Abstract
The main aim of this study was to assess the differences in the chemical composition of essential oil from biocultivated Lavandula angustifolia in the Thracian Lowland floristic region, Bulgaria, and commercially available products from Bulgarian markets. Following the analytical results conducted with gas chromatography-mass spectrometry, we have established some differences in the chemical composition of the tested samples. The essential oil of biocultivated lavender contained 35 compounds, which represent 94.13% of the total oil. Samples from commercial products contained 28–42 compounds that represent 93.03–98.69% of the total oil. All the examined samples were rich in monoterpene hydrocarbons (1.68–12.77%), oxygenated monoterpenes (70.42–87.96%), sesquiterpene hydrocarbons (4.03–13.78%), and oxygenated sesquiterpenes (0.14–0.76%). The dominant components in all examined samples were linalool (20.0–45.0%) and linalyl acetate (20.79–39.91%). All the examined commercial samples contained linalool and linalyl acetate as was described in the European Pharmacopoeia, but in one of the samples, the quality of linalyl acetate is lower than that recommended in the European Pharmacopoeia.
1. Introduction
Lavender (Lavandula angustifolia Mill.) is one of the most popular aromatic plants in the Lamiaceae family with origin in the Mediterranean region and is cultivated worldwide for medicinal and commercial purposes [1, 2]. Lavandula angustifolia (L. angustifolia) has great economic values as an essential oil-producing plant. Because of its characteristic and pleasant aroma, as well as its therapeutic properties, the essential oil of lavender is of considerable importance in pharmaceutical, cosmetics, perfume, food, and flavor industries [3–5]. The floral essential oil of lavender is documented to have therapeutic effects such as antibacterial, antioxidant, antifungal, carminative, sedative, antidepressive, analgesic, and anti-inflammatory [3, 6, 7]. In addition, according to Basch et al., the aroma of lavender is one of the most widely utilized in aromatherapy, considered to be relaxing, with anxiolytic effects [8]. The multiple therapeutic applications of L. angustifolia are attributed mainly to the presence of volatile bioactive substances contained in the essential oil [4].
Lavender essential oil is usually produced by steam or hydrodistillation from flowering tops, and other used methods for oil extraction are supercritical CO2 fluid extraction, microwave, ultrasound, and turbohydrodistillation [9]. The chemical composition of the EO differs according to the extraction technique [5, 10]. However, essential oil from L. angustifolia is composed of various constituents, including esters: linalyl acetate, lavandulyl acetate, and geranyl acetate; alcohols: linalool, α-terpineol, and terpinen-4-ol; sesquiterpenes: β-caryophyllene; and monoterpene: cis-β-ocimene [2, 4, 10–13]. The greater proportion is linalyl acetate and linalool which are considered active constituents, but linalool is considered one of the most examined odorant molecules [14]. Both components are responsible for therapeutic effects. Moreover, all constituents contribute to the synergism of the total therapeutic effect. Today, lavender is cultivated around the world and enjoys continuing popularity for various therapeutic and cosmetic purposes [3, 12].
According to Stanev et al., Bulgaria has a long tradition of lavender cultivation and essential oil production dating since the 1900s [15]. Moreover, according to Stanev et al., Bulgarian lavender populations are characterized by high adaptability to the geographic, climate, and soil conditions of the country and consequently high essential oil content and quality [16]. This placed Bulgaria as one of the main lavender-growing and essential oil-producing countries, along with France [1, 13, 17]. Due to the economic value of lavender essential oil, interest in the development of new commercial products has increased. This study compares the chemical composition of essential oils from biocultivated L. angustifolia and commercial products in Bulgaria.
2. Materials and Methods
2.1. Chemicals and Reagents
For determination of retention indices (RIs), heptane (99%), octane (≥99%), nonane (99%), decane (≥99%), undecane (≥99%), dodecane (99%), tridecane (≥99%), tetradecane (≥99%), hexadecane (≥99%), heptadecane (99%), octadecane (99%), nonadecane (99%), and eicosane (99%) purchased from Merck KGaA (Darmstadt, Germany) were used. For diluting essential oils, hexane purchased from Thermo Fisher Scientific GmbH (Bremen, Germany) was used.
2.2. Plant Material and Oil Extraction
L. angustifolia was cultivated in the area of Belashtitsa, Thracian Lowland floristic region, Bulgaria. The cultivated plant has grown in a continental climate with an average annual temperature of 12.3°C and rendzina soil. The essential oil was obtained from the air-dried flowering tops of lavender by hydrodistillation using the Clevenger apparatus for 3 h according to the standard procedure described in the European Pharmacopoeia 9 (07/2018:1534) [18]. After completion of distillation, the collected oil was dried over anhydrous sodium sulfate and stored in dark glass vials at 4°C until GC-MS analysis.
2.3. Chromatographic Conditions
Gas chromatography-mass spectrometry (GC-MS) was used for the analysis. GC-MS analyses were carried out using Bruker Scion 436-GC SQ MS, Bremen, Germany. The column used was a Bruker BR-5 ms fused silica capillary (0.25 μm film thickness and 15 m × 0.25 mm i.d.). The oven temperature was initially held at 45°C for 1 min and then increased to 140 at 3°C/min, and after that, it was increased to 250°C at 17°C/min and then held for 1 min. The flow rate of helium (carrier gas) was 1 mL/min. The injector split ratio was 1 : 50, and the injection volume was 1 μL. The range of m/z was 50–350 in the full-scan mode. To compare the spectral data and retention indices of compounds, the Wiley NIST11 Mass Spectral Library (NIST11/2011/EPA/NIH) and the literature were used. Retention index values were calculated and compared with reported values for a C7–C20 series of n-alkane standards.
3. Results and Discussion
The distilled lavender EO had a strong odour of linalool and linalyl acetate. Its colour was yellow. The extracted essential oil and commercial essential oils were diluted with hexane and analyzed with GC-MS. Figure 1 shows the chromatogram of the EO of the cultivated lavender.
Figure 1.
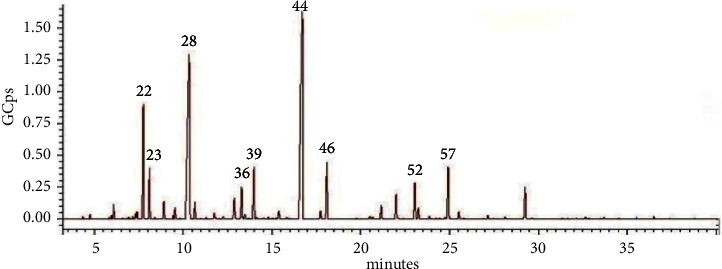
Chromatogram of the cultivated L. angustifolia essential oil. The numbers are related to 22, trans-β- ocimene; 23, cis-β-ocimene; 28, β-linalool; 36, (-)-terpinen-4-ol; 39, α-terpineol; 44, linalyl acetate; 46, lavandulyl acetate; 52, caryophyllene; 57, cis-β- farnesene.
The essential oil of the cultivated lavender contained 35 compounds, which represent 94.13% of the total oil. Samples from commercial products contained 28–42, which represent 93.03–98.69% of the total oil. Table 1 shows the chemical composition found in essential oils from biocultivated L. angustifolia (CLA) and those from commercial products (CP 1–7).
Table 1.
The GC data for essential oil components identified in cultivated L. angustifolia and commercial products, where tr indicates traces, less than 0.05%.
| No. | Compound | RI | Class | CLA | CP 1 | CP 2 | CP 3 | CP 4 | CP 5 | CP 6 | CP 7 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 937 | MH | 0.14 | 0.35 | 0.14 | 0.21 | 0.11 | 0.22 | 0.55 | 0.31 |
| 2 | Camphene | 947 | MH | 0.2 | tr | 0.17 | 0.17 | tr | 0.19 | 0.39 | 0.19 |
| 3 | β-Pinene | 967 | MH | tr | — | — | tr | 0.11 | 0.11 | 0.38 | tr |
| 4 | 1-Octen-3-ol | 973 | O | 0.10 | tr | 0.33 | 0.16 | 0.21 | 0.26 | 0.15 | 0.20 |
| 5 | 3-Octanone | 976 | O | 0.16 | 0.13 | 1.75 | 0.29 | 0.20 | 0.44 | tr | 1.38 |
| 6 | β-Myrcene | 980 | MH | 0.74 | 0.11 | 0.48 | 0.43 | 0.55 | 0.34 | 0.42 | 0.65 |
| 7 | 3-Octanol | 988 | O | — | — | 0.66 | — | — | — | tr | 0.43 |
| 8 | 3-Carene | 993 | MH | — | — | tr | 0.13 | — | tr | — | 0.13 |
| 9 | Acetic acid, hexyl ester | 1001 | O | 0.1 | 1.86 | 0.73 | 0.34 | 0.13 | 0.26 | 0.16 | 0.69 |
| 10 | p-Cymene | 1003 | MH | tr | 0.54 | — | tr | — | 0.25 | 0.14 | tr |
| 11 | o-Cymene | 1007 | MH | 0.13 | — | 0.24 | 0.18 | tr | 0.10 | — | 0.20 |
| 12 | Limonene | 1010 | MH | 0.33 | — | 1.21 | 0.55 | 0.33 | 0.40 | 0.74 | 0.93 |
| 13 | Eucalyptol | 1013 | MO | 0.42 | 5.38 | 1.58 | 0.86 | 2.17 | 0.79 | 3.57 | 0.90 |
| 14 | Trans-β- ocimene | 1022 | MH | 6.70 | 0.29 | 1.21 | 7.19 | 4.93 | 3.87 | 1.46 | 5.81 |
| 15 | cis-β-ocimene | 1031 | MH | 2.89 | 0.15 | 1.21 | 2.47 | 1.86 | 3.84 | 0.37 | 4.06 |
| 16 | γ-Terpinene | 1039 | MH | tr | 0.11 | 0.13 | 0.15 | tr | 0.11 | 0.14 | 0.30 |
| 17 | Linalool oxide | 1051 | MO | 1.10 | — | 0.21 | 0.16 | tr | 0.33 | 0.19 | 0.23 |
| 18 | α-Terpinolene | 1064 | MH | 0.20 | 0.14 | tr | tr | 0.11 | tr | 0.29 | 0.14 |
| 19 | β-Linalool | 1087 | MO | 23.13 | 34.04 | 35.99 | 24.34 | 35.32 | 26.60 | 24.68 | 26.92 |
| 20 | 1-Octen-3-yl acetate | 1095 | O | 1.01 | — | 0.46 | 0.68 | 0.36 | 0.76 | 0.25 | 0.82 |
| 21 | Camphor | 1123 | MO | 0.34 | 2.78 | 0.47 | 0.29 | 0.13 | 0.46 | 8.29 | 0.28 |
| 22 | Isoborneol | 1140 | MO | — | 0.64 | — | — | — | — | — | — |
| 23 | Lavandulol | 1150 | MO | — | — | 2.34 | — | 1.64 | — | — | 1.68 |
| 24 | Endo-borneol | 1151 | MO | 1.68 | 1.62 | — | 1.44 | — | 2.44 | 2.82 | — |
| 25 | (-)-Terpinen-4-ol | 1161 | MO | 2.28 | 2.15 | 2.64 | 3.97 | 1.54 | 3.36 | 3.48 | 5.34 |
| 26 | Cryptone | 1166 | MO | 0.37 | — | 0.35 | 0.28 | — | — | — | 0.22 |
| 27 | α-Terpineol | 1179 | MO | 3.95 | 0.66 | 1.29 | 0.98 | — | 0.75 | 0.64 | 1.15 |
| 28 | Cis-geraniol | 1214 | MO | 0.17 | — | 0.16 | tr | 0.21 | 0.10 | — | 0.13 |
| 29 | Cuminal | 1215 | MO | — | — | 0.20 | 0.15 | — | tr | — | — |
| 30 | Linalyl acetate | 1249 | MO | 31.46 | 39.91 | 20.79 | 36.97 | 35.39 | 36.03 | 36.47 | 30.79 |
| 31 | Bornyl acetate | 1273 | MO | 0.57 | 0.26 | — | 0.22 | 0.13 | 0.14 | tr | 0.15 |
| 32 | Lavandulyl acetate | 1282 | MO | 4.21 | 0.41 | 3.57 | 3.87 | 2.88 | 3.67 | 2.40 | 3.53 |
| 33 | Nerol acetate | 1359 | MO | 1.01 | tr | 0.32 | 0.31 | 0.43 | 0.12 | 0.14 | 0.28 |
| 34 | Geranyl acetate | 1380 | MO | 1.85 | 0.11 | 0.51 | 0.51 | 0.81 | 0.44 | 0.23 | 0.45 |
| 35 | Zingiberene | 1440 | SH | — | — | 0.18 | tr | tr | tr | 0.11 | tr |
| 36 | Caryophyllene | 1407 | SH | 2.87 | 4.64 | 5.15 | 5.72 | 3.85 | 6.09 | 1.96 | 4.06 |
| 37 | α- Santalene | 1412 | SH | 0.87 | tr | — | 0.69 | 0.44 | 0.78 | 0.33 | 0.50 |
| 38 | Trans-α-bergamotene | 1427 | SH | 0.18 | — | 0.17 | 0.20 | 0.12 | 0.20 | — | 0.13 |
| 39 | Humulene | 1442 | SH | Tr | 0.28 | 0.13 | 0.14 | tr | 0.20 | tr | tr |
| 40 | Cis-β-farnesene | 1454 | SH | 3.99 | 0.23 | tr | 3.26 | 2.77 | — | — | 3.78 |
| 41 | Trans-β-farnesene | 1455 | SH | — | — | 6.85 | 0.14 | — | 2.39 | 1.37 | — |
| 42 | Gemacrene D | 1468 | SH | — | — | 0.89 | 0.61 | 0.59 | — | tr | 0.69 |
| 43 | β-Copaene | 1469 | SH | 0.56 | tr | — | — | — | — | tr | — |
| 44 | γ-Cadinene | 1490 | SH | — | — | 0.26 | 0.29 | tr | 0.25 | 0.26 | 0.19 |
| 45 | β-Sesquiphellandrene | 1510 | SH | — | — | 0.15 | — | tr | 0.14 | — | tr |
| 46 | Caryophyllene oxide | 1563 | SO | — | 0.15 | 0.50 | 0.34 | 0.14 | 0.65 | 0.10 | 0.26 |
| 47 | Tau-cadinol | 1607 | SO | tr | — | 0.13 | tr | — | 0.11 | 0.17 | tr |
| 48 | α-Bisabolol | 1615 | SO | — | tr | — | — | — | — | 0.38 | — |
|
| |||||||||||
| Terpene classes | |||||||||||
| Monoterpene hydrocarbons (MHs) | 11.33 | 1.68 | 4.79 | 1148 | 8.00 | 9.43 | 4.88 | 12.77 | |||
| Oxygenated monoterpenes (MOs) | 72.94 | 87.96 | 70.42 | 74.35 | 80.45 | 75.23 | 82.91 | 72.05 | |||
| Sesquiterpene hydrocarbons (SHs) | 8.47 | 5.15 | 13.78 | 11.05 | 7.77 | 10.05 | 4.03 | 9.35 | |||
| Oxygenated sesquiterpenes (SOs) | — | 0.15 | 0.63 | 0.34 | 0.14 | 0.76 | 0.65 | 0.26 | |||
| Others (O) | 1.37 | 1.99 | 3.93 | 1.47 | 0.90 | 1.72 | 0.56 | 3.52 | |||
| Total identified | 94.13 | 96.93 | 93.55 | 98.69 | 97.26 | 97.19 | 93.03 | 97.95 | |||
The results show the mean values of the three independent samples of each sample, CLA and CP 1–7. The standard error of the mean does not exceed 2% of it and has been removed to simplify reporting.
The essential oil obtained from the biocultivated lavender was characterized by monoterpene hydrocarbons (11.33%), oxygenated monoterpenes (72.94%), and sesquiterpenes hydrocarbons (8.47%). Among the monoterpene hydrocarbons, the main components were trans-β- ocimene (6.70%) and cis-β-ocimene (2.89%). Oxygenated monoterpenes were detected in higher amounts. The prevailing components from oxygenated monoterpenes were linalyl acetate (31.46%), β-linalool (23.13%), lavandulyl acetate (4.21%), α-terpineol (3.95%), and (-)-terpinen-4-ol (2.28%). The presence of linalool and linalyl acetate in lavender essential oil determined its anti-inflammatory, cytotoxic, antimicrobial, repellent effect, sedative, local anaesthetic, analgesic, antioxidant, antimicrobial, and other activities [14, 19–22]. The main volatile components of sesquiterpene hydrocarbons were caryophyllene (2.87%) and cis-β-farnesene (3.99%).
All analyzed commercial essential oils were rich in monoterpene hydrocarbons (1.68–12.77%), oxygenated monoterpenes (70.42–87.96%), sesquiterpenes hydrocarbons (4.03–13.78%), and oxygenated sesquiterpenes (0.14–0.76%). The representatives of the monoterpenes with the highest content were trans-β- ocimene (0.29–7.19%) with higher amounts in CP 3 and cis-β-ocimene (0.15–4.06%) with higher amounts in CP 7. Oxygenated monoterpenes, especially β-linalool (24.34–35.99%) and linalyl acetate (20.79–39.91%), were in higher amounts in all of the commercial products. They were followed by (-)- terpinen-4-ol (1.54–5.34%), lavandulyl acetate (0.41–3.87%), and eucalyptol (0.79–5.38%). The sesquiterpene hydrocarbons caryophyllene (1.96–6.09%), cis-β-farnesene (0.08–3.78%) and trans-β-farnesene (0.14–6.85%) were also isolated. Oxygenated sesquiterpenes (0.14–0.76%) were in the lower concentration in all of the analyzed commercial products.
According to the European Pharmacopoeia, the relative content of lavender oil compounds should be in the following ranges: limonene (maximum 1.0%), 1,8-cineole (maximum 2.5%), 3-octanone (0.1–5.0%), camphor (maximum 1.2%), linalool (20.0–45.0%), linalyl acetate (25.0–47.0%), terpinen-4-ol (0.1–8.0%), lavandulyl acetate (minimum 0.2%), lavandulol (minimum 0.1%), and α-terpineol (maximum 2.0%) [18]. Only one sample contained more than 1% lemonene, and the rest corresponded to the requirements of the European Pharmacopoeia. In our results, only in two of the commercial products, the amount of 1,8-cineole was higher than 2.5%. According to the requirements of the European Pharmacopoeia, isolated 3-octanone was in the described ranges in all of the examined samples, but in two of the examined samples (CP 2 and CP 6), camphor did not correspond to the requirements. All the samples contained the recommended amounts of linalool and terpinen-4-ol. Only sample 2 contained less than 25% linalyl acetate. Not all the samples corresponded to the requirements for lavandulyl acetate, lavandulol, and α-terpineol.
The obtained results can be compared with these by other researchers. In research conducted with Ukrainian cultivars, Pokajewicz et. al. found that linalool (11.42–44.05%), (-)-terpinen-4-ol (1.17–11.25%), and linalyl acetate (15.79–35.27%) as the main chemical compounds [23]. Moreover, Dong et al., researchers from China, reported linalool (19.71%), linalyl acetate (26.61%), α-terpineol (3.61%), and lavandulyl acetate (12.68%) as the main compounds [24]. In addition, Adaszynska et al., researchers from Poland, reported that linalool (15.85–23.88%) and linalyl anthranilate (1.58–12.78%) are the main ingredients together with geraniol acetate (2.37–10.61%), caryophyllene (2.78–6.24%), 1-terpinen-4-ol (5.53–9.73%), and p-menth-1-en-8-ol (3.98–7.94%) [25].
The difference in the composition of lavender essential oils may be due to influence of cultivation methods and different geographic regions on the accumulation of chemical compounds [26–28]. According to Bara, some of the exogenous factors such as light and soil (pH and constituents) may increase the concentration of terpenes [29]. It is considered that many enzymes of secondary pathways are UV-B-dependent [29, 30]. Hassiotis et al. reported that temperature and the flowering stage have a positive influence on the EO composition, but rainfall during the flowering period has a negative influence on EO content [31]. The linalool content is influenced by temperature, flower development, and rainfalls, and rainfalls during the harvest period decrease linalool production [31]. Also, the addition of synthetic compounds would affect the differences in the chemical composition and its concentrations [6, 32]. Moreover, according to Filly et. al., the different isolation methods of essential oil may also lead to differences in composition [33]. For further studies, it is recommended conducting a survey on adding synthetic and part synthetic compounds to commercial products containing essential oils. The method used for analyses of the essential oil chemical profile is also important, and the GC-MS analysis represents well separation and identification of volatile compounds [34]. The method described above could also be used for further analysis.
4. Conclusions
A total of 50 volatile compounds were found in lavender biocultivated essential oil, which represents 93.17% of the total oil. The following terpene classes were found in the essential oil from lavender biocultivated essential oil: monoterpene hydrocarbons (11.33%), oxygenated monoterpenes (72.94%), and sesquiterpene hydrocarbons (8.47%). Oxygenated monoterpenes were detected in higher amounts. The prevailing components from them were linalool (23.13%) and linalyl acetate (31.46%). Volatile compounds found in the commercial products were 28–42, which represent 93.55–98.69% of the total oil. Commercial products were rich in oxygenated monoterpenes, and especially, linalool (24.34–35.32%) and linalyl acetate (20.79–39.91%) were in higher amounts. The results of this study indicate that the essential oil content and quality of the analyzed commercial products corresponded to the recommendations given in the European Pharmacopoeia.
Data Availability
All data generated and analyzed during this study are included in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Héral B., Stierlin É., Fernandez X., Michel T. Phytochemicals from the genus Lavandula: a review. Phytochemistry Reviews . 2021;20(4):751–771. doi: 10.1007/s11101-020-09719-z. [DOI] [Google Scholar]
- 2.Perra M., Fancello L., Castangia I., et al. Formulation and testing of antioxidant and protective effect of hyalurosomes loading extract rich in rosmarinic acid biotechnologically produced from Lavandula angustifolia miller. Molecules . 2022;27(8):p. 2423. doi: 10.3390/molecules27082423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavanagh H. M. A., Wilkinson J. M. Biological activities of lavender essential oil. Phytotherapy Research . 2002;16(4):301–308. doi: 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]
- 4.Erland L. A. E., Mahmoud S. S. Essential Oils in Food Preservation, Flavor and Safety . Amsterdam, Netherlands: Elsevier; 2016. Lavender (Lavandula angustifolia) oils; pp. 501–508. [Google Scholar]
- 5.Lesage-Meessen L., Bou M., Sigoillot J.-C., Faulds C. B., Lomascolo A. Essential oils and distilled straws of lavender and lavandin: a review of current use and potential application in white biotechnology. Applied Microbiology and Biotechnology . 2015;99(8):3375–3385. doi: 10.1007/s00253-015-6511-7. [DOI] [PubMed] [Google Scholar]
- 6.Prusinowska R., Śmigielski K. B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L). A review. Herba Polonica . 2014;60(2):56–66. doi: 10.2478/hepo-2014-0010. [DOI] [Google Scholar]
- 7.Silva G. L. D., Luft C., Lunardelli A., et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. An. Acad. Bras. Ciênc . 2015;87(2):1397–1408. doi: 10.1590/0001-3765201520150056. [DOI] [PubMed] [Google Scholar]
- 8.Basch E., Foppa I., Liebowitz R., et al. Lavender (Lavandula angustifolia miller) Journal of Herbal Pharmacotherapy . 2004;4(2):63–78. doi: 10.1080/J157v04n02_07. [DOI] [PubMed] [Google Scholar]
- 9.Périno-Issartier S., Ginies C., Cravotto G., Chemat F. A comparison of essential oils obtained from lavandin via different extraction processes: ultrasound, microwave, turbohydrodistillation, steam and hydrodistillation. Journal of Chromatography A . 2013;1305:41–47. doi: 10.1016/j.chroma.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Rathore S., Kumar R. Essential oil content and compositional variability of Lavandula species cultivated in the mid hill conditions of the western himalaya. Molecules . 2022;27(11):p. 3391. doi: 10.3390/molecules27113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Białoń M., Krzyśko-Łupicka T., Nowakowska-Bogdan E., Wieczorek P. P. Chemical composition of two different lavender essential oils and their effect on facial skin microbiota. Molecules . 2019;24(18):p. 3270. doi: 10.3390/molecules24183270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denner S. S., Lavender E. Lavandula angustifolia miller. Holistic Nursing Practice . 2009;23(1):57–64. doi: 10.1097/01.HNP.0000343210.56710.fc. [DOI] [PubMed] [Google Scholar]
- 13.Smigielski K., Prusinowska R., Stobiecka A., Kunicka-Styczyñska A., Gruska R. Biological properties and chemical composition of essential oils from flowers and aerial parts of lavender (Lavandula angustifolia) Journal of Essential Oil Bearing Plants . 2018;21(5):1303–1314. doi: 10.1080/0972060X.2018.1503068. [DOI] [Google Scholar]
- 14.Aprotosoaie A. C., Hăncianu M., Costache I.-I., Miron A. Linalool: a review on a key odorant molecule with valuable biological properties. Flavour and Fragrance Journal . 2014;29(4):193–219. doi: 10.1002/ffj.3197. [DOI] [Google Scholar]
- 15.Stanev S., Zagorcheva T., Atanassov I. Lavender cultivation in Bulgaria - 21st century developments, breeding challenges and opportunities. Bulgarian Journal of Agricultural Science . 2016;22:584–590. [Google Scholar]
- 16.Stanev S. Evaluation of the stability and adaptability of the Bulgarian lavender (Lavandula angustifolia Mill.) sorts yield. Agricultural Science and Technology . 2010;2:121–123. [Google Scholar]
- 17.Giray F. H. An analysis of world lavender oil markets and lessons for Turkey. Journal of Essential Oil Bearing Plants . 2018;21(6):1612–1623. doi: 10.1080/0972060X.2019.1574612. [DOI] [Google Scholar]
- 18.Lavender F. European Pharmacopoeia, Monograph 07/2018:1534 . Strasburg, France: European Directorate for the Quality of Medicines & Health Care; 2019. European directorate for the quality of medicines & health care. [Google Scholar]
- 19.Peana A. T., D’Aquila P. S., Panin F., Serra G., Pippia P., Moretti M. D. L. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine . 2002;9(8):721–726. doi: 10.1078/094471102321621322. [DOI] [PubMed] [Google Scholar]
- 20.Prashar A., Locke I. C., Evans C. S. Cytotoxicity of lavender oil and its major components to human skin cells. Cell Proliferation . 2004;37(3):221–229. doi: 10.1111/j.1365-2184.2004.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sfara V., Zerba E. N., Alzogaray R. A. Fumigant insecticidal activity and repellent effect of five essential oils and seven monoterpenes on first-instar nymphs of Rhodnius prolixus. Journal of Medical Entomology . 2009;46(3):511–515. doi: 10.1603/033.046.0315. [DOI] [PubMed] [Google Scholar]
- 22.Tadtong S., Suppawat S., Tintawee A., Saramas P., Jareonvong S., Hongratanaworakit T. Antimicrobial activity of blended essential oil preparation. Natural Product Communications . 2012;7(10) doi: 10.1177/1934578X1200701041.1934578X1200701 [DOI] [PubMed] [Google Scholar]
- 23.Pokajewicz K., Białoń M., Svydenko L., Fedin R., Hudz N. Chemical composition of the essential oil of the new cultivars of Lavandula angustifolia Mill. Bred in Ukraine. Molecules . 2021;26(18):p. 5681. doi: 10.3390/molecules26185681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong G., Bai X., Aimila A., Aisa H. A., Maiwulanjiang M. Study on lavender essential oil chemical compositions by GC-MS and improved PGC. Molecules . 2020;25(14):p. 3166. doi: 10.3390/molecules25143166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adaszyńska M., Swarcewicz M., Dzięcioł M., Dobrowolska A. Comparison of chemical composition and antibacterial activity of lavender varieties from Poland. Natural Product Research . 2013;27(16):1497–1501. doi: 10.1080/14786419.2012.724408. [DOI] [PubMed] [Google Scholar]
- 26.Gruda N., Bisbis M., Tanny J. Influence of climate change on protected cultivation: impacts and sustainable adaptation strategies - a review. Journal of Cleaner Production . 2019;225:481–495. doi: 10.1016/j.jclepro.2019.03.210. [DOI] [Google Scholar]
- 27.Najar B., Demasi S., Caser M., et al. Cultivation substrate composition influences morphology, volatilome and essential oil of Lavandula angustifolia Mill. Agronomy . 2019;9(8):p. 411. doi: 10.3390/agronomy9080411. [DOI] [Google Scholar]
- 28.Giannoulis K. D., Evangelopoulos V., Gougoulias N., Wogiatzi E. Could bio-stimulators affect flower, essential oil yield, and its composition in organic lavender (Lavandula angustifolia) cultivation? Industrial Crops and Products . 2020;154 doi: 10.1016/j.indcrop.2020.112611.112611 [DOI] [Google Scholar]
- 29.Barra A. Factors affecting chemical variability of essential oils: a review of recent developments. Natural Product Communications . 2009;4(8) doi: 10.1177/1934578X0900400827.1934578X0900400 [DOI] [PubMed] [Google Scholar]
- 30.Kuhn D. N., Chappell J., Boudet A., Hahlbrock K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase MRNAs in cultured plant cells by UV light or fungal elicitor. Proceedings of the National Academy of Sciences . 1984;81(4):1102–1106. doi: 10.1073/pnas.81.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassiotis C. N., Ntana F., Lazari D. M., Poulios S., Vlachonasios K. E. Environmental and developmental factors affect essential oil production and quality of Lavandula angustifolia during flowering period. Industrial Crops and Products . 2014;62:359–366. doi: 10.1016/j.indcrop.2014.08.048. [DOI] [Google Scholar]
- 32.Imre S., Tero-Vescan A., Muntean D.-L., Oprean R. Qualitative assay of essential oils of lavender and peppermint in commercial products through spectral and chromatographic methods. Farmacia . 2016;64:p. 6. [Google Scholar]
- 33.Filly A., Fabiano-Tixier A. S., Louis C., Fernandez X., Chemat F. Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers. Comptes Rendus Chimie . 2016;19(6):707–717. doi: 10.1016/j.crci.2016.01.018. [DOI] [Google Scholar]
- 34.Virgiliou C., Zisi C., Kontogiannopoulos K. N., et al. Headspace gas chromatography-mass spectrometry in the analysis of lavender’s essential oil: optimization by response surface methodology. Journal of Chromatography B . 2021;1179 doi: 10.1016/j.jchromb.2021.122852.122852 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are included in the manuscript.


