Abstract
Dimethyl nitrosamine (DMN) is a known hepatotoxin, carcinogen, and mutagen. This study is therefore carried out to investigate the therapeutic effects of syringic acid (SYRA) and ascorbic acid (ASCA) in DMN-induced hepatic injury in rats. Following DMN administrations, malondialdehyde (MDA), nitric oxide (NO) and reduced glutathione (GSH) as well as activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD) were significantly increased. Also significantly increased were levels of tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Following treatment with SYRA and ASCA, the activities of ALT, AST, GPx, CAT and SOD, as well as MDA, GSH, TNF-α, IL-1β, and NFkB levels were significantly reduced. Overall, both treatments were effective, but SYRA had a better therapeutic effect than ASCA. Therefore, this promising potential of SYRA can be taken advantage of in the treatment of DMN-induced hepatic injury.
Keywords: Liver injury, Inflammation, Oxidative stress, Dimethyl nitrosamine, Syringic acid
Highlights
-
•
DMN significantly increased MDA, GSH levels and activities of GPx, CAT and SOD.
-
•
DMN significantly increased Bax, caspase-3, NF-κB, TNF-α, and IL-1β levels.
-
•
SYR & ASC significantly reduced NF-κB, caspase-3, TNF-α, IL-1β, GSH, and MDA.
-
•
SYR & ASC significantly reduced GPx, CAT and SOD activities.
1. Introduction
DMN is in a class of the N-nitroso compounds seen in some industrial products as well as processed meats. It is an extremely toxic compound, capable of causing hepatic fibrosis and cancer [1]. Nitrosamines are carcinogens that are formed and found in human environment and are known to promote the formation of reactive species (free radicals) that can initiate oxidative stress, undermine the protection of cells by antioxidants, and causing cellular injuries. All these are among the factors that can lead to cancer initiation [2,3]. Chemical-induced free radical generation leads to oxidative stress in biological systems, causing DNA damage. Free radicals oxidize cellular thiols and compete with lipid components (unsaturated fatty acids) of biological membrane for electrons, a process that undermine the membrane integrity [4]. In a normal cellular state, free radical formation is overwhelmed by the action of antioxidant proteins, vitamins, and enzymes. Overproduction of free radicals must be promptly mopped by antioxidants (such as glutathione reductase, SOD, GPx, CAT, GST, GSH, α-tocopherol, ascorbic acid, etc.), thereby shielding the cells from their deleterious effects [5].
Phenolic compounds are secondary metabolites, widely found in most plant fruits and vegetables. They are biosynthesized via the phenylpropanoid and shikimic acid pathways. These natural antioxidants reduce oxidative stress by mopping cellular reactive oxygen species (ROS) [6]. Natural compounds having antioxidant properties have been utilized to alleviate different kinds of disorders like cardiovascular diseases, liver damage, cancer, neurodegenerative diseases, and diabetes [[7], [8], [9]]. One of such natural compounds is syringic acid (SYRA). It is a phenolic compound abundantly present in pumpkin, grapes, olives, spices [10], red wine, honey, acai palm [11], and other plants. SYRA possesses useful properties capable of treating cancer, diabetes, inflammation, microbial infection, and possesses anti-oxidative, cardioprotective, hepatoprotective, and neuroprotective properties [12]. SYRA is better than p-hydroxybenzoic acid in scavenging ROS due to the presence of two methoxy groups at positions 3 and 5 of the aromatic ring [13]. SYRA has been reported to protect the liver against CCl4 and concanavalin-A-induced hepatic damage in mice. SYRA was also reported to significantly decrease the liver function transaminases activities and cytokine levels. SYRA also decreased high blood pressure and blocks hypertension-induced organ damage in rats [14]. Non-enzymatic antioxidants vitamins including ascorbic acid and alpha-tocopherol levels were significantly elevated by SYRA and activities of liver function markers such as AST and ALT are also reduced by SYRA in N-nitro-l-arginine methyl ester (l-NAME)-treated rats. Also, SYRA was reported to protect neuronal cells against cerebral ischemic damage [15].
In the light of the above, this study investigated the antioxidative, anti-inflammatory, and anti-apoptotic effects of SYRA and ASCA treatments in DMN-induced hepatic injury in rats.
2. Materials and methods
2.1. Chemicals and enzyme linked immunosorbent assay (ELISA) kits
The dimethyl nitrosamine (DMN; C2H6N2O; 98% purity) used in this study was produced by Sigma Chemical Co., Saint Louis, MO, USA. Syringic acid (SYRA; 98% purity; C9H10O5) is produced by AK Scientific, USA. Tablets of ascorbic acid are produced by Kunimed Pharmachem Ltd, Ikeja, Lagos, Nigeria. Enzyme linked immunosorbent assay (ELISA) kits for the quantification of hepatic levels of TNF-α, Bax, IL-1β, Bcl-2, and caspase-3 were produced by Cusabio Technology Llc, Houston, TX, USA. Primers (Oligonucleotide sequence) for NF-κB and β-actin for the reverse transcriptase-polymerase chain reaction (RT-PCR) analyses were designed by Shanghai ShineGene Molecular Bio-Technologies, Inc., China.
2.2. Experimental rats
Thirty male Wistar rats that weighed 200 g were sourced from a local commercial farmer. These rats were kept in cages where water and food were supplied unrestricted.
2.3. Experimental design
Before the study commenced, approval to conduct this research was granted by the Research Ethics Committee of the department, which oversees the handling and use of experimental animals. Acclimatization (for 2 weeks) of animals was ensured, after which they were grouped into 6 that comprised 5 animals each. Rats in group I were the control and were intraperitoneally administered 1 ml/kg of 0.9% sodium chloride (normal saline). Hepatotoxicity was induced in group II rats following intraperitoneally injection of 10 mg/kg of 1% w/v of DMN dissolved in normal saline, three (3) consecutive days only per week for four (4) weeks [16,17]. Rats in groups III and IV were treated the same as rats in group II but were immediately treated orally with 50 mg/kg and 100 mg/kg of SYRA and ascorbic acid (ASCA) respectively [18,19], every day for 4 weeks, while rats in groups V and VI were orally administered SYRA and ASCA only respectively, every day for 4 weeks. This experimental design is summarized in the table below. Table 1
Table 1.
Experimental design/setup.
| Groups | Treatment |
|---|---|
| Group 1 | Control (1 ml/kg of 0.9% sodium chloride) for 4 weeks |
| Group 2 | DMN only (10 mg/kg of 1% w/v of DMN) for 3 consecutive days only per week for four (4) weeks |
| Group 3 | DMN only (10 mg/kg of 1% w/v of DMN) as in group 2 + 50 mg/kg SYRA everyday for 4 weeks |
| Group 4 | DMN only (10 mg/kg of 1% w/v of DMN) as in group 2 + 100 mg/kg ASCA everyday for 4 weeks |
| Group 5 | 50 mg/kg SYRA everyday for 4 weeks |
| Group 6 | 100 mg/kg ASCA everyday for 4 weeks |
2.4. Sacrifice of animals and collection of samples
After the final administration of test substances, rats were left for 24 h before they were sacrificed. Sacrifice was carried out humanely by adhering strictly to the documented guidelines provided for the handling and utilization of animals [20]. Blood samples were obtained into clean plain tubes, from which serum was taken after centrifugation for 10 min at 3000 rpm, for the estimation of liver function markers. Harvested liver from each rat was washed in normal saline, dried, and each of their weight was noted. Part of the collected liver sample was homogenized in buffer solution (0.1 M phosphate buffer of pH 7.4). The homogenized samples were centrifuged at 5000 rpm for 10 min, and the supernatant of each sample was collected for the quantification of apoptotic, pro-inflammatory, and oxidative stress markers. A portion of each liver was also collected in trizol reagent for the preservation and extraction of liver mRNA needed for the RT-PCR analysis.
2.5. Estimation of serum ALT and AST activities
Activities of serum transaminases (ALT and AST) were estimated using the methods described in commercial assay kits manufactured by Randox Laboratories Ltd, United Kingdom.
2.6. Determination of liver MDA, NO and GSH levels
Assay to quantify the liver level of MDA was done using the method described by Buege and Aust [21], liver level of NO (detection of nitrite ion) was quantified using the Griess reagent, as established by Green et al. [22], while liver level of GSH was quantified using the protocol of Moron et al. [23].
2.7. Quantification of liver activities of SOD, GPx, CAT, and GST
SOD activity in the liver was quantified as described by Misra and Fridovich [24], GPx activity in the liver was estimated using Rotruck et al. [25] method, quantification of liver CAT activity was done according to Sinha [26] method, while the activity of liver GST was determined using the Habig et al. [27] method.
2.8. ELISA quantification of liver IL-1β, TNF-α, Bcl-2, caspase-3, and bax
ELISA method as highlighted in the Cusabio ELISA kits for IL-1β, TNF-α, Bcl-2, caspase-3, and Bax was followed and used [28].
2.9. Reverse transcriptase (RT) gene expressions of liver NF-κB
From the liver samples collected in trizol reagent, mRNA was extracted. The extracted mRNA was used for the synthesis of complementary DNA (cDNA). Polymerase chain reaction technique was used to amplify the synthesized cDNA in a PCR thermocycler where 30 amplification cycles was done, which also requires the availability of DNA templates and the backward and forward primers of interested genes as depicted in Table 2.
Table 2.
Reverse (R) and forward (F) primer sequences of the genes of interest.
| Gene | Sequences | |
|---|---|---|
| NFkB | F | 5′-TCCCACAAGGGGACATTAAGC-3′ |
| R | 5′-CAATAGGCCTCTAGTAGTAGCCC-3′ | |
| β-Actin | F | 5′-CCCGCGAGTACAACCTTCTT-3′ |
| R | 5′-CATCGGTAGGTCCGACACAA-3′ |
Amplified genes were run on gel (gel electrophoresis). Bands of cDNA were captured, and their degree of intensity were measured quantitatively compared to that of β-actin, which served as the gate-keeper gene, using a software for image analysis [29].
2.10. Total protein estimation
Liver concentration of total protein was determined using the Gornall et al. [30] method. Liver total protein concentration of each animal was used to calculate the antioxidant enzyme activities.
2.11. Liver histopathology
Liver sections fixed in 10% p-formaldehyde were processed on glass slides, dehydrated using decreasing concentrations of ethanol, stained with eosin-hematoxylin dye, and observed at x4 magnification under the microscope.
2.12. Statistical analyses
Generated data were all analyzed by one way analysis of variance, followed by Tukey's test that checked for the significance that existed among the six groups, using Graph Pad Prism v6.0. All results were written as mean ± standard error of mean, and P-values above 0.05 were accepted to be significant.
3. Results
3.1. Treatment effects of ASCA and SYRA on relative liver weight of DMN-administered rats
The results of body weight (Fig. 1A) and relative liver weight (Fig. 1B) of rats are shown in Fig. 1. Administrations of DMN and other treatments had no effect on the relative liver weight (Fig. 1B), except for rats administered SYRA only, which resulted in a significant (p < 0.05) decrease the relative liver weight by 17.79% compared with rats exposed to DMN only.
Fig. 1.
Treatment outcomes of SYRA and ASCA on body weights (Fig. 1A) and relative weights of liver (Fig. 1B). Bars depict mean ± SEM. Bars sharing similar labels are not significant statistically (p > 0.05). DMN = dimethyl nitrosamine; SYRA = syringic acid; ASCA = ascorbic acid.
3.2. Treatment effects of ASCA and SYRA on activities of serum ALT and AST
In Fig. 2, administration of DMN to rats significantly (p < 0.05) increased the activities of serum ALT (Fig. 2A) and AST (Fig. 2B) by 214.11% and 57.06% respectively compared with control. Treatment with SYRA significantly (p < 0.05) decreased the activities of the two enzymes by 37.16% (AST) and 68.81% (ALT), while treatment with ASCA significantly (p < 0.05) reduced the activities of the two enzymes by 36.18% (AST) and 67.60% (ALT) compared with DMN only.
Fig. 2.
Treatment effects of SYRA and ASCA on activities of serum ALT (Fig. 2A) and AST (Fig. 2B) in DMN-induced hepatic injury. Bars depict mean ± SEM. Bars sharing similar labels are not significant statistically (p > 0.05). DMN = dimethyl nitrosamine; SYRA = syringic acid; ASCA = ascorbic acid.
3.3. Treatment effects of ASCA and SYRA on liver concentrations of MDA, NO, and GSH
Concentration of hepatic MDA (Fig. 3A) was elevated significantly (p < 0.05) following the DMN only exposure (by 101.52%) compared with rats that served as control. The elevated liver MDA concentration was significantly lowered following treatments with SYRA (by 49.65%) and ASCA (by 49.44%) compared with DMN only administered rats. Liver NO (Fig. 3B) was significantly decreased by 24.10% following administration of DMN to rats compared with control. No significant difference in NO level was recorded after SYRA treatment but there was a significant decrease by 45.84% after ASCA administration, compared with DMN. For liver GSH (Fig. 3C), the increase in the level of the non-enzymatic antioxidant in DMN administered rats compared with control was significant by 65.32%. Both SYRA and ASCA significantly (p < 0.05) lowered the protein by 30.04% and 20.83% respectively, compared with DMN only.
Fig. 3.
Treatment effects of SYRA and ASCA on concentrations of MDA (Fig. 3A), NO (Fig. 3B), and GSH (Fig. 3C) in DMN-induced hepatic injury. Bars depict mean ± SEM. Bars sharing similar labels are not significant statistically (p > 0.05). DMN = dimethyl nitrosamine; SYRA = syringic acid; ASCA = ascorbic acid.
3.4. Treatment effects of ASCA and SYRA on hepatic activities of SOD, GPx, CAT, and GST
The intraperitoneal injection of DMN to rats caused a significant (p < 0.05) elevation in the liver activity of GPx (Fig. 4A) by 32.03% compared with control. ASCA intervention had no significant effect, but SYRA intervention yielded a significant reduction by 17.23% compared with rats administered DMN only. In Fig. 4B, liver GST activity was reduced by 24.14% due to DMN administrations, compared with the rats in control group. ASCA and SYRA treatments significantly raised in the hepatic activity of GST by 65.08% and 55.13% respectively compared with DMN only exposed rats. Hepatic CAT activity (Fig. 4C) was elevated (by 88.36%) significantly due to DMN administrations compared with rats in control group. Following SYRA treatments, a reduction in CAT activity by 28.94% compared with DMN only exposed rats was recorded. Similarly, liver SOD activity (Fig. 4D) was elevated (by 97.19%) significantly by DMN administrations compared with control rats. At the end of treatments, both SYRA and ASCA effectively decreased the liver SOD activity by 28.95% and 17.25% respectively, compared with DMN only exposed rats.
Fig. 4.
Treatment effects of SYRA and ASCA on activities of GPx (Fig. 4A), GST (Fig. 4B), CAT (Fig. 4C), and SOD (Fig. 4D) in DMN-induced hepatic injury. Bars depict mean ± SEM. Bars sharing similar labels are not significant statistically (p > 0.05). DMN = dimethyl nitrosamine; SYRA = syringic acid; ASCA = ascorbic acid.
3.5. Treatment effects of SYRA and ASCA on liver levels of pro-inflammatory and apoptotic parameters
Liver level of TNF-α was significantly increased in rats intraperitoneally injected with DMN (by 121.32%) compared with control rats (Fig. 5A). At the end of ASCA and SYRA treatments, a significant reduction by 23.88% and 36.84% respectively was seen compared with DMN only exposed rats. Like TNF-α, IL-1β level in the liver (Fig. 5B) was elevated significantly by 173.37% compared with rats in control group. ASCA and SYRA interventions significantly reduced liver IL-1β level by 26.30% and 35.97% respectively, compared with DMN only. For liver caspase-3 level (Fig. 5C), a significant increase by 168.07% was recorded compared with control. This significant elevation in caspase-3 was significantly brought down by SYRA (33.21%) only, compared with DMN only. Similar result was recorded for Bax (Fig. 5D), a significant increase by 99.23% was recorded compared with control. This significant elevation in hepatic Bax was significantly lowered by SYRA (by 36.17%) only, compared with DMN only. Contrarily, Bcl-2 level (Fig. 5E) was significantly decreased by DMN administrations (by 32.48%) compared with control. At the end of treatments with SYRA and ASCA, there was a significant increase in Bcl-2 level by 45.12% for ASCA only, compared with DMN only. For the Bax/Bcl-2 ratio (Fig. 5F), which serves as an apoptotic index, a significant rise in this ratio by 192.10% was seen in DMN administered rats compared with rats designated as control. Administrations of SYRA and ASCA significantly brought down the ratio by 48.88% and 33.06%, respectively compared with DMN only.
Fig. 5.
Treatment effects of SYRA and ASCA on levels of pro-inflammatory and apoptotic parameters in DMN-induced hepatic injury. Bars depict mean ± SEM. Bars sharing similar labels are not significant statistically (p > 0.05). DMN = dimethyl nitrosamine; SYRA = syringic acid; ASCA = ascorbic acid.
3.6. Treatment effects of SYRA and ASCA on liver NF-κB mRNA expressions
For liver NFkB, a significant increase by 48.42% was seen compared with control (Fig. 7E). Also, 19.15% and 40.43% significant reduction were seen after SYRA and ASCA treatments respectively, compared with DMN only (Fig. 6).
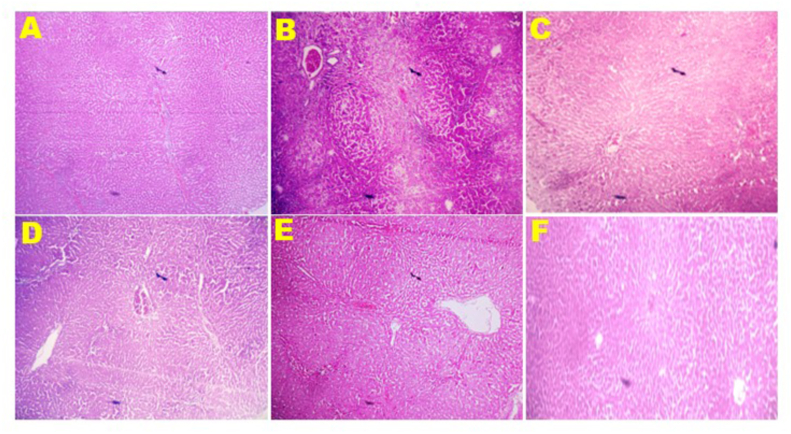
Fig. 7.
Treatment effects of SYRA and ASCA on liver architecture (magnification x4). Control (A) showing no visible lesion; DMN (B) showing absolute architecture disruption, presence of fibrotic bands around the nodules, presence of lymphocytes at the edge of the nodules and central vein. There is absolute disruption of hepatic parenchyma cells; DMN + SYRA (C) showing congestion, mild disseminated periportal infiltration by inflammatory cells, and the parenchyma cells are intact; DMN + ASCA (D) showing congestion, marked disseminated periportal infiltration by inflammatory cells, trabecular-like fibrous connective tissue with few parenchyma cells; SYRA (E) showing no visible lesion; ASCA (F) showing no visible lesion. DMN = dimethyl nitrosamine; SYRA = syringic acid; ASCA = ascorbic acid.
Fig. 6.
Treatment effects of SYRA and ASCA on relative mRNA expressions of NFkB in DMN-induced hepatic injury. Bars depict mean ± SEM. Bars sharing similar labels are not significant statistically (p > 0.05). DMN = dimethyl nitrosamine; SYRA = syringic acid; ASCA = ascorbic acid.
3.7. Effect of SYRA and ASCA treatments on hepatic architectures of DMN administered rats
As a result of DMN administration only, liver of rats showed absolute architecture disruption, presence of fibrotic bands around the nodules, presence of lymphocytes at the edge of the nodules and central vein, and absolute disruption of hepatic parenchyma cells (Fig. 7). After treatments of DMN-induced injury with SYRA, liver of rats showed congestion, mild disseminated periportal infiltration by inflammatory cells, and intact parenchyma cells (Fig. 7). Treatments of DMN-induced injury by ASCA also showed congestion, marked disseminated periportal infiltration by inflammatory cells, and trabecular-like fibrous connective tissue with few parenchyma cells (Fig. 7). Liver of rats administered only SYRA and ASCA separately showed no visible lesion (Fig. 7).
4. Discussion
This present study looked at the therapeutic effects of SYRA and ASCA on DMN-induced hepatic oxidative stress, inflammation, and apoptosis in rats.
Hepatocellular damage is marked by elevated activities of enzyme markers such as ALP, ALT and AST [31]. Following liver cell membrane destruction, these markers find their way into the blood circulation where they raised the serum levels [32,33]. Administrations of DMN to rats significantly increased the serum levels of the ALT and AST compared with control. The elevated serum levels of both transaminases in this study after administration of DMN is an indication of hepatocellular damage, since these enzymes are localized within the tissue and can only find their way into the blood if the cellular membrane integrity is compromised, as previously reported by Fukawa et al. [34], Abdu and Al-Bogami [35] and Somade et al. [32]. The liver damaging effect of DMN was abrogated by treatments with SYRA and ASCA, which brought the levels of both enzymes back to normal (when compared with control), a revelation that the two compounds may possess antioxidant as well as cell membrane and integrity-protecting properties against DMN-induced hepatotoxicity [32].
Oxidative stress can result into the disorganization of redox signals, thereby leading to tissue damage. In oxidative stress, the endogenous antioxidant armory is overwhelmed, causing the excessive production of free radicals [36]. To prevent oxidative stress, there are standby defense and protective systems that can detoxify or render lethal chemicals unharmful via the inhibition of ROS production. In hepatocytes, the generated free radicals can lead to hepatic damage, providing a cellular signal to switch on Ito cells activation [37]. In this study, the elevated levels of MDA and GSH, as well as activities of GPx, SOD, and CAT in rats only challenged with DMN compared with control, is an indication of DMN-induced oxidative stress in the rats [32,38]. The elevated levels signify an adaptive mechanism to respond and tackle the cellular overproduction of ROS in the liver of DMN-administered rats. However, SYRA and ASCA were able to exert their antioxidant properties and protected the liver cells against ROS production that can elicit hepatic oxidative stress in the rats [32,38,39]. NO is generated by nitric oxide synthase from l-arginine. There are 3 types of nitric oxide synthase, but the NO formed by the catalysis of inducible nitric oxide synthase (iNOS) is known to be reactive nitrogen species. The generation of peroxynitrite from NO usually happens when NO has only attained toxic levels, a point where it begins to compete with SOD for superoxide radical scavenging, and this may be responsible for the reduced level of liver NO in the toxicant only group compared to the control group.
NF-κB is a nuclear transcriptional factor that is indirectly under the control of Akt. Indirectly because Akt does not directly phosphorylate NF-κB but IKKα, an enzyme that phosphorylate IκB, which is found sequestered to NF-κB in the cytosol. Production of free radicals or cytokines (IL-1β and TNF-α) stimulates IKKα to phosphorylate IκB, enabling the separated and activated NF-κB to translocate to the nucleus [40] and associate with other transcription factors to express large amounts of chemokines and pro-inflammatory cytokines like IL-6, IL-1β, and TNF-α [41], leading to an uncontrolled tissue inflammation. These pro-inflammatory cytokines are known to be players in the progression of chronic liver diseases that lead to liver disorders including fibrosis and cirrhosis [42,43]. In this study, DMN administrations significantly increased the levels of pro-inflammatory cytokines compared with control animals. This significant increase in the levels of TNF-α and IL-1β as well as mRNA expression of NF-κB recorded following DMN-induced hepatic injury is a clear indication of severe injury that may have led to hepatic inflammation in the animals. SYRA and ASCA demonstrated anti-inflammatory actions by blocking NF-κB pathway activation via the downregulation of pro-inflammatory cytokine productions, thereby restoring them (TNF-α) to levels that are comparable to the control. Thus, we propose that the anti-inflammatory mechanism of action of SYRA and ASCA may be through the inhibition of pro-inflammatory cytokines and NF-κB pathway.
Bcl-2 and Bax participate in apoptosis, as well as caspase-3, a downstream executor of apoptosis. While bax and caspase-3 are pro-apoptotic, bcl-2 blocks apoptosis (anti-apoptotic), and they all participate in the apoptotic process by controlling of mitochondrial function [44,45]. This they do by creating pores on mitochondrial membrane, causing leakage and release of cytochrome c into the cytosol [46,47]. Upregulated Bcl-2 level in the cell inhibits the formation of mitochondria pore and so, the inhibition of Bcl-2 potentiates cytochrome c release [44,48]. Administration of DMN only increased the hepatic Bax and caspase-3 levels, and decreased hepatic Bcl-2 level, when this group is compared with the control, suggesting DMN-induced hepatic damage, which may have occurred beyond repair and necessitated the downregulation of antiapoptotic Bcl2 protein and, activation of the Bax-caspase-3 apoptotic pathway in the liver of rats. The decreased levels of liver Bax and caspase-3, as well as elevated level of liver Bcl-2 following SYRA and ASCA interventions is an indication of their antiapoptotic potentials in liver of DMN-challenged rats.
Hepatotoxicity induced by DMN in this study was further corroborated by the results of liver histopathology that revealed absolute architecture disruption, presence of fibrotic bands around the nodules, presence of lymphocytes at the edge of the nodules and central vein, and absolute disruption of hepatic parenchyma cells. These severe pathological alterations and disorders were corrected by SYRA and ASCA treatments, as marked by intact parenchyma cells and mild disseminated periportal infiltration by inflammatory cells.
5. Conclusion
Findings from this study have revealed that SYRA demonstrated a better antioxidative, anti-inflammatory, and antiapoptotic effects than ASCA in the liver of DMN-administered rats. Thus, SYRA could be a promising candidate for the treatment of DMN-induced hepatic injury.
Funding
We received none.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.George J., Rao K.R., Stern R., Chandrakasan G. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156:129–138. doi: 10.1016/s0300-483x(00)00352-8. [DOI] [PubMed] [Google Scholar]
- 2.Bansal A.K., Bansal M., Soni G., Bhatnagar D. Protective role of Vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem. Biol. Interact. 2005;156(2–3):101–111. doi: 10.1016/j.cbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Mittal G., Brar A.P., Soni G. Impact of hypercholesterolemia on toxicity of N-nitrosodiethylamine: biochemical and histopathological effects. Pharmacol. Rep. 2006;58(3):413–419. [PubMed] [Google Scholar]
- 4.Halliwell B., Gutteridge J.M.C. 4nd edn. Oxford University Press; Oxford: 1998. Free Radicals in Biology and Medicine; p. 851. [Google Scholar]
- 5.Ozsoy N., Can A., Yanardag R., Akev N. Antioxidant activity of Smilax excelsa leaf extracts. Food Chem. 2008;110(3):571–583. [Google Scholar]
- 6.Prior R.L., Cao G., Martin A., Sofic E., McEwen J., O'Brien C., Lischner N., Ehlenfeldt M., Kalt W., Krewer G., Mainland C.M. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity nd variety of Vaccinium species. J. Agric. Food Chem. 1998;46:2686–2693. [Google Scholar]
- 7.Kayahara H., Miao Z., Fujiwara G. Synthesis and biological activities of ferulic acid derivatives. Anticancer Res. 1999;19:3763–3768. [PubMed] [Google Scholar]
- 8.Kim H.K., Jeong T.S., Lee M.K., Park Y.B., Choi M.S. Lipid lowering efficacy of hesperidin metabolites in high-cholesterol fed rats. Clin. Chim. Acta. 2003;327:129–137. doi: 10.1016/s0009-8981(02)00344-3. [DOI] [PubMed] [Google Scholar]
- 9.Soobrattee M.A., Neergheen V.S., Luximonramma A., Aruoma O.I., Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat. Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Pezzuto J.M. Grapes and human health: a perspective. J. Agric. Food Chem. 2008;56:6777–6784. doi: 10.1021/jf800898p. [DOI] [PubMed] [Google Scholar]
- 11.Pacheco-Palencia L.A., Mertens-Talcott S., Talcott S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.) J. Agric. Food Chem. 2008;56:4631–4636. doi: 10.1021/jf800161u. [DOI] [PubMed] [Google Scholar]
- 12.Kiran P., Denni M., Daniel M. Antidiabetic principles, phospholipids and fixed oil of kodo millet (Paspalum scrobiculatum Linn.) Indian J. Appl. Res. 2014;4:13–15. [Google Scholar]
- 13.Karamaæ M., Kosinska A., Pegg R.B. Comparison of radical–scavenging activities of selected phenolic acids. Pol. J. Food Nutr. Sci. 2005;14:165–170. [Google Scholar]
- 14.Jalili T., Carlstrom J., Kim S., Freeman D., Jin H., Wu T.C., Litwin S.E., David Symons J. Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. J. Cardiovasc. Pharmacol. 2006;47:531–541. doi: 10.1097/01.fjc.0000211746.78454.50. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasulu C., Ramgopal M., Ramanjaneyulu G., Anuradha C.M., Suresh K.C. Syringic acid (SA) – a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018;108:547–557. doi: 10.1016/j.biopha.2018.09.069. [DOI] [PubMed] [Google Scholar]
- 16.Lee E.S., Lee H.E., Shin J.Y., Yoon S., Moon J.O. The flavonoid quercetin inhibits dimethylnitrosamine-induced liver damage in rats. J. Pharmacol. Pharmacother. 2003;55:1169–1174. doi: 10.1211/0022357021396. [DOI] [PubMed] [Google Scholar]
- 17.Shin D.S., Kim K.W., Chung H.Y., Yoon S., Moon J.O. Effect of sinapic acid against dimethylnitrosamine-induced hepatic fibrosis in rats. Arch Pharm. Res. (Seoul) 2013;36:608–618. doi: 10.1007/s12272-013-0033-6. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S., Prahalathan P., Raja B. Syringic acid ameliorates (L)-NAME-induced hypertension by reducing oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012;385:1175–1184. doi: 10.1007/s00210-012-0802-7. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran V., Raja B. Protective effects of syringic acid against acetaminophen-induced hepatic damage in albino rats. J. Basic Clin. Physiol. Pharmacol. 2010;21:369–385. doi: 10.1515/jbcpp.2010.21.4.369. [DOI] [PubMed] [Google Scholar]
- 20.NRC . National Academy Press; Washington, DC: 1996. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 21.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 22.Green L.C., Wagner D.A., Glogowski J., Skiper P.L., Wishnock J.S., Tannenbaum S.R. Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 23.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 24.Misra H.P., Fridovich I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 25.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 26.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 27.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 28.Somade O.T., Ajayi B.O., Adeyi O.E., Aina B.O., David B.O., Sodiya I.D. Activation of NF-kB mediates up-regulation of cerebellar and hypothalamic pro-inflammatory chemokines (RANTES and MCP-1) and cytokines (TNF-α, IL-1β, IL-6) in acute edible camphor administration. Sci. Afri. 2019;5 doi: 10.1016/j.toxrep.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gornall A.G., Bardawill C.J., David M.M. Determination of serum protein by biuret method. J. Biol. Chem. 1949;117:751–766. [PubMed] [Google Scholar]
- 31.Wegwu M.O., Ayalogu E.O., Sule O.J. Anti-oxidant protective effects of Cassia alata in rats exposed to carbon tetrachloride. J. Appl. Sci. Environ. Manag. 2005;9:77–80. [Google Scholar]
- 32.Somade O.T., Akinloye O.A., Ugbaja R.N., Idowu M.A. Cnidoscolus aconitifolius leaf extract exhibits comparable ameliorative potentials with ascorbate in dimethylnitrosamine induced bone marrow clastogenicity and hepatotoxicity. Clin. Nutri. Exp. 2020;29:36–48. [Google Scholar]
- 33.Rajesh M.G., Latha M.S. Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J. Ethnopharmacol. 2004;91:99–104. doi: 10.1016/j.jep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Fukawa A., Kobayashi O., Yamaguchi M., Uchida M., Hosono A. Bovine milk-derived α-lactalbumin prevents hepatic fibrosis induced by dimethylnitrosamine via nitric oxide pathway in rats. Biosci. Biotechnol. Biochem. 2017;81(10):1941–1947. doi: 10.1080/09168451.2017.1356215. [DOI] [PubMed] [Google Scholar]
- 35.Abdu S.B., Al-Bogami F.M. Influence of resveratrol on liver fibrosis induced by dimethylnitrosamine in male rats. Saudi J. Biol. Sci. 2019;26:201–209. doi: 10.1016/j.sjbs.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones D.P. Redefining oxidative stress. Antioxidants Redox Signal. 2006;8(9–10):1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 37.Novo E., Busletta C., Bonzo L.V., Povero D., Paternostro C., Mareschi K., Ferrero I., David E., Bertolani C., Caligiuri A., Cannito S., Tamagno E., Compagnone A., Colombatto S., Marra F., Fagioli F., Pinzani M., Parola M. Intracellular reactive oxygen species are required for directional migration of resident and bone marrow-derived hepatic pro-fibrogenic cells. J. Hepatol. 2011;54:964–974. doi: 10.1016/j.jhep.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 38.Somade O.T., Ugbaja R.N., Idowu M.A., Akinloye O.A. Cnidoscolus aconitifolius leaf extract and ascorbate confer amelioration and protection against dimethylnitrosamine-induced renal toxicity and testicular abnormalities in rats. Toxicol Rep. 2021;8:1098–1108. doi: 10.1016/j.toxrep.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Somade O.T., Oyinloye B.E., Ajiboye B.O., Osukoya O.A., Adeyi O.E. Effect of syringic acid on steroid and gonadotropic hormones, hematological indices, sperm characteristics and morphologies, and markers of tissue damage in methyl cellosolve-administered rats. Biochem. Biophys. Rep. 2022;32 doi: 10.1016/j.bbrep.2022.101360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aghai Z.H., Kode A., Saslow J.G., Nakhla T., Farhath S., Stahl G.E., Eydelman R., Strande L., Leone P., Rahman I. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr. Res. 2007;62:483–488. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 41.Rius-Perez S., Perez S., Marti-Andres P., Monsalve M., Sastre J. Nuclear factor kappa B signaling complexes in acute inflammation. Antioxidants Redox Signal. 2020;33:145–165. doi: 10.1089/ars.2019.7975. [DOI] [PubMed] [Google Scholar]
- 42.Robinson M.W., Harmon C., O’farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016;13(3):267–276. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greuter T., Shah V.H. Hepatic sinusoids in liver injury, inflammation, and fibrosis: new pathophysiological insights. J. Gastroenterol. 2016;51(6):511–519. doi: 10.1007/s00535-016-1190-4. [DOI] [PubMed] [Google Scholar]
- 44.Hou Q., Cymbalyuk E., Hsu H.C., Xu M., Hsu Y.T. Apoptosis modulatory activities of transiently expressed Bcl-2: roles in cytochrome c release and Bax regulation. Apoptosis. 2003;8:617–629. doi: 10.1023/A:1026187526113. [DOI] [PubMed] [Google Scholar]
- 45.Degli E.M., Dive C. Mitochondrial membrane permeabilisation by Bax/Bak. Biochem. Biophys. Res. Commun. 2003;304:455–461. doi: 10.1016/s0006-291x(03)00617-x. [DOI] [PubMed] [Google Scholar]
- 46.Somade O.T., Ajayi B.O., Adeyi O.E., Adeshina A.A., Adekoya M.O., Abdulhameed R.O. Oxidative stress-mediated induction of pulmonary oncogenes, inflammatory, and apoptotic markers following time-course exposure to ethylene glycol monomethyl ether in rats. Metab. Open. 2021;9 doi: 10.1016/j.metop.2020.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somade O.T., Ajayi B.O., Olunaike O.E., Jimoh L.A. Hepatic oxidative stress, up-regulation of pro-inflammatory cytokines, apoptotic and oncogenic markers following 2-methoxyethanol administrations in rats. Biochem. Biophy. Rep. 2020;24 doi: 10.1016/j.bbrep.2020.100806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kluck R.M., Bossy-Wetzel E., Green D.R., Newmeyer D.D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]