Abstract
Vaccine‐induced immune thrombotic thrombocytopenia (VITT) is a rare clinical condition that has emerged during the mass immunization against SARS‐CoV‐2. Reports indicate that VITT may also be induced by other vaccines, such as the human papillomavirus vaccine, or occur independently of vaccination. Its recognition requires a high index of suspicion, especially in patients presenting with thrombocytopenia and thrombosis several days after vaccination with an adenoviral vector‐based vaccine against SARS‐CoV‐2. Bleeding manifestations do not exclude VITT, as initially assumed. It is of great importance to perform the appropriate diagnostic tests early in the course of the disease, as false‐negative results may occur and many aspects of VITT are not fully understood. These two cases from Germany demonstrate unusual presentations of VITT.
Keywords: COVID‐19, SARS‐CoV‐2 vaccination, vaccine‐induced immune thrombocytopenia and thrombosis
Vaccine‐induced immune thrombotic thrombocytopenia (VITT) is a rare clinical condition that has emerged during mass vaccination against SARS‐CoV‐2. The diagnosis of VITT requires a high index of suspicion because many aspects of the syndrome are not yet fully understood, and unusual presentations may occur.

1. INTRODUCTION
Vaccine‐induced immune thrombotic thrombocytopenia or vaccine‐induced thrombocytopenia and thrombosis (VITT) has been described as a rare adverse event mainly following immunization with adenoviral vector‐based vaccines against SARS‐CoV‐2. After understanding the pathophysiological mechanisms connected to the development of VITT and with the availability of diagnostic assays, patients with VITT manifestations have been increasingly recognized. 1 Recently, VITT has been observed after human papillomavirus vaccination 2 and evidence indicates that VITT‐like anti‐platelet antibodies may also appear independently of vaccination. For example, recurrent thrombosis was described in a patient with monoclonal gammopathy, as a paraprotein bound to the same epitope as platelet factor 4 (PF4) and caused intravascular platelet activation. 3 In addition, symptoms in patients with autoimmune heparin‐induced thrombocytopenia (aHIT) or spontaneous HIT‐like syndrome could resemble VITT‐like autoantibodies and technically simple functional testing is necessary to enable rapid and accurate differential diagnosis. 4
The incidence of VITT is low with 15.5 per million after first or unknown dose and 2.0 per million after second dose with an overall case fatality of 22%. 1 , 5 VITT is caused by an anti‐PF4 antibody‐mediated platelet activation leading to thrombotic complications and thrombocytopenia. Hallmarks of VITT are thrombosis at unusual sites such as cerebral venous sinus (CVST) or splanchnic vasculature, but deep vein thrombosis and/or pulmonary embolism may also occur. 6 , 7 , 8 Leading symptoms are persistent and severe headache, focal neurological symptoms, seizures, blurred vision, shortness of breath, chest or abdominal pain, swelling and redness in a limb, or pallor and coldness in a limb. 9 According to Pavord S et al. VITT should be considered as a definite diagnosis when all five criteria are fulfilled: onset of symptoms 5–30 days after vaccination against SARS‐CoV‐2 (or ≤42 days in patients with isolated deep vein thrombosis or pulmonary embolism), presence of thrombosis, thrombocytopenia (platelet count < 150,000 per cubic millimeter), D‐dimer level > 4000 FEU, and positive anti‐PF4 antibodies on ELISA. 1
Bleeding symptoms, other than intracerebral bleeding secondary to CVST, were previously considered to argue against a diagnosis of VITT. The suspected diagnosis is serologically confirmed by a positive anti‐PF4/heparin‐IgG enzyme‐linked immunosorbent assay (ELISA) and if positive a functional test (HIPA, heparin‐induced platelet activation assay or SRA, serotonin‐release assay). 9 Appropriate imaging should be obtained to support diagnosis and guide treatment.
We report two cases of confirmed VITT were clinical features and laboratory results are unusual, indicating that some aspects of VITT are not still fully understood .
2. CASE PRESENTATION
2.1. Case 1: cerebral venous sinus thrombosis (CVST)
A 43‐year‐old male patient with uneventful medical history was admitted 23 days after receiving a first dose of ChAdOx1 nCoV‐19 vaccine. Symptoms of headache, falls, and unsteadiness began 7 days after vaccination. At admission, he presented with reduced vigilance, multiple blue, or purple bruises in all extremities and left‐sided hemiparesis. The bruises had the typical clinical characteristics of hematomas after subcutaneous bleeding and were painful, reflecting their extent, location, and size. Unfortunately, no lesion biopsy was performed, in order to exclude dermal vessel thrombosis, vasculitis, or necrotic changes.
Platelet count was 108 × 109/L (norm: 150–400 × 109/L), slightly increased fibrinogen levels (441 mg/dl, norm: 180–350) and massively increased D‐dimers of 20.7 mg/L (norm: <0.55) were present. The platelet count nadir was 97 × 109/L. A computed tomography (CT) angiography revealed atypical intracranial bleeding in the presence of massive pansinus CVST and jugular venous thrombosis (Figures 1A–C). Initial treatment with heparin was changed to argatroban upon suspicion of VITT. 10 The patient received high‐dose intravenous immune globulin (IVIG; 1 g/kg body weight on two consecutive days) 11 which led to rapid normalization of platelet counts. The anti‐PF4/heparin IgG ELISA was highly positive (optical density [OD] > 3). The PF4‐dependent washed platelet activation assay, however, was negative. Together with the bleeding symptoms, especially the skin hematomas, this was considered evidence against the diagnosis of VITT.
FIGURE 1.
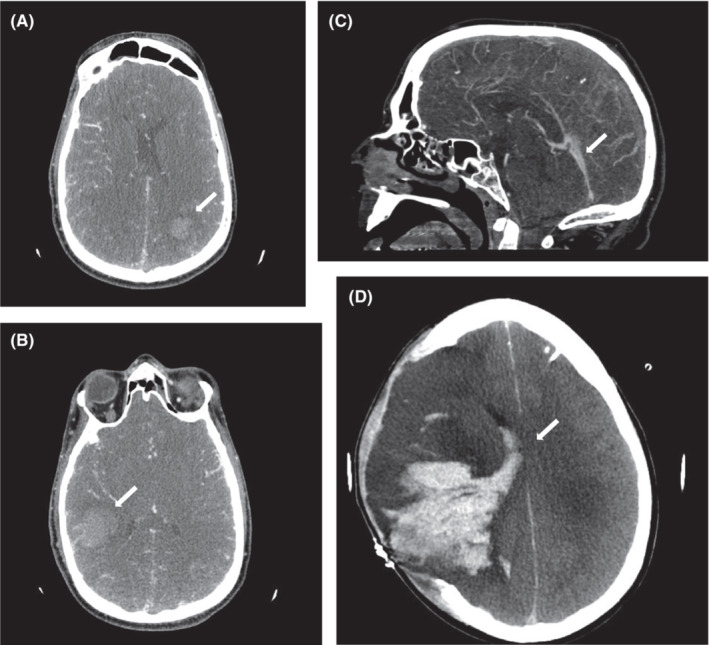
(A–C) Cranial CT on the day of admission to neurological ICU showing pansinus thrombosis with accompanying atypical intracranial bleeding (white arrows). (D) Cranial CT showing progression of intracranial bleeding and midline shift despite decompressive hemicraniectomy and external ventricular drainage 7 days after admission (white arrow).
Heparin was restarted and platelet counts remained stable. The patient's neurological status deteriorated due to intracranial hemorrhage (Figure 1D) with signs of elevated intracranial pressure (ICP). Despite all rescuing efforts, 31 days after vaccination, brain death was confirmed and organ donation followed. The autopsy revealed a widespread thrombosis of the superior sagittal sinus, transverse sinus, and confluence of sinuses with patchy hemorrhagic infarction and global cerebral edema, leading to tonsillar herniation. Additionally, disseminated thrombosis of small and medium‐sized vessels was identified at autopsy. Further genetic screening and coagulation diagnostics including vasculitis serology were all negative. The patient's original serum was retested using repository material and the washed platelet‐activation assay revealed platelet‐activating anti‐PF4 antibodies, confirming VITT. The assay at first time has been obviously false negative.
2.2. Case 2: pulmonary embolism, pulmonary hemorrhage, portal vein thrombosis, and deep vein thrombosis of the left leg
A 34‐year‐old male patient with uneventful medical history was admitted with a 1‐week history of shortness of breath, hemoptysis, chills, headache, and a low platelet count 12 days after the first dose of ChAdOx1 nCoV‐19 vaccine. The symptoms began 7 days after vaccination. His physical examination on admission showed no abnormalities except for a respiratory rate of 33 breaths per minute. The initial laboratory analysis notably showed an increase in D‐dimer (34.4 mg/L, norm: <0.55), a reduced platelet count (14 × 109/L, norm: 150–400), increased inflammatory biomarkers such as C‐reactive protein (68 mg/L, norm: <5), and lactate dehydrogenase (349 U/L, norm: 125–220) as well as elevated liver enzymes. The SARS‐CoV‐2 PCR test was negative as well as the urine dipstick and blood culture. The nadir of platelet count in this patient was 7 × 109/L (norm: 150–400) 2 days after admission.
A chest CT scan revealed pulmonary embolism and widespread ground‐glass opacities. Abdominal CT scan showed complete portal and mesenteric vein thrombosis (Figure 2A,B). Duplex Doppler sonography of the leg vessels indicated deep vein thrombosis (Figure 2C). Abdominal ultrasonography showed slight splenomegaly but no further pathology. Magnetic resonance imaging (MRI) angiography showed no pathology other than a small cavernoma (Figure 2D).
FIGURE 2.

(A, B) CT of the abdomen showing complete portal venous thrombosis and thrombosis of mesenteric veins (white arrows). (C) Doppler and duplex sonography showing a 3‐level deep vein thrombosis of the left leg (white arrow). (D) Cranial MRI showing a small cavernoma but no other relevant pathology (white arrow).
Vaccine‐induced immune thrombotic thrombocytopenia was suspected and confirmed by a strongly positive anti‐PF4/heparin IgG ELISA and a positive PF4‐dependent platelet activation assay. Anticoagulation with argatroban (2 μg/kg/h) was initiated, and high‐dose IVIG (1 g/kg on two consecutive days) were administered. Intravenous levofloxacin was started because of suspected community‐acquired pneumonia.
The patient's condition improved, and platelet counts rapidly rose from a minimum of 7 × 109–147 × 109/L within 5 days. At day 10 of admission the platelet count decreased again to 66 × 109/L, but responded well to a second course of 1 g/kg immunoglobulin therapy on two consecutive days. After clinical stabilization, anticoagulation was switched to apixaban. The patient was discharged 20 days after admission.
Upon discharge, the patient was monitored with weekly platelet counts by his general practitioner, which showed a persistent mild thrombocytopenia of 100 × 109 ± 20 × 109/L. The follow‐up of anti‐PF4‐antibodies is shown in Graph 1. The patient received a second and third vaccination with Comirnaty (BioNTech/Pfizer), which was uneventful. Platelet‐activating antibodies persisted until week 13, the optical density of the anti‐PF4/heparin ELISA remained stable over the time. Gastroscopy ruled out esophageal varices and other relevant signs of portal hypertension. Nine months after VITT the patient remains stable, but therapeutic anticoagulation has not been discontinued yet.
GRAPH 1.

Follow up of PAA status, platelet count and OD anti‐PF4/Heparin IgG ELISA after discharge.
3. DISCUSSION
The two presented cases (compared in Table 1) highlight the complexity and challenges in recognizing and treating suspected VITT, especially demonstrating that bleeding manifestations and false‐negative anti‐PF4‐antibodies do not exclude an underlying VITT. This knowledge is relevant for clinicians, as VITT‐like phenotype may occur independently of a SARS‐CoV‐2 vaccination.
TABLE 1.
Comparison of key demographics, clinical features, and laboratory data
| Case 1 | Case 2 | |
|---|---|---|
| Sex | Male | Male |
| Age (years) | 43 | 34 |
| Time between vaccination and onset of symptoms (days) | 7 | 7 |
| Time between vaccination and admission to hospital (days) | 23 | 12 |
| Site(s) of thrombosis | Cerebral venous sinuses | Portal vein, mesenteric vein, deep peripheral veins |
| Site(s) of bleeding | Subcutaneous hematomas, intracranial bleeding | Pulmonary bleeding |
| Nadir of platelet count (norm: 150–400 × 109/L) | 97 × 109/L | 7 × 109/L |
| Anti‐PF4/heparin IgG ELISA | Highly positive (optical density [OD] > 3) | Highly positive (optical density [OD] > 3) |
| PF4‐dependent washed platelet‐activation assay | Positive | Positive |
| VITT probability according to Pavord et al. 1 | Definite | Definite |
| Outcome | Fatal | Full recovery |
Abbreviations: Anti‐PF4, anti‐platelet factor 4; ELISA, enzyme‐linked immunosorbent assay; VITT, vaccine‐induced immune thrombotic thrombocytopenia.
Both patients presented with bleeding symptoms. Bleeding manifestations, other than secondary bleeding due to CVST or pulmonary embolism, are considered to be atypical in the setting of VITT. In the first case a massive subcutaneous bleeding occurred. This cannot be explained by the moderately decreased platelet count and may even indicate the presence of additional antibodies interfering with the platelet function. In the second case, the patient presented with pulmonary embolism and hemoptysis. It remains unclear if there was a primary pulmonary bleeding or secondary by underlying pulmonary embolism. The very low platelet count with rapid response to IVIG and steroids may indicate the co‐incidence of VITT and idiopathic thrombocytopenic purpura (ITP). Nevertheless, the monoclonal antibody‐specific immobilization of platelet antigens (MAIPA) was negative against glycoprotein‐specific antiplatelet antibodies to glycoproteins IIbIIIa, IbIX, V, or IaIIa, which are the main target glycoproteins in ITP.
We recently identified free anti‐platelet antibodies in about 25% of patients with VITT. 12 The sensitivity of the current anti‐platelet antibody tests for free antibodies is only 10%. It is, therefore, likely that the prevalence of these plated autoantibodies was much higher than 25%. Unfortunately, this information was not available when the patients were originally diagnosed and we did not perform anti‐platelet antibody tests using the patients' own platelets. We now have the rationale for assuming that plated autoantibodies might have been involved.
While our clinical observation cannot prove the contribution of several mechanisms to the clinical phenotype of the two patients reported, such a coincidence should be taken into account and bleeding manifestations should not rule out an underlying VITT. The diagnostic workup of our cases indicates that our current understanding of VITT is still incomplete and more studies are needed to elucidate possible concomitant effects of anti‐PF4 and other anti‐platelet antibodies.
A second important observation is the false‐negative functional test in the first patient on admission. This cannot be attributed to preceding treatment with IVIG, as the blood sample was obtained before the administration of IVIG. When we retested the patient's serum in the dilution of 1:4 it was consistently strongly positive. We now know that the stoichiometric ratio of PF4 and antibodies is highly important and in fact this case was one of the driving forces to further modify the PIPA test. 13 This led to restart heparin without consecutive decrease in platelet count, which may provide some evidence that heparin can be safely used as anticoagulant in VITT cases. 14
4. CONCLUSION
Vaccine‐induced immune thrombotic thrombocytopenia is now recognized as a rare adverse effect of vaccination against SARS‐CoV‐2 with adenoviral vector DNA‐based vaccines. Patients who develop an identical syndrome independent of a SARS‐CoV‐2 vaccination have been increasingly recognized. Presentation and diagnosis of VITT might be delayed due to unspecific signs and symptoms. The presented cases indicate that bleeding symptoms do not exclude the diagnosis of an underlying VITT.
AUTHOR CONTRIBUTIONS
F. Schuppert and S. Habben conceived and presented the idea and began collating data. R. Edmonds collated the data, conducted a literature review, and wrote the manuscript in consultation with F. Schuppert. A. Greinacher contributed to the interpretation of the results and highlighted relevant supporting research. L. Schönborn contributed to the interpretation of the results and created Graph 1. A. Greinacher and L. Schönborn carried out further testing on the patient's material. M. Paparoupa revised the manuscript and provided consultation regarding intellectual interpretation. All authors provided critical feedback and helped to shape the manuscript.
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
CONSENT
Written informed consent was obtained from the patients and/or their legal guardian to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
We thank Dr N. Bradtke (St.‐Marien‐Hospital, Marsberg, Germany) for referring a patient to our hospital (case 1) for further evaluation, and Dr J. Bösel, chief of the Department of Neurology at the Klinikum Kassel for granting us access the clinical data of this patient after being treated in the Department of Neurology. We gratefully acknowledge the evaluation of the CT and MRI scans by Dr R. Loeschhorn‐Becker from the Department of Radiology.
Edmonds R, Schönborn L, Habben S, Paparoupa M, Greinacher A, Schuppert F. Vaccine‐induced immune thrombotic thrombocytopenia (VITT) after SARS‐CoV‐2 vaccination: Two cases from Germany with unusual presentation. Clin Case Rep. 2023;00:e6883. doi: 10.1002/ccr3.6883
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine‐induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680‐1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johansen S, Laegreid IJ, Ernstsen SL, et al. Thrombosis and thrombocytopenia after HPV vaccination. J Thromb Haemost. 2022;20(3):700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greinacher A, Langer F, Schonborn L, et al. Platelet‐activating anti‐PF4 antibodies mimic VITT antibodies in an unvaccinated patient with monoclonal gammopathy. Haematologica. 2022;107(5):1219‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanack AJ, Jones C, Singh B, et al. Off‐the‐shelf cryopreserved platelets for the detection of HIT and VITT antibodies. Blood. 2022;140:2722‐2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medicines and Healthcare products Regulatory Agency (MHRA) . Coronavirus vaccine. Summary of the yellow card reporting; 2021.
- 6. Furie KL, Cushman M, Elkind MSV, Lyden PD, Saposnik G. Diagnosis and management of cerebral venous sinus thrombosis with vaccine‐induced immune thrombotic thrombocytopenia. Stroke. 2021;52(7):2478‐2482. [DOI] [PubMed] [Google Scholar]
- 7. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384(22):2124‐2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384(23):2202‐2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franchini M, Liumbruno GM, Pezzo M. COVID‐19 vaccine‐associated immune thrombosis and thrombocytopenia (VITT): diagnostic and therapeutic recommendations for a new syndrome. Eur J Haematol. 2021;107(2):173‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greinacher A, Langer F, Makris M, et al. Vaccine‐induced immune thrombotic thrombocytopenia (VITT)‐update on diagnosis and management considering different resources: response to comment from Yamada et al. J Thromb Haemost. 2022;20(2):542‐543. [DOI] [PubMed] [Google Scholar]
- 11. Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and Management of Vaccine‐Related Thrombosis following AstraZeneca COVID‐19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021;41(3):184‐189. [DOI] [PubMed] [Google Scholar]
- 12. Nicolai L, Leunig A, Pekayvaz K, et al. Thrombocytopenia and splenic platelet‐directed immune responses after IV ChAdOx1 nCov‐19 administration. Blood. 2022;140(5):478‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schönborn L, Thiele T, Esefeld M, et al. Quantitative interpretation of PF4/heparin‐EIA optical densities in predicting platelet‐activating VITT antibodies. J Thromb Haemost. 2022;20:2579‐2586. doi: 10.1111/jth.15862 [DOI] [PubMed] [Google Scholar]
- 14. Gabarin N, Arnold DM, Nazy I, Warkentin TE. Treatment of vaccine‐induced immune thrombotic thrombocytopenia (VITT). Semin Hematol. 2022;59(2):89‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


