Abstract
Background
Over the past four decades, the Chinese government has conducted three surveys on the distribution of causes of death and built cancer registration. In order to shine a new light on better cancer prevention strategies in China, we evaluated the profile of cancer mortality over the forty years and analyzed the policies that have been implemented.
Methods
We described spatial and temporal changes in both cancer mortality and the ranking of major cancer types in China based on the data collected from three national surveys during 1973‐1975, 1990‐1992, 2004‐2005, and the latest cancer registration data published by National Central Cancer Registry of China. The mortality data were compared after conversion to age‐standardized mortality rates based on the world standard population (Segi's population). The geographical distribution characteristics were explored by marking hot spots of different cancers on the map of China.
Results
From 1973 to 2016, China witnessed an evident decrease in mortality rate of stomach, esophageal, and cervical cancer, while a gradual increase was recorded in lung, colorectal, and female breast cancer. A slight decrease of mortality rate has been observed in liver cancer since 2004. Lung and liver cancer, however, have become the top two leading causes of cancer death for the last twenty years. From the three national surveys, similar profiles of leading causes of cancer death were observed among both urban and rural areas. Lower mortality rates from esophageal and stomach cancer, however, have been demonstrated in urban than in rural areas. Rural areas had similar mortality rates of the five leading causes of cancer death with the small urban areas in 1973‐1975. Additionally, rural areas in 2016 also had approximate mortality rates of the five leading causes with urban areas in 2004‐2005. Moreover, stomach, esophageal, and liver cancer showed specific geographical distributions. Although mortality rates have decreased at most of the hotspots of these cancers, they were still higher than the national average levels during the same time periods.
Conclusions
Building up a strong primary public health system especially among rural areas may be one critical step to reduce cancer burden in China.
Keywords: cancer control strategy, cancer mortality, cancer registry, China, national cause of death survey, risk factor
List of Abbreviations
- NCC
National Cancer Center
- ASMR
age‐standardized mortality rate
- ASMRW
age‐standardized mortality rate by world standard population
- H. pylori
Helicobacter pylori
- ESCC
esophageal squamous cell carcinoma
- GBD
Global Burden of Disease
- HBV
hepatitis B virus
- AFP
alpha‐fetoprotein
- CT
computed tomography
- SNP
single nucleotide polymorphism
- LDCT
low‐dose computed tomography
- FIT
fecal immunochemical test
- FOBT
fecal occult blood test
1. BACKGROUND
Over the past four decades in China, three retrospective surveys on causes of death have been conducted, and the latest cancer registration data was published by the China National Cancer Center (NCC). During the first survey in the 1970s, detailed information for causes of death during 1973‐1975 was collected across 395 cities and 2392 counties, covering 96.7% of the population in China [1, 2]. Since then, some guidelines have been formulated accordingly to establish health care facilities and to set public health priorities, for example, Chinese government started performing some pilot programs of early screening and early treatment among demonstration sites with high cancer mortality rates since 1973. Then the second and the third national surveys were performed during periods of 1990‐1992 and 2004‐2005, respectively [3, 4], with the aim of assessing changes of cancer burden for a long run. After the third survey, the Chinese government gradually set up some cancer registry sites for regularly collecting cancer incidence and mortality data, and then established National Cancer Registration, and the number of its cancer registries increased from 43 in 2008 to 574 in 2019 [5]. Using the data from qualified 487 cancer registries, earlier in 2022, the China NCC reported results of nationwide cancer incidence and mortality of 2016 [5]. Therefore, we could evaluate and compare the profile of causes of death and cancer burden about per 10 years over the forty years in China. In addition, some areas with high mortality of specific cancer types reported in the first survey were tracked during the three national surveys and the latest cancer registration, which provides a chance to track changes in cancer burden in the targeted hotspots and to evaluate effectiveness of the applied strategies for cancer prevention and control.
In this study, we aimed to establish the cancer mortality patterns during the four time periods by analyzing published data from three national cause of death surveys [2, 3, 4] and the data from the latest national cancer registration [5]. In addition, we highlighted some effective cancer prevention strategies for major cancer types, especially from areas with intervention measures, which could shine a new light on better cancer prevention strategies in China.
2. MATERIALS AND METHODS
Chinese Ministry of Health organized and executed totally three nationwide retrospective surveys on causes of death from 1973 to 2005 (not including Taiwan, Hong Kong and Macau areas) [2, 3, 4]. During the first survey, approximately all cities and counties were included. For the second and third nationwide surveys, a multistage stratified cluster sampling method was used to select the survey sites and to achieve good population representativeness during the two surveys. Finally, 189 rural and 74 urban sites in the second national survey and 63 rural and 97 urban sites in the third survey were selected. The detailed information on the study settings, methods and mortality rate has been described in the three national reports of causes of death [2, 3, 4, 5]. The China NCC also gave the detailed method for data collecting in cancer registries, and it reported the latest results of nationwide cancer mortality in 2016 covering 200 rural and 287 urban areas with a 27.6% coverage rate [5].
All summarized cancer mortality rates, stratified by residential area (urban or rural area) and sex for five major cancer types, were obtained from three national cause‐of‐death surveys [2, 3, 4] and the latest national cancer registration [5]. Age‐standardized mortality rates (ASMRs) were calculated by world standard population (Segi's population; ASMRWs) for the four time periods. High‐risk geographical areas (hotspot sites) for different cancer types (where the mortality rate was found two or more times of that of the national level) were found out during the first national survey. Furthermore, spatial and temporal changes in both cancer mortality and ranking of major cancer types were also evaluated across different calendar periods. The mortality profiles for those hotspots of major cancer types were compared by analyzing the data from the same sites across the four periods.
3. RESULTS
3.1. The five major cancer types among both sexes from 1973 to 2016
Table 1 lists the ASMRWs of the top five cancer types by sex during the four time periods. During the period of 1973–1975, stomach and esophageal cancers were the top two causes of cancer death for both sexes, followed by cervical, liver, and lung cancers. Stomach cancer remained as the top cause of cancer death in the 1990s, which had been replaced by lung cancer since 2004. During the four periods, the ASMRWs of stomach and liver cancers first displayed an increase then a drop, the ASMRWs of esophageal cancer showed a continuous decline, while an opposite trend of steady increase was found in those of lung cancer (from 7.41 to 28.09 per 100 000 population from 1973 to 2016) and colorectal cancer (from 5.49 to 8.13 per 100 000 population from 1973 to 2016).
TABLE 1.
Mortality of five leading cancer types by sex in China during four time periods
| 1973‐1975 | 1990‐1992 | 2004‐2005 | 2016 | ||||
|---|---|---|---|---|---|---|---|
| Tumor location | ASMRW (per 100 000 population) | Tumor location | ASMRW (per 100 000 population) | Tumor location | ASMRW (per 100 000 population) | Tumor location | ASMRW (per 100 000 population) |
| Both sexes | |||||||
| All sites | 99.46 | All sites | 124.46 | All sites | 121.58 | All sites | 105.19 |
| Stomach | 23.86 | Stomach | 29.35 | Lung | 27.62 | Lung | 28.09 |
| Esophagus | 23.40 | Liver | 22.99 | Liver | 23.48 | Liver | 15.07 |
| Cervix | 14.61 | Lung | 20.41 | Stomach | 22.14 | Stomach | 12.30 |
| Liver | 14.01 | Esophagus | 20.40 | Esophagus | 13.73 | Esophagus | 8.28 |
| Lung | 7.41 | Colorectum | 6.08 | Colorectum | 6.57 | Colorectum | 8.13 |
| Male | |||||||
| All sites | 119.64 | All sites | 163.98 | All sites | 159.89 | All sites | 138.14 |
| Stomach | 32.36 | Stomach | 40.80 | Lung | 39.06 | Lung | 40.58 |
| Esophagus | 31.66 | Liver | 33.67 | Liver | 34.61 | Liver | 22.90 |
| Liver | 19.99 | Lung | 29.69 | Stomach | 30.78 | Stomach | 17.77 |
| Lung | 10.25 | Esophagus | 27.73 | Esophagus | 19.66 | Esophagus | 12.73 |
| Colorectum | 6.35 | Colorectum | 7.15 | Colorectum | 7.92 | Colorectum | 10.04 |
| Female | |||||||
| All sites | 80.69 | All sites | 87.19 | All sites | 84.84 | All sites | 73.95 |
| Stomach | 15.94 | Stomach | 18.60 | Lung | 16.73 | Lung | 16.24 |
| Esophagus | 15.92 | Esophagus | 13.63 | Stomach | 13.80 | Liver | 7.27 |
| Cervix | 14.61 | Liver | 12.25 | Liver | 12.34 | Stomach | 7.13 |
| Liver | 8.09 | Lung | 11.72 | Esophagus | 8.02 | Breast | 6.39 |
| Lung | 4.75 | Colorectum | 5.18 | Breast | 5.34 | Colorectum | 6.36 |
Abbreviations: ASMRW, age‐standardized mortality rate by world standard population (Segi's population).
Since 2004, the top five causes of cancer death have been lung, liver, stomach, esophageal and colorectal cancers, although their mortality rates have been changing over time. For females, cervical cancer dropped out of the top five in the 1990s, while female breast cancer has ranked as one of the top five leading causes of cancer death since 2004. Strikingly, males demonstrated much higher ASMRWs in liver and lung cancers than females throughout the four periods.
3.2. The five major cancer types among urban and rural areas from 1973 to 2016
The comparison of cancer mortality patterns between urban and rural areas in China during the four periods is shown in Figure 1. Distribution of leading causes of cancer death changed from 1973 to 2016 across urban and rural areas. Urban areas displayed a gradual increase in mortality from lung and colorectal cancers but a rapid decrease from esophageal and stomach cancers. Rural areas displayed the lowest lung cancer mortality rate in 1973–1975, later it increased rapidly and reached that of urban areas in 2016. Rural areas also demonstrated a decreased mortality from esophageal and stomach cancers, although their mortality rates were always higher than those in urban areas.
FIGURE 1.

Age‐standardized mortality rates (according to world standard population [Segi's population]) of five leading cancer types by geographic areas in China during four time periods. Large urban, population size > 750 000; medium urban, 750 000 ≥ population size >250 000; small urban, population size ≤ 250 000
Lung, liver, stomach, colorectal and female breast cancer became the top five causes of cancer death in urban areas in 2016, and differently, esophageal cancer but not female breast cancer was one of main causes of cancer death in rural areas. Rural areas had the approximate mortality rates of the five leading causes of cancer death with small urban areas in 1973–1975; and rural areas in 2016 had approximate mortality rates of the five leading causes with urban areas in 2004–2005.
3.3. Cancer map and high‐risk areas for top five causes of cancer death stratified by sex
The national report of causes of death from the first survey [2] shows the mortality map of stomach, esophageal and liver cancers in 1973–1975 stratified by sex, and lists their hotspot sites showing exceptionally high mortality rate. Supplementary Table S1 and Supplementary Table S2 also summarized the mortality rate and the number of hotspot sites of each province in China during the first survey. Figure 2 also displays the specific hotspots with mortality data of these three cancers during three time periods and compares their mortality rates since the 1990s. These three cancers had their specific geographical distribution.
FIGURE 2.
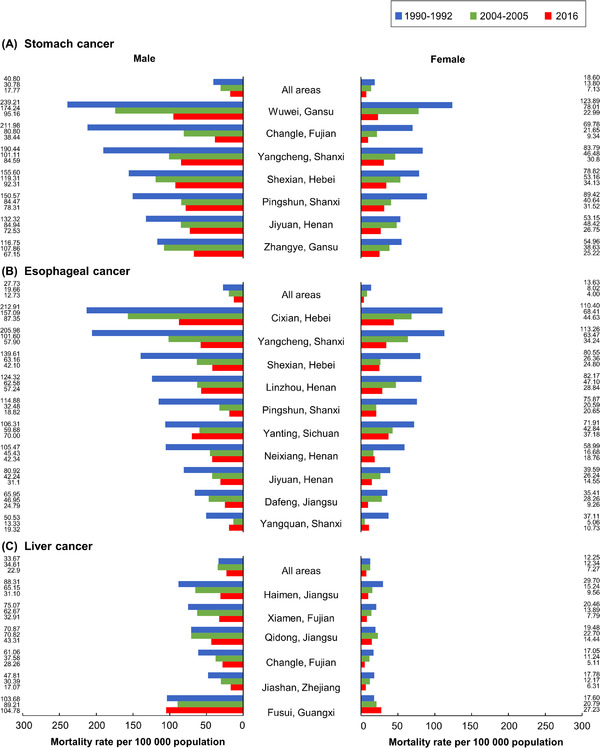
Age‐standardized mortality rate by the world standard population (Segi's population) of stomach (A), esophagus (B), and liver cancers (C) in ever high‐risk areas (counties with high mortality rates in 1973‐1975) in China during three time periods
3.3.1. Stomach cancer
The first survey revealed several stomach cancer hotspots, such as the Liaodong peninsula (e.g., Dandong and Yingkou cities in Liaoning province), Shandong peninsula (e.g., Qingdao and Yantai cities), Yangtze delta (e.g., Shanghai city, areas of Zhejiang, Jiangsu and Anhui provinces), Taihang mountains (e.g., areas of Hebei, Shanxi, and Henan provinces), Hexi corridor (e.g., Wuwei city in Gansu province), and eastern coastal Fujian province (e.g., Changle and Putian counties) (Supplementary Table S1 , Supplementary Table S2 ). Stomach cancer mortality rate from individual high‐risk areas decreased distinctly from 1990 to 2004 (Figure 2A), where a more significant decrease was found in Changle in Fujian province, Wuwei and Zhangye in Gansu province, and Shexian in Hebei province either among the males or among the females. However, stomach cancer mortality rates of these hotspot sites were still more than twice that of the whole country in 2016.
3.3.2. Esophageal cancer
The highest mortality rate of esophageal cancer was identified in counties in geographically adjacent areas of Henan, Shanxi and Hebei provinces, such as Linzhou, Shexian, Yangcheng, and Cixian (Supplementary Table S1 , Supplementary Table S2 ) [2]. Moving away from these areas, a gradual decrease of mortality rates was observed. Henan province had the highest mortality from esophageal cancer in China in 1973–1975 (age‐standardized mortality rate was 22.5 per 100 000 population), followed by Jiangsu, Shanxi, Fujian, Anhui, and Hebei provinces (21.7, 19.3, 14.7, 13.1 per 100 000 population, respectively) [2]. By 2016, either among the males or among the females, most of these high‐risk areas, however, had shown a sharp decrease in esophageal cancer mortality rates, with a significant decrease in Cixian in Hebei province, Yangcheng and Pingshun in Shanxi from 2004 to 2016 (Figure 2B). However, except Pingshun and Yangcheng in Shanxi, the esophageal cancer mortality rates in other sites were still twice that of the country in 2016.
3.3.3. Liver cancer
The southeastern coastal areas had high mortality rates from liver cancer, among which Shanghai city had the highest rate (17.7 per 100 000 population), followed by Fujian (17.5 per 100 000 population), Guangxi (16.5 per 100 000 population), Jiangsu (16.1 per 100 000 population), Zhejiang (15.3 per 100 000 population) and Jilin provinces (12.2 per 100 000 population) in 1973–1975 [2]. On one hand, some cities demonstrated an obvious decrease of liver cancer mortality rates from 1990 to 2016, among which are Haimen and Qidong of Jiangsu province, Xiamen and Changle of Fujian province, and Jiashan of Zhejiang province. These cities had approximate liver cancer mortality rates with the whole country in 2016. On the other hand, Fusui of Guangxi Zhuang Autonomous region showed an upward trend, and its liver cancer mortality rate was 4.6‐fold higher among males and 3.7‐fold higher among females than the national average level in 2016 (Figure 2C).
3.3.4. Lung, colorectal and female breast cancers
In the first nationwide survey, China's northeast and east areas showed a higher risk for lung cancer or female breast cancer than the average national level [2]. However, these areas were discretely distributed. While China's eastern coastal area (Zhejiang, Shanghai, Fujian, and Jiangsu) and northern China (Inner Mongolia and Shanxi) had slightly higher mortality from colorectal cancer (Supplementary Table S1 , Supplementary Table S2 ), the ASMRWs in the former areas fell between 4.5 and 6.2 per 100 000 population, and the ASMRWs in the latter were about 4.2 per 100 000 population [2]. However, when coming to 2016, there was no obvious difference across various areas either for lung cancer, female breast cancer, or colorectal cancer [5]. Because there were limited hotspot areas with high mortality rate for the three cancers during the four time periods, we couldn't compare their mortality rate changings of the relative hotspots during the forty years.
4. DISCUSSION
4.1. Changing mortality pattern of the top five causes of cancer death
These three national surveys and the latest cancer registration illustrate a cancer mortality pattern of China in a transitional stage between developing and developed countries. Particularly, cancer mortality from major cancer types (stomach, esophageal, and liver cancers) has remained a relatively high level since 1975, and they started declining since recent 10 years, while other common cancer types (lung, female breast, and colorectal cancers) generally observed in developed countries have been mushrooming in China within a short period. China rural areas had similar top five major causes of cancer death with urban areas of 10 years ago; while China urban areas in 2016 had the same top five causes of cancer death with worldwide region in 2020 [6]. It seems that cancer mortality pattern in China was changing along with the urbanization.
4.2. Epidemiological studies among hotspot sites of the top five causes of cancer death and updated prevention strategies in China
4.2.1. Stomach cancer
Helicobacter pylori (H. pylori) infection, genetic susceptibility, environmental and behavioral factors are still the important risk factors contributing to stomach cancer, such as intake of high‐salted food, moldy food, or exposure to N‐nitroso compounds from diet (processed meats, smoked preserved foods, pickled and salty preserved foods, and foods dried at high temperatures)[7, 8, 9, 10]. Correspondingly, epidemiological data from three hotspots including Zhuanghe county of Liaodong Peninsula [11], Linqu county of Shandong Peninsula [12], Changle county of Fujian province [13], have shown that the prevalence of H. pylori infection was above 60%, which was 60.4% in Zhuanghe [11], 74.7% in Linqu and 80.9% in Changle [12, 13]. Two randomized controlled trials in Linqu and Changle have confirmed that eradication of H. pylori effectively reduced the incidence of stomach cancer, especially among those without precancerous lesions at baseline [14, 15]. Concurrently, several local special dietary habits, including consumption of fermented sour pancake in Linqu, processed salted pork in Zhuanghe, and shrimp sauce in Changle were strongly associated with stomach cancer risk [16, 17, 18]. The exposure levels of these factors have changed over time, due to such as change of dietary pattern and sanitary condition. However, there are few prospective studies to confirm the potential effect of these changes on stomach cancer incidence and mortality.
Although the infection rate of H. pylori in China has decreased over the past few decades, the current infection rate stays as high as 44.2% [19]. Owing to the large population base, widespread eradication of H. pylori infection in China may encounter many obstacles, one of which is the substantial economic burden. In China, the current national guideline recommends an opportunistic screening among hospital outpatients, in which a combined impact of age, sex, the status of H. pylori infection, level of gastrin‐17, and pepsinogen is investigated to identify the high‐risk people, who are then subjected to endoscopy [20]. However, there still lacks population‐based studies to verify the effectiveness of this screening option [20].
Over 80% of stomach cancer cases are diagnosed at an advanced stage [21]. However, due to the widespread implementation of national screening programs, some high‐incidence countries (such as Japan and South Korea) have demonstrated a relative high 5‐year survival rate of stomach cancer through early cancer detection (64.6% in Japan; 71.5% in South Korea) compared with 35% in China [22, 23]. As a result, a national screening program with a high coverage may help to reduce stomach cancer mortality.
4.2.2. Esophageal cancer
Esophageal squamous cell carcinoma (ESCC) accounts for nearly 90% of all esophageal cancer cases in China [24]. In areas with low and medium incidence of ESCC, its incidence can be mainly attributed to excessive smoking and consumption of alcohol, along with chewing areca nut, low intake of fruits and vegetables. The attributable proportion for the above‐mentioned risk factors of ESCC was reported elsewhere as high as 89% [25], much higher than that of 60% reported in China [26]. To investigate its potential risk factors, a large‐scale population‐based case‐control study has been conducted in Taixing of Jiangsu province, one of the hotspots of esophageal cancer from the first survey. The findings indicate a strong correlation between esophageal cancer occurrence and factors such as hot drinking, alcohol drinking and its interaction with the level of ethanol dehydrogenase, which confirms alcohol drinking as an obvious incentive for this cancer [27, 28].
Comprehensive screening for upper gastrointestinal cancer has been conducted in Cixian since 2005, which has led to a significant increase of 5‐year relative survival of esophageal cancer [29]. One long‐term cohort study performed among China's communities with a high burden of ESCC showed that the endoscopic screening and intervention program could significantly reduce mortality from esophageal cancer [30]. Another study also confirmed different cost‐effectiveness of several endoscopic screening strategies [31]. The current guideline for early screening and early treatment of upper gastrointestinal cancer in China has a relatively wide definition for the high‐risk people [32], and there exist several risk prediction models in China to guide risk stratification among the general population [33, 34]. However, there is little solid evidence to confirm their effectiveness. More efforts are needed to develop highly feasible and reliable strategies before they could be implemented among vast rural areas.
4.2.3. Liver cancer
According to the Global Burden of Disease (GBD) 2015 study, 84% of liver cancer deaths was attributed to infection of hepatitis B virus (HBV) or hepatitis C virus, and excessive alcohol use at the global level [35]. Marked differences of ASMRWs of liver cancer across sexes may be partly explained by the different levels of HBV infection and alcohol use in males and females [35]. Epidemiological and molecular biological studies consistently confirmed that HBV infection, exposure to aflatoxin and microcystins, as well as polluted drinking water were the main risk factors of liver cancer in Qidong, Fusui and Haimen in China [36, 37, 38, 39]. A systematic review and meta‐analysis showed that population attributable risk of aflatoxin‐related liver cancer was about 17% [40]. Dietary exposure to aflatoxin B1 amplified the risk of HBV‐related liver cancer [40, 41, 42]. The pioneering experiences from Qidong tell us that the primary prevention strategies could be summarized as following: reduced exposure of aflatoxin by changing storage method of corn, government policy‐induced switch of dietary staple from corn to rice in mid‐1980s [38], and vaccination against HBV across the whole area. Although China has become an intermediate endemic area for HBV infection with 5%–8% prevalence since the introduction of the HBV vaccination policy among infants [43, 44], Fusui still holds a high burden of liver cancer even after introducing similar strategies.
Various dietary patterns exist across China's vast areas, but some main food items in eastern coastal areas are similar, such as corn and peanuts, which can be more likely polluted by aflatoxin than other food staples [45]. Apart from similar dietary patterns, these areas also share the same sea coastline. Other studies may be needed to investigate the unknown factors from seafood exposures. One randomized controlled trial in Qidong showed that, even after earlier diagnosis of liver cancer, it may not reduce overall liver cancer mortality with a screening strategy using serum α‐fetoprotein detection for first‐line screening and then B‐mode ultrasound scan for suspicious cases [46]. Similarly, in another large‐scale population study in Zhongshan city of Guangdong, no significant reduction of liver cancer mortality was achieved by the similar screening strategy [47]. In recent years, biomarkers, such as GALAD (gender × age × log alpha‐fetoprotein [AFP] × des‐gamma‐carboxy prothrombin), have been applied in the surveillance of cirrhosis to detect early‐stage liver cancer, which provides alternative approaches for non‐invasive and effective screening [48].
4.2.4. Lung cancer
Smoking is a well‐known major risk factor or cause of lung cancer, followed by occupational or environmental exposures, such as radon, coal tar, asbestos, air pollution, as well as passive smoking from indoors or working place and so on [49]. An exposure to household burning of solid fuels for heating might be responsible for some hotspots of lung cancer identified in the first survey such as in Xuanwei, Yunnan province [50]. According to the GBD study in 2017, the tobacco‐attributable age‐standardized death rates of tracheal, bronchus and lung cancer increased by above 45% either in females or in males from 1990 to 2017 [51, 52]. In contrast, according to the recent Global Cancer Statistics in 2020, a continuously declining cancer burden has been reported in the United States, and lung cancer‐related death has decreased by 41% from 1991 to 2019, due largely to the success of the smoking cessation campaign since 1965 [53]. Tobacco control should thus be the primary strategy to reduce the high cancer burden in China.
It is puzzling that both high burden of lung cancer and low smoking rate (2.1%) are found among Chinese women [54]. The difference and rapid change of distribution of histological types of lung cancer across sexes may contribute to this puzzle. Temporal analysis of lung cancer in Shenzhen or Beijing showed annual increase percentage of lung adenocarcinoma of 5%–15% since 2000, and between 2000 and 2018, 45%–60% and 77%–83% of incident lung cancer was adenocarcinoma in males and in females, respectively [55, 56]. Several studies tried to correlate this high lung cancer burden with the exposures to outdoor ambient air pollution or other forms of smoke such as cooking fumes [57].
Further studies have identified high‐risk groups for computed tomography (CT) lung cancer screening according to a series of conventional risk factors, such as smoking status and duration, cumulative pack‐years of cigarette smoking, and various other exposures [58, 59, 60]. One study from China has developed a novel lung cancer risk prediction model based on the Chinese population, which was based on excavated 19 single nucleotide polymorphisms (SNPs) plus some conventional risk factors to identify the high‐risk population [61]. Its cost‐effectiveness, however, needs to be further assessed from the public health perspective. Importantly, both the low smoking rate and the fact that it is difficult to accurately measure the impact of second‐hand smoking make it challenging to identify the true risk factors among Chinese women. Low‐dose computed tomography (LDCT) has gradually become a screening method for lung cancer. One cohort study (median follow‐up of 3.6 years between 2013 and 2018) from the China National Lung Cancer Screening program suggests that one‐off LDCT screening could significantly reduce lung cancer mortality and all‐cause mortality in a large population [59]. However, LDCT is hardly applicable in rural areas. As a result, mobile CT screening along with early treatment has become a new promising strategy.
4.2.5. Colorectal cancer
Convincing evidence has shown that factors such as less physical activity, processed meat, alcoholic drinks, body fatness, and tobacco smoking could increase the risk of colorectal cancer. Still, the estimation of the pooled relative risk to associate these factors with colorectal cancer risk varies from 1.1 to 1.25 [62]. These insignificant relative risks categorize them as general risk factors rather than specific ones. On the contrary, the consumption of dietary fibers, dairy products, whole grains, or calcium supplements can probably reduce its risk [62]. So far, following factors have been attributed to the increasing secular trend of the colorectal cancer burden in China: change of lifestyle and dietary pattern, for instance, a more sedentary working mode, an increased intake of various meat products, accompanied with overweight or obesity.
After the first survey, regions with the highest mortality rate of colorectal cancer were identified. Following the first survey, large‐scale population‐based colorectal cancer screening or studies were initiated in the 1980s in Haining county and Jiashan county of Zhejiang province. As a result, Jiashan has become one of the demonstration sites for the early diagnosis and treatment of colorectal cancer. Rectal cancer mortality in Jiashan dropped by 31.7% from 1980 to 1990 [63]. Different screening methods, such as fecal occult blood test (FOBT), fecal immunochemical test (FIT), and questionnaire, have been generally adopted for various age groups. Specifically in Jiashan, a two‐stage strategy was adopted: questionnaire‐based risk assessment was followed by FOBT, and the high‐risk group was further referred to colonoscopy and necessary biopsy [62]. This is also currently considered as a general model for the cancer screening program in China. Furthermore, cost‐effectiveness analysis for colorectal cancer screening in Shanghai, China showed that the plan was more favorable for the rural than the urban population, primarily due to the relatively low participation rate in urban areas [64, 65]. More and more studies have recently involved the use of circulating tumor DNA methylation profiles for the early screening or diagnosis [66]. However, large population‐based evaluation studies are crucially required to ensure the reliability and cost‐effectiveness of this emerging tool as a primary screening method.
4.2.6. Female breast cancer
Ever since 2004, female breast cancer has been ranked as one of the top five cancer types concerning mortality rates, although its current age‐standardized incidence and mortality rates of female breast cancer were still lower than the global level [67]. It is generally understood that, breast cancer is associated with the age at menarche or menopause, childbearing and breastfeeding history and so on [68]. China's One‐child Policy was put into effect in the 1980s, which directly affected childbearing and breastfeeding history among Chinese women especially in urban areas [69]. A relatively low total fertility rate and relatively high mortality rate of breast cancer was observed among the wealthy eastern regions, such as urban Shanghai [68, 70]. In this sense, the recent Second‐child Policy may perturb breast cancer burden especially among urban women. Other risk factors in lifestyle and behavior habits include overweight or obesity, physical inactivity [68] and so on, which are more and more common in urbanized areas in China. Along with rapid urbanization in China, this vast country may be threatened with further rising breast cancer incidence and mortality among young women. Similar trends have been reported in young women in Taiwan and Hong Kong, China [70], which has been similarly associated with the changing distribution of risk factors mentioned above.
Patients with early female breast cancer (stage I‐II) demonstrated a high 5‐year survival rate of over 80% [71]. Therefore, breast screening is highly recommended among developed regions. For this purpose, the Gail model and several updated Gail models were developed and used in identifying high‐risk women for screening [72, 73, 74]. However, its application in China is limited due to the lack of a definite method for identification of high‐risk groups for screening among Chinese women [75]. China is the only country where both ultrasound and clinical breast examination are recommended as the primary screening method [76]. Launched in 2012, the risk‐based breast cancer screening program, for instance, recommends mammography in conjunction with ultrasound and clinical breast examination to be the primary screening methods among women aged 45–69 years in urban China. Nevertheless, the cost‐effectiveness of screening using combined methods, compared with mammography alone, is uncertain yet [77]. In rural settings, a big concern is insufficient equipment or lack of well‐trained, experienced medical personnel [78].
5. OPPORTUNITIES AND CHALLENGES
It is observed that the general cancer mortality pattern in China has been moving towards that of more developed and urbanized countries or regions in the world. However, China is a vast country with unbalanced development and diverse cultures. It is therefore necessary to adopt flexible procedures and multiple measures to promote healthier lifestyles as well as participation in early screening programs among the general public.
One point that should not be neglected is the fact of insufficient facilities for cancer screening as well as few qualified health‐care staff especially in rural areas. To reduce cancer burden in China, it is critical to build up a strong primary public health system particularly in rural areas. This new era brings us new opportunities such as screening based on artificial intelligence and emerging new biomarkers. It is worth mentioning, however, that all prevention strategies should be systematically assessed based on large‐scale population‐based studies.
AUTHOR CONTRIBUTIONS
Ruimei Feng, Qingling Su, and Xiaoyin Huang contributed to the conception and design of the study and data analysis. Ruimei Feng, Qingling Su, Xiaoyin Huang, and Xin Xu drafted the paper and interpreted the results. Ruimei Feng, Qingling Su, Xiaoyin Huang, Xin Xu, Til Basnet, and Weimin Ye contributed to the data interpretation and rewriting. All authors read and approved the final manuscript.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
Supporting information
Supplementary Information
ACKNOWLEDGEMENTS
We would like to thank the Chinese Ministry of Health who organized and reported the data of the three nationwide retrospective surveys on causes of death from 1973 to 2005. We thank the staff from the government and healthcare organization in each county or city across the whole China who executed three nationwide retrospective surveys. We also thank the China National Cancer Center and the local registries of China who provided and published the cancer registration data.
Feng R, Su Q, Huang X, Basnet T, Xu X, Ye W. Cancer situation in China: what does the China cancer map indicate from the first national death survey to the latest cancer registration? Cancer Commun. 2023;43:75–86. 10.1002/cac2.12393
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author.
REFERENCES
- 1. Li JY, Liu BQ, Li GY, Chen ZJ, Sun XI, Rong SD. Atlas of cancer mortality in the People's Republic of China. An aid for cancer control and research. Int J Epidemiol. 1981;10(2):127–33. [DOI] [PubMed] [Google Scholar]
- 2. Office for Cancer Prevention and Control, Ministry of Health, P. R. China . Malignant tumor mortality survey report. 1980. 1st ed.
- 3. Office for Cancer Prevention Control MoH, P. R. China . Malignant tumor mortality survey report (2004–2005), 1st ed. Beijing: People's Medical Publishing House; 2007. [Google Scholar]
- 4. National Office for Cancer Prevention and Control . Survey of cancer mortality in China 1990–1992. People Health Publishing House; 1993. [Google Scholar]
- 5. Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO . Global Health Estimates 2020: Deaths by cause, age, sex, by country and by region, 2000–2019. 2020. [Google Scholar]
- 7. Konturek PC, Konturek SJ, Brzozowski T. Helicobacter pylori infection in gastric cancerogenesis. J Physiol Pharmacol. 2009;60(3):3–21. [PubMed] [Google Scholar]
- 8. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52(24):6735–40. [PubMed] [Google Scholar]
- 9. Ye W, Olof N, H‐O A. Stomach Cancer. In: Textbook of cancer epidemiology. Hans‐Olov A, David John H, Pagona L, M L, editors, Oxford University Press; 2018. 69 p. [Google Scholar]
- 10. Lu L, Mullins CS, Schafmayer C, Zeißig S, Linnebacher M. A global assessment of recent trends in gastrointestinal cancer and lifestyle‐associated risk factors. Cancer Commun (Lond). 2021;41(11):1137–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gong YH, Wang Y, Yuan Y. Distribution of Helicobacter pylori in north China. World J Gastroenterol. 2005;11(23):3523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. You WC, Zhang L, Gail MH, Chang YS, Liu WD, Ma JL, et al. Gastric dysplasia and gastric cancer: Helicobacter pylori, serum vitamin C, and other risk factors. J Natl Cancer Inst. 2000;92(19):1607–12. [DOI] [PubMed] [Google Scholar]
- 13. Wong BC, Lam SK, Ching CK, Hu WH, Ong LY, Chen BW, et al. Seroprevalence of cytotoxin‐associated gene A positive Helicobacter pylori strains in Changle, an area with very high prevalence of gastric cancer in south China. Aliment Pharmacol Ther. 1999;13(10):1295–302. [DOI] [PubMed] [Google Scholar]
- 14. Yan L, Chen Y, Chen F, Tao T, Hu Z, Wang J, et al. Effect of helicobacter pylori eradication on gastric cancer prevention: updated report from a randomized controlled trial with 26.5 years of follow‐up. Gastroenterology. 2022;163(1):154–62.e3. [DOI] [PubMed] [Google Scholar]
- 15. Li WQ, Zhang JY, Ma JL, Li ZX, Zhang L, Zhang Y, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow‐up of a randomized intervention trial. BMJ. 2019;366:l5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ye WM, Yi YN, Luo RX, Zhou TS, Lin RT, Chen GD. Diet and gastric cancer: a casecontrol study in Fujian Province, China. World J Gastroenterol. 1998;4(6):516–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai L, Yu SZ, Ye WM, Yi YN. Fish sauce and gastric cancer: an ecological study in Fujian Province, China. World J Gastroenterol. 2000;6(5):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–48. [DOI] [PubMed] [Google Scholar]
- 19. Ren S, Cai P, Liu Y, Wang T, Zhang Y, Li Q, et al. Prevalence of Helicobacter pylori infection in China: A systematic review and meta‐analysis. J Gastroenterol Hepatol. 2022;37(3):464–70. [DOI] [PubMed] [Google Scholar]
- 20. Fan X, Qin X, Zhang Y, Li Z, Zhou T, Zhang J, et al. Screening for gastric cancer in China: Advances, challenges and visions. Chin J Cancer Res. 2021;33(2):168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. [DOI] [PubMed] [Google Scholar]
- 22. Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388(10060):2606. [DOI] [PubMed] [Google Scholar]
- 23. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population‐based cancer registries. Lancet Glob Health. 2018;6(5):e555–e67. [DOI] [PubMed] [Google Scholar]
- 24. Zeng H, Zheng R, Zhang S, Zuo T, Xia C, Zou X, et al. Esophageal cancer statistics in China, 2011: Estimates based on 177 cancer registries. Thorac Cancer. 2016;7(2):232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murphy G, McCormack V, Abedi‐Ardekani B, Arnold M, Camargo MC, Dar NA, et al. International cancer seminars: a focus on esophageal squamous cell carcinoma. Ann Oncol. 2017;28(9):2086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Islami F, Chen W, Yu XQ, Lortet‐Tieulent J, Zheng R, Flanders WD, et al. Cancer deaths and cases attributable to lifestyle factors and infections in China, 2013. Ann Oncol. 2017;28(10):2567–74. [DOI] [PubMed] [Google Scholar]
- 27. Suo C, Yang Y, Yuan Z, Zhang T, Yang X, Qing T, et al. Alcohol intake interacts with functional genetic polymorphisms of aldehyde dehydrogenase (ALDH2) and alcohol dehydrogenase (ADH) to increase esophageal squamous cell cancer risk. J Thorac Oncol. 2019;14(4):712–25. [DOI] [PubMed] [Google Scholar]
- 28. Yu C, Tang H, Guo Y, Bian Z, Yang L, Chen Y, et al. Hot tea consumption and its interactions with alcohol and tobacco use on the risk for esophageal cancer: a population‐based cohort study. Ann Intern Med. 2018;168(7):489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li D, Li D, Song G, Liang D, Chen C, Zhang Y, et al. Cancer survival in Cixian of China, 2003–2013: a population‐based study. Cancer Med. 2018;7(4):1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei WQ, Chen ZF, He YT, Feng H, Hou J, Lin DM, et al. Long‐term follow‐up of a community assignment, one‐time endoscopic screening study of esophageal cancer in China. J Clin Oncol. 2015;33(17):1951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xia R, Zeng H, Liu W, Xie L, Shen M, Li P, et al. Estimated cost‐effectiveness of endoscopic screening for upper gastrointestinal tract cancer in high‐risk areas in China. JAMA Netw Open. 2021;4(8):e2121403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen R, Liu Y, Song G, Li B, Zhao D, Hua Z, et al. Effectiveness of one‐time endoscopic screening programme in prevention of upper gastrointestinal cancer in China: a multicentre population‐based cohort study. Gut. 2021;70(2):251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Z, Guo C, He Y, Chen Y, Ji P, Fang Z, et al. A clinical model predicting the risk of esophageal high‐grade lesions in opportunistic screening: a multicenter real‐world study in China. Gastrointest Endosc. 2020;91(6):1253–60.e3. [DOI] [PubMed] [Google Scholar]
- 34. Yang X, Suo C, Zhang T, Yin X, Man J, Yuan Z, et al. A nomogram for screening esophageal squamous cell carcinoma based on environmental risk factors in a high‐incidence area of China: a population‐based case‐control study. BMC Cancer. 2021;21(1):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ueno Y, Nagata S, Tsutsumi T, Hasegawa A, Watanabe MF, Park HD, et al. Detection of microcystins, a blue‐green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis. 1996;17(6):1317–21. [DOI] [PubMed] [Google Scholar]
- 37. Ruan CC, Chen YH, Zhang ZQ. Drinking water and liver cancer. World J Gastroenterol. 1997;3(1):47–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen J, Zhu J, Wang G, Groopman JD, Kensler TW. Qidong: a crucible for studies on liver cancer etiology and prevention. Cancer Biol Med. 2019;16(1):24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng D, Deng W, Huang T, Li X, Li Z. Relationship between hepatitis B virus genotype, BCP/Pre‐C region mutations and risk of hepatocellular carcinoma in Guangxi Zhuang Autonomous Region. Zhonghua Liu Xing Bing Xue Za Zhi. 2015;36(7):725–9. (Chinese). [PubMed] [Google Scholar]
- 40. Liu Y, Chang CC, Marsh GM, Wu F. Population attributable risk of aflatoxin‐related liver cancer: systematic review and meta‐analysis. Eur J Cancer. 2012;48(14):2125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–62. [DOI] [PubMed] [Google Scholar]
- 42. Chen JG, Zhang SW. Liver cancer epidemic in China: past, present and future. Semin Cancer Biol. 2011;21(1):59–69. [DOI] [PubMed] [Google Scholar]
- 43. Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97(3):230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang H, Men P, Xiao Y, Gao P, Lv M, Yuan Q, et al. Hepatitis B infection in the general population of China: a systematic review and meta‐analysis. BMC Infect Dis. 2019;19(1):811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kensler TW, Roebuck BD, Wogan GN, Groopman JD. Aflatoxin: a 50‐year odyssey of mechanistic and translational toxicology. Toxicol Sci. 2011;120(Suppl 1):S28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10(4):204–9. [DOI] [PubMed] [Google Scholar]
- 47. Ji M, Liu Z, Chang ET, Yu X, Wu B, Deng L, et al. Mass screening for liver cancer: results from a demonstration screening project in Zhongshan City, China. Sci Rep. 2018;8(1):12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sumida Y, Yoneda M, Seko Y, Ishiba H, Hara T, Toyoda H, et al. Surveillance of hepatocellular carcinoma in nonalcoholic fatty liver disease. Diagnostics (Basel). 2020;10(8):579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schabath MB, Cote ML. Cancer progress and priorities: Lung cancer. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1563–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim C, Chapman RS, Hu W, He X, Hosgood HD, Liu LZ, et al. Smoky coal, tobacco smoking, and lung cancer risk in Xuanwei, China. Lung Cancer. 2014;84(1):31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wen H, Xie C, Wang F, Wu Y, Yu C. Trends in disease burden attributable to tobacco in China, 1990–2017: Findings from the Global Burden of Disease Study 2017. Front Public Health. 2020;8:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu X, Yu Y, Wang M, Mubarik S, Wang F, Wang Y, et al. The mortality of lung cancer attributable to smoking among adults in China and the United States during 1990–2017. Cancer Commun (Lond). 2020;40(11):611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Williams PA, Zaidi SK, Sengupta R. AACR Cancer Disparities Progress Report 2022. Cancer Epidemiol Biomarkers Prev. 2022;31(7):1249–50. [DOI] [PubMed] [Google Scholar]
- 54. Prevention CCfDCa . 2018 China Adult Tobacco Survey Report [internet]. Available Online at: http://www.notc.org.cn/gzdt/201908/t20190814_204617.html [cited October 22, 2019]
- 55. Yang L, Wang N, Yuan Y, Liu S, Li H, Tian J, et al. Secular trends in incidence of lung cancer by histological type in Beijing, China, 2000–2016. Chin J Cancer Res. 2019;31(2):306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lei L, Huang A, Cai W, Liang L, Wang Y, Liu F, et al. Spatial and temporal analysis of lung Cancer in Shenzhen, 2008–2018. Int J Environ Res Public Health. 2020;18(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mu L, Liu L, Niu R, Zhao B, Shi J, Li Y, et al. Indoor air pollution and risk of lung cancer among Chinese female non‐smokers. Cancer Causes Control. 2013;24(3):439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dyabd E, Yang LC, Cba E, Xwd E, Cap B. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Lett. 2020;468:82–7. [DOI] [PubMed] [Google Scholar]
- 59. Li N, Tan F, Chen W, Dai M, Wang F, Shen S, et al. One‐off low‐dose CT for lung cancer screening in China: a multicentre, population‐based, prospective cohort study. Lancet Respir Med. 2022;10(4):378–91. [DOI] [PubMed] [Google Scholar]
- 60. Lim KP, Marshall H, Tammemägi M, Brims F, McWilliams A, Stone E, et al. Protocol and rationale for the international lung screening trial. Ann Am Thorac Soc. 2020;17(4):503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dai J, Lv J, Zhu M, Wang Y, Qin N, Ma H, et al. Identification of risk loci and a polygenic risk score for lung cancer: a large‐scale prospective cohort study in Chinese populations. Lancet Respir Med. 2019;7(10):881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Lett. 2021;522:255–68. [DOI] [PubMed] [Google Scholar]
- 63. Cao M, Li H, Sun D, He S, Yu Y, Li J, et al. Cancer screening in China: The current status, challenges, and suggestions. Cancer Lett. 2021;506:120–7. [DOI] [PubMed] [Google Scholar]
- 64. Li XP, Chen HM, Lei XH, Dou GS, Chen YC, Chen LP, et al. Cost‐effectiveness analysis of a community‐based colorectal cancer screening program in Shanghai, China. J Dig Dis. 2021;22(8):452–62. [DOI] [PubMed] [Google Scholar]
- 65. Chen H, Li N, Ren J, Feng X, Lyu Z, Wei L, et al. Participation and yield of a population‐based colorectal cancer screening programme in China. Gut. 2019;68(8):1450–7. [DOI] [PubMed] [Google Scholar]
- 66. Luo H, Zhao Q, Wei W, Zheng L, Yi S, Li G, et al. Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer. Sci Transl Med. 2020;12(524):eaax7533. [DOI] [PubMed] [Google Scholar]
- 67. Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, et al. Global patterns of breast cancer incidence and mortality: A population‐based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond). 2021;41(11):1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Fan L, Strasser‐Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–89. [DOI] [PubMed] [Google Scholar]
- 69. Lan M, Kuang Y. Evolutionary trends in fertility among Chinese women, 1990–2015. Reprod Health. 2021;18(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huang Z, Wen W, Zheng Y, Gao YT, Wu C, Bao P, et al. Breast cancer incidence and mortality: Trends over 40 years among women in Shanghai, China. Ann Oncol. 2016;27(6):1129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. National Cancer Institute . Division of cancer prevention and control, surveillance program, cancer statistics branched [internet]. Available from: https://seer.cancer.gov/ [cited 2018 Jun 25]
- 72. Maas P, Barrdahl M, Joshi AD, Auer PL, Gaudet MM, Milne RL, et al. Breast cancer risk from modifiable and nonmodifiable risk factors among white women in the United States. JAMA Oncol. 2016;2(10):1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–86. [DOI] [PubMed] [Google Scholar]
- 74. McCarthy AM, Keller B, Kontos D, Boghossian L, McGuire E, Bristol M, et al. The use of the Gail model, body mass index and SNPs to predict breast cancer among women with abnormal (BI‐RADS 4) mammograms. Breast Cancer Res. 2015;17(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang X, Huang Y, Li L, Dai H, Song F, Chen K. Assessment of performance of the Gail model for predicting breast cancer risk: a systematic review and meta‐analysis with trial sequential analysis. Breast Cancer Res. 2018;20(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sun L, Sadique Z, Dos‐Santos‐Silva I, Yang L, Legood R. Cost‐effectiveness of breast cancer screening programme for women in rural China. Int J Cancer. 2019;144(10):2596–604. [DOI] [PubMed] [Google Scholar]
- 77. Sun L, Legood R, Sadique Z, Dos‐Santos‐Silva I, Yang L. Cost‐effectiveness of risk‐based breast cancer screening programme, China. Bull World Health Organ. 2018;96(8):568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Qin C, Liu M, Guo X, Liu J. Human resources in primary health‐care institutions before and after the new health‐care reform in China from 2003 to 2019: an interrupted time series analysis. Int J Environ Res Public Health. 2022;19(10):6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author.


