Abstract
Cytokine-mediated host defense against Candida glabrata infection was compared to that against C. albicans, using immunocompetent murine models of systemic candidiasis. The pathogenesis of infection was evaluated morphologically and by culture of target organs, while the kinetics of induction of cytokine mRNAs and corresponding proteins were determined in kidneys by real-time reverse transcription-PCR and cytokine-specific murine enzyme-linked immunosorbent assays, respectively. Systemic infection with C. glabrata resulted in a chronic, nonfatal infection with recovery of organisms from kidneys, while intravenous inoculation with C. albicans resulted in rapid mortality with logarithmic growth of organisms in kidneys and recovery of C. albicans from the spleen, liver, and lungs. Survival of C. glabrata-infected mice was associated with rapid induction of mRNAs and corresponding immunoreactive proteins for the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-12 (IL-12), and gamma interferon (IFN-γ) and the lack of induction of protein for the anti-inflammatory cytokine IL-10. In contrast, mortality in C. albicans-infected mice was associated with induction of mRNA and corresponding protein for IL-10 but delayed (i.e., TNF-α) or absent (i.e., IL-12 and IFN-γ) induction of immunoreactive proinflammatory cytokines. Mice were subsequently treated with cytokine-specific neutralizing monoclonal antibodies (MAbs) to TNF-α, IL-12, or IFN-γ, and the effect on growth of C. glabrata in kidneys was assessed. Neutralization of endogenous TNF-α resulted in a significant increase in C. glabrata organisms compared to similarly infected mice administered an isotype-matched control MAb, while neutralization of endogenous IL-12 or IFN-γ had no significant effect on C. glabrata replication. These results demonstrate that in response to intravenous inoculation of C. glabrata, immunocompetent mice develop chronic nonfatal renal infections which are associated with rapid induction of the proinflammatory cytokines TNF-α, IL-12, and IFN-γ. Furthermore, TNF-α plays a key role in host defense against systemic candidiasis caused by either C. glabrata or C. albicans, as the absence of endogenous TNF-α activity was associated with enhanced tissue burden in both infection models.
Candida glabrata, a monomorphic haploid yeast, has historically been considered a nonpathogenic saprophyte of normal flora of healthy individuals (22, 27, 77). However, due to the widespread use of immunosuppressive agents and/or broad-spectrum antimycotic therapy, the frequency of mucosal and systemic C. glabrata infections has increased significantly, making it the second or third most common cause of candidiasis after C. albicans (22, 24, 35, 36, 52, 72, 73, 84). Infections caused by C. glabrata are particularly difficult to treat, as resistance to azole antifungal agents including fluconazole is common (22, 31, 36, 69, 79, 82, 83). Consequently, C. glabrata infections often result in high morbidity and mortality in immunocompromised hospitalized patients (22).
Despite increasing clinical significance, there are few reports regarding host response to C. glabrata infections (22). Previous studies have demonstrated that innate immunity, mediated by granulocytes (polymorphonuclear leukocytes) and monocytes/macrophages, is crucial to containment and resolution of systemic candidiasis caused by other Candida species, including C. albicans (5, 13, 18, 55, 66). Phagocytic cells kill C. albicans yeast, hyphae, and pseudohyphae, using both oxidative and nonoxidative mechanisms (20, 28, 41, 81). Previous in vitro and in vivo studies have demonstrated that polymorphonuclear leukocytes and/or macrophage antifungal activities are modulated by cytokines (1, 61, 62). Specifically, stimulation of phagocytic cells in vitro with proinflammatory cytokines including gamma interferon (IFN-γ) and/or tumor necrosis factor alpha (TNF-α) enhanced anti-C. albicans activity, while anti-inflammatory cytokines including interleukin-10 (IL-10) and IL-4 had the opposite effect (6, 15–17, 47, 48, 57–59, 85, 86). Likewise, murine resistance to systemic C. albicans infections was associated with induction of TNF-α, IL-12, and IFN-γ, while susceptibility to infection was associated with induction of IL-4 and IL-10 (38, 61, 65, 76). Furthermore, mice depleted of endogenous IL-10 (by administration of cytokine-specific neutralizing monoclonal antibody [MAb], receptor antagonists, or IL-10 knockout mice), developed protective immune responses to systemic C. albicans infection, while inhibition of endogenous TNF-α, IL-12, or IFN-γ had the opposite effect (9, 12, 38, 40, 46, 53, 63, 64, 67, 78, 80).
To gain insight into cytokine-mediated host defense against systemic C. glabrata infection, immunocompetent Crl:CF-1 mice were inoculated intravenously (i.v.) with either C. glabrata or C. albicans. The pathogenesis of infection was evaluated morphologically and by culture of target organs, while the kinetics of induction of cytokine mRNAs and corresponding proteins for TNF-α, IL-12, IFN-γ, and IL-10 were determined in tissues by real-time reverse transcription-PCR (RT-PCR) and cytokine-specific murine enzyme-linked immunosorbent assays (ELISAs), respectively. Subsequently, the biological relevance of induced proinflammatory cytokine activity in host resistance to systemic C. glabata infection was assessed using cytokine-specific neutralizing MAbs.
MATERIALS AND METHODS
Mice.
Male specific-pathogen-free outbred immunocompetent Crl:CF-1 mice (11 to 13 g; Charles River) were used for all experiments. Animals were housed in microisolator cages and were cared for in accordance with standard guidelines. All in vivo experiments were approved by the institutional Animal Care and Use Committee.
Fungal inoculum and animal inoculation.
Clinical isolates of C. glabrata and C. albicans were grown on Sabouraud's dextrose agar (SDA) (7, 14). For preparation of the inocula, C. glabrata and C. albicans were quantified from SDA plates that had been incubated for 48 h at 35°C and resuspended in phosphate-buffered saline at the desired concentration. Crl:CF-1 mice were inoculated i.v. with C. glabrata or with C. albicans (104 to 108 CFU/mouse) via the lateral tail vein.
Quantification of Candida sp. in infected tissue homogenates.
At 0, 1, 2, 3, 7, 10, 14, and 21 days postinfection (p.i.), mice were euthanized, and target organs (brain, heart, lung, liver, spleen, and kidney) were excised and homogenized in 10 ml of sterile phosphate-buffered saline. Tissue homogenates from individual mice were serially diluted on SDA plates and incubated for 48 h at 35°C prior to quantifying C. glabrata or C. albicans. Results are expressed as CFU log10 per organ.
Pathology.
The inflammatory response in target organs of C. glabrata- and C. albicans-infected mice was assessed by light microscopy. Mice were inoculated i.v. with C. glabrata (108 CFU/mouse) or with C. albicans (5 × 106 CFU/mouse). At 0, 4, 24, 48, 72, 168, 240, 336, and/or 504 h p.i., groups of three surviving mice were euthanized, and tissues were excised and fixed in 10% buffered formalin. Fixed tissues were sectioned, embedded in paraffin, and stained with hematoxylin-eosin and Gomori's silver stain.
Quantitation of cytokine transcripts by real-time RT-PCR.
Real-time RT-PCR assays were performed to specifically quantify murine TNF-α, IL-12, IL-10, and IFN-γ transcripts. Briefly, kidneys were excised from C. glabrata- and C. albicans-infected mice at specific times p.i. and flash-frozen in liquid nitrogen. Total RNA was extracted using TriReagent (Molecular Research Center, Cincinnati, Ohio) according to the manufacturer's directions and stored at −80°C in nuclease-free water containing 0.1 mM EDTA. Isolated RNA (5 μg) was incubated with 10 U of DNase I (Boehringer Mannheim) in the presence of RNasin (Promega) for 30 min at 37°C. Samples were heat inactivated at 70°C for 10 min, chilled, and reverse transcribed with Superscript II reverse transcriptase (Gibco/BRL) with 1 μg each of random hexamers and oligo(dT) 12–18. PCR primers were obtained from Perkin-Elmer as predeveloped assay reagents (TNF-α, IL-12 p35, IL-12 p40, IL-10, and IFN-γ). Samples were then subjected to 40 cycles of amplification at 95°C for 15 s followed by 60°C for 1 min using an ABI Geneamp 7700 sequence detection system as specified by the manufacturer (Perkin-Elmer). PCR amplification of the housekeeping ubiquitin gene was performed for each sample to control for sample loading and allow normalization between samples as instructed by the manufacturer (Perkin-Elmer). Water controls were included to ensure specificity. Each data point was examined for integrity by analysis of the amplification plot. The ubiquitin-normalized data were expressed as fold induction of gene expression in infected mice compared to that in uninfected mice.
Immunoreactive cytokine analysis of tissue homogenate supernatants.
Kidney supernatants were procured from uninfected and infected mice by filtering tissue homogenates (prepared as described above) through a 0.22-μm-pore-size filter (Gelman Sciences, Ann Arbor, Mich.). Protein levels of TNF-α, IL-12, IL-10, and IFN-γ were subsequently measured using commercially available cytokine-specific murine ELISA kits (Quantikine mouse TNF-α, mouse IL-12 p70, mouse IFN-γ, and mouse IL-10; R&D systems, Minneapolis, Minn.) according to the manufacturer's directions.
Interventional studies.
Endogenous TNF-α, IL-12, and IFN-γ activities were blocked by administration of cytokine-specific neutralizing MAbs directed toward TNF-α (30, 32), IL-12 (51), and IFN-γ (10). Each neutralizing antibody was given intraperitoneally at a dose of 1 mg of antibody/mouse 24 h prior to systemic infection with C. glabrata. Similarly infected mice administered an isotype-matched immunoglobulin G2a (IgG2a) served as controls.
Statistical analysis.
The average cytokine mRNA expression for all time points p.i. was compared against that of the initial time point. Analysis of variance was performed to model dCt against time, using the following equation: dCtT = dCt0 − log2(fold expression), where T is time. The P values were computed using a Dunnett adjustment for multiple comparisons.
The average immunoreactive cytokine protein was measured for all time points p.i. and compared to that of the initial time point. Due to the clearly nonnormal nature of the data, a permutation test was performed to make these comparisons.
All P values were compared to a significance level of α = 0.05. Results are presented as mean ± standard error of the mean (SEM).
RESULTS
Comparison of virulence of C. glabrata and C. albicans in immunocompetent Crl:CF-1 mice.
Initial experiments were conducted to compare the pathogenesis of C. glabrata and C. albicans systemic infection in immunocompetent Crl:CF-1 mice. Mice were inoculated with C. glabrata or with C. albicans (104 to 108 organisms in 100 μl of saline i.v. via the lateral tail vein) and observed once daily for 7 days for morbidity and mortality. As shown in Table 1, all mice inoculated with C. glabrata (≤108 organisms/mouse) survived and appeared clinically normal. In contrast, systemic infection with C. albicans (≥5 × 106 organisms/mouse) resulted in 100% mortality within 4 days p.i., which was associated with clinical signs of disease including weight loss, lethargy, and a ruffled appearance. In all subsequent experiments, mice were inoculated systemically with either C. glabrata (108 organisms/mouse) or C. albicans (5 × 106 organisms/mouse).
TABLE 1.
Assessment of virulence of C. glabrata and C. albicans in systemically infected Crl:CF-1 micea
| Dose (organisms/mouse) | Organism | No. of survivors | % Survival |
|---|---|---|---|
| 108 | C. glabrata | 5 | 100 |
| C. albicans | 0 | 0 | |
| 5 × 107 | C. glabrata | 5 | 100 |
| C. albicans | 0 | 0 | |
| 107 | C. glabrata | 5 | 100 |
| C. albicans | 0 | 0 | |
| 5 × 106 | C. glabrata | 5 | 100 |
| C. albicans | 0 | 0 | |
| 106 | C. glabrata | 5 | 100 |
| C. albicans | 2 | 40 | |
| 5 × 105 | C. glabrata | 5 | 100 |
| C. albicans | 5 | 100 | |
| 105 | C. glabrata | 5 | 100 |
| C. albicans | 5 | 100 | |
| 5 × 104 | C. glabrata | 5 | 100 |
| C. albicans | 5 | 100 | |
| 104 | C. glabrata | 5 | 100 |
| C. albicans | 5 | 100 |
Crl:CF-1 mice were inoculated i.v. with the indicated doses of C. glabrata or C. albicans as described in Materials and Methods. Mice were observed once daily for 7 days for clinical signs of illness or mortality. Results are representative of five mice per inoculum.
Pathogenesis of systemic C. glabrata and C. albicans infection in immunocompetent Crl:CF-1 mice.
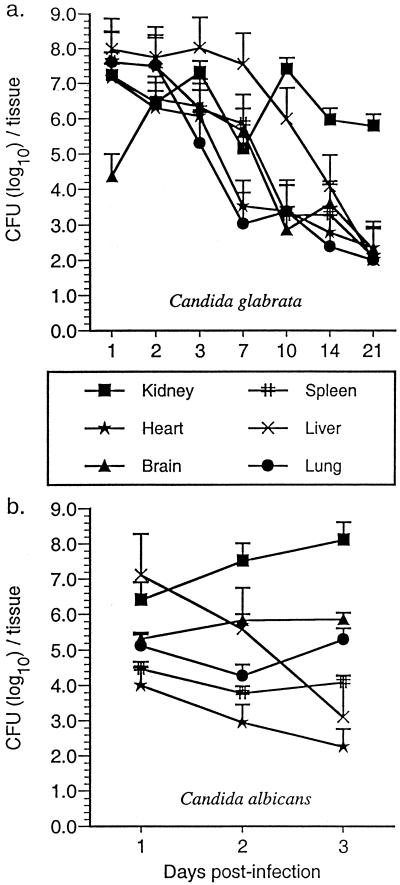
The tissue distribution of C. glabrata and C. albicans in systemically infected Crl:CF-1 mice was subsequently determined by culturing brains, hearts, lungs, livers, kidneys, and spleens. As shown in Fig. 1a, C. glabrata was recovered from all organs within 1 day p.i., and there was no significant net growth of the yeast in any organ over the 21-day duration of infection. However, while the number of C. glabrata organisms in the hearts, brains, spleens, livers, and lungs of infected mice declined over the duration of infection (<103 C. glabrata organisms/organ at 21 days p.i.), ≥105 organisms were consistently recovered from kidneys of infected mice for 21 days p.i.
FIG. 1.
Growth of C. glabrata and C. albicans in tissues of immunocompetent Crl:CF-1 mice. Mice were inoculated i.v. with C. glabrata (108 organisms/mouse) or with C. albicans (5 × 106 organisms/mouse). At specific time points p.i., mice were euthanized, and net growth of C. glabrata and C. albicans was quantified in kidneys, hearts, lungs, brains, spleens, livers, and lungs by culture of tissue homogenates. Results represent the mean ± SEM of CFU (log10)/tissue of three mice per time point.
As shown in Fig. 1b, C. albicans was also recovered from all organs within 1 day p.i. However, while the number of C. albicans organisms in the heart and liver declined rapidly with time (≤103 organisms at 3 days p.i.), there was no significant change in the fungal load in the brains, spleens, or lungs of infected mice over the duration of infection (104 to 105 organisms/tissue at 3 days p.i.). Furthermore, the number of C. albicans organisms in kidneys of infected mice increased by nearly 2 logs within 3 days p.i. Taken together, these results demonstrate systemic infection with a relatively large inoculum (108 CFU/mouse) of C. glabrata resulted in a chronic nonfatal infection with persistent recovery of the yeast from the kidneys, while systemic C. albicans infection, induced by 100-fold fewer organisms, was rapidly fatal (100% mortality within 4 days p.i.) and was associated with logarithmic growth of the organism in the kidneys and persistent recovery from the brain, spleen, and lungs.
Pathology.
Gross lesions were not apparent in organs from Crl:CF-1 mice inoculated with C. glabrata. In contrast, within 72 h p.i., kidneys from mice infected with C. albicans appeared tan and mottled in color with an irregular surface.
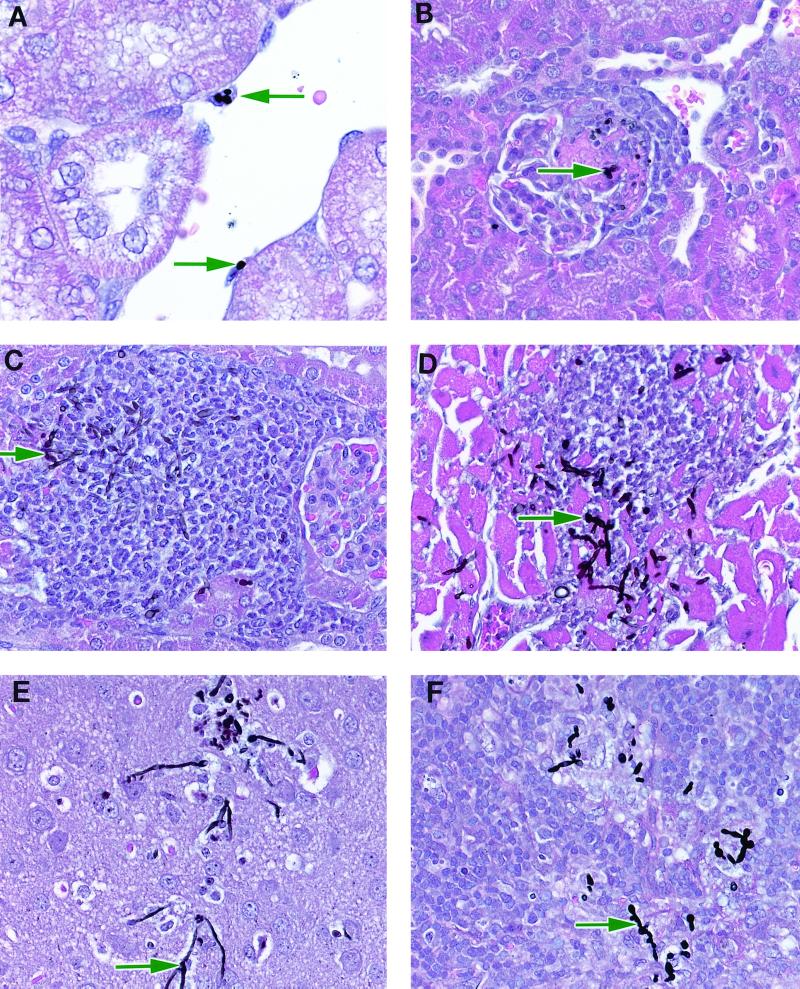
Histopathological lesions in C. glabrata-infected mice were most prominent in kidneys. Within 4 h p.i., organisms were apparent in the glomerular tuft and within macrophages attached to the renal vascular endothelium (Fig. 2A). The presence of organisms at 4 h p.i. was associated with minimal inflammation. By 48 to 72 h p.i. there were perivascular and periglomerular foci of mixed mononuclear cell infiltrates scattered primarily throughout the renal cortex. These foci infrequently contained organisms (Fig. 2B). The renal mononuclear cell infiltrate persisted until 10 days p.i.; however, organisms were not observed after 7 days p.i. Organisms were also infrequently demonstrated in arterioles and capillaries of the brains, spleens, hearts, and livers of infected mice at 24 to 72 h p.i. These foci were associated with minimal mononuclear cell inflammatory infiltrates which resolved within 10 days p.i.
FIG. 2.
Pathology in Crl:CF-1 mice systemically inoculated with C. glabrata or C. albicans. (A) Mouse kidney 4 h after infection with C. glabrata. Note organisms (arrows) in macrophages attached to the renal vessel endothelium. (B) Mouse kidney 72 h after infection with C. glabrata. Note organisms (arrow) in renal glomeruli with a mixed mononuclear cell infiltrate. (C) Mouse kidney 24 h after infection with C. albicans. Note organisms (arrow) in glomeruli with moderate mononuclear cell infiltrate. (D) Mouse heart 24 h after infection with C. albicans. Note organisms (arrow) and polymorphonuclear infiltrate. (E) Mouse brain 48 h after infection with C. albicans. Note organisms (arrow) with minimal inflammatory response. (F) Mouse spleen 48 h after infection with C. albicans. Note organisms (arrow) with mild mononuclear cell infiltrate. Magnification, ×370.
In sharp contrast, histopathological lesions in mice systemically infected with C. albicans were extensive, with multiple foci of hyphal invasion in kidneys, hearts, brains, and spleens of infected mice. Foci were largest and most numerous in kidneys, with a cortical perivascular or periglomerular distribution, and were associated with minimal to severe mononuclear cell inflammatory infiltrates within 24 h p.i. (Fig. 2C). Multiple foci of perivascular oriented hyphae and polymorphonuclear inflammatory cell infiltrates were also apparent in hearts of infected mice within 24 h p.i. (Fig. 2D). These lesions were associated with foci of minimal mononuclear cell inflammatory infiltrates and cardiac myodegeneration and/or necrosis within 72 h p.i. Hyphae were also visible in brains (Fig. 2E) and spleens (Fig. 2F) of infected mice within 48 h p.i. and were associated with mild inflammatory cell infiltrates.
Temporal induction of cytokine mRNA in kidneys of infected mice.
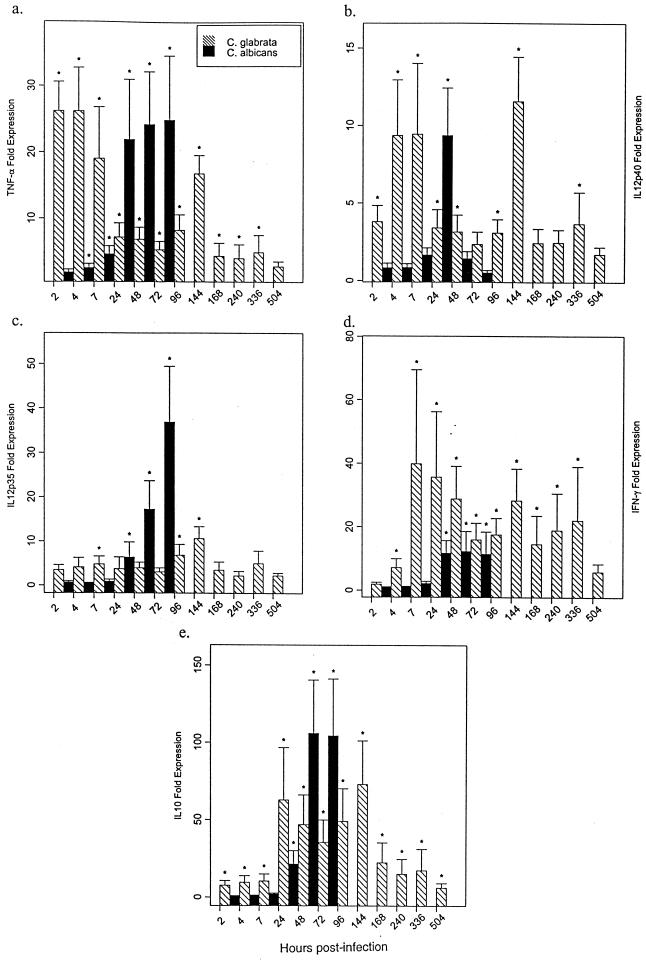
To gain insight into the potential role of pro- and anti-inflammatory cytokines in the pathogenesis of systemic C. glabrata infection, the kinetics of induction of cytokine mRNAs including TNF-α, IL-12 p40, IL-12 p35, IFN-γ, and IL-10 were assessed in kidneys of infected mice by real-time RT-PCR. This methodology allowed a rapid, accurate, and precise quantitation of gene transcripts (25, 29). For comparison, these cytokine mRNAs were also assessed in tissues from mice systemically infected with C. albicans. TNF-α (Fig. 3a), IL-12 p40 (Fig. 3b), IL-12 p35 (Fig. 3c), IFN-γ (Fig. 3d), and IL-10 (Fig. 3e) mRNAs were significantly enhanced in kidneys from C. glabrata-infected mice within 2 to 7 h p.i., with maximal induction of mRNAs for TNF-α and IFN-γ at ≤7 h p.i. and for IL-12 p40, IL-12 p35, and IL-10 at 144 h p.i. These cytokine mRNAs were also significantly enhanced in kidneys from C. albicans-infected mice; however, significant induction occurred later, at 4 to 24 h p.i., with maximal induction of mRNAs for TNF-α (Fig. 3a) at 72 h p.i., IL-12 p40 (Fig. 3b) at 24 h p.i., and IL-12 p35 (Fig. 3c), IFN-γ (Fig. 3d), and IL-10 (Fig. 3e) at 48 to 72 h p.i.
FIG. 3.
Temporal expression of TNF-α, IL-12, IFN-γ, and IL-10 mRNAs in kidneys of C. glabrata- or C. albicans-infected mice. Crl:CF-1 mice were infected i.v. with virulent C. glabrata (108 CFU/mouse) or C. albicans (5 × 106 CFU/mouse). At specific time points p.i., mice were euthanized, kidneys were excised, and total RNA was extracted. Transcript levels for TNF-α (a), IL-12 p40 (b), IL-12 p35 (c), IFN-γ (d), and IL-10 (e) were quantified in kidneys by real-time RT-PCR. For mRNA quantification, PCR amplification of the housekeeping ubiquitin gene was performed for each sample to control for sample loading and facilitate normalization between samples. The ubiquitin-normalized data were expressed as fold induction of gene expression in C. glabrata- or C. albicans-infected mice compared to uninfected mice. Results represent the mean ± SEM of two separate experiments, four to nine mice per treatment group. ∗, significantly greater than control mice, P < 0.05.
Temporal induction of immunoreactive cytokine activity in kidneys of infected mice.
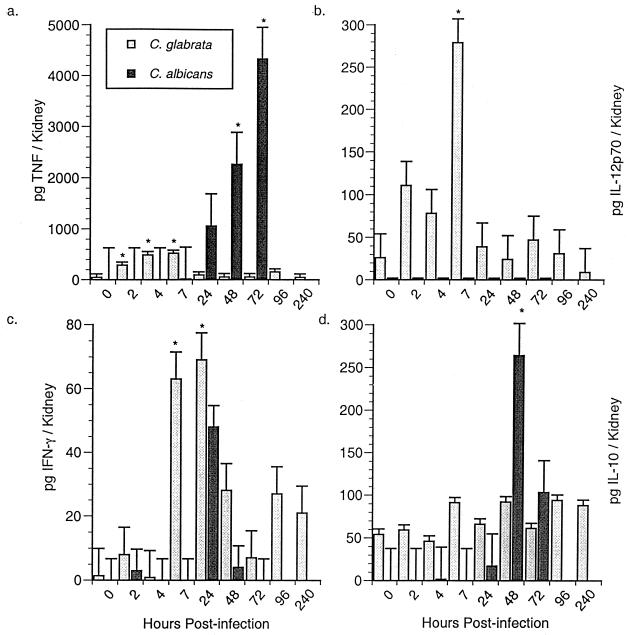
In subsequent studies, the kinetics of induction of the corresponding cytokine proteins were assessed in kidneys of infected mice by cytokine-specific ELISAs. TNF-α (Fig. 4a), IL-12p70 (Fig. 4b), and IFN-γ (Fig. 4c) proteins were significantly enhanced in kidney homogenates of C. glabrata-infected mice within 2 to 7 h p.i., with maximal induction within 4 to 24 h p.i. In contrast, IL-10 protein was not significantly increased in kidneys of C. glabrata-infected mice at any time point p.i. (Fig. 4d). Immunoreactive TNF-α (Fig. 4a) was also significantly increased in kidney homogenates of C. albicans-infected mice; however, this was not apparent until ≥48 h p.i. In contrast, IL-12 p70 (Fig. 4b) and IFN-γ (Fig. 4c) proteins were not significantly induced in kidneys of C. albicans-infected mice at any time point p.i., while IL-10 protein was significantly enhanced in kidneys of similarly infected mice within 48 h p.i. (Fig. 4d).
FIG. 4.
Temporal expression of TNF-α, IL-12, IFN-γ, and IL-10 proteins in kidneys during C. glabrata and C. albicans infection. Crl:CF-1 mice were infected with virulent C. glabrata or C. albicans as described for Fig. 3. At specific time points p.i., the mice were euthanized, and kidneys were excised and homogenized. Levels of the immunoreactive cytokines TNF-α (a), IL-12 p70 (b), IFN-γ (c), and IL-10 (d) were quantified in kidney homogenates. Results represent the mean ± SEM of two separate experiments, 8 to 14 mice per treatment group. ∗, significantly greater than control mice, P < 0.05.
Role of proinflammatory cytokines in host defense against systemic C. glabrata infection.
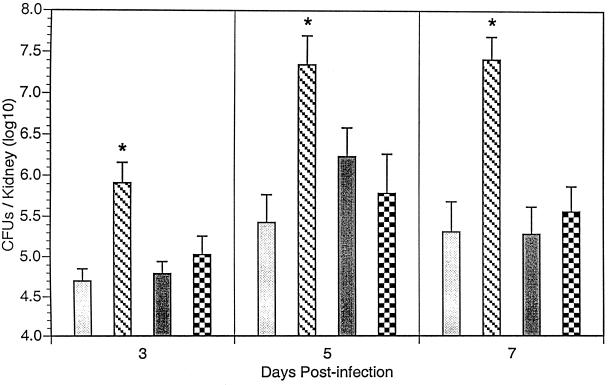
To assess the biological relevance of TNF-α, IL-12 p70, and IFN-γ in host defense against systemic C. glabrata infection, mice were administered cytokine-specific neutralizing MAbs prior to infection. At 3, 5, and 7 days p.i., mice were humanely euthanized, kidneys were excised, and C. glabrata organisms were quantified in kidney homogenates by culture. As shown Fig. 5, treatment of mice with anti-TNF-α MAb resulted in a significant increase in C. glabrata organisms in infected kidney at all time points p.i. compared to infected mice administered control MAb (IgG2a). In contrast, there was no significant increase in the number of organisms recovered from kidneys of similarly infected mice administered either anti-IL-12 or anti-IFN-γ MAb compared to infected mice administered control MAb.
FIG. 5.
Role of endogenous TNF-α, IL-12, and IFN-γ in resolution of primary systemic C. glabrata infection. Crl:CF-1 mice were administered control (IgGa) ( ), TNF-α (
), TNF-α ( ), anti-IL-12 (
), anti-IL-12 ( ), or anti-IFN-γ (
), or anti-IFN-γ ( ) MAb (1 mg/mouse intraperitoneally) 1 day prior to infection with C. glabrata. At 3, 5, and 7 days p.i., mice were euthanized, kidneys were excised and homogenized, and growth of C. glabrata was quantified by culture of kidney homogenates. Results represent the pooled mean ± SEM of two separate experiments, 10 to 15 animals per treatment group. *, significantly greater than similarly infected mice administered control MAb, P < 0.05.
) MAb (1 mg/mouse intraperitoneally) 1 day prior to infection with C. glabrata. At 3, 5, and 7 days p.i., mice were euthanized, kidneys were excised and homogenized, and growth of C. glabrata was quantified by culture of kidney homogenates. Results represent the pooled mean ± SEM of two separate experiments, 10 to 15 animals per treatment group. *, significantly greater than similarly infected mice administered control MAb, P < 0.05.
DISCUSSION
Despite its increasing clinical significance, relatively little is known about the pathogenesis of systemic C. glabrata infection, and virtually nothing is known about host responses to infection with this organism (22). To gain insight into these issues, we infected immunocompetent Crl:CF-1 mice i.v. with C. glabrata and compared tissue distribution and the morphologic response and cytokine activity with those in mice similarly infected with C. albicans. Results of these studies demonstrated C. glabrata was considerably less pathogenic than C. albicans, as injection of a relatively large number of C. glabrata organisms (108 CFU/mouse) resulted in a chronic nonfatal infection with recovery of C. glabrata from kidneys for 21 days p.i. In contrast, inoculation with approximately 100-fold-fewer C. albicans organisms (i.e., 5 × 106 CFU/mouse) resulted in 100% mortality within 4 days p.i., with logarithmic growth of the pathogen in infected kidneys. Our results, demonstrating persistent recovery of C. glabrata from immunocompetent mice, are in sharp contrast to a previous study by Atkinson et al. (2), who concluded that immunocompromisation was required to achieve a sustained C. glabrata infection in mice inoculated i.v. with a relatively large number of C. glabrata organisms (i.e., 108 blastoconidia). While the reason for these discrepancies in host susceptibility to infection between the two studies is not clear, it may be due to the use of different murine and/or C. glabrata strains.
Subsequent morphologic examination of tissues confirmed that the kidney is the preferred target organ in systemic C. glabrata of C. albicans infections. Furthermore, both Candida species were most frequently observed in the periglomerular cortical tissue rather than in the glomerular mesangium, suggesting that the glomerular mesangium may possess more innate candicidal potential than does the surrounding cortical vasculature. In agreement with previously published studies, our morphologic studies also demonstrated that while C. albicans is dimorphic in vivo, C. glabrata is monomorphic. Likewise, the presence of hyphae in tissues of C. albicans-infected mice was associated with a significant inflammatory response, characterized by a neutrophilic infiltrate initially, followed by a mononuclear cell infiltrate composed primarily of macrophages. The minimal inflammatory cell infiltrate into tissues of C. glabrata-infected mice was composed primarily of macrophages. These results are consistent with those of previous studies (22, 49, 74, 75) and suggest that formation of pseudohyphae and/or hyphae promotes neutrophil infiltration and is key to Candida virulence. The potential role of other factors known to be important in C. albicans pathogenicity, including proteinases and phospholipases (3, 4, 8, 33, 68), have not been investigated with regard to C. glabrata.
Proinflammatory cytokines including TNF-α, IL-12, and/or IFN-γ have previously been shown to play key roles in host defense against C. albicans infections, due to their ability enhance phagocytosis of C. albicans blastoconidia and increase oxygen-dependent and independent candicidal activity (45, 60). In contrast, the production of anti-inflammatory cytokines such as IL-10 impairs development of a protective immune response to C. albicans, due to downregulation of phagocytic cell effector mechanisms (46, 54, 57, 67, 86). Our findings agree with those of previous studies, which demonstrate a dose-dependent induction of anti-inflammatory cytokines in response to C. albicans (45) and suggest that rapid induction of proinflammatory cytokines is essential for prompt control of C. albicans or C. glabrata growth and host survival. Our findings also indicate that the rapid induction of both mRNAs and corresponding proteins for proinflammatory cytokines, including TNF-α, IL-12 p70 and IFN-γ, and lack of induction of immunoreactive IL-10 may play a significant role in the lack of relative pathogenicity of systemic C. glabrata infection.
To characterize the biological relevance of induced proinflammatory cytokines in the pathogenesis of C. glabrata infection, mice were subsequently administered cytokine-specific neutralizing antibodies, and the effect on fungal growth was assessed. Our results demonstrate a key role of TNF-α, rather than IL-12 and IFN-γ, in innate resistance to systemic C. glabrata infection, as neutralization of endogenous TNF-α activity alone resulted in significant increase in C. glabrata growth in infected tissues. These results complement those of previous studies which have shown that murine resistance to primary C. albicans infections was dependent on TNF-α and independent of IFN-γ and IL-12 (38, 40, 44, 45, 56, 62). The mechanism(s) by which TNF-α inhibits growth of C. glabrata has not been thoroughly investigated. However, TNF-α has multiple regulatory effects, exerting endocrine, paracrine, and autocrine control of inflammatory responses (37, 71). Furthermore, TNF-α facilitates phagocytic cell activation, resulting in altered cell functional responses including increased adherence, enhanced generation of reactive oxygen and nitrogen species, enhanced degranulation of azurophilic granules, and increased phagocytosis (21, 26, 34, 39, 42, 43, 70), all of which could facilitate control of C. glabrata replication in vivo.
Despite induction of a polarized proinflammatory cytokine response, kidneys of immunocompetent Crl:CF-1 mice remained persistently infected with C. glabrata, while the organism was cleared from the brain, heart, lungs, liver, and spleen. Mechanisms by which C. glabrata selectively resists destruction by innate immune responses in the kidney are incompletely understood. However, our morphometric examination of infected tissues demonstrated that neutrophils, which are essential for resolution of C. albicans infections, are much less numerous in tissues from C. glabrata-infected mice. Previous studies have demonstrated that leukocytes are recruited from the bloodstream into infected tissues at least in part by chemotactic cytokines and leukocyte adhesion molecules expressed by the vascular endothelium (11, 23, 50). Recent studies have demonstrated that while both chemotactic cytokines and leukocyte adhesion molecules are induced in cultured vascular endothelium in response to endocytosis of C. albicans, neither are expressed by similar cells following endocytosis of C. glabrata (23). The relative absence of these mediators likely contributes to the minimal inflammatory cell response in tissues from C. glabrata-infected mice. Furthermore, because chemotactic cytokines facilitate release of azurophilic granules, thereby contributing to phagocytic cell candicidal activity (19), a potential lack of these mediators may also contribute to persistent C. glabrata infection. Our results also support conclusions of previous studies which demonstrate that C. albicans and C. glabrata differ phenotypically, as C. albicans is dimorphic whereas C. glabrata is monomorphic in vivo. Because phenotypic differences profoundly influence the efficacy of phagocyte fungicidal responses (13, 85), it is likely that these different Candida species differ in both their capacities to trigger phagocytic cell activation and their susceptibilities to fungicidal activity. Future studies to identify interactions between C. glabrata and host cells including endothelial cells and phagocytic cells are warranted.
In summary, we have demonstrated that immunocompetent mice following systemic inoculation with C. glabrata develop chronic nonfatal renal infections which are associated with rapid induction of proinflammatory cytokines including TNF-α, IL-12, and IFN-γ. Furthermore, endogeneous TNF-α plays a dominant role in controlling growth of C. glabrata in vivo, as neutralization of TNF-α activity resulted in enhanced tissue burden. Future in vivo studies to elucidate the role of endogenous cytokines in host resistance to C. glabrata are warranted, as manipulation of cytokine gene expression may provide an important adjuvant therapy for prevention and/or treatment of systemic candidiasis in immunocompromised patients.
ACKNOWLEDGMENTS
We thank Ferdous Gheyas and Steven Novick, Biostatistics Department, Schering Plough Research Institute, for assistance with analysis of the data. We also thank Frank Sabatelli, Department of Chemotherapy, Schering Plough Research Institute, for assistance with graphics.
REFERENCES
- 1.Ashman R B, Papadimitriou J M. Production and function of cytokines in natural and acquired immunity to Candida albicans infections. Microbiol Rev. 1995;59:646–672. doi: 10.1128/mr.59.4.646-672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson B A, Bouthet C, Bocanegra R, Correa A, Luther M F, Graybill J R. Comparison of fluconazole, amphotericin B, and flucytosine in treatment of a murine model of disseminated infection with Candida glabrata in immunocompromised mice. J Antimicrob Chemother. 1995;35:631–640. doi: 10.1093/jac/35.5.631. [DOI] [PubMed] [Google Scholar]
- 3.Barret-Bee K, Hayes Y, Wilson R G, Ryley J F. A comparison of phospholipase activity, cellular adherence and pathogenicity of yeasts. J Gen Microbiol. 1985;131:1217–1221. doi: 10.1099/00221287-131-5-1217. [DOI] [PubMed] [Google Scholar]
- 4.Bennet D E, McCreary C E, Coleman D C. Genetic characterization of a phospholipase C gene from Candida albicans: presence of homologous sequences in Candida species other than Candida albicans. Microbiology. 1998;144:55–72. doi: 10.1099/00221287-144-1-55. [DOI] [PubMed] [Google Scholar]
- 5.Blasti E, Manuela P, Pitzurra L, Bartoli A, Bistoni F. Heterogeneous secretory responses of phagocytes from different anatomical districts to the dimorphic fungus Candida albicans. Cell Immunol. 1994;153:239–247. doi: 10.1006/cimm.1994.1021. [DOI] [PubMed] [Google Scholar]
- 6.Brummer E, Morrison C J, Stevens D A. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing against phagocytizable and nonphagocytizable fungi. Infect Immun. 1985;49:724–730. doi: 10.1128/iai.49.3.724-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cacciapuoti A, Loebenberg D, Corcoran E, Menzel F, Moss E L, Norris C, Michalski M, Raynor K, Halpern J, Mendrick C, Arnold B, Antonacci B, Parmegiani R, Yarosh-Tomaine T, Miller G H, Hare R S. In vitro and in vivo activities of SCH56592 (Posaconazole), a new triazole antifungal agent, against Aspergillus and Candida. Antimicrob Agents Chemother. 2000;44:2017–2022. doi: 10.1128/aac.44.8.2017-2022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassone A, DeBernardis F, Mondello F, Ceddia T, Agatensi L. Evidence for a correlation between proteinase secretion and vulvovaginal candidosis. J Infect Dis. 1987;156:777–783. doi: 10.1093/infdis/156.5.777. [DOI] [PubMed] [Google Scholar]
- 9.Cenci E, Romani L, Vecchiarelli A, Puccetti P, Bistoni F. T cell subsets and IFN-γ production in resistance to systemic candidiasis in immunized mice. J Immunol. 1990;144:4333–4339. [PubMed] [Google Scholar]
- 10.Cherwsinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clones. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemons K V, Calich V L G, Burger E, Filler S G, Graziutti M, Murphy J, Roilides E, Campa A, Dias M R, Edwards J E, Fu Y, Fernandes-Bordignon G, Ibrahim A, Katsifa H, Lamaignere C G, Meoni-Bruneri L H, Rex J, Savary C A, Xidieh C. Pathogenesis. I. Interactions of host cells and fungi. Med Mycol. 2000;38:99–111. [PubMed] [Google Scholar]
- 12.Del Sero G, Mancacci A, Cenci E, d'Ostiani C F, Montagnoli C, Bacci A, Mosci P, Kopf M, Romani L. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect. 1999;1:1169–1180. doi: 10.1016/s1286-4579(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 13.Diamond R D. Interactions of phagocytic cells with Candida and other opportunistic fungi. J Arch Med Res. 1993;24:361–369. [PubMed] [Google Scholar]
- 14.Dick J D, Merz W G, Saral R. Incidence of polyene-resistant yeasts recovered from clinical specimens. Antimicrob Agents Chemother. 1980;18:158–163. doi: 10.1128/aac.18.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djeu J Y. Role of tumor necrosis factor and colony-stimulating factors in phagocyte function against Candida albicans. Diagn Microbiol Infect Dis. 1990;13:383–386. doi: 10.1016/0732-8893(90)90007-i. [DOI] [PubMed] [Google Scholar]
- 16.Djeu J Y, Blanchard D K, Halkias D, Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon γ and tumor necrosis factor. J Immunol. 1986;137:2980–2984. [PubMed] [Google Scholar]
- 17.Djeu J Y. Tumor necrosis factor and Candida albicans. Behring Inst Mitt. 1991;88:222–227. [PubMed] [Google Scholar]
- 18.Djeu J Y, Blanchard D K. Regulation of human polymorphonuclear neutrophil (PMN) activity against Candida albicans by large granular lymphocytes via release of a PMN-activating factor. J Immunol. 1987;139:2761–2767. [PubMed] [Google Scholar]
- 19.Djeu J Y, Matsushima K, Oppenheim J J, Shiotsuki K, Blanchard D K. Functional activation of human neutrophils by recombinant monocyte-derived neutrophil chemotactic factor/IL-8. 1990. J Immunol. 1990;144:2205–2210. [PubMed] [Google Scholar]
- 20.Domer J E, Lehrer R I. Introduction to Candida. In: Murphy J W, Friedman H, Berdinjelli H, editors. Systemic candidiasis. Fungal infections and immune responses. New York, N.Y: Plenum Press; 1993. pp. 49–116. [Google Scholar]
- 21.Ferrante A. Tumor necrosis factor alpha potentiates neutrophil antimicrobial activity: increased fungicidal activity against Torulopsis glabrata and Candida albicans and associated increases in oxygen radical production and lysosomal enzyme release. Infect Immun. 1989;57:2115–2122. doi: 10.1128/iai.57.7.2115-2122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fidel P L, Jr, Vazquez J A, Sobel J D. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12:80–96. doi: 10.1128/cmr.12.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filler S G, Pfunder A S, Spellberg B J, Spellverg J P, Edwards J E. Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–2617. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiger A M, Foxman B, Sobel J D. Chronic vulvovaginal candidiasis: characteristics of women with Candida albicans, Candida glabrata and no Candida. Genitourin Med. 1995;71:304–307. doi: 10.1136/sti.71.5.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson U E, Heid C A, Williams P M. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 26.Hachicha M, Rathanaswami P I, Naccache P H, McColl S R. Regulation of chemokine gene expression in human peripheral blood neutrophils phagocytosing microbial pathogens. J Immunol. 1998;160:449–454. [PubMed] [Google Scholar]
- 27.Haley L D. Yeasts of medical importance. Am J Clin Pathol. 1961;36:227–234. doi: 10.1093/ajcp/36.3.227. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto T. In vitro study of contact mediated killing of Candida albicans hyphae by activated murine peritoneal macrophages in a serum-free medium. Infect Immun. 1991;59:3555–3561. doi: 10.1128/iai.59.10.3555-3561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heid C A, Stevens J, Livak K J, Williams P M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Caselles T, Stutman O. Immune functions of tumor necrosis factor. I. Tumor necrosis factor induces apoptosis of mouse thymocytes and can also stimulate or inhibit IL-6-induced proliferation depending on the concentration of mitogenic costimulation. J Immunol. 1993;151:3999–4012. [PubMed] [Google Scholar]
- 31.Hitchcock C A, Pye G W, Troke P F, Johnson E M, Warnock D W. Fluconazole resistance in Candida glabrata. Antimicrob Agents Chemother. 1993;37:1962–1965. doi: 10.1128/aac.37.9.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter C A, Abrams J S, Beaman M H, Remington J S. Cytokine mRNA in the central nervous system of SCID mice infected with Toxoplasma gondii: importance of T-cell-independent regulation of resistance to T. gondii. Infect Immun. 1993;61:4038–4044. doi: 10.1128/iai.61.10.4038-4044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim A S, Mirbod F, Filler S G, Yoshiko B, Cole G, Kitajima Y, Edwards J E, Jr, Nosawa Y, Ghannoum M A. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect Immun. 1995;63:1993–1998. doi: 10.1128/iai.63.5.1993-1998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klebanoff S J, Vadas M A, Harlan J M, Sparks L H, Gamble J R, Agosti J M, Waltersdorph A M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986;136:4220–4225. [PubMed] [Google Scholar]
- 35.Knoke M, Schultz K, Bernhardt H. Dynamics of Candida isolates from humans from 1992–1995 in Greifswald, Germany. Mycoses. 1997;40:105–110. doi: 10.1111/j.1439-0507.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 36.Komshian S V, Uwaydah A K, Sobel J D. Fungemia caused by Candida species and Torulopsis glabrata in the hospitalized patient: frequency, characteristics and evaluation of factors influencing outcome. Rev Infect Dis. 1989;11:379–390. doi: 10.1093/clinids/11.3.379. [DOI] [PubMed] [Google Scholar]
- 37.Le J, Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Investig. 1987;56:234–248. [PubMed] [Google Scholar]
- 38.Louie A, Baltch A L, Smith R P, Franke M A, Ritz W A, Singh J K, Gordon M A. Tumor necrosis factor alpha has a protective role in a murine model of systemic candidiasis. Infect Immun. 1994;62:2761–2772. doi: 10.1128/iai.62.7.2761-2772.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandell G L. Cytokines, phagocytes and pentoxifylline. J Cardiovasc Pharmacol. 1995;25:S20–S22. doi: 10.1097/00005344-199500252-00005. [DOI] [PubMed] [Google Scholar]
- 40.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marquis G, Garzon S, Montplausir S, Strykowski H, Benhamou N. Histochemical and immunochemical study of the fate of Candida albicans inside human neutrophil phagolysosomes. J Leukoc Biol. 1991;50:587–599. doi: 10.1002/jlb.50.6.587. [DOI] [PubMed] [Google Scholar]
- 42.McColl S R, Bbeauseigle D, Gilbert C, Naccache P H. Priming of the human neutrophil respiratory burst by granulocyte-macrophage colony stimulating factor and tumor necrosis factor-alpha involves regulation at a post-cell surface receptor level: enhancement of the effect of agents which directly activate G proteins. J Immunol. 1990;145:3047–3053. [PubMed] [Google Scholar]
- 43.McColl S R, Paquin R, Menard C, Beaulieu A D. Human neutrophils produce high levels of the interleukin 1 receptor antagonist in response to granulocyte/macrophage colony stimulating factor and tumor necrosis factor alpha. J Exp Med. 1992;176:593–598. doi: 10.1084/jem.176.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mencacci A, Cenci E, DelSero G, Ge d'Ostiani C, Mosci P, Montagnoli C, Bacci A, Bistoni F, Quesniaux V F, Ryffel B, Romani L. Defective co-stimulation and impaired Th1 development in tumor necrosis factor/lymphotoxin-alpha double deficient mice infected with Candida albicans. Int Immunol. 1998;10:37–48. doi: 10.1093/intimm/10.1.37. [DOI] [PubMed] [Google Scholar]
- 45.Mencacci A, Spaccapelo R, Del Sero G, Enssle K-H, Cassone A, Bistoni F, Romani L. CD4+ T helper responses in mice with low-level Candida albicans infection. Infect Immun. 1996;64:4907–4914. doi: 10.1128/iai.64.12.4907-4914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mencacci A, Perruccio K, Bacci A, Cenci E, Benedett R, Martelli M F, Bistoni F, Coffman R, Velardi A, Romani L. Defective antifungal T-helper 1 (Th1) immunity in a murine model of allogeneic T-cell-depleted bone marrow transplantation and its restoration by treatment with cytokine antagonists. Blood. 2001;97:1483–1490. doi: 10.1182/blood.v97.5.1483. [DOI] [PubMed] [Google Scholar]
- 47.Milton Gaviria J, van Burik J H, Dale D C, Root R K, Liles W C. Comparison of IFN-γ, GCSF, and GMCSF for priming leukocyte-mediated hyphal damage of opportunistic fungal pathogens. J Infect Dis. 1999;179:1038–1044. doi: 10.1086/314679. [DOI] [PubMed] [Google Scholar]
- 48.Morrison C J, Brummer E, Isnberg R A, Stevens D A. Activation of murine polymorphonuclear neutrophils for fungicidal activity by recombinant gamma interferon. J Leukoc Biol. 1987;41:434–440. doi: 10.1002/jlb.41.5.434. [DOI] [PubMed] [Google Scholar]
- 49.Odds F C. Candida and candidosis. Baltimore, Md: University Park Press; 1988. pp. 104–110. [Google Scholar]
- 50.Orozco A S, Xhou X, Filler S G. Mechanisms of the proinflammatory response of endothelial cells to Candida albicans infection. Infect Immun. 2000;68:1134–1141. doi: 10.1128/iai.68.3.1134-1141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozmen L, Pericin M, Hakimi J, Chizzonite R A, Wysocka M, Trinchieri G, Tagely M, Garotta G. Interleukin 12, interferon gamma, and tumor necrosis factor-alpha are the key cytokines of the generalized Shwartzman reaction. J Exp Med. 1994;180:907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfaller M A. Nosocomial candidiasis: emerging species, reservoirs, and modes. Clin Infect Dis. 1996;22:S89–S94. doi: 10.1093/clinids/22.supplement_2.s89. [DOI] [PubMed] [Google Scholar]
- 53.Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Enssle K-H, Romani L, Bistoni F. Cure of murine candidiasis by recombinant soluble interleukin 4 receptor. J Infect Dis. 1994;169:1325–1331. doi: 10.1093/infdis/169.6.1325. [DOI] [PubMed] [Google Scholar]
- 54.Puccetti P, Romani L, Bistoni F. A Th1-Th2 like switch in candidiasis: new perspectives for therapy. Trends Microbiol. 1995;3:237–240. doi: 10.1016/s0966-842x(00)88931-3. [DOI] [PubMed] [Google Scholar]
- 55.Qian Q, Jutila M, A, Van Rooijen N, Cutler J E. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol. 1994;152:5000–5008. [PubMed] [Google Scholar]
- 56.Qian Q, Cutler J E. Gamma interferon is not essential in host defense against disseminated candidiasis in mice. Infect Immun. 1997;65:1748–1753. doi: 10.1128/iai.65.5.1748-1753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roilides E, Anatoasiou-Katsardani A, Dimitriadou-Georgiadou A, Kadiltsoglou I, Tsaparidou S, Panteloadis C, Walsh T J. Suppressive effects of interleukin 10 on human mononuclear phagocyte functions against Candida albicans and Staphylococcus aureus. J Infect Dis. 1998;178:1734–1742. doi: 10.1086/314479. [DOI] [PubMed] [Google Scholar]
- 58.Roilides E, Katsifa H, Tsaparidou S, Stergiopoulouy T, Panteliadis C, Walsh T J. Interleukin 10 suppresses phagocytic and antihyphal activities of human neutrophils. Cytokine. 2000;12:379–387. doi: 10.1006/cyto.1999.0567. [DOI] [PubMed] [Google Scholar]
- 59.Roilides E, Kadiltsoglou I, Dimitriaou A, Hatzistilianou M, Manitsa A, Karpouzas J, Pizzo P A, Walsh T J. Interleukin-4 suppresses antifungal activity of human mononuclear phagocytes against Candida albicans in association with decreased uptake of blastoconidia. FEMS Immunol Med Microbiol. 1997;19:169–180. doi: 10.1111/j.1574-695X.1997.tb01086.x. [DOI] [PubMed] [Google Scholar]
- 60.Romani L, Howard D H. Mechanism and resistance to fungal infections. Curr Opin Immunol. 1995;7:517–523. doi: 10.1016/0952-7915(95)80097-2. [DOI] [PubMed] [Google Scholar]
- 61.Romani L, Puccetti P, Bistoni F. Biological role of helper T-cell subsets in candidiasis. Chem Immunol. 1996;63:115–137. [PubMed] [Google Scholar]
- 62.Romani L. Immunity to Candida albicans Th1, Th2 cells and beyond. Curr Opin Microbiol. 1999;2:363–367. doi: 10.1016/S1369-5274(99)80064-2. [DOI] [PubMed] [Google Scholar]
- 63.Romani L, Cenci E, Mencacci A, Spaccapelo R, Grohmann U, Puccetti P, Bistoni F. Gamma interferon modifies CD4+ subset expression in murine candidiasis. Infect Immun. 1992;60:4950–4952. doi: 10.1128/iai.60.11.4950-4952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Puccetti P, Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romani L. Innate and adaptive immunity in Candida albicans infections and saprophytism. J Leukoc Biol. 2000;68:175–179. [PubMed] [Google Scholar]
- 66.Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol. 1997;158:2356–2362. [PubMed] [Google Scholar]
- 67.Romani L, Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Tommelli L, Grohmann U, Bistoni F. Neutralization of IL-10 upregulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J Immunol. 1994;152:3514–3521. [PubMed] [Google Scholar]
- 68.Ross I K, De Bernardis F, Emerson G W, Cassone A, Sullivan P A. The secreted aspartate proteinase of Candida albicans: physiology of secretion and virulence of a proteinase-deficient mutant. J Gen Microbiol. 1990;136:687–694. doi: 10.1099/00221287-136-4-687. [DOI] [PubMed] [Google Scholar]
- 69.Schwab U, Chernomas F, Larcom L, Weems J. Molecular typing and fluconazole susceptibility of urinary Candida glabrata isolates from hospitalized patients. Diagn Microbiol Infect Dis. 1997;29:11–17. doi: 10.1016/s0732-8893(97)00076-x. [DOI] [PubMed] [Google Scholar]
- 70.Shalaby M R, Aggarwal B B, Rinderknecht E, Svedersky L P, Finkle B S, Palladino M A. Activation of human polymorphonuclear neutrophil functions by interferon gamma and tumor necrosis factors. J Immunol. 1985;135:2096–2073. [PubMed] [Google Scholar]
- 71.Sherry B, Cerami A. Cachectin/tumor necrosis factor exerts endocrine, paracrine and autocrine control of inflammatory responses. J Cell Biol. 1988;107:1269–1277. doi: 10.1083/jcb.107.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinnott J T. Candida (Torulopsis) glabrata. Infect Control. 1987;8:334–336. doi: 10.1017/s0195941700066443. [DOI] [PubMed] [Google Scholar]
- 73.Sobel J D. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann N Y Acad Sci. 1988;544:547–557. doi: 10.1111/j.1749-6632.1988.tb40450.x. [DOI] [PubMed] [Google Scholar]
- 74.Sobel J D, Muller G, Buckley H R. Critical role of germ tube formation in the pathogenesis of candidal vaginitis. Infect Immun. 1984;44:576–580. doi: 10.1128/iai.44.3.576-580.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soll D R. High-frequency switching in Candida albicans. Clin Microbiol Rev. 1992;5:183–203. doi: 10.1128/cmr.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steinshamn S, Bemelmans M H, van Tits L J, Bergh K, Buurman W A, Waage A. TNF receptors in murine Candida albicans infection: evidence for an important role of TNF receptor p55 in antifungal defense. J Immunol. 1996;157:2155–2159. [PubMed] [Google Scholar]
- 77.Stenderup A, Pederson G T. Yeasts of human origin. Acta Pathol Microbiol Scand. 1962;54:462–472. doi: 10.1111/j.1699-0463.1962.tb05088.x. [DOI] [PubMed] [Google Scholar]
- 78.Tavares D, Ferreira P, Arala-Chaves M. Increased resistance to systemic candidiasis in athymic or interleukin-10-depleted mice. J Infect Dis. 2000;182:266–273. doi: 10.1086/315674. [DOI] [PubMed] [Google Scholar]
- 79.Vanden-Bossche H, Marichal P, Odds F C, LeJeune L, Coene M C. Characterization of an azole-resistant Candida glabrata isolate. Antimicrob Agents Chemother. 1992;36:2602–2610. doi: 10.1128/aac.36.12.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vazquez-Torres A, Jones-Carson J, Wagner R D, Warner T, Balish E. Early resistance of interleukin 10 knockout mice to acute systemic candidiasis. Nature. 1999;67:670–674. doi: 10.1128/iai.67.2.670-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vazquez-Torres A, Balish E. Macrophages and resistance to candidiasis. Microbiol Mol Biol Rev. 1997;61:170–192. doi: 10.1128/mmbr.61.2.170-192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Willocks L, Leen C L, Brettle R P, Urquhart D, Russell T B, Milne L J. Fluconazole resistance in AIDS patients. J Antimicrob Chemother. 1991;28:937–939. doi: 10.1093/jac/28.6.937. [DOI] [PubMed] [Google Scholar]
- 83.Wingard J R, Merz W G, Rinaldi M G, Miller C B, Karp J E, Saral R. Association of Torulopsis glabrata infections with fluconazole prophylaxis in neutropenic bone marrow transplant patients. Antimicrob Agents Chemother. 1993;37:1847–1849. doi: 10.1128/aac.37.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wingard J R. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin Infect Dis. 1995;20:115–125. doi: 10.1093/clinids/20.1.115. [DOI] [PubMed] [Google Scholar]
- 85.Xiong J, Kang K, Liu L, Yoshida Y, Cooper K D, Ghannoum M A. Candida albicans and Candida krusei differentially induce human blood mononuclear cell interleukin-12 and gamma interferon production. Infect Immun. 2000;68:2464–2469. doi: 10.1128/iai.68.5.2464-2469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ziege S U, Geerdes-Fenge H F, Rau M, Buchwald U, Lode H. In vitro effects of interleukin 10, prednisolone, and GM-CSF on the non-specific immune function of human polymorphonuclear leukocytes and monocytes. Eur J Med Res. 2000;5:369–374. [PubMed] [Google Scholar]