Abstract
Background
The risk factors and clinical outcomes of quantitative interstitial abnormality progression over time have not been characterized.
Research Questions
What are the associations of quantitative interstitial abnormality progression with lung function, exercise capacity, and mortality? What are the demographic and genetic risk factors for quantitative interstitial abnormality progression?
Study Design and Methods
Quantitative interstitial abnormality progression between visits 1 and 2 was assessed from 4,635 participants in the Genetic Epidemiology of COPD (COPDGene) cohort and 1,307 participants in the Pittsburgh Lung Screening Study (PLuSS) cohort. We used multivariable linear regression to determine the risk factors for progression and the longitudinal associations between progression and FVC and 6-min walk distance, and Cox regression models for the association with mortality.
Results
Age at enrollment, female sex, current smoking status, and the MUC5B minor allele were associated with quantitative interstitial abnormality progression. Each percent annual increase in quantitative interstitial abnormalities was associated with annual declines in FVC (COPDGene: 8.5 mL/y; 95% CI, 4.7-12.4 mL/y; P < .001; PLuSS: 9.5 mL/y; 95% CI, 3.7-15.4 mL/y; P = .001) and 6-min walk distance, and increased mortality (COPDGene: hazard ratio, 1.69; 95% CI, 1.34-2.12; P < .001; PLuSS: hazard ratio, 1.28; 95% CI, 1.10-1.49; P = .001).
Interpretation
The objective, longitudinal measurement of quantitative interstitial abnormalities may help identify people at greatest risk for adverse events and most likely to benefit from early intervention.
Key Words: 6-min walk test, interstitial lung disease, pulmonary fibrosis, pulmonary function test, radiology
Abbreviations: 6MWD, 6-min walk distance; COPDGene, Genetic Epidemiology of COPD; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HR, hazard ratio; ILA, interstitial lung abnormality; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; PLuSS, Pittsburgh Lung Screening Study; PRISm, preserved ratio impaired spirometry; QIA, quantitative interstitial abnormality
The parenchymal changes associated with interstitial lung diseases (ILDs) have also been found on the CT scans of smokers and community dwellers in large cohorts without a diagnosis of ILD. Prior studies have demonstrated that such visually identified parenchymal changes, often termed interstitial lung abnormalities (ILAs), are associated with spirometric and exercise limitations, increased mortality, and the MUC5B polymorphism associated with idiopathic pulmonary fibrosis (IPF), leading to the conclusion that such changes may represent early ILD in some patients.1, 2, 3, 4, 5, 6
We previously developed an automated, machine learning-based objective approach to identifying these parenchymal changes on CT images, and these findings are called quantitative interstitial abnormalities (QIAs; previously called interstitial features). We have shown that the objective presence and quantity of QIAs are associated with the presence of visually defined ILAs, as well as functional and survival outcomes similar to those associated with ILAs.7, 8, 9, 10 In the current study, we hypothesized that the progression of objectively measured QIAs over time would be associated with worsening disease severity, including poorer lung function, exercise capacity, and mortality. We sought to determine the demographic and genetic risk factors associated with the progression of QIAs in smokers.
In addition to these primary analyses, we performed secondary analyses in two specific spirometrically defined subgroups: smokers with reduced FEV1 and FVC, but with an FEV1-to-FVC ratio ≥ 0.7, suggestive of a restrictive phenotype (often termed preserved ratio impaired spirometry [PRISm]), and those with normal spirometry (often termed Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 0).11,12 The former was selected because of spirometric similarities to IPF, and the latter because one of the larger goals of this work is to identify smokers at risk for disease progression and to intervene before it occurs.13
Study Design and Methods
We performed our analysis of QIA progression on chest CT scans of participants from the Genetic Epidemiology of COPD (COPDGene) and the Pittsburgh Lung Screening Study (PLuSS), described previously and in e-Appendix 1.14, 15, 16 This study was approved by the institutional review board at Brigham and Women’s Hospital (IRB 2007P000554).
COPDGene is a prospective cohort of more than 10,300 non-Hispanic White and Black ever-smokers with at least a 10-pack-year smoking history, aged 45 to 80 years, without prior bronchiectasis or ILD, from 21 study centers in the United States.14 At baseline visit 1 (2006-2011) and visit 2 (2013-2017) approximately 5 years later, participants underwent collection of inspiratory and expiratory chest CT scans, pre- and postbronchodilator spirometric testing, 6-min walk distance (6MWD) measurements, questionnaires, and genotyping of the MUC5B polymorphism (rs35705950).8,17 Participants with a postbronchodilator FEV1 ≥ 80% predicted and FEV1-to-FVC ratio ≥ 0.7 were defined as in GOLD stage 0, and those with a postbronchodilator FEV1 ≤ 80% predicted and a FEV1-to-FVC ratio ≥ 0.7 were defined as having PRISm. CT scans were obtained at inspiration (200 mA) and after expiration (50 mA) with submillimeter slice reconstruction.14 Mortality was assessed through the longitudinal follow-up program conducted every 3 to 6 months, as previously described, and the Social Security Death Index, with deaths adjudicated through January 31, 2018.18,19
PLuSS is a prospective cohort of 3,642 ever-smokers with at least a 12.5-pack-year history, aged 50 to 79 years, without prior history of lung cancer, from one study center in the United States.16 Between 2002 and 2005, participants underwent collection of inspiratory low-dose chest CT scans, spirometric testing, and questionnaires. CT scans were obtained at breath hold (40-60 mA) with 2.5-mm reconstruction.16 Participants were monitored by serial imaging as per PLuSS protocols, as well as by spirometry, annual telephone surveys, and/or mailed questionnaires.16 For this study, we included only CT scans acquired within 90 days of spirometry and limited the analyses to participants’ first and last CT imaging. Therefore, although many PLuSS participants had several imaging visits, for the purposes of this study only two CT scans were included, with the first referred to as visit 1 and the last as visit 2. Mortality was assessed by acquisition of death certificates from the surveys and questionnaires, with deaths adjudicated through February 28, 2020.16
The percentages of lung with QIAs and emphysema were measured by a local density classification approach, which uses a k-nearest neighbors classifier applied to the local histogram measurements combined with the distance from the pleural surface as features on the inspiratory CT scans, described previously (Fig 1, e-Appendix 2).7,8 All analyses were based on the annualized rates of change in QIAs and emphysema between visits 1 and 2.
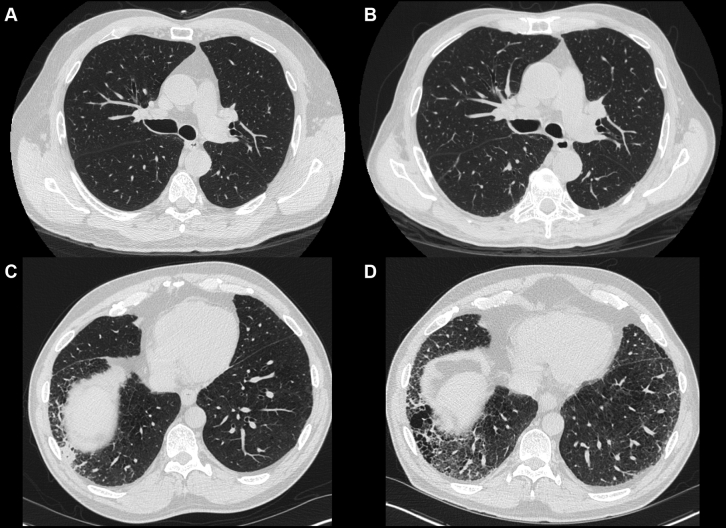
Figure 1.
Representative CT scan images of patients with quantitative interstitial abnormality (QIA) progression. A and B, A patient with no visible QIAs at baseline (A) who developed subtle evidence of QIAs at the bilateral bases after 5 years of follow-up (B). The percentage of this patient’s lung occupied by QIAs increased by 8.5% over 5 years, and he died approximately 6 months after his second CT scan was acquired. C and D, A patient with visually defined interstitial lung abnormalities (ILAs) at baseline (C) who showed progression of QIAs over the 5 years of follow-up (D). The percentage of this patient’s lung occupied by QIAs increased by 11.6% over the 5 years of follow-up, and he died shortly after his follow-up CT scan.
We used multivariable linear regression to determine the longitudinal association between QIA progression and the changes in FVC and 6MWD (COPDGene only). We used Cox proportional hazards regression models for the association between QIA progression and subsequent mortality. All analyses were adjusted for age; sex; self-reported race (except for the association between MUC5B and QIA progression); baseline: percentage of lung with QIA, percentage of lung with emphysema, airway wall thickness (COPDGene), smoking status, pack-years, and BMI; and change in: emphysema, smoking status (COPDGene), BMI, and scanner manufacturer. The association between MUC5B and QIA progression was adjusted for principal components of genetic ancestry.20 Associations with change in 6MWD and mortality also were adjusted for baseline and change in FEV1. Secondary analysis included a stratified analysis of participants in GOLD stage 0, with PRISm, and in GOLD stages 1 through 4 (COPDGene). Secondary “volume-adjusted” analysis was also performed on the participants whose FVC on spirometry and inspiratory lung volume on CT images changed in the same direction (either both increased or both decreased) between visits 1 and 2. Last, we performed secondary analyses using additional models adjusted for self-reported cardiac risk factors at visit 1 (in COPDGene: diabetes, high BP, high cholesterol, coronary artery disease, and congestive heart failure; in PLuSS: history of heart attack, coronary artery bypass surgery, and lower extremity edema).
All statistical tests were two-sided, and a P value < .05 was taken to indicate statistical significance. Analyses were performed with R software (version 4.0.3) implemented with RStudio version 1.3.21,22
Results
Baseline Characteristics
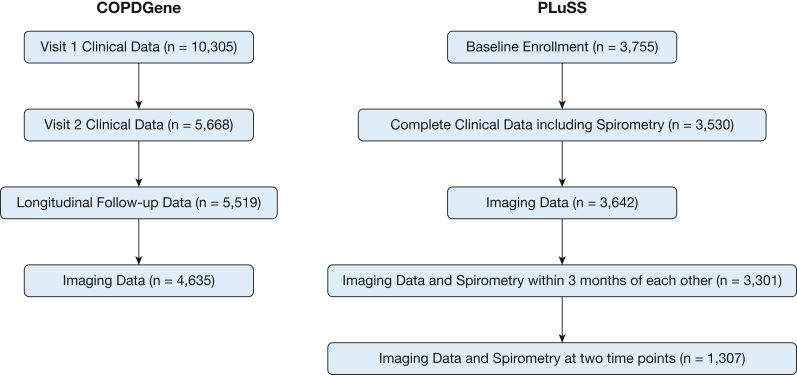
In the COPDGene cohort, 4,635 participants had complete clinical data, CT scans, and spirometry from visits 1 and 2 for analysis (Fig 2, Table 1). The mean time between visits 1 and 2 was 5.6 ± 0.8 years. At visit 1, participants had a mean age of 59.9 ± 8.7 years, 52.3% were female, 29.1% were self-reported Black and 70.9% were self-reported non-Hispanic White, 47.6% were current smokers, and the cohort had a mean pack-year history of 41.9. The mean prebronchodilator FEV1 decreased over the follow-up period, from 2.2 ± 0.8 to 2.0 ± 0.8 L between visits 1 and 2. The mean prebronchodilator FVC decreased from 3.2 ± 1.0 to 3.0 ± 0.9 L between visits 1 and 2. At baseline, 563 participants (12.2%) had PRISm, 2,122 (46.0%) were in GOLD stage 0 (similar to previously published data12), and 1,874 (40.6%) were in GOLD stages 1 through 4. The mean percentage of lung occupied by QIAs was 6.0% ± 4.6% at visit 1 and 5.7% ± 4.6% at visit 2, and by emphysema was 6.8% ± 12.9% at visit 1 and 6.2% ± 13.4% at visit 2.
Figure 2.
CONSORT diagrams for the COPDGene and PLuSS cohorts. CONSORT = Consolidated Standards of Reporting Trials; COPDGene = Genetic Epidemiology of COPD; PLuSS = Pittsburgh Lung Screening Study.
Table 1.
Baseline Characteristics
| Characteristic | COPDGene (n = 4,635) |
PLuSS (n = 1,307) |
||
|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | |
| Time from visit 1 (mean ± SD), y | … | 5.6 ± 0.8 | … | 8.6 ± 2.8 |
| Age (mean ± SD), y | 59.9 ± 8.7 | 65.5 ± 8.7 | 61.0 ± 6.6 | 69.6 ± 6.4 |
| Sex | ||||
| Female | 2,426 (52.3) | 506 (38.7) | ||
| Male | 2,209 (47.7) | 801 (61.3) | ||
| Self-reported race | ||||
| American Indian/Alaskan Native | … | 2 (0.2) | ||
| Asian | … | 2 (0.2) | ||
| Black | 1,351 (29.1) | 55 (4.2) | ||
| Pacific Islander | … | 1 (0.1) | ||
| Non-Hispanic White | 3,284 (70.9) | 1,247 (95.4) | ||
| Smoking | ||||
| Former | 2,431 (52.4) | 2,840 (61.4) | 484 (40.8) | …a |
| Current | 2,204 (47.6) | 1,785 (38.6) | 701 (59.2) | …a |
| Pack-years, mean ± SD | 41.9 ± 23.9 | 43.3 ± 24.3 | 65.0 ± 22.5 | …a |
| BMI (mean ± SD), kg/m2 | 29.1 ± 6.1 | 29.0 ± 6.4 | 28.5 ± 5.2 | 28.7 ± 5.4 |
| Pulmonary function | ||||
| Time between CT scan and PFTs (mean ± SD), d | 1.1 ± 25.7 | 0.6 ± 12.0 | 29.4 ± 16.0 | 34.1 ± 20.3 |
| Prebronchodilator FEV1 (mean ± SD), L | 2.2 ± 0.8 | 2.0 ± 0.8 | 2.5 ± 0.8 | 2.1 ± 0.8 |
| Prebronchodilator FVC (mean ± SD), L | 3.2 ± 1.0 | 3.0 ± 0.9 | 3.6 ± 1.0 | 3.2 ± 1.0 |
| Baseline lung function | ||||
| Preserved ratio impaired spirometry (PRISm) | 563 (12.2) | …a | … | |
| Without spirometric limitations (GOLD stage 0) | 2,122 (46.0) | …a | … | |
| With COPD (GOLD stages 1-4) | 1,874 (40.6) | … | … | |
| 6-Min walk distance, m | 437.2 ± 112.6 | 393.8 ± 132.3 | … | … |
| Quantitative CT measures | ||||
| Percentage of lung occupied by QIAs, mean ± SD | 6.0 ± 4.6 | 5.7 ± 4.6 | 16.2 ± 7.8 | 15.9 ± 6.5 |
| Percentage of lung occupied by emphysema, mean ± SD | 6.8 ± 12.9 | 6.2 ± 13.4 | 10.2 ± 4.2 | 11.7 ± 4.2 |
| Percentage of lung occupied by normal parenchyma, mean ± SD | 87.2 ± 13.3 | 87.8 ± 14.1 | 66.6 ± 7.3 | 65.6 ± 6.4 |
| Mortality | ||||
| Alive | 4,386 (94.6) | 1,033 (79.0) | ||
| Dead | 249 (5.4) | 274 (21.0) | ||
| Survival duration from enrollment (mean ± SD), y | 8.3 ± 1.2 | 12.0 ± 1.9 | ||
Data displayed as No. (%) unless otherwise specified. COPDGene = Genetic Epidemiology of COPD; PFTs = pulmonary function tests; PLuSS = Pittsburgh Lung Screening Study; QIAs = quantitative interstitial abnormalities.
Information unavailable.
In the PLuSS cohort, 1,307 had complete imaging and spirometry data, within 3 months of each other, available from visits 1 and 2 for analysis (Fig 2, Table 1). The mean time between visits 1 and 2 was 8.6 ± 2.8 years. During visit 1, participants had a mean age of 61.0 ± 6.6 years; 38.7% were female, 0.2% were American Indian/Alaskan Native, 0.2% were Asian, 4.2% were Black, 0.1% were Pacific Islander, and 95.4% were White by self-reporting; 59.2% were current smokers; and the cohort had a mean pack-year history of 65.0. The mean prebronchodilator FEV1 decreased from 2.5 ± 0.8 to 2.1 ± 0.8 L between visits 1 and 2. The mean prebronchodilator FVC decreased from 3.6 ± 1.0 to 3.2 ± 1.0 L between visits 1 and 2. GOLD stage data were not available. The mean percentage of lung occupied by QIAs was 16.2% ± 7.8% at visit 1 and 15.9% ± 6.5% at visit 2, and by emphysema was 10.2% ± 4.2% at visit 1 and 11.7% ± 4.2% at visit 2.
Risk Factors for Progression
The demographic and genetic risk factors associated with the progression of QIAs are shown in Table 2 and are expressed as the absolute annual rate of change in QIAs over the course of follow-up. Every additional year of baseline age was associated with a higher annual increase in QIAs by 0.01%/y (95% CI, 0.01%-0.02%; P < .001) in COPDGene and 0.02% (95% CI, 0.01%-0.02%; P < .001) in PLuSS. Female sex was associated with a 0.07%/y (95% CI, 0.02%-0.12%; P = .003) higher annual increase in QIAs compared with male sex in COPDGene and 0.14% (95% CI, 0.02%-0.26%; P = .025) in PLuSS. Current smoking status was associated with higher annual QIA progression only in COPDGene (0.10%/y [0.06%/y-0.15%/y]; P < .001). Every copy of the minor allele of the MUC5B promoter polymorphism was associated with a 0.12%/y (95% CI, 0.07%/y-0.16%/y; P < .001) annual increase in QIAs in COPDGene.
Table 2.
Predictors of Quantitative Interstitial Abnormality Progression Between Visits 1 and 2
| Predictor | COPDGene |
PLuSS |
||
|---|---|---|---|---|
| Effect,a Mean (95% CI) | P Value | Effect,a Mean (95% CI) | P Value | |
| Age at enrollment (per year of age) | 0.01 (0.01-0.02) | < .001 | 0.02 (0.01-0.02) | < .001 |
| Female sex | 0.07 (0.02-0.12) | .003 | 0.14 (0.02-0.26) | .025 |
| Current smoker (vs former) | 0.10 (0.06-0.15) | < .001 | 0.001 (–0.10 to 0.10) | .98 |
| MUC5B minor allele (per copy) | 0.12 (0.07-0.16) | < .001 | …b | …b |
Adjusted for age; sex; baseline: quantitative interstitial abnormalities, emphysema, airway wall thickness (COPDGene only), smoking status, pack-years, BMI, and FEV1; and change in: emphysema, smoking status (COPDGene only), BMI, FEV1, and scanner manufacturer. Analyses of age at enrollment, female sex, and current smoking status were additionally adjusted for self-reported race. Analysis of MUC5B was adjusted for principal components of genetic ancestry. COPDGene = Genetic Epidemiology of COPD; PLuSS = Pittsburgh Lung Screening Study.
Effect expressed as the association between the noted exposure and the annual rate of change (percent per year) in quantitative interstitial abnormalities.
Information unavailable.
Clinical Outcomes
As seen in Table 3, each percent increase in QIAs per year was associated with an 8.5-mL/y decline in FVC in COPDGene (95% CI, 4.7-12.4 mL/y; P < .001) and a 9.5-mL/y decline in FVC in PLuSS (95% CI, 3.7-15.4 mL/y; P = .001). Each percent increase in QIAs per year was associated with a 1.2 m/y decrease in 6MWD (95% CI, 0.2-2.3 m/y; P = .021) in COPDGene.
Table 3.
Associations Between Rate of Quantitative Interstitial Abnormality Progression and Rate of Change in FVC and 6-Min Walk Distance Between Visits 1 and 2
| FVC (mL/y) |
6-Min Walk Distance (m/y) |
|||
|---|---|---|---|---|
| Cohort | Effect,a Mean (95% CI) | P Value | Effect,a Mean (95% CI) | P Value |
| COPDGene | –8.5 (–12.4 to –4.7) | < .001 | –1.2 (–2.3 to –0.2) | .021 |
| PLuSS | –9.5 (–15.4 to –3.7) | .001 | …b | …b |
Adjusted for age; sex; self-reported race; baseline: quantitative interstitial abnormalities, emphysema, airway wall thickness (COPDGene only), smoking status, pack-years, and BMI; and change in: emphysema, smoking status (COPDGene only), BMI, and scanner manufacturer. Associations with change in 6-min walk distance also adjusted for baseline and change in FEV1. COPDGene = Genetic Epidemiology of COPD; PLuSS = Pittsburgh Lung Screening Study.
Effect expressed as the association between the annual rate of change (percent per year) in quantitative interstitial abnormalities and rate of change in the clinical outcome.
Information unavailable.
QIA progression was associated with higher subsequent mortality in both COPDGene and PLuSS (Table 4), with each percent increase in QIA progression per year associated with a 69% increase in the expected risk of mortality in COPDGene (hazard ratio [HR], 1.69; 95% CI, 1.34-2.12; P < .001) and a 28% increase in the expected risk of mortality in PluSS (HR, 1.28; 95% CI, 1.10-1.49; P = .001), while holding the covariates constant.
Table 4.
Association Between Rate of Quantitative Interstitial Abnormality Progression Between Visits 1 and 2 and All-Cause Mortality After Visit 2
| Cohort | Mortality (Hazard Ratio) |
|
|---|---|---|
| Effect,a Mean (95% CI) | P Value | |
| COPDGene | 1.69 (1.34-2.12) | < .001 |
| PLuSS | 1.28 (1.10-1.49) | .001 |
Adjusted for age; sex; self-reported race; baseline: quantitative interstitial abnormalities, emphysema, airway wall thickness (COPDGene only), smoking status, pack-years, BMI, and FEV1; and change in: emphysema, smoking status (COPDGene only), BMI, FEV1, and scanner manufacturer. COPDGene = Genetic Epidemiology of COPD; PLuSS = Pittsburgh Lung Screening Study.
Effect expressed as the association between the annual rate of change (percent per year) in quantitative interstitial abnormalities and the hazard ratio for all-cause mortality.
PRISm and GOLD 0
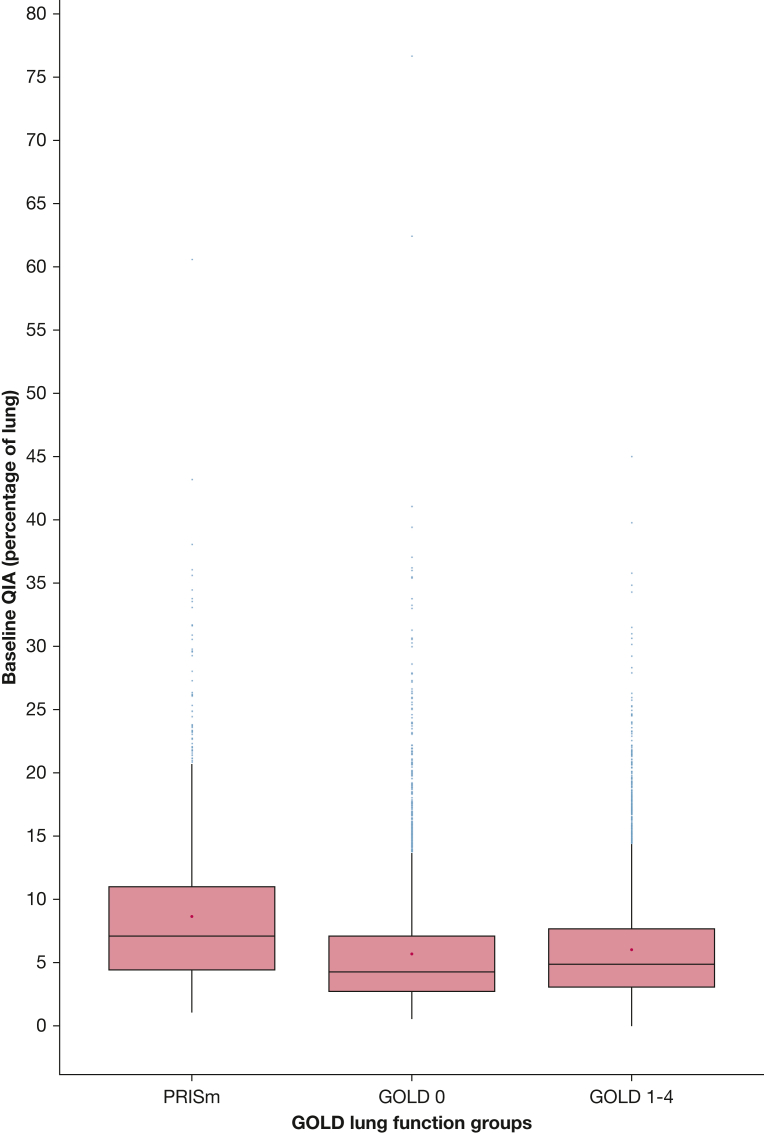
As shown in e-Table 1, at baseline, 563 participants had PRISm and 2,122 were in GOLD stage 0. The mean percentage of lung with QIAs in participants with PRISm was 8.4% ± 5.9% at visit 1 and 7.6% ± 6.1% at visit 2; and in patients in GOLD stage 0 it was 5.6% ± 4.4% at visit 1 and 5.3% ± 4.3% at visit 2 (e-Table 1, Fig 3). Mean emphysema in participants with PRISm was 2.3% ± 4.8% at visit 1 and 1.3% ± 3.1% at visit 2; similarly, mean emphysema in participants in GOLD stage 0 was 2.0% ± 3.6% at visit 1 and 1.1% ± 2.5% at visit 2. As shown in Table 5, each percent increase in QIAs per year was associated with a faster decline in FVC in both subgroups, with a 13.2-mL/y decline in FVC in PRISm (95% CI, 5.5-20.8 mL/y; P < .001) and a 9.9-mL/y decline in GOLD stage 0 (95% CI, 5.2-14.5 mL/y; P < .001). Similar associations between QIA progression and 6MWD in the subgroup analyses of PRISm and GOLD stage 0 participants were not statistically significant. There was a trend toward QIA progression and mortality in PRISm participants; in GOLD stage 0 participants, each percent increase in QIA progression per year was associated with a 54% increase in the expected risk of mortality (HR, 1.54; 95% CI, 1.00-2.38; P = .049), while holding the covariates constant.
Figure 3.
Box plots of baseline quantitative interstitial abnormalities (QIAs) in the participants with preserved ratio impaired spirometry (PRISm), participants in Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 0, or participants in GOLD stages 1 through 4. Boxes show medians and interquartile ranges; asterisks show means.
Table 5.
Associations Between Rate of Quantitative Interstitial Abnormality Progression and Rates of Change in Clinical Outcomes Stratified by GOLD Groups
| Group | FVC (mL/y) |
6-Min Walk Distance (m/y) |
Mortality (Hazard Ratio) |
|||
|---|---|---|---|---|---|---|
| Effect,a Mean (95% CI) | P Value | Effect,a Mean (95% CI) | P Value | Effect,a Mean (95% CI) | P Value | |
| PRISm (n = 563) | –13.2 (–20.8 to –5.5) | < .001 | 0.2 (–2.3 to 2.6) | .90 | 1.39 (0.69 to 2.82) | .36 |
| GOLD stage 0 (n = 2,122) | –9.9 (–14.5 to –5.2) | < .001 | –1.2 (–2.7 to 0.3) | .11 | 1.54 (1.00 to 2.38) | .049 |
| GOLD stages 1-4 (n = 1,874) | –6.4 (–14.5 to 1.7) | .12 | –2.1 (–3.9 to –0.3) | .02 | 1.87 (1.41 to 2.47) | < .001 |
Adjusted for age; sex; self-reported race; baseline: quantitative interstitial abnormalities, emphysema, airway wall thickness (COPDGene only), smoking status, pack-years, and BMI; and change in: emphysema, smoking status (COPDGene only), BMI, and scanner manufacturer. GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = preserved ratio impaired spirometry.
Effect expressed as the association between the annual rate of change (percent per year) in quantitative interstitial abnormalities and rate of change in the clinical outcome or the hazard ratio for all-cause mortality.
Volume-Adjusted Secondary Analyses
To adjust for possible noise on the CT images caused by an incomplete breath hold on the inspiratory scan, a secondary analysis of the cohort was performed, limited to participants whose FVC on spirometry and inspiratory lung volume on CT images both changed in the same direction between visits 1 and 2. In this volume-adjusted cohort (e-Table 2), each percent increase in QIAs per year was associated with a 29.2-mL/y decline in FVC in COPDGene (95% CI, 24.5-33.9 mL/y; P < .001) and a 10.7-mL/y decline in PLuSS (95% CI, 3.0-18.3 mL/y; P = .006). Each percent increase in QIAs per year in the volume-adjusted cohort was associated with a 42% increase in the expected risk of mortality in COPDGene (HR, 1.42; 95% CI, 1.04-1.96; P = .03) and a 32% increase in PLuSS (HR, 1.32; 95% CI, 1.09-1.58; P = .003), while holding the covariates constant. The relationship between QIA progression and 6MWD was not significant in the volume-adjusted cohorts.
This volume-adjusted cohort was also used to study the PRISm and GOLD stage 0 participants. One reason was to account for the fact that the PRISm participants had a higher amount of QIAs and higher BMI at baseline. Although prior studies of restrictive disease like PRISm have identified extreme high body mass and central obesity to be associated factors,11,23,24 we wanted to adjust for any effects of obesity-related atelectasis contributing to CT noise. In this volume-adjusted cohort, each percent increase in QIAs per year was associated with an even faster decline in FVC than the nonadjusted cohort in both subgroups, with a 24.9-mL/y decline in FVC in PRISm (95% CI, 15.8-34.0 mL/y; P < .001) and a 27.8-mL/y decline in FVC in GOLD stage 0 (95% CI, 22.1-33.6 mL/y; P < .001).
Cardiac-Adjusted Secondary Analyses
To account for any contributions of pulmonary edema-related noise on the inspiratory scan, secondary analyses adjusting for cardiac risk factors were performed (e-Table 3). These cardiac-adjusted models showed that the relationship between QIA progression and our clinical outcomes of interest remained significant, with each percent increase in QIAs per year associated with an 8.6-mL/y decline in FVC in COPDGene (95% CI, 4.8-12.4 mL/y; P < .001), a 9.5-mL/y decline in FVC in PLuSS (95% CI, 3.6-15.4 mL/y; P = .002), and a 1.2 m/y decline in 6MWD in COPDGene (95% CI, 0.1-2.2 m/y; P = .03). In these models, each percent increase in QIAs per year was associated with a 61% increase in the expected risk of mortality in COPDGene (HR, 1.61; 95% CI, 1.27-2.02; P < .001) and a 29% increase in PLuSS (HR, 1.29; 95% CI, 1.11-1.51; P = .001).
Discussion
Multiple prior studies have demonstrated the clinical relevance of subtle interstitial findings on CT scans, such as areas of higher attenuation or QIAs, which have been measured by a variety of techniques and assessed in a range of cohorts.8, 9, 10,25, 26, 27 For example, in previous work, we had shown that objectively measured QIAs (previously called interstitial features), measured by a machine learning-based approach that uses lung density and distance from the pleural surface, are associated with decreased lung function, a shorter 6MWD, worse subjective dyspnea, and increased mortality.8, 9, 10 However, these and other studies have largely focused on cross-sectional associations or predictions of change in clinical metrics, rather than on the changes in the CT measures themselves over time. In this study, we found that in two large cohorts of smokers, the progression of objectively measured QIAs is associated with faster declines in lung function and exercise capacity, and higher mortality. Our findings demonstrate there is clinical significance not only in the presence of QIAs in cross-sectional studies, but also in the gain of QIAs over time.
We found that the risk factors for QIA progression include advanced age at enrollment, current smoking status, female sex, and the MUC5B promoter polymorphism. The MUC5B promoter polymorphism is a known risk factor for IPF, and prior cross-sectional studies have shown that the MUC5B polymorphism is also associated with higher odds of ILAs and QIAs, suggesting that, for some, ILAs and QIAs represent early pulmonary fibrosis.4,10,28,29 Our findings show an association of the MUC5B polymorphism with longitudinal gain in QIAs, suggesting that increased MUC5B expression confers ongoing longitudinal risk and may play an important role in the development of IPF from QIAs. Additional risk factors for IPF are older age and cigarette smoking, and we similarly found that advanced age at enrollment and current smoking status are associated with QIA progression.30 However, the association of female sex with QIA progression was somewhat surprising, as male sex is a risk factor of IPF.30 It is possible that QIA progression in women in our cohort represents a different disease pathway, or a non-IPF ILD, given their overlap with IPF in histopathology and genetic mutations31,32; further studies are needed to evaluate these hypotheses further.
This longitudinal study suggests that QIA measurements capture areas that can progress and are associated with subsequent changes in lung function and mortality. The associations between QIA progression and lung function measured by FVC as well as mortality remained consistent when the model was limited to volume-limited participants, further strengthening the conclusion that QIA progression reflects areas of active disease. The associations between QIA progression and FVC, 6MWD, and mortality remained significant when the model was adjusted for cardiac risk factors, or in some, the presence of congestive heart failure, further strengthening the conclusion that QIA progression represents changes in parenchymal disease rather than artifact or pulmonary edema.
Regarding mortality, the association between QIA progression and mortality is important in light of prior work that suggests that both visually identified interstitial lung abnormalities (ILAs) and objectively identified QIAs may represent early or subtle evidence of future advanced ILD, such as IPF, in some people.4,5,8, 9, 10,25, 26, 27,33, 34, 35 The fact that QIA progression is associated with mortality in patients without known ILD, independent of other factors such as emphysema progression and change in lung function, highlights that those people with progression should be studied further to understand and possibly prevent the development of more advanced disease. Additional work is needed to establish more clearly the pathways of progression from QIAs to IPF. This is a particularly important area of study, given that the current treatment options for IPF only slow disease progression and do not reverse prior fibrosis.36, 37, 38
In our secondary analysis of QIAs in participants with PRISm, we found that these people have a higher amount of QIAs at baseline (8.4% QIAs; P < .001) compared with other lung function groups, especially GOLD stage 0 (5.6% QIAs), despite similar amounts of visual emphysema at baseline (2.3% and 2.0% emphysema, respectively). PRISm participants had an increased rate of FVC decline (13.2 mL/y) associated with every annual increase in QIAs, as did GOLD stage 0 participants (9.9 mL/y). The FVC decline was even faster in the volume-adjusted participants; PRISm participants showed an FVC decline of 24.9 mL/y associated with every annual increase in QIAs, and GOLD stage 0 participants showed an FVC decline of 27.8 mL/y associated with every annual increase in QIAs. The decline in FVC associated with QIA progression is particularly interesting, as prior studies have shown that PRISm participants actually have lower rates of FVC decline compared with participants in GOLD stage 0 or GOLD stages 1-4.12 Our findings suggest that the presence and progression of QIAs in PRISm participants may independently be a contributing reason to the worse clinical findings seen in these patients. It is also possible that the resolution or stability of QIAs seen in some participants may be related to the transitional nature of PRISm, in which participants improve to GOLD stage 039; further analysis is needed to test this hypothesis. Given that PRISm, IPF, and emphysema are all smoking-related diseases, it is possible that PRISm encapsulates a group of patients with early disease in the form of QIAs that can progress to IPF or combined pulmonary fibrosis and emphysema, rather than pure emphysema in the future.
Of special interest is the application of these objectively identified QIAs from research cohorts to the clinical setting. The CT scans in the PLuSS cohort were obtained through low-dose radiation protocols and are closer to the lung cancer screening scans that patients with a smoking history are likely to receive more readily in the real world.16 A strength of our study is that we applied our automated, machine learning-based objective measure to the PLuSS low-dose CT scans, and we found consistent associations between QIA progression and decreased spirometric and survival outcomes. Given that low-dose cancer screening CT scans are already standard-of-care, there is potential in broadening their use to detect and perhaps even screen for QIAs; this potential should be studied further.
Our study has several limitations in addition to those already mentioned. First, this is a prospective cohort study of exposures and outcomes that were evaluated longitudinally. Although we adjusted for known and possible confounders of the relationship between QIA progression and the outcomes of interest, we cannot prove a causal relationship with complete certainty. Second, our participants were all ever-smokers, which is a population at risk for pulmonary fibrosis; the impact of the presence and progression of QIAs in nonsmokers remains to be seen. Although our study of two smoker cohorts focused on risk factors and outcomes related to IPF, as it is a smoking-related disease, it is likely that QIAs encompass early stages of other fibrosing ILDs in addition to IPF, as one can find histopathologic changes and genetic mutations of end-stage IPF in other fibrosing ILDs.31,32 Given the functional and survival implications of QIA progression, future studies should explore and confirm these results in other cohorts. In addition, given the relationship between age and cross-sectional QIAs from prior studies, as well as the association between age and QIA progression in our study, it was surprising that in both cohorts the percentage of lung with QIAs decreased over the duration of follow-up.10 It is possible that this may be due to survival bias, although the changes in other measures such as emphysema argue somewhat against that. Another possibility is regression to the mean, although again this is not a finding seen with the other radiographic and clinical measures in this study. Additional work in other cohorts is needed to better understand this finding. Finally, although the clinical associations were similar, there was a large difference in the absolute amount of lung with QIAs between the two cohorts. Although some of this difference is likely due to differences in baseline characteristics between the two groups, the bulk of the difference is likely due to differences in CT imaging protocol, particularly in the use of high vs low radiation doses. This highlights the need for structured approaches to the application of measurement tools such as the one employed in this study, including the use of standardized and consistent CT imaging protocols and the application of image harmonization techniques to enable cross-protocol analyses.
Interpretation
In conclusion, in two cohorts of ever-smokers, longitudinal progression of QIAs is independently associated with decreased lung function and increased all-cause mortality. Identified risk factors for progression include advanced age, female sex, and the MUC5B minor allele. Although additional work is needed, the objective, longitudinal measurement of QIAs may help identify people at greatest risk for adverse events and perhaps most likely to benefit from early intervention to prevent the development of advanced ILDs such as IPF.
Take-home Points.
StudyQuestions: What are the associations of quantitative interstitial abnormality (QIA) progression with lung function, exercise capacity, and mortality, and what are the demographic and genetic risk factors for QIA progression?
Results: Older age, female sex, current smoking status, and the MUC5B minor allele are associated with QIA progression; QIA progression over time is associated with poorer lung function, worse exercise capacity, and increased mortality, including in low-dose screening CTs.
Interpretation: The longitudinal measurement of QIA progression may help identify people at greatest risk for adverse events and perhaps most likely to benefit from early intervention to prevent the development of advanced pulmonary fibrosis.
Acknowledgments
Author contributions: All authors approved the final version of this manuscript and agree to be accountable for all aspects of the work. B. C.: design, analysis, interpretation, writing, revision; N. A.: acquisition, interpretation, revision; T. J. D.: interpretation, writing, revision; Ru. S. J. E.: analysis, interpretation, revision; R. H.: analysis, acquisition, revision; S. M. H.: interpretation, revision; M. M.: analysis, interpretation, revision; M. H. C.: analysis, interpretation, revision; R. K. P.: interpretation, revision; G. M. H.: interpretation, writing, revision; R. K.: interpretation, revision; G. Y. L.: interpretation, revision; A. A. D.: interpretation, revision; S. E. M.: interpretation, revision; F. N. R.: interpretation, revision; C. L. P.: interpretation, revision; N. E.: interpretation, revision; C. P.: interpretation, revision; G. V. S.-F.: analysis, interpretation, revision; J. C. R.: interpretation, revision; D. A. L.: design, interpretation, revision; F. J. M.: interpretation, revision; M.L. K. H.: interpretation, revision; R. P. B.: interpretation, revision; D. O. W.: design, interpretation, revision; I. O. R.: design, interpretation, revision; G. R. W.: design, analysis, interpretation, revision; Ra. S. J. E.: design, analysis, interpretation, writing, revision; S. Y. A.: design, analysis, interpretation, writing, revision.
Funding/support: The COPDGene study (NCT00608764) is supported by NHLBI U01 HL089897 and U01 HL089856, as well as by the COPD Foundation through contributions made to an Industry Advisory Board composed of AstraZeneca, Bayer Pharmaceuticals, Boehringer Ingelheim, Genentech, GlaxoSmithKline, Novartis, Pfizer, and Sunovion. The PLuSS study is supported by the University of Pittsburgh Lung Cancer SPORE: NCI P50-CA90440, the University of Pittsburgh Cancer Institute, and the University of Pittsburgh Medical Center. Additional funding for this work includes National Institutes of Health grants: K08-HL145118 (S. Y. A.), NIH: R03-HL148484, K23-HL119558 (T. J. D.), K23-HL141651 (C. L. P.), R01-HL111024, R01-30974 (G. M. H.), R01-HL135142 (M. H. C. and G. M. H.), K23-HL136905 (F. N. R.), T32-HL007633 (B. C.), T32-HL007427 (M. M.), R01-HL137927, R01-HL147148 (M. H. C.), K24-HL138188 (M. L. K. H.), R01-HL116931 (Ra. S. J. E. and G. R. W.), R21-HL140422, R01HL149877 (R. S. J. E.), and P01-HL114501 (G. R. W.), as well as from Boehringer Ingelheim Pharmaceuticals, Inc. (G. R. W.), DOD W81XWH1810772 (T. J. D., I. O. R., and G. R. W.), and the Pulmonary Fibrosis Foundation (S. Y. A.).
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: B. C. received grant support from the NIH and consulting fees from Quantitative Imaging Solutions. T. J. D. received support from US Army Medical Research Acquisition Activity, the NIH, and Bristol Myers Squibb, consulting fees from Boehringer Ingelheim, and part of a clinical trial funded by Genentech. Ru. S. J. E. received consulting fees from Quantitative Imaging Solutions. M. M. received grant support from the NIH and Bayer. M. H. C. received grant support from the NIH, Bayer, GlaxoSmithKline, consulting fees from AstraZeneca; payment/honoraria from Illumina. G. M. H. received consulting through Boehringer Ingelheim and the Gerson Lehrman Group. A. A. D. received support from the NIH. S. E. M. is employed at Sarepta Therapeutics unrelated to current work. F. N. R. received support from the NIH. C. L. P. received support from the NIH. G. V. S.-F. received support from the NIH. J. C. R. received support from the NIH. F. J. M. received support from the NIH, grants and consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, CsL Behring, Gala, GlaxoSmithKline, Novartis, Polarean, Pulmonx, Sanofi/Regeneron, Sunovion, Teva, Verona, UpToDate, MedTronic. M. L. K. H. received personal fees from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Cipla, Chiesi, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, Regeneron, United Therapeutics, Medscape, and Integrity. M. L. K. H. has received either in-kind research support or funds paid to the institution from the NIH, Novartis, Sunovion, Nuvaira, Sanofi, AstraZeneca, Boehringer Ingelheim, Gala Therapeutics, Biodesix, the COPD Foundation, and the American Lung Association. M. L. K. H. has participated in Data Safety Monitoring Boards for Novartis and Medtronic with funds paid to the institution. M. L. K. H. has received stock options from Meissa Vaccines. I. O. R. received support from the NIH, US Army Medical Research Acquisition Activity. G. R. W. received support from the NIH, US Army Medical Research Acquisition Activity, Boehringer Ingelheim, Janssen; consulting fees from Boehringer Ingelheim, Janssen, Vertex, Pulmonx, Novartis, Philips; founder and co-owner of Quantitative Imaging Solutions; spouse works for Biogen. Ra. S. J. E. received support from the NIH founder and co-owner of Quantitative Imaging Solutions. S. Y. A. received support from the NIH, Pulmonary Fibrosis Foundation; member of Quantitative Imaging Solutions. None declared (N. A., R. H., S. M. H., R. K. P., R. K., G. Y. L., N. E., C. P., D. A. L., R. P. B., D. O. W.)
Role of sponsors: This was an independent, investigator-initiated study supported by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI had no role in the design, analysis, or interpretation of the results in this study; BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BIPI substances, as well as intellectual property considerations.
Additional information: The e-Appendixes and e-Tables are available online under “Supplementary Data.”
Footnotes
Ra. San José Estépar and S. Y. Ash contributed equally (co-senior authors) to this manuscript.
Part of this article has been presented as an oral presentation at the 2021 ATS International Conference [virtual meeting], May 14-19, 2021.
Supplementary Data
References
- 1.Washko G.R., Hunninghake G.M., Fernandez I.E., et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle T.J., Washko G.R., Fernandez I.E., et al. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med. 2012;185(7):756–762. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle T.J., Hunninghake G.M., Rosas I.O. Subclinical interstitial lung disease: why you should care. Am J Respir Crit Care Med. 2012;185(11):1147–1153. doi: 10.1164/rccm.201108-1420PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunninghake G.M., Hatabu H., Okajima Y., et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368(23):2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putman R.K., Hatabu H., Araki T., et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315(7):672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller E.R., Putman R.K., Vivero M., et al. Histopathology of interstitial lung abnormalities in the context of lung nodule resections. Am J Respir Crit Care Med. 2018;197(7):955–958. doi: 10.1164/rccm.201708-1679LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross J.C., San Jose Estepar R., Kindlmann G., et al. Automatic lung lobe segmentation using particles, thin plate splines, and maximum a posteriori estimation. Med Image Comput Comput Assist Interv. 2010;13(Pt 3):163–171. doi: 10.1007/978-3-642-15711-0_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ash S.Y., Harmouche R., Ross J.C., et al. The objective identification and quantification of interstitial lung abnormalities in smokers. Acad Radiol. 2017;24(8):941–946. doi: 10.1016/j.acra.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ash S.Y., Harmouche R., Ross J.C., et al. Interstitial features at chest CT enhance the deleterious effects of emphysema in the COPDGene cohort. Radiology. 2018;288(2):600–609. doi: 10.1148/radiol.2018172688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ash S.Y., Harmouche R., Putman R.K., et al. Clinical and genetic associations of objectively identified interstitial changes in smokers. Chest. 2017;152(4):780–791. doi: 10.1016/j.chest.2017.04.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan E.S., Castaldi P.J., Cho M.H., et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan E.S., Fortis S., Regan E.A., et al. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene Study. Am J Respir Crit Care Med. 2018;198(11):1397–1405. doi: 10.1164/rccm.201804-0663OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauwels R.A., Buist A.S., Calverley P.M., Jenkins C.R., Hurd S.S., GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 14.Regan E.A., Hokanson J.E., Murphy J.R., et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson D.O., Weissfeld J.L., Balkan A., et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178(7):738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson D.O., Weissfeld J.L., Fuhrman C.R., et al. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med. 2008;178(9):956–961. doi: 10.1164/rccm.200802-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz A.A., Strand M., Coxson H.O., et al. Disease severity dependence of the longitudinal association between CT lung density and lung function in smokers. Chest. 2018;153(3):638–645. doi: 10.1016/j.chest.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart J.I., Moyle S., Criner G.J., et al. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD. 2012;9(5):466–472. doi: 10.3109/15412555.2012.690010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe K.E., Regan E.A., Anzueto A., et al. COPDGene® 2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chronic Obstr Pulm Dis. 2019;6(5):384–399. doi: 10.15326/jcopdf.6.5.2019.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price A.L., Patterson N.J., Plenge R.M., Weinblatt M.E., Shadick N.A., Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 21.RStudio . RStudio, Inc.; Boston, MA: 2015. Integrated Development for R. [computer program] [Google Scholar]
- 22.R . R Foundation for Statistical Computing; Vienna, Austria: 2020. A Language and Environment for Statistical Computing [computer program] Version 4.0.3. [Google Scholar]
- 23.Mannino D.M., Holguin F., Pavlin B.I., Ferdinands J.M. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9(6):613–621. [PubMed] [Google Scholar]
- 24.Vatrella A., Calabrese C., Mattiello A., et al. Abdominal adiposity is an early marker of pulmonary function impairment: findings from a Mediterranean Italian female cohort. Nutr Metab Cardiovasc Dis. 2016;26(7):643–648. doi: 10.1016/j.numecd.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Podolanczuk A.J., Oelsner E.C., Barr R.G., et al. High-attenuation areas on chest computed tomography and clinical respiratory outcomes in community-dwelling adults. Am J Respir Crit Care Med. 2017;196(11):1434–1442. doi: 10.1164/rccm.201703-0555OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podolanczuk A.J., Oelsner E.C., Barr R.G., et al. High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J. 2016;48(5):1442–1452. doi: 10.1183/13993003.00129-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lederer D.J., Enright P.L., Kawut S.M., et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180(5):407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seibold M.A., Wise A.L., Speer M.C., et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364(16):1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ash S.Y., Harmouche R., Putman R.K., et al. Association between acute respiratory disease events and the MUC5B promoter polymorphism in smokers. Thorax. 2018;73(11):1071–1074. doi: 10.1136/thoraxjnl-2017-211208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lederer D.J., Martinez F.J. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 31.Renzoni E.A., Poletti V., Mackintosh J.A. Disease pathology in fibrotic interstitial lung disease: is it all about usual interstitial pneumonia? Lancet. 2021;398(10309):1437–1449. doi: 10.1016/S0140-6736(21)01961-9. [DOI] [PubMed] [Google Scholar]
- 32.Borie R., Le Guen P., Ghanem M., et al. The genetics of interstitial lung diseases. Eur Respir Rev. 2019;28(153):190053. doi: 10.1183/16000617.0053-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araki T., Putman R.K., Hatabu H., et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016;194(12):1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Putman R.K., Rosas I.O., Hunninghake G.M. Genetics and early detection in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;189(7):770–778. doi: 10.1164/rccm.201312-2219PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Putman R.K., Hatabu H., Araki T., et al. Association between interstitial lung abnormalities and all-cause mortality. J Am Med Assoc. 2016;315(7):672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King T.E., Jr., Bradford W.Z., Castro-Bernardini S., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 37.Flaherty K.R., Wells A.U., Cottin V., et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 38.Richeldi L., du Bois R.M., Raghu G., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 39.Wan E.S., Hokanson J.E., Regan E.A., et al. Significant spirometric transitions and preserved ratio impaired spirometry among ever smokers. Chest. 2022;161(3):651–661. doi: 10.1016/j.chest.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.