Abstract
Introduction
Despite current standard of care (SoC), there is an unmet need for the treatment of active systemic lupus erythematosus (SLE). The study assessed the cost-effectiveness of Acthar® Gel (repository corticotropin injection) versus SoC treatment in patients with active, moderate-to-severe SLE from the US payer and societal perspectives over 2 and 3 years.
Methods
Cost-effectiveness model was developed using a probabilistic cohort-level state-transition approach. Patients received Acthar Gel in an exacerbation state, and the outcomes were assessed at the end of a 3-month cycle for response achievement based on the probability of treatment success with Acthar Gel. Patients may sustain the response or experience an exacerbation. For the base case scenario, moderate-to-severe SLE was defined as British Isles Lupus Assessment Group (BILAG)-2004 ≥ 20 or SLE Disease Activity Index 2000 (SLEDAI-2K) ≥ 10 and clinical response was based on SLE responder index (SRI)-4. Clinical response, productivity loss, and utility were derived from a phase 4 SLE trial; cost and disutility estimates were sourced from the literature.
Results
From a payer perspective, Acthar Gel versus SoC resulted in an incremental cost-effectiveness ratio (ICER) of $133,110 per quality-adjusted life-year (QALY) and $94,818 per QALY over 2 and 3 years, respectively. From a societal perspective, Acthar Gel versus SoC results in an ICER of $70,827 per QALY and $32,525 per QALY over 2 and 3 years, respectively. Results from the sensitivity and scenario analyses are consistent with those of the base case model.
Conclusions
Acthar Gel is a cost-effective, value-based treatment option for appropriate patients with moderate-to-severe SLE at a willingness-to-pay threshold of $150,000 over 2–3 years from the US payer and societal perspectives. Acthar Gel results in the reduction of direct medical and indirect costs.
Keywords: Acthar® Gel, Cost-effectiveness analysis, Repository corticotropin injection, Systemic lupus erythematosus
Key Summary Points
| Why carry out this study? |
| Acthar® Gel (repository corticotropin injection) is approved by the US Food and Drug Administration for use during an exacerbation or as maintenance therapy in systemic lupus erythematosus (SLE). |
| Acthar Gel has been shown to be safe and provides durable benefits among patients with moderate-to-severe SLE who have persistently active SLE despite aggressive treatment. However, data on the economic benefit of Acthar Gel in moderate-to-severe SLE are limited. |
| Assessment of the economic benefit of Acthar Gel for treatment-experienced patients with moderate-to-severe SLE by integrating the information on efficacy, effectiveness, cost, and patient outcomes is important to support decision-making. |
| What was learned from the study? |
| Treatment with Acthar Gel is a cost-effective, value-based strategy for active, moderate-to-severe SLE versus standard of care at a willingness-to-pay threshold of $150,000 over 2 and 3 years from the US payer and societal perspectives. These findings suggest that the use of Acthar Gel may considerably improve clinical and health outcomes among patients with moderate-to-severe SLE with a reduction in direct medical and indirect costs. |
Introduction
Systemic lupus erythematosus (SLE) is a chronic non-organ specific autoimmune inflammatory disease and is characterized by a dysregulation of the immune system [1]. SLE involves many vital organs and systems such as the kidneys, brain, heart, dermatologic manifestations, and blood [1, 2]. SLE is characterized by heterogeneity of clinical manifestations and periods of remission and relapse and may present with various constitutional and organ-specific symptoms. Approximately 204,295 people in the US have definite or suspected SLE based on the American College of Rheumatology 1997 revised classification criteria for SLE [3]. Reported incidence rates for SLE in North America range from 1.2 to 8.7 per 100,000 person-years [4], and prevalence rates in studies of US populations range from 5 to 241 per 100,000 people [5]. About 90% of people living with SLE are women [6]. Approximately three-fourths of patients experience moderate-to-severe SLE [7]. SLE results in functional impairment, reduced productivity, poor quality of life (QoL), unemployment, increased mortality, and increased healthcare utilization [8–10]. Furthermore, an increase in SLE disease severity results in greater flare frequency and severity, thereby adding to the existing economic burden on patients and the healthcare system [7]. Increase in flare frequency is related to impaired functional and psychologic well-being, family functioning, and the number of monthly healthy days [11] as well as increased productivity loss and healthcare utilization [12].
The goal of available SLE treatments is the management of symptoms and disease flares [8]. Effective management of chronically active disease and disease flare is crucial to reduce the risk of accumulated organ and tissue damage over time, thereby reducing end-organ damage related to morbidity and mortality and the economic burden of SLE on patients [2, 13–16]. In addition, SLE treatments should also improve patient QoL [2]. A change of treatment should be considered based on the disease activity (flares) and severity [15, 16]. Treatments include the use of steroidal and nonsteroidal anti-inflammatory drugs, glucocorticoids, immunosuppressives, antimalarials, and biologic agents. Despite treatment with these agents, there is an unmet need for value-based treatments in managing persistently active SLE [16].
Acthar® Gel (repository corticotropin injection) is approved by the US Food and Drug Administration for treatment during an exacerbation or as maintenance therapy in selected cases of systemic lupus erythematosus. The therapy is also indicated for inducing a diuresis or remission of proteinuria in nephrotic syndrome without uremia of the idiopathic type or due to lupus erythematosus [17]. It is unclear or there are limited data available examining gender or racial differences in the effectiveness of this treatment for this indication. Acthar Gel is a naturally sourced complex mixture of adrenocorticotropic hormone analogues and other pituitary peptides that interacts with all five melanocortin receptors. Its therapeutic effects in SLE may be attributed to the activation of several potential anti-inflammatory pathways through both glucocorticoid-dependent and -independent mechanisms [17]. Acthar Gel has been shown to be safe and provide durable and beneficial effects among patients with moderate-to-severe SLE who have persistently active SLE despite aggressive treatment with conventional medications [2, 18, 19]. Furthermore, in an efficacy trial of Acthar Gel in patients with moderate-to-severe SLE, the findings supported the utility of Acthar Gel for treating persistently active SLE [20]. Acthar Gel treatment resulted in a reduction in 28-point swollen joint count and/or tender joint count and Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI)-Activity score in a proliferation-inducing ligand cytokine. Post hoc analyses demonstrated a greater proportion of British Isles Lupus Assessment Group (BILAG)-based Combined Lupus Assessment (BICLA) responders for Acthar Gel compared to placebo as early as Week 4 and sustained response through Week 24 [20]. Furthermore, patients in the Acthar Gel group had greater SLE Responder Index (SRI)-4 response compared to the placebo group with better SLE Disease Activity Index-2000 (SLEDAI 2K) and CLASI-Activity [20]. Improvement in the Lupus QoL, specifically pain, planning, and fatigue domains, as well as the Work Productivity and Activity Impairment (WPAI)-Lupus percent impairment while the Working domain was observed among patients with high baseline disease activity on Acthar Gel versus placebo [21]. Acthar Gel therapy also led to reductions in B cell activating factor and IL-6 cytokines, total B-cells, and atypical activated memory B cells, particularly in patients with high baseline disease activity [22].
There is substantial evidence suggesting a favorable clinical profile of Acthar Gel; however, data on the economic benefit of Acthar Gel in moderate-to-severe SLE are limited. Only one study utilizing administrative claims data reported that patients receiving Acthar Gel had lower utilization and costs for medical services [8]. It is important to evaluate the economic benefit of Acthar Gel for treatment-experienced patients with moderate-to-severe SLE integrating the information on efficacy, effectiveness, cost, and patient outcomes to support decision-making. To address this knowledge gap, the objective of the current analysis was to estimate the cost-effectiveness of Acthar Gel versus standard of care (SoC) in patients with active, moderate-to-severe SLE despite aggressive treatment from the US payer and societal perspectives over 3 years.
Methods
Model Structure
A probabilistic cohort-level state-transition approach was used to develop the cost-effectiveness model in Microsoft® Excel 2019. This is a novel method to evaluate the short-term cost-effectiveness of Acthar Gel in persistently active, moderate-to-severe SLE. The incremental cost-effectiveness ratio (ICER) of Acthar Gel versus SoC was assessed using the direct medical costs, indirect costs, and quality-adjusted life-years (QALYs) over 3 years from the US payer and societal perspectives.
All patients entered the model cycle 0 in the exacerbation state and initiated treatment with Acthar Gel or SoC. The model used a natural history matrix and applied the probability of treatment success with Acthar Gel during each treatment cycle based on the clinical trial. Treatments were administered consistent with the current recommendations and clinical practices; Acthar Gel is administered to patients in an exacerbation state. Patients were monitored at the end of a 3-month cycle for the achievement of response. Following the achievement of response, patients could have had a durable response or experienced an exacerbation. Patients who did not achieve a response were assumed to discontinue Acthar Gel. Patients who experienced exacerbation received Acthar Gel and moved into a response or non-response state, based on the probability of treatment success with Acthar Gel. Patients in the response or non-response state were allowed to experience an exacerbation in subsequent cycles. Costs and utilities are calculated for each state every 3 months over a 3-year time horizon. The model assumed that patients in the non-response and exacerbation states experience an additional decrement in utilities for the duration of one cycle due to the ongoing disease burden (Fig. 1).
Fig. 1.
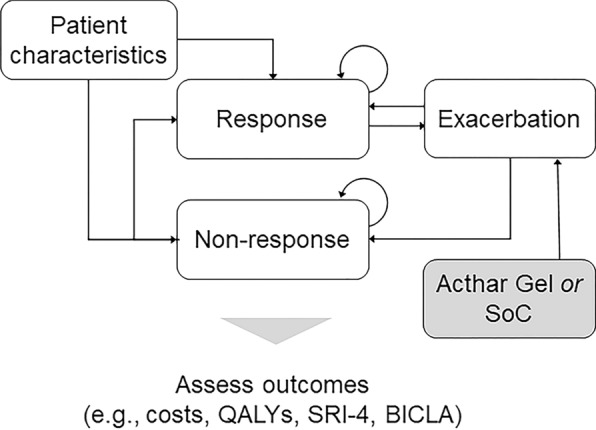
Schematic of the probabilistic cohort-level state-transition model. BICLA British Isles Lupus Assessment Group-based Composite Lupus Assessment, QALY quality-adjusted life-year, SLE systemic lupus erythematosus, SoC standard of care, SRI-4 SLE Responder Index
This study does not involve any human participants, human data, and/or human material. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Key Inputs
Clinical Inputs
The principal evidence source used to derive clinical parameter values was a phase 4 multicenter, randomized, double-blind, placebo-controlled study. This trial was conducted across 54 study sites in Argentina, Chile, Mexico, Peru, and the US. Adults aged ≥ 18 years with active SLE (≥ 4 of 11 American College of Rheumatology criteria; SLEDAI-2K score ≥ 6 at screening; and clinical SLEDAI-2K [excluding laboratory results] score ≥ 4 at both screening and randomization) and with moderate-to-severe rash and/or arthritis by BILAG-2004 scores A or B in the mucocutaneous or musculoskeletal domains at both screening and randomization were enrolled in the study. Patients were administered 80 U of Acthar Gel or matching placebo subcutaneously every other day for 4 weeks and then twice per week for an additional 20 weeks. The modified intent-to-treat population, defined as patients who received at least one dose of the study drug and contributed any post-baseline efficacy data, comprised 169 patients; 84 patients received Acthar Gel and 85 received placebo. Details of this clinical trial have been described elsewhere [20].
Moderate-to-severe SLE was defined as patients with BILAG-2004 ≥ 20 or SLEDAI-2K ≥ 10. These benchmarks were considered to align with the moderate-to-severe SLE defined in the Phase 4 SLE trial [20]. There are no established benchmarks for defining moderate-to-severe SLE based on BILAG-2004 and SLEDAI-2K. Both SLEDAI ≥ 6 [23, 24] and SLEDAI ≥ 10 [25] have been used; however, SLEDAI-2K ≥ 10 provides a more conservative definition for this population. No specific benchmark has been defined for BILAG-2004. Furthermore, the relationship between these clinical measures is not known; thus, each of these measures was considered to define moderate-to-severe SLE.
The clinical response was based on the proportion of SRI-4 or BICLA responders sourced from the Phase 4 SLE trial [20]. The probability of exacerbation was based on the rate of flares among adults with SLE sourced from the literature; the overall rate was 3.5 flares per year [7]. A 3-month probability of an exacerbation in SLE was 0.58 [7]. The relative risk reduction in exacerbation was calculated based on the type of clinical measure to define moderate-to-severe SLE and the type of clinical response evaluated. The relative risk reduction was applied to SRI-4 or BICLA responders on Acthar Gel and SoC. The annual rates were converted to the monthly probability of exacerbation (Table 1). The transition probabilities from exacerbation to response were based on the probability of treatment success at the end of the 3-month cycle to align with the clinical trial assessment. Transition probabilities varied by type of clinical measure to define moderate-to-severe SLE and the type of clinical response evaluated.
Table 1.
Clinical parameters among patients with moderate-to-severe SLE
| Parameter | SRI-4 responders | BICLA responders | |
|---|---|---|---|
| Proportion of respondersa | |||
| Acthar Gel | |||
| BILAG-2004 | |||
| 3 months | 40.0% | 60.0% | |
| 6 months | 52.5% | 65.0% | |
| SLEDAI-2K | |||
| 3 months | 48.9% | 58.7% | |
| 6 months | 61.7% | 58.7% | |
| Placebo | |||
| BILAG-2004 | |||
| 3 months | 27.8% | 27.8% | |
| 6 months | 38.9% | 58.3% | |
| SLEDAI-2K | |||
| 3 months | 38.6% | 23.3% | |
| 6 months | 40.9% | 34.9% | |
| Exacerbation risk reduction with Acthar Gelb | |||
| BILAG-2004 | 0.200 | 0.148 | |
| SLEDAI-2K | 0.297 | 0.306 | |
BILAG British Isles Lupus Assessment Group, BICLA BILAG-based Composite Lupus Assessment, SLE systemic lupus erythematosus, SLEDAI-2K SLE Disease Activity Index 2000, SRI-4 SLE Responder Index
aDerived from data on file (Phase 4 SLE trial) [20]
bCalculation based on annual flare rate (Hammond 2021) [7] and response rate on the type of clinical measure to define moderate-to-severe SLE and the type of clinical response evaluated (Phase 4 SLE trial) [20]
Healthcare Resource Utilization and Costs
Costs considered in the present model included treatment costs, direct medical costs, and indirect costs (Table 2). Treatment costs included the cost of Acthar Gel as well as concomitant medications (corticosteroids, disease-modifying anti-rheumatic drug [DMARDs], and biologics) for patients on Acthar Gel. Wholesale acquisition costs for Acthar Gel were obtained from the IBM Micromedex® Red Book [26]. The dose strength and dosing of Acthar Gel were based on dispensing data from specialty pharmacies, from the last 12 months as of March 29, 2019. The proportion of patients using corticosteroids, DMARDs, and biologics was sourced from the literature [27]. Wholesale acquisition costs were used for the cost of biologics (specifically, belimumab) [26], and the costs of corticosteroids and DMARDs were sourced from the literature [28].
Table 2.
Healthcare resource use and costs
| Parameter | Value | References | ||
|---|---|---|---|---|
| Healthcare resource use and costs (2022 USD) | ||||
| Treatment costs | ||||
| Cost of Acthar Gel | $41,459 | Red Book 2022 [26] | ||
| Acthar Gel use (12 months) | 8.57 packs | Data on Filea | ||
| Medication utilization | ||||
| Proportion of patients | Acthar Gel | SoC | ||
| Corticosteroids | 58.0% | 100.0% | Myung 2017 [27] | |
| DMARDs | 37.0% | 50.0% | Myung 2017 [27] | |
| Biologics | 9.0% | 14.0% | Myung 2017 [27] | |
| Cost of medications | ||||
| Corticosteroids | $410 | AHRQ 2007 [28] | ||
| DMARDs | $2909 | AHRQ 2007 [28] | ||
| Biologics | $55,312 | Red Book 2022 [26] | ||
| Healthcare costs | Mild | Moderate | Severe | |
| Inpatient | $3444 | $5325 | $16,609 | Murimi-Worstell 2021 [9] |
| Outpatient | $11,589 | $13,669 | $27,208 | Murimi-Worstell 2021 [9] |
| ER | $399 | $825 | $1046 | Murimi-Worstell 2021 [9] |
| Physician office | $2314 | $3446 | $4346 | Murimi-Worstell 2021 [9] |
| Surgery | ||||
| Surgery rate | ||||
| Kidney Transplant | 2.4% | Lionaki 2014 [29] | ||
| Splenectomy | 3.1% | You 2004 [30] | ||
| Total hip or knee replacement | 33.2% | Mukherjee 2015 [58] | ||
| Surgery-related costs | ||||
| Kidney Transplant | $127,337 | Axelrod 2016 [31] | ||
| Splenectomy | $21,923 | Hamlat 2012 [32] | ||
| Total hip or knee replacement | $47,412 | Clair 2016 [33] | ||
| Organ damage | ||||
| Organ damage rate | ||||
| Cardiovascular | 30.1% | Pierotti 2015 [34] | ||
| Diabetes | 19.0% | Pierotti 2015 [34] | ||
| Gastrointestinal | 22.2% | Pierotti 2015 [34] | ||
| Malignancy | 36.3% | Pierotti 2015 [34] | ||
| Musculoskeletal | 64.5% | Pierotti 2015 [34] | ||
| Neuropsychiatric | 46.9% | Pierotti 2015 [34] | ||
| Ocular | 60.5% | Pierotti 2015 [34] | ||
| Peripheral vascular | 20.9% | Pierotti 2015 [34] | ||
| Pulmonary | 34.3% | Pierotti 2015 [34] | ||
| Renal | 26.0% | Pierotti 2015 [34] | ||
| Organ damage-related costs | ||||
| Cardiovascular | $2729 | Pierotti 2015 [34] | ||
| Diabetes | $5726 | Pierotti 2015 [34] | ||
| Gastrointestinal | $505 | Pierotti 2015 [34] | ||
| Malignancy | $1601 | Pierotti 2015 [34] | ||
| Musculoskeletal | $23,310 | Pierotti 2015 [34] | ||
| Neuropsychiatric | $8998 | Pierotti 2015 [34] | ||
| Ocular | $556 | Pierotti 2015 [34] | ||
| Peripheral vascular | $1860 | Pierotti 2015 [34] | ||
| Pulmonary | $51,775 | Pierotti 2015 [34] | ||
| Renal | $15,842 | Pierotti 2015 [34] | ||
| Pain-related costs | ||||
| Opioid use for pain | ||||
| Opioid use | 23.0% | Somers 2019 [35] | ||
| Cost of opioid use | $24,722 | Luo 2021 [36] | ||
| Substance use disorder | ||||
| SLE-related opioid abuse (> 1 year of opioid use) | 68.0% | Somers 2019 [35] | ||
| SLE patients on ≥ 2 opioids | 22.0% | Somers 2019 [35] | ||
| Opioid abuse and overdose | $24,503 | Luo 2021 [36] | ||
| Work Productivity Lossb | Acthar Gel | SoC | ||
| Absenteeism | 7.8% | 17.6% | Phase 4 SLE trial [21] | |
| Presenteeism | 27.3% | 51.8% | Phase 4 SLE trial [21] | |
| Activity impairment | 34.3% | 58.1% | Phase 4 SLE trial [21] | |
| SF-6D utility | ||||
| Response | 0.654 | Phase 4 SLE trial [21] | ||
| Non-response | 0.595 | Phase 4 SLE trial [21] | ||
| Disutilities | ||||
| Disutilities: patient outcomes | ||||
| Chronic OCS use | − 0.023 | ICER 2018 [40] | ||
| Exacerbation (new flare) | − 0.360 | Pollard 2015 [41] | ||
| Exacerbation Requiring Steroid Burst | − 0.100 | ICER 2018 [40] | ||
| Planning | − 0.106 | Pollard 2015 [41] | ||
| Body image | − 0.102 | Pollard 2015 [41] | ||
| Intimate relationships | − 0.020 | Pollard 2015 [41] | ||
| Burden to others | − 0.059 | Pollard 2015 [41] | ||
| Disutilities: surgery | ||||
| Kidney transplant | − 0.170 | Li 2017 [42] | ||
| Splenectomy | − 0.168 | Synder 2008 [43] | ||
| Total hip or knee replacement | − 0.261 | Benson 2016 [44] | ||
| Disutilities: organ damage | ||||
| Cardiovascular | − 0.076 | Di Tanna 2021 [45] | ||
| Diabetes (type 2) | − 0.110 | Matza 2007 [46] | ||
| Gastrointestinal | − 0.240 | Worbes-Cerezo 2019 [47] | ||
| Malignancy | − 0.110 | Choi 2015 [48] | ||
| Musculoskeletal | − 0.030 | Törmälehto 2018 [49] | ||
| Neuropsychiatric | − 0.640 | Pollard 2015 [41] | ||
| Ocular | − 0.029 | Brown 2009 [50] | ||
| Peripheral vascular | − 0.076 | Assumption [same as cardiovascular] | ||
| Pulmonary | − 0.327 | Moayeri 2016 [51] | ||
| Renal | − 0.260 | Cooper 2020 [52] |
DMARD disease-modifying anti-rheumatic drug, ER emergency department, OCS oral corticosteroid, SF-6D Short form-six dimension, SLE systemic lupus erythematosus, USD United States dollar
aUsing dispensing data from specialty pharmacies, from the last 12 months as of March 29, 2019
bBased on the Work Productivity and Activity Impairment Questionnaire
Direct medical costs comprised inpatient, outpatient, emergency department, and physician office visit-related costs [9]. SLE affects multiple organs and may result in organ damage that may require surgery and/or transplant. The risk of organ damage is higher for patients who have persistently active SLE. The model also considered both organ damage and surgical costs for patients who did not respond to Acthar Gel and those who experienced a new exacerbation. Organ damage and surgery rates as well as related costs were sourced from the literature [29–34]. Costs related to the use of opioids for pain and opioid abuse were also applied to the model. Estimates from the literature suggest that up to 23% of patients with SLE are regular users of opioids and the effects of DMARDs are minimal in reducing opioid use [35]. Although the literature supports the efficacy of short-term opioids for the improvement of pain, long-term use is associated with reduced efficacy and increased safety concerns. Opioid use for pain management and opioid abuse costs were sourced from the literature [36] and applied to patients in the non-response and exacerbation state.
Furthermore, indirect costs due to the productivity loss for the patients and the additional cost of caregiving were applied in the model from the societal perspective. The proportion of work loss was derived from WPAI scores from the Phase 4 SLE trial [21]. For estimating the indirect costs in the US, the model used $50,910 as the per capita income in the US [37], $4514 for the annual cost of caregiving for SLE [38], and $22,883 for the cost of work-related training [38].
Health Utilities
Both health utilities and disutilities were considered given the multi-organ involvement in SLE (Table 2). Lupus QoL scores were derived from the Phase 4 SLE clinical trial [21]; four domains (emotional health, fatigue, pain, and physical health) from the Lupus QoL measure were mapped to Short form-Six dimension utilities based on the method used in the literature [39]. In addition, disutility for chronic oral corticosteroid use [40], exacerbation (new flare) [41], exacerbation requiring steroid burst [40], surgery [42–44], organ damage [41, 45–52], and health outcomes based on Lupus QoL domains (body image, burden to others, intimate relationships, and planning) [41] were also considered. Disutilities were sourced from the literature. The disutilities were applied based on the scores for each patient in the cycle and were additive.
Analyses
Base Case Analysis
For the base case scenario, moderate-to-severe SLE was defined as BILAG-2004 ≥ 20 or SLEDAI-2K ≥ 10. BILAG-2004 and SLEDAI-2K were selected to define moderate-to-severe SLE as these measures are primarily used in clinical trials for the selection and classification of patients with SLE [53, 54]. Furthermore, BILAG-2004 assesses different organs/systems, and SLEDAI-2K is a global assessment measure, and thus they complement each other well, thereby providing a comprehensive assessment [54]. Clinical response was based on the SRI-4 as it is a recommended measure in clinical trials [53] and was also the primary endpoint of the Phase 4 SLE trial [20].
The primary outcome in the present model was the discounted incremental ICER defined as the difference in costs divided by the difference in QALYs of Acthar Gel and SoC at 2 and 3 years from both US payer and societal perspectives. From a payer perspective, total costs comprised direct medical costs (costs paid by third party-payers), and from a societal perspective, total costs comprised both direct and indirect costs (productivity loss, work-related training, and caregiving). The secondary outcome included the cost per SRI-4 response. Unless otherwise specified, the costs and QALYs were discounted at 3.0% annually and all costs were adjusted to 2022 US dollars (USD). For the costs obtained from ex-US studies, purchasing power parity exchange rates were used, which adjust for the different costs of buying a similar basket of goods and services in each country, a most commonly used method in economics.
Sensitivity Analyses
The base case assumptions and alternative values for these assumptions were tested and fully explored in the deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA). The full range of uncertainty is tested for each variable with anticipated uncertainty. A PSA combined bootstrapping with random draws from uncertainty distributions. By bootstrapping the data from the randomized clinical trial using normal and Poisson distributions, we obtained uncertainty margins surrounding the parameters. The full range of uncertainty is tested for each variable with anticipated uncertainty.
Scenario Analyses
Four scenario analyses were conducted varying the definition of moderate-to-severe SLE (BILAG-2004 ≥ 20 or SLEDAI-2K ≥ 10) and a clinical measure of response (SRI-4 or BICLA) in each scenario. BICLA was assessed as a secondary outcome in the Phase 4 SLE trial [20].
Results
Base Case Analyses
The use of Acthar Gel in moderate-to-severe SLE (defined as BILAG-2004 ≥ 20 or SLEDAI-2K ≥ 10) results in an incremental cost of $117,270 and an incremental QALY gain of 0.881, resulting in an ICER of $133,110 per QALY compared to that of SoC from the payer perspective over 2 years. From the societal perspective over 2 years, Acthar Gel has an incremental cost of $62,399 and an incremental QALY gain of 0.881, resulting in an ICER of $70,827 per QALY compared to that of SoC. The ICER was lower from the payer ($94,818 per QALY) and societal ($32,525 per QALY) perspective over 3 years (Table 3).
Table 3.
Base case results for incremental cost-effectiveness among patients with moderate-to-severe SLE (2022 USD)
| Acthar Gel versus SoCa | Incremental costs | Incremental QALYs | ICER (cost/QALY) |
|---|---|---|---|
| Payer | |||
| 2 years | $117,270 | 0.881 | $133,110 |
| 3 years | $129,047 | 1.361 | $94,818 |
| Societal | |||
| 2 years | $62,399 | 0.881 | $70,827 |
| 3 years | $44,266 | 1.361 | $32,525 |
BILAG British Isles Lupus Assessment Group, BICLA BILAG-based Composite Lupus Assessment, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-years, SLE systemic lupus erythematosus, SLEDAI-2K SLE Disease Activity Index 2000, SoC standard of care, SRI-4 SLE Responder Index, USD United States dollar
aFor the base case scenario, moderate-to-severe SLE was defined as BILAG-2004 ≥ 20 or SLEDAI-2K ≥ 10. Clinical response was based on the SRI-4
From the payer perspective, the incremental cost per SRI-4 response achieved was $30,750 and $21,452 compared to SoC over 2 and 3 years, respectively. From the societal perspective, the incremental cost per SRI-4 response achieved was $16,362 and $7358 compared to SoC over 2 and 3 years, respectively. The breakdown of costs over 3 years by each cost component is provided in Table 4.
Table 4.
Base case per patient-year costs among patients with moderate-to-severe SLE from the payer perspective (2022 USD)
| Cost componenta | Acthar Gel | SoC |
|---|---|---|
| Treatment | $77,228 | – |
| Direct medical costs | ||
| Concomitant medication | $1533 | $7033 |
| Inpatient | $5139 | $10,378 |
| Outpatient | $13,230 | $19,431 |
| Emergency department | $516 | $804 |
| Physician visit | $2605 | $3491 |
| Surgery | $2380 | $9902 |
| Organ damage | $3153 | $9022 |
| Pain-related costs | $1455 | $4162 |
| Total direct medical | $30,011 | $64,223 |
| Indirect costs | ||
| Pain-related | $5667 | $16,168 |
| Productivity loss | $4596 | $13,103 |
| Activity impairment | $2428 | $11,681 |
| Total indirect | $12,691 | $40,951 |
Bold is used to differentiate between “total of all individual costs” from “individual costs” listed under direct and indirect cost categories.
BILAG British Isles Lupus Assessment Group, BICLA BILAG-based Composite Lupus Assessment, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-years, SLE systemic lupus erythematosus, SLEDAI-2K SLE Disease Activity Index 2000, SoC standard of care, SRI-4 SLE Responder Index, USD United States dollar
aFor the base case scenario, moderate-to-severe SLE was defined as BILAG-2004 ≥ 20 or SLEDAI-2K ≥ 10. Clinical response was based on the SRI-4
Sensitivity Analyses
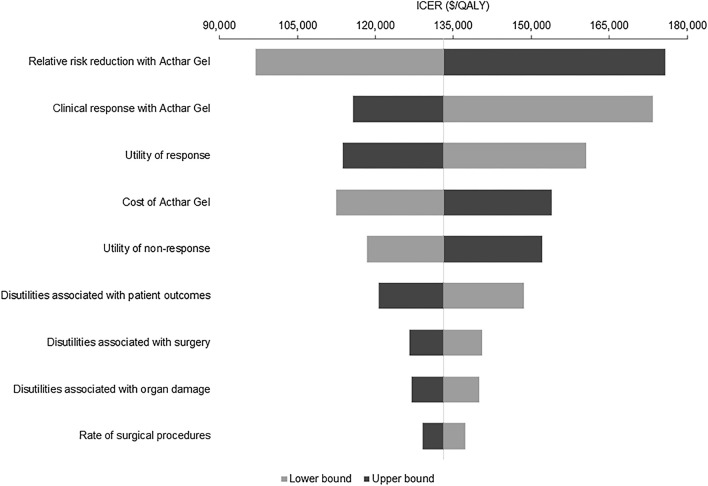
DSA findings are consistent with the base case analysis; Acthar Gel is a cost-effective strategy over SoC at a threshold of $150,000 per QALY. The relative risk reduction with Acthar Gel, clinical response with Acthar Gel, the utility of response and non-response, and the cost of Acthar Gel are major influencers of the ICER (Fig. 2).
Fig. 2.
Deterministic sensitivity analyses. Base case: Moderate-to-severe SLE was defined as BILAG-2004 ≥ 20 or SLEDAI-2K ≥ 10; Clinical response was based on the SRI-4; 2-year payer perspective. BILAG British Isles Lupus Assessment Group, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-years, SLE systemic lupus erythematosus, SLEDAI-2K SLE Disease Activity Index 2000, SoC standard of care, SRI-4 SLE Responder Index
PSA randomly sampled parameters from within chosen distributions over 1000 iterations. The PSA shows that Acthar Gel is cost-effective for 63.1% of the iterations at a willingness-to-pay threshold of $150,000 per QALY over 3 years from a payer perspective. The findings from the PSA are consistent with the base case analysis; Acthar Gel is cost-effective compared to the SoC (ICER: $129,677 per QALY; 95% confidence interval [CI]: $121,476, $137,878 per QALY) at a willingness-to-pay threshold of $150,000 per QALY.
Scenario Analyses
For all scenarios, ICER for Acthar Gel versus SoC was within the willingness-to-pay threshold of $150,000 per QALY over 3 years from both payer and societal perspectives (Table 5).
Table 5.
Scenario analyses results for incremental cost-effectiveness among patients with moderate-to-severe SLE (2022 USD)
| Acthar Gel versus SoC | Incremental costs | Incremental QALYs | ICER (cost/QALY) | Cost/response |
|---|---|---|---|---|
| Scenario 1: Moderate-to-severe SLE: BILAG-2004 ≥ 20; clinical response: SRI-4 | ||||
| Payer | ||||
| 2 years | $112,213 | 0.919 | $122,103 | $28,060 |
| 3 years | $114,639 | 1.456 | $78,736 | $17,896 |
| Societal | ||||
| 2 years | $54,664 | 0.919 | $59,482 | $13,669 |
| 3 years | $24,710 | 1.456 | $16,971 | $3857 |
| Scenario 2: Moderate-to-severe SLE: SLEDAI-2K ≥ 10; clinical response: SRI-4 | ||||
| Payer | ||||
| 2 years | $128,389 | 0.833 | $154,128 | $35,143 |
| 3 years | $145,271 | 1.292 | $112,439 | $25,116 |
| Societal | ||||
| 2 years | $75,229 | 0.833 | $90,311 | $20,592 |
| 3 years | $62,929 | 1.292 | $48,707 | $10,880 |
| Scenario 3: Moderate-to-severe SLE: BILAG-2004 ≥ 20; clinical response: BICLA | ||||
| Payer | ||||
| 2 years | $75,456 | 1.021 | $73,904 | $16,954 |
| 3 years | $65,656 | 1.568 | $41,872 | $9513 |
| Societal | ||||
| 2 years | $13,162 | 1.021 | $12,891 | $2957 |
| 3 years | − $29,113 | 1.568 | Dominant | −$4,219 |
| Scenario 4: Moderate-to-severe SLE: SLEDAI-2K ≥ 10; clinical response: BICLA | ||||
| Payer | ||||
| 2 years | $123,693 | 0.869 | $142,339 | $30,713 |
| 3 years | $142,428 | 1.316 | $108,228 | $23,051 |
| Societal | ||||
| 2 years | $65,416 | 0.869 | $75,277 | $16,243 |
| 3 years | $54,591 | 1.316 | $41,483 | $8835 |
| Scenario 5: Moderate-to-severe SLE: BILAG-2004 ≥ 20 or SLEDAI-2K ≥ 10; clinical response: BICLA | ||||
| Payer | ||||
| 2 years | $109,030 | 0.849 | $128,422 | $28,166 |
| 3 years | $121,222 | 1.282 | $94,557 | $20,495 |
| Societal | ||||
| 2 years | $53,752 | 0.849 | $63,312 | $13,886 |
| 3 years | $38,184 | 1.282 | $29,785 | $6456 |
BILAG British Isles Lupus Assessment Group, BICLA BILAG-based Composite Lupus Assessment, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-years, SLE systemic lupus erythematosus, SLEDAI-2K SLE Disease Activity Index 2000, SoC standard of care, SRI-4 SLE Responder Index, USD United States dollar
Discussion
Effective management of chronically active SLE is crucial to reducing the associated clinical and economic burden on patients as well as improving patient QoL [2, 13–16]. Acthar Gel has been shown to be safe and effective treatment among patients with moderate-to-severe SLE who have persistently active SLE despite aggressive treatment [2, 18–20]. It is important to assess both economic and health outcomes to understand the value of the interventions. The current analysis was conducted to understand the potential health-economic implications of using Acthar Gel for the short-term treatment of patients with persistently active moderate-to-severe SLE. To the best of our knowledge, this is the first economic analysis to compare the cost-effectiveness of Acthar Gel versus SoC for persistently active moderate-to-severe SLE despite aggressive treatment with conventional medications.
The findings from the current base case analysis indicate that Acthar Gel is cost-effective compared to SoC at a willingness-to-pay threshold of USD 150,000 per QALY over 2 years from both payer and societal perspectives. The findings were consistent in the sensitivity analysis. The use of Acthar Gel results in reduced direct medical (excluding treatment) and indirect costs with gain in QALYs. The relative risk reduction and clinical response with Acthar Gel primarily influenced variation in ICER estimates. The probable reason could be that achievement of the clinical response is important for reducing organ damage and improving patient QoL. The cost-effectiveness of Acthar Gel may further improve with rebates and drug price discounts.
The findings from the current study on lower direct medical costs are consistent with published economic evaluation, where patients with SLE receiving Acthar Gel had lower healthcare resource utilization and costs for medical services; lower per person per member hospitalization costs ($3192 vs $799, p = 0.04) after initiating Acthar Gel [8]. Furthermore, these findings showed that these lower medical costs partially offset the increased prescription costs by 37% [8]. However, these prior economic evaluations only focused on the direct medical costs and did not consider indirect costs. Furthermore, this prior analysis did not examine the cost-effectiveness of Acthar Gel, integrating clinical, economic, and patient-related health outcomes. This study adds to the nascent literature on economic assessments in moderate-to-severe SLE.
The current analysis also assessed the cost-effectiveness of Acthar Gel from the societal perspective as patients with SLE experience functional impairment, reduced productivity, poor QoL, unemployment, and increased mortality [8], which may further add to the overall economic burden for the patient and caregivers. It is crucial to consider indirect costs due to productivity loss and caregiver burden in addition to the direct medical costs in an economic evaluation. The current cost-effectiveness analysis includes costs accrued because of increased disability, caregiver costs, and costs due to the lost productivity of patients. The findings indicate that Acthar Gel is a cost-effective strategy compared to SoC over 2 years from the societal perspective at a willingness-to-pay threshold of USD 150,000 per QALY.
Cost of treatment is central to issues of access and affordability; however, it is important to assess the value of treatment based on the clinical, economic, and humanistic components. Interventions that are intended for a special population or offer substantial other benefits are considered high “Care Value” within the cost/QALYs range of USD 100,000–150,000 [55, 56]. Based on the recommendations of the World Health Organization’s Choosing Interventions that are Cost-Effective (WHO-CHOICE), this ICER threshold range is estimated to be three times the nation’s per capita income [57]. Acthar Gel may provide value for the patients with persistently active moderate-to-severe SLE at a willingness-to-pay threshold of USD 150,000 per QALY.
The findings in the present model should be interpreted considering the following limitations. First, the efficacy, work productivity, and QoL data for the model were based on the data from a single Phase 4 SLE clinical trial, which may not reflect real-world outcomes. In addition, the sample size considered for the model was small and may have introduced bias to the findings to some extent. However, we conducted sensitivity analysis to account for uncertainty in the data used. Furthermore, the analysis was considered for patients with moderate-to-severe SLE and may not be generalizable to patients with mild SLE. Second, the presence of heterogeneity in the SLE population and other inflammatory comorbidities may further exacerbate SLE and enhance the value of Acthar Gel. Third, a simplified care paradigm was implemented for the model, which may not capture the complexity of SLE. Real-world treatment pathways in SLE are complex, dependent on multiple factors, and highly individualized. Fourth, the clinical measures used to define active, moderate-to-severe SLE have their strengths and limitations; thus, this might result in variation in cost-effectiveness estimates considering other cut-offs for the definition. Fifth, Phase 4 SLE clinical trial examined short-term outcomes, i.e., at 24 weeks in the RCT; the model assessed the cost-effectiveness of Acthar Gel versus SoC in a longer term (1–3 years). Lastly, the data on healthcare utilization and costs as well as health disutility were obtained from various published sources and may result in under- or over-estimation. However, a PSA was conducted to account for uncertainty in the parameters, and the findings were consistent with base-case analyses.
Conclusions
Acthar Gel is a cost-effective, value-based treatment option for appropriate patients with moderate-to-severe SLE at a willingness-to-pay threshold of $150,000 over 2–3 years from the US payer and societal perspectives. Further research is required to examine the long-term clinical effectiveness and cost-effectiveness of Acthar Gel for active SLE.
Acknowledgements
Funding
This study and the journal’s Rapid Service and Open Access Fee was funded by Mallinckrodt Pharmaceuticals.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
GJW, JB, JN, KH, and MP contributed to the conceptualization of the study. GJW and KH contributed to data acquisition. GJW, IC, JB, JN, KH, and MP contributed to the methodology. IC and JB contributed to analysis and data interpretation. GJW, IC, JB, JN, KH, and MP were involved in drafting the manuscript.
Disclosures
George J. Wan, Kyle Hayes, and John Niewoehner are employees of Mallinckrodt Pharmaceuticals and declare that they have no other disclosures. Ishveen Chopra, Jas Bindra, and Mary Panaccio were independent research consultants for the study and have nothing to disclose.
Compliance with ethics guidelines
This study does not involve any human participants, human data, and/or human material. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data availability
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
References
- 1.Fortuna G, Brennan MT. Systemic lupus erythematosus: epidemiology, pathophysiology, manifestations, and management. Dent Clin N Am. 2013;57(4):631–655. doi: 10.1016/j.cden.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Fiechtner JJ, Montroy T. Treatment of moderately to severely active systemic lupus erythematosus with adrenocorticotropic hormone: a single-site, open-label trial. Lupus. 2014;23(9):905–912. doi: 10.1177/0961203314532562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izmirly PM, Parton H, Wang L, et al. Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the centers for disease control and prevention National Lupus Registries. Arthritis Rheumatol. 2021;73(6):991–996. doi: 10.1002/art.41632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–395. doi: 10.1111/joim.12395. [DOI] [PubMed] [Google Scholar]
- 5.Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology (Oxford) 2017;56(11):1945–1961. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- 6.Pons-Estel GJ, Alarcon GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39(4):257–268. doi: 10.1016/j.semarthrit.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond ER, Desta B, Near AM, Wang X, Jiang M. Frequency, severity and costs of flares increase with disease severity in newly diagnosed systemic lupus erythematosus: a real-world cohort study, United States. Lupus Sci Med. 2021;8(1):e000504. doi: 10.1136/lupus-2021-000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu B, Deshpande G, Gu T, Popelar B, Philbin M, Wan GJ. Demographics, treatment patterns, and healthcare utilization and cost of repository corticotropin injection in patients with systemic lupus erythematosus or rheumatoid arthritis. J Med Econ. 2017;20(11):1170–1177. doi: 10.1080/13696998.2017.1362411. [DOI] [PubMed] [Google Scholar]
- 9.Murimi-Worstell IB, Lin DH, Kan H, et al. Healthcare utilization and costs of systemic lupus erythematosus by disease severity in the United States. J Rheumatol. 2021;48(3):385–393. doi: 10.3899/jrheum.191187. [DOI] [PubMed] [Google Scholar]
- 10.Jiang M, Near AM, Desta B, Wang X, Hammond ER. Disease and economic burden increase with systemic lupus erythematosus severity 1 year before and after diagnosis: a real-world cohort study, United States. Lupus Sci Med. 2021;8(1):e000503. doi: 10.1136/lupus-2021-000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz P, Wan GJ, Daly P, et al. Patient-reported flare frequency is associated with diminished quality of life and family role functioning in systemic lupus erythematosus. Qual Life Res. 2020;29(12):3251–3261. doi: 10.1007/s11136-020-02572-9. [DOI] [PubMed] [Google Scholar]
- 12.Katz P, Nelson WW, Daly RP, Topf L, Connolly-Strong E, Reed ML. Patient-reported lupus flare symptoms are associated with worsened patient outcomes and increased economic burden. J Manag Care Spec Pharm. 2020;26(3):275–283. doi: 10.18553/jmcp.2020.26.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panopalis P, Yazdany J, Gillis JZ, et al. Health care costs and costs associated with changes in work productivity among persons with systemic lupus erythematosus. Arthritis Rheum. 2008;59(12):1788–1795. doi: 10.1002/art.24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turchetti G, Yazdany J, Palla I, Yelin E, Mosca M. Systemic lupus erythematosus and the economic perspective: a systematic literature review and points to consider. Clin Exp Rheumatol. 2012;30(4 Suppl 73):S116–122. [PMC free article] [PubMed] [Google Scholar]
- 15.Guidelines for referral and management of systemic lupus erythematosus in adults. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Arthritis Rheum. 1999;42(9):1785–1796. [DOI] [PubMed]
- 16.Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- 17.Mallinckrodt Pharmaceuticals. Acthar® Gel prescribing information. 1952 (Revised October 2021). https://www.acthar.com/pdf/Acthar-PI.pdf.
- 18.Furie RA, Mitrane M, Zhao E, Becker PM. Repository corticotropin injection in patients with persistently active SLE requiring corticosteroids: post hoc analysis of results from a two-part, 52-week pilot study. Lupus Sci Med. 2017;4(1):e000240. doi: 10.1136/lupus-2017-000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furie R, Mitrane M, Zhao E, Das M, Li D, Becker PM. Efficacy and tolerability of repository corticotropin injection in patients with persistently active SLE: results of a phase 4, randomised, controlled pilot study. Lupus Sci Med. 2016;3(1):e000180. doi: 10.1136/lupus-2016-000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Askanase AD, Zhao E, Zhu J, Bilyk R, Furie RA. Repository corticotropin injection for persistently active systemic lupus erythematosus: results from a phase 4, multicenter, randomized, double-blind, placebo-controlled trial. Rheumatol Ther. 2020;7(4):893–908. doi: 10.1007/s40744-020-00236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Askanase AD, Wan GJ, Panaccio MP, et al. Patient-reported outcomes from a phase 4, multicenter, randomized, double-blind, placebo-controlled trial of repository corticotropin injection (Acthar((R)) Gel) for persistently active systemic lupus erythematosus. Rheumatol Ther. 2021;8(1):573–584. doi: 10.1007/s40744-021-00294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Askanase AD, Wright D, Zhao E, Zhu J, Bilyk R, Furie RA. Post Hoc biomarker analyses from a phase 4, multicenter, randomized, double-blind, placebo-controlled trial of repository corticotropin injection (Acthar(R) Gel) for persistently active systemic lupus erythematosus. Rheumatol Ther. 2021;8(4):1871–1886. doi: 10.1007/s40744-021-00351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furie RA, Bruce IN, Dorner T, et al. Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus. Rheumatology (Oxford) 2021;60(11):5397–5407. doi: 10.1093/rheumatology/keab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khamashta M, Merrill JT, Werth VP, et al. Sifalimumab, an anti-interferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75(11):1909–1916. doi: 10.1136/annrheumdis-2015-208562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10143):222–231. doi: 10.1016/S0140-6736(18)31363-1. [DOI] [PubMed] [Google Scholar]
- 26.IBM Micromedex RED BOOK. In: IBM, ed2021.
- 27.Myung G, Nelson WW, McMahon MA. Effects of repository corticotropin injection on medication use in patients with rheumatologic conditions: a claims data study. J Pharm Technol. 2017;33(4):151–155. doi: 10.1177/8755122517709825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agency for Healthcare Research and Quality. Choosing Medications for Rheumatoid Arthritis. 2007. [PubMed]
- 29.Lionaki S, Skalioti C, Boletis JN. Kidney transplantation in patients with systemic lupus erythematosus. World J Transplant. 2014;4(3):176–182. doi: 10.5500/wjt.v4.i3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You YN, Tefferi A, Nagorney DM. Outcome of splenectomy for thrombocytopenia associated with systemic lupus erythematosus. Ann Surg. 2004;240(2):286–292. doi: 10.1097/01.sla.0000133182.92780.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Axelrod DA, Schnitzler MA, Xiao H, et al. The changing financial landscape of renal transplant practice: a national cohort analysis. Am J Transplant. 2017;17(2):377–389. doi: 10.1111/ajt.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamlat CA, Arbabi S, Koepsell TD, Maier RV, Jurkovich GJ, Rivara FP. National variation in outcomes and costs for splenic injury and the impact of trauma systems: a population-based cohort study. Ann Surg. 2012;255(1):165–170. doi: 10.1097/SLA.0b013e31823840ca. [DOI] [PubMed] [Google Scholar]
- 33.Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a bundled payment care improvement initiative. J Arthroplasty. 2016;31(9):1862–1865. doi: 10.1016/j.arth.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Pierotti F, Palla I, Treur M, Pippo L, Turchetti G. Assessment of the economic impact of belimumab for the treatment of systemic lupus erythematosus in the italian setting: a cost-effectiveness analysis. PLoS One. 2015;10(10):e0140843. doi: 10.1371/journal.pone.0140843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somers EC, Lee J, Hassett AL, et al. Prescription opioid use in patients with and without systemic lupus erythematosus—Michigan Lupus Epidemiology and Surveillance Program, 2014–2015. MMWR Morb Mortal Wkly Rep. 2019;68(38):819–824. doi: 10.15585/mmwr.mm6838a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo F, Li M, Florence C. State-level economic costs of opioid use disorder and fatal opioid overdose—United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70(15):541–546. doi: 10.15585/mmwr.mm7015a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.US Bureau of Labor Statistics. Usual Weekly Earnings Summary. 2022.
- 38.Birnbaum HG, Ivanova JI, Samuels S, et al. Economic impact of multiple sclerosis disease-modifying drugs in an employed population: direct and indirect costs. Curr Med Res Opin. 2009;25(4):869–877. doi: 10.1185/03007990902743869. [DOI] [PubMed] [Google Scholar]
- 39.Meacock R, Harrison M, McElhone K, et al. Mapping the disease-specific LupusQoL to the SF-6D. Qual Life Res. 2015;24(7):1749–1758. doi: 10.1007/s11136-014-0892-4. [DOI] [PubMed] [Google Scholar]
- 40.Institute for Clinical and Economic Review. Biologic Therapies for Treatment of Asthma Associated with Type 2 Inflammation: Effectiveness, Value, and Value-Based Price Benchmarks. 2018.
- 41.Pollard C, Hartz S, Leage SL, Paget MA, Cook J, Enstone A. Elicitation of health state utilities associated with varying severities of flares in Systemic Lupus Erythematosus. Health Qual Life Outcomes. 2015;13:66. doi: 10.1186/s12955-015-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B, Cairns JA, Draper H, et al. Estimating health-state utility values in kidney transplant recipients and waiting-list patients using the EQ-5D-5L. Value Health. 2017;20(7):976–984. doi: 10.1016/j.jval.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snyder CF, Mathias SD, Cella D, Isitt JJ, Wu AW, Young J. Health-related quality of life of immune thrombocytopenic purpura patients: results from a web-based survey. Curr Med Res Opin. 2008;24(10):2767–2776. doi: 10.1185/03007990802377461. [DOI] [PubMed] [Google Scholar]
- 44.Benson T, Williams DH, Potts HW. Performance of EQ-5D, howRu and Oxford hip & knee scores in assessing the outcome of hip and knee replacements. BMC Health Serv Res. 2016;16(1):512. doi: 10.1186/s12913-016-1759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Tanna GL, Urbich M, Wirtz HS, et al. Health state utilities of patients with heart failure: a systematic literature review. Pharmacoeconomics. 2021;39(2):211–229. doi: 10.1007/s40273-020-00984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res. 2007;16(7):1251–1265. doi: 10.1007/s11136-007-9226-0. [DOI] [PubMed] [Google Scholar]
- 47.Worbes-Cerezo M, Nafees B, Lloyd A, Gallop K, Ladha I, Kerr C. Disutility study for adult patients with moderate to severe Crohn's disease. J Health Econ Outcomes Res. 2019;6(2):47–60. doi: 10.36469/9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi I, Park S, Song J, Jun JH, Suh D. Utilities for health states in patients with relapsed/refractory non-hodgkin lymphoma and factors influending utility values. Value Health. 2015;18:A1–A307. doi: 10.1016/j.jval.2015.03.1206. [DOI] [Google Scholar]
- 49.Tormalehto S, Mononen ME, Aarnio E, Arokoski JPA, Korhonen RK, Martikainen J. Health-related quality of life in relation to symptomatic and radiographic definitions of knee osteoarthritis: data from Osteoarthritis Initiative (OAI) 4-year follow-up study. Health Qual Life Outcomes. 2018;16(1):154. doi: 10.1186/s12955-018-0979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown MM, Brown GC, Brown HC, Peet J, Roth Z. Value-based medicine, comparative effectiveness, and cost-effectiveness analysis of topical cyclosporine for the treatment of dry eye syndrome. Arch Ophthalmol. 2009;127(2):146–152. doi: 10.1001/archophthalmol.2008.608. [DOI] [PubMed] [Google Scholar]
- 51.Moayeri F, Hsueh YS, Clarke P, Hua X, Dunt D. Health State utility value in chronic obstructive pulmonary disease (COPD); the challenge of heterogeneity: a systematic review and meta-analysis. COPD. 2016;13(3):380–398. doi: 10.3109/15412555.2015.1092953. [DOI] [PubMed] [Google Scholar]
- 52.Cooper JT, Lloyd A, Sanchez JJG, Sorstadius E, Briggs A, McFarlane P. Health related quality of life utility weights for economic evaluation through different stages of chronic kidney disease: a systematic literature review. Health Qual Life Outcomes. 2020;18(1):310. doi: 10.1186/s12955-020-01559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohmura K. Which is the best SLE activity index for clinical trials? Mod Rheumatol. 2021;31(1):20–28. doi: 10.1080/14397595.2020.1775928. [DOI] [PubMed] [Google Scholar]
- 54.Arora S, Isenberg DA, Castrejon I. Measures of adult systemic lupus erythematosus: disease activity and damage. Arthritis Care Res (Hoboken) 2020;72(Suppl 10):27–46. doi: 10.1002/acr.24221. [DOI] [PubMed] [Google Scholar]
- 55.Dubois RW. Cost-effectiveness thresholds in the USA: are they coming? Are they already here? J Comp Eff Res. 2016;5(1):9–11. doi: 10.2217/cer.15.50. [DOI] [PubMed] [Google Scholar]
- 56.Institute for Clinical and Economic Review. 2020–2023 Value Assessment Framework. 2020.
- 57.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 58.Mukherjee S, Culliford D, Arden N, Edwards C. What is the risk of having a total hip or knee replacement for patients with lupus? Lupus. 2015;24(2):198–202. doi: 10.1177/0961203314547894. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.