10 patients received monoclonal antibody therapy with bamlanivimab. The median age of the patients was 81 years, and all of them were older than 65 years. Among the 10 patients, 60% were female, and 40% were male (Table 1 ). The treatment with the antibody cocktail casirivimab/imdevimab was performed from February to May 2021, and 16 patients received the treatment. The median age of the patients was 67 years, 56.3 % were over 65 years, 56,3 % were female, and 43.7 % were male. The two intervention groups were compared to a control group that did not receive any treatment with monoclonal antibodies with a total of seven patients, median age of 70 years and 57.1% over 65 years, 42.1% were female, and 57.1% were male (Table 1).
Table 1.
Patient demographics and baseline clinical characteristics.
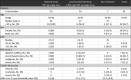 |
All 33 patients had at least one medical comorbidity as a risk factor for the development of severe COVID-19 progression. The most common medical comorbidity was hypertension (66.7%), followed by a malignoma (30.3%) and diabetes mellitus (21.2%). Furthermore, increased body mass index (BMI) (over 30) could be observed in 15% and over 35 in 9% of all patients.
Primary outcome
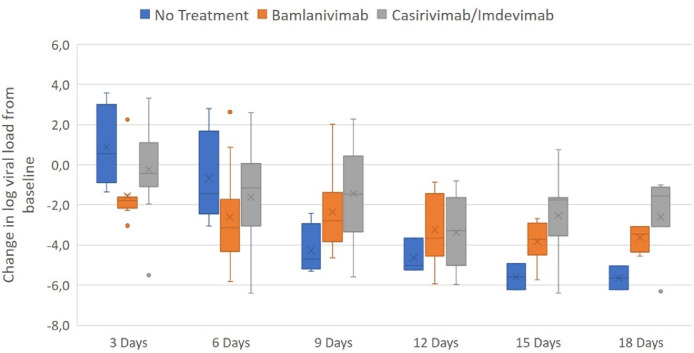
The decrease in log viral load from baseline to day 3 in the bamlanivimab was statistically significant with -1.5 (95% confidence interval [CI], 0.60 to 4.22), P-value = 0.01 compared to the control group. A value of -0.2 (95% CI, -0.94 to 3.16), P-value = 0.27 in the combination therapy group casirivimab/imdevimab and 0.9 in the control group, could be observed. After all other investigation times, no significant differences in change log viral load from baseline could be observed. Nevertheless, after 6 days, the change in log viral load from baseline in the bamlanivimab group was -2.6 (95% CI, -0.76 to 4.62), P-value = 0.15, -1.6 (95% CI, -1.54 to 3.40), P-value = 0.44 in the casirivimab/imdevimab group compared to the control with -0.7. After 9 days, both intervention groups showed lower differences with -2.4 (95% CI, -4.85 to 0.23) P-value = 0.07 for bamlanivimab and a statistically significant difference of - 1.4 (95% CI, -6.05 to 0.40) P-value = 0.02 for the combination therapy compared to the control group with -4.3. Similar results were observed after later investigation times. For day 12, the intervention groups showed changes in log viral load with -3.2 (95% CI, -3.76 to 0.94) P-value = 0.21 and -3.4 (95% CI, -3.85 to 1.30) P-value = 0.31 for bamlanivimab and casirivimab/imdevimab, respectively. The control group showed a more distinct change with -4.4. After 15 days, the bamlanivimab group showed a change in viral load of -3.8 (95% CI, -3.58 to 0.13) P-value = 0.06, the combination therapy with - 2.5 (95% CI, -7.03 to 0.99) P-value = 0.12, compared to the control group with -5.6. On day 18, the bamlanivimab group showed a value change of -3.6 (95% CI, -9.89 to 3.42) P-value = 0.31, -2.6 (95% CI, -8.35 to 2.25) P-value = 0.19 for the casirivimab/imdevimab group compared to the control group with -5.6 (Figure 2 ).
Figure 2.
Change of log viral load from baseline after 3, 6, 9, 12, 15, and 18 days. The boxes represent the interquartile range with the mean values (squares). The dots outside the boxes represent the observed values outside the range. After day 3 and 6 the change in log viral load from baseline is highest after the treatment with both antibody therapies. Only for the therapy with bamlanivimab after 3 days a statistically significant difference can be observed compared to the control group with a P-value of 0.01 (95% confidence interval, 0.60 to 4.22). Due to earlier discharge of patients and one death case in the control group, the number of measurements declined over time and only two patients could be included at day 15 and 18.
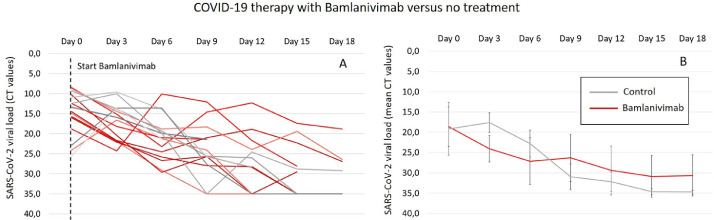
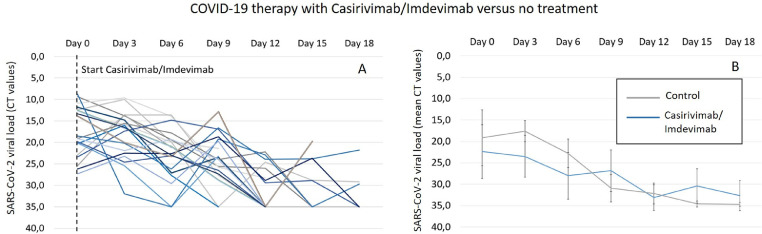
With respect to the viral load based on the mean CT (Figures 3 b and 4 b), it can be observed that after the administration of both antibody therapies, there is a distinct decrease in the mean CT values, especially at early time points (day 3 and day 6) compared to the control group. Interestingly, in the control group, there is an increase in the mean CT value after 3 days and, thus, a higher viral load compared to the intervention groups. Nevertheless, after later investigation times, a stagnation of the decreasing effect on the viral loads can be observed in both intervention groups, whereas a continuous decrease is seen in the control group, resulting in lower viral loads from day 9. With exception to the mean CT values in the casirivimab/imdevimab group with slightly lower viral loads compared to the control group, after all, later investigation times until day 18, higher viral loads are observed in both intervention groups (bamlanivimab and casirivimab/Imdevimab). Although a continuous decrease in viral loads can be seen in the bamlanivimab group, after 12 days, a clear increase in the mean CT values can be observed after the administration of the antibody combination therapy (Figures 3b and 4b). The increasing effect of the viral loads observed in the intervention groups can be explained by looking at the spaghetti plots (Figures 3a and 4a). Here, a distinct increase in the viral loads in individual patients can be observed.
Figure 3.
(a) Single cycle threshold values of every patient as change in viral load after 3, 6, 9, 12, 15, and 18 days for the treatment with bamlanivimab (red line) compared to no treatment (gray line). The CT value is defined as number of cycles that are necessary to generate a fluorescent signal during a polymerase chain reaction that exceeds a critical threshold such as the background signal. Because the generated signals are inversely proportional to the copy number of the coronavirus, it can be used as indirect measure for the viral load and thus for the potential to develop a severe SARS-CoV-2 infection. (b) Mean CT values as change in viral load after 3, 6, 9, 12, 15, and 18 days for the treatment with bamlanivimab (red dashed line) compared to no treatment (gray line).
CT, cycle threshold.
Figure 4.
(a) Single CT values of every patient as change in viral load after 3, 6, 9, 12, 15, and 18 days for the treatment with casirivimab/imdevimab (blue line) compared to no treatment (gray line). The CT value is defined as number of cycles that are necessary to generate a fluorescent signal during a polymerase chain reaction that exceeds a critical threshold such as the background signal. Because the generated signals are inversely proportional to the copy number of the coronavirus, it can be used as indirect measure for the viral load and thus for the potential to develop a severe SARS-CoV-2 infection. (b) Mean CT values as change in viral load after 3, 6, 9, 12, 15, and 18 days for the treatment with casirivimab/imdevimab (blue dashed line) compared to no treatment (gray line).
CT, cycle threshold.
Secondary outcome
With respect to COVID-19 severity, 10% (1 of 10 patients) in the bamlanivimab group, 6.3% (1 of 16 patients) in the casirivimab/imdevimab and no patient (0 of 7 patients) in the control group developed mild symptoms. Moderate symptoms were observed in 18.8 % of patients in casirivimab/imdevimab group (th3ree of 16 patients), 14.2 % (1 of 7 patients) in the control group, and no patient in the bamlanivimab group (0 of 10 patients). Severe symptoms were observed only in the control group with 14.2 % (1 of 7 patients), and one death case was reported in this group.
Discussion
In this retrospective cohort study, the efficacy of two different neutralizing antibody therapies against COVID-19 was evaluated in 26 hospitalized patients that were diagnosed with COVID-19 in the early stages with no or only mild symptoms. The primary end point was the mean change in log viral load from baseline over an investigation period of 18 days.
During the first 6 days, the decline of the viral load was more pronounced and accelerated in patients who received antibody therapies in comparison to the control group, and this difference reached statistical significance for bamlanivimab at day 3 after treatment.
It can be suggested that, especially in the early stages of the COVID-19 infection, the neutralizing effect of the used antibodies against the virus was highest, most likely due to high antibody concentrations in the body. However, after later investigation times, the virus neutralization attenuated since the viral loads stagnated in the bamlanivimab group compared to the control group. For casirivimab/imdevimab, stagnation, or even an increase in the mean viral load, was observed over time. Interestingly, neither in studies of monotherapy with bamlanivimab nor with casirivimab/imdevimab could these effects be observed [21,24,25].
The reason for these findings might be the emergence of resistant mutants, as described in earlier studies. Peiffer-Smadja et al. demonstrated the emergence of E484K mutations among five out of six patients after the treatment with bamlanivimab, yielding antibody resistance [26].
Although the emergence of resistance mutations also seems to be a possible explanation for the treatment with casirivimab/imdevimab, it has been demonstrated in an earlier study that especially this antibody cocktail does not lead to escape mutants [27]. Another possibility could be a proteolytic degradation of the used antibodies, resulting in elimination and loss of the clearance efficacy [28,29]. Furthermore, the half-life of the used antibodies associated with a weakened neutralization efficacy over time could have led to a stagnation of the virus load elimination. For bamlanivimab, a half-life of 17.6 days, for casirivimab 8.35 days, and for imdevimab 6.8 days was reported. The faster elimination and, thus, the lower concentration of the antibody cocktail casirivimab/imdevimab over time could lead to a plateau and even an increase of the virus load after day 12.
Regarding secondary outcomes, the results discussed here should be understood as preliminary results because of the very low number of patients included in this study. In general, no clear differences comparing both intervention groups with the control group were observed regarding the development of severe COVID-19 progression. For bamlanivimab, similar findings were described in the ACTIVE-3/TICO clinical trial in hospitalized patients. Here, after treatment with bamlanivimab, no better clinical outcomes were seen compared to the placebo group. Nevertheless, it was suggested that although no clinical effects have been observed, patients who received bamlanivimab have had a slightly increased viral clearance from the nasopharynx resulting in a lower risk of hospitalization [30].
Severity after infection was measured by recording different symptoms such as fever, dyspnea, cough, and myalgias. Although most of the patients only developed mild or moderate symptoms, interestingly, no mild progression was observed in the control group. However, one patient in the control group developed severe symptoms, resulting in the only COVID-19-related death in this study. It was already observed that a higher initial viral load leads to increased disease severity resulting in higher mortality or percentage of intubation [31]. Furthermore, it was postulated that viral dynamics are associated with mortality in hospitalized patients and that an accelerated viral clearance could lead to a reduction in mortality in patients > 65 years [32].
With respect to the clinical outcomes, such as resolution of symptoms and deaths, similar findings as in this study were already observed in two phase III studies. In these studies, a combination of bamlanivimab and etesevimab has been administered to a higher number of patients with a high risk for severe progression [33,34]. It could be demonstrated that the treatment with monoclonal antibodies reduced the incidence of hospitalization and resulted in a more rapid resolution of symptoms after the treatment with monoclonal antibodies. Furthermore, no deaths occurred in the intervention groups compared to the control. Although there were differences in the study design and the evaluation of the clinical outcomes comparing the mentioned phase III studies and this study, our results may confirm the correlation between an accelerated reduction in viral loads and better clinical outcomes, especially in the first week after monoclonal antibody administration.
Because this study is based on a very low number of patients, additional studies are needed to further investigate whether there is a significant clinical benefit after administration of monoclonal antibodies in early stages of COVID-19 infection. Especially the emergence of escape mutants and the half-lives of neutralizing antibodies can influence the clinical outcome of longer COVID-19 progression.
Only patients who had one or more risk factors, as described above, were included in this study. Although the most common comorbidity reported in this study was advanced age >65 and hypertension, especially malignant diseases such as bronchial carcinoma, ovarian carcinoma, or colon carcinoma, were included as high-risk factors. In total, two out of six included cancer patients were on chemotherapy during this study. Because chemotherapy might have an immune-suppressing effect, it was expected that there might be a prolongated viral clearance due to a decreased endogenous antibody response. Because the two patients under chemotherapy showed a complete viral clearance on day 12 after treatment with monoclonal antibodies and developed no symptoms after COVID-19 infection, it can be suggested that there might be a clinical benefit, especially for immunosuppressed patients.
There were no consistent results emphasizing specific comorbidities for the development of severe symptoms. Further studies are needed to investigate the influence of certain comorbidities on COVID-19 progression and, thus, to determine priority groups for the treatment with monoclonal antibodies.
Conclusion
Due to the fact that his study was performed from January to May 2021, the used monoclonal antibodies are not up to date anymore at the time point of publication. However, we assume that our results are generalizable and hypothesize that our findings can be transferred to other therapies with monoclonal antibodies e.g., against novel variants.
The treatment with monoclonal antibodies against SARS-CoV2 in early stages after infection is a promising treatment to prevent severe COVID-19 progression. Especially in the first days after treatment, the most distinct reduction in viral loads could be observed. Because higher viral loads can be correlated to worse clinical outcomes and severe progression in the early stages after infection, fast treatment with monoclonal antibodies might prevent a severe COVID-19 disease, especially in high-risk patients. Further studies are needed to investigate the effects of the described viral load reduction to increase the efficacy of antibody treatments against novel variants and to improve the therapies for immunocompromised patients.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Author contributions
MH und CS contributed to the conception and design of the study. CH, JB, SL-A, IT, PP, AH, IS performed tests and collected data. MH analyzed the data. MH, EH, MS, MD and CS interpreted the data. MH wrote the manuscript. All authors reviewed and approved the manuscript.
References
- 1.Tabata S, Imai K, Kawano S, Ikeda M, Kodama T, Miyoshi K, et al. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis. Lancet Infect Dis. 2020;20:1043–1050. doi: 10.1016/S1473-3099(20)30482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020;58:299–301. doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 3.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlin DA, Gulick RM, Martinez FJ. Severe COVID-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 5.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA. 2020;324:1292–1295. doi: 10.1001/jama.2020.16747. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garibaldi BT, Wang K, Robinson ML, Zeger SL, Bandeen-Roche K, Wang MC, et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer WA, 2nd, Eron JJ, Jr, Holman W, Cohen MS, Fang L, Szewczyk LJ, et al. A Phase 2a clinical trial of Molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Sci Transl Med. 2022;14:eabl7430. doi: 10.1126/scitranslmed.abl7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macchiagodena M, Pagliai M, Procacci P. Characterization of the non-covalent interaction between the PF-07321332 inhibitor and the SARS-CoV-2 main protease. J Mol Graph Model. 2022;110 doi: 10.1016/j.jmgm.2021.108042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bariola JR, McCreary EK, Wadas RJ, Kip KE, Marroquin OC, Minnier T, et al. Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect Dis. 2021;8:ofab254. doi: 10.1093/ofid/ofab254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falcone M, Tiseo G, Valoriani B, Barbieri C, Occhineri S, Mazzetti P, et al. Efficacy of Bamlanivimab/Etesevimab and Casirivimab/Imdevimab in preventing progression to severe COVID-19 and role of variants of concern. Infect Dis Ther. 2021;10:2479–2488. doi: 10.1007/s40121-021-00525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Jang YR, Hong JH, Jung JG, Park JH, Streinu-Cercel A, et al. Safety, virologic efficacy, and pharmacokinetics of CT-P59, a neutralizing monoclonal antibody against SARS-CoV-2 spike receptor-binding protein: two randomized, placebo-controlled, phase I studies in healthy individuals and patients with mild SARS-CoV-2 infection. Clin Ther. 2021;43:1706–1727. doi: 10.1016/j.clinthera.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen B, Luebke N, Feldt T, Keitel V, Brandenburger T, Kindgen-Milles D, et al. Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany. Lancet Reg Health Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert-Koch Institute . Robert-Koch Institute.; Germany: 2021. Second official report of SARS-CoV-2 virus variants in Germany, especially the variant of concern (VOC) B.1.1.7. Date of report 17th of February 2021. [Google Scholar]
- 23.Kluge S, Janssens U, Welte T, Weber-Carstens S, Schälte G, Spinner CD, et al. AWMF; Standard. (German) Germany: 2022. S3-Leitlinie-Empfehlungen zur stationären Therapie von Patienten mit COVID-19. [DOI] [PubMed] [Google Scholar]
- 24.Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peiffer-Smadja N, Bridier-Nahmias A, Ferré VM, Charpentier C, Garé M, Rioux C, et al. Emergence of E484K mutation following bamlanivimab monotherapy among high-risk patients infected with the alpha variant of SARS-CoV-2. Viruses. 2021;13:1642. doi: 10.3390/v13081642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baum A, Fulton BO, Wloga E, Copin R, Pascal KE, Russo V, et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–1018. doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eli Lilly and Company. Fact sheet for health care providers emergency use authorization (EUA) of Bamlanivimab. Indianapolis: Eli Lilly and Company,
- 29.Regeneron Pharmaceuticals, Inc. Fact sheet for health care providers emergency use authorization (EUA) of Regen-Cov® (Casirivimab and Imdevimab). New York: Regeneron Pharmaceuticals, Inc,
- 30.ACTIV-3/TICO LY-CoV555 Study Group. Lundgren JD, Grund B, Barkauskas CE, Holland TL, Gottlieb RL, Sandkovsky U, Brown SM, Knowlton KU, Self WH, Files DC, Jain MK, Benfield T, Bowdish ME, Leshnower BG, Baker JV, Jensen JU, Gardner EM, Ginde AA, Harris ES, Johansen IS, Markowitz N, Matthay MA, Østergaard L, Chang CC, Davey VJ, Goodman A, Higgs ES, Murray DD, Murray TA, Paredes R, Parmar MKB, Phillips AN, Reilly C, Sharma S, Dewar RL, Teitelbaum M, Wentworth D, Cao H, Klekotka P, Babiker AG, Gelijns AC, Kan VL, Polizzotto MN, Thompson BT, Lane HC, Neaton JDA. Neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021:384905–384914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Zein S, Chehab O, Kanj A, Akrawe S, Alkassis S, Mishra T, et al. SARS-CoV-2 infection: initial viral load (iVL) predicts severity of illness/outcome, and declining trend of iVL in hospitalized patients corresponds with slowing of the pandemic. PLoS One. 2021;16 doi: 10.1371/journal.pone.0255981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Néant N, Lingas G, Le Hingrat Q, Ghosn J, Engelmann I, Lepiller Q, et al. Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2017962118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dougan M, Azizad M, Mocherla B, Gottlieb RL, Chen P, Hebert C, et al. A randomized, placebo-controlled clinical trial of bamlanivimab and etesevimab together in high-risk ambulatory patients with COVID-19 and validation of the prognostic value of persistently high viral load. Clin Infect Dis. 2022;75:e440–e449. doi: 10.1093/cid/ciab912. [DOI] [PMC free article] [PubMed] [Google Scholar]