Biotechnological exploitation of the isoprenoidal iridoid biosynthetic pathway is crucial for cheap access to many drugs and food production, specifically in the provision of loganin, the substrate for secologaninin synthase (1) in the route to the anticancer drug vinblastine (2), and for sustainable management of the aphid vectors of food plant viral pathogens, as specific isomers of nepetalactone and nepetalactol comprising the aphid sex pheromones (3, 4). The newly reported elucidation of the iridoid pathway in aphids (5) (Fig. 1) plus the earlier elucidation in plants in work also led by Sarah O’Connor (see references in ref. 5) (Fig. 1) conclusively demonstrate the independent evolution of this pathway in plants and insects. Thus, as representing separate biosynthetic pathways to the same compounds from the respective kingdoms of plants and animals, this discovery is a unique evolutionary example for further study and also of value in biotechnological exploitation by offering alternative enzymology for constructing production systems including by synthetic biology.
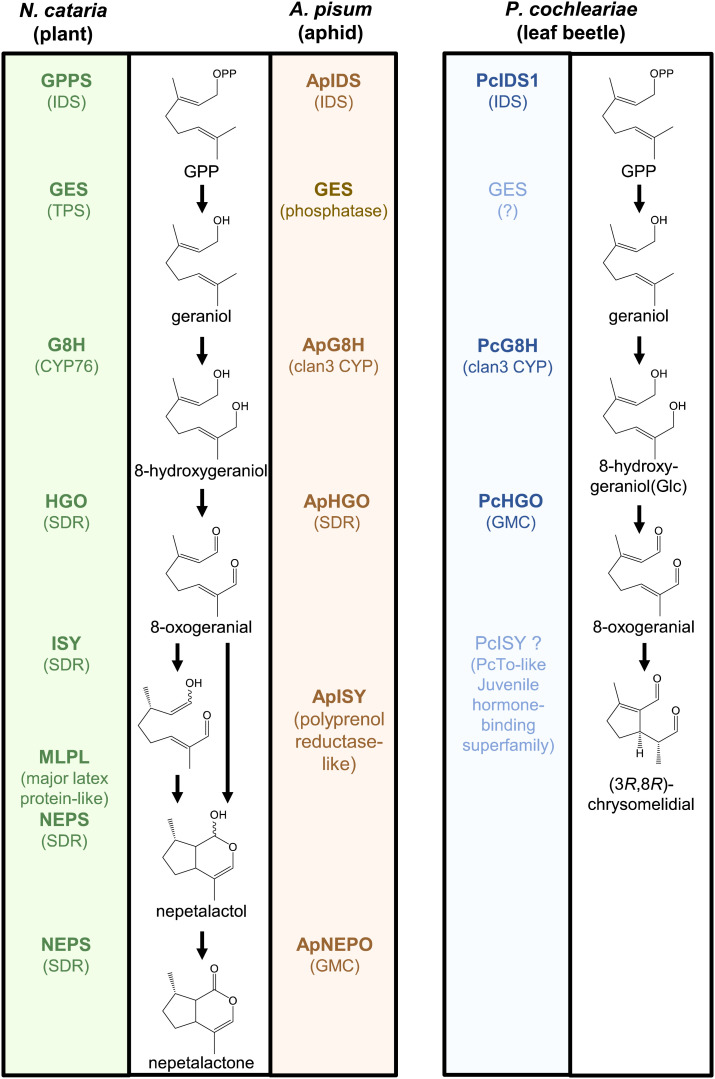
Fig. 1.
The iridoid pathways in plants, aphids, and beetles evolved independently from each other. While the plant and aphid pathways both lead to nepetalactol, the beetle Phaedon cochleariae produces a chrysomelidial. Enzymes, identified and characterized (5), are shown in bold. IDS, isoprenyl diphosphate synthase; TPS, terpene synthase; CYP, cytochrome P450 monooxygenase; SDR, short-chain dehydrogenase/reductase; and GMC, glucose–methanol–choline oxidoreductase. From ref. 5 and SI Appendix, Fig. S15.
The aphid sex pheromones were chemically characterized in 1987 and the report in Nature (3), published on St Valentine’s Day, stimulated a great deal of public media interest. This is related also to the component, (4aS,7S,7aR)-nepetalactone, common to plants in the Nepeta genus (Lamiaceae) being previously known to confer excitatory behavior in cats and most other members of the Felidae. The absolute stereochemistry of the second pheromonal compound, (1R,4aS,7S,7aR)-nepetalactol, formally the reduced nepetalactone and essential for behavioral activity, was established via X-ray crystallography on the 3,5-dinitrobenzoate ester (4). Following the growing number of aphid sex pheromones identified as comprising this chemistry (6), the possibility was widely raised at scientific meetings that sequestration from plants was the origin of this chemistry in aphids. The refutation of such ideas has now culminated in the unequivocal establishment of the aphid biosynthetic pathway as exemplified by the pea aphid, Acyrthosiphon pisum (5). Thus, by a transcriptomics approach from expression in the female hindlegs, known as the site of pheromone production (3), and coupled phylogenetic analysis, candidate genes for the aphid iridoid pathway were tentatively identified. Functional characterization of these genes by heterologous expression and rigorous chemical analysis of the products then confirmed the biosynthetic pathway to the nepetalactone. Surprisingly the route involves the same intermediate compounds as in plants but with all of the six enzymes involved being different from those in the plant pathway (Fig. 1). The intermediate compounds include 8-oxogeranial, and then as part of the reductive cyclization reaction, the reduction to what is formally the enol tautomer of 8-oxocitronellal clearly set for the cyclization to the nepetalactol. This step involves concomitant establishment of the ubiquitous absolute stereochemistry at carbon 7 as 7S, prior to oxidation to the nepetalactone. When formally reported in the plant pathway, the reductive cyclization created quite a stir and yet is confirmed by the previous work of the O’Connor group (see references in ref. 5) and now appears analogously in the aphid pathway. However, the enzymology is radically different as summarized (Fig. 1) and involving the membrane-bound enzyme, ApISY. Indeed, the aphid GES is also membrane bound, whereas there are no such membrane-bound enzymes in the plant pathway (5).
There are a plethora of ways forward, and these have been discussed both in the direction of lipophilic metabolite production further in insects and also in higher animals. For further work on insects, and aphids in particular, the authors (5) suggest studies on the biosynthetic pathway for the damson-hop aphid Phorodon humili sex pheromone which comprises both (1S,4aR,7S,7aS)- and (1R,4aR,7S,7aS)-nepetalactols (7) and could thereby provide additional insight into the chemistry underlying the formation of the iridoid carbon skeleton in animals. Another iridoid, (1S,2R,3S)-dolichodial, not discussed in ref. 5, is found also in association with aphid sex pheromones, e.g., from the rosy apple aphid, Dysaphis plantaginea (8). However, the biosynthesis of dolichodial is difficult to rationalize from the iridoid pathway now reported (5) and invites investigation. Wider entomological investigations are also suggested particularly arising from studies on production of (3R,8R)-chrysomelidial by the leaf beetle, Phaedon cochleariae (9), which is shown in a number of ways to diverge from the aphid iridoid pathway in its biosynthesis (Fig. 1). Thus, there is expected to be independent evolution in the different animal order of the Coleoptera, comprising the beetles, as distinct from the aphid order of Hemiptera through convergent evolution. The detailed study of the enzymes involved in the iridoid pathway of aphids has led the authors to speculate (5) that these enzymes might be organized in a membrane-associated metabolon. Thus, while the aphid HGO (Fig. 1) is expected to be localized as a cytosolic protein and the aphid NEPO (Fig. 1) in the lumen of the endoplasmic reticulum, the ApGES and the ApISY, as already mentioned, are transmembrane proteins and could thereby form the speculated metabolon as they describe in their figure 6 (5). This metabolon model can now be investigated in a wider sense and extended to studies in higher animals for the production of related lipophilic biochemicals. However, if this approach were considered for human studies, it would be well also to apply the rigorous use of analytical chemistry exploited here and to view critically the provenance of many of the compounds often claimed to be components of the human metabolome that sadly include compounds most likely comprising contaminants from industrial, cosmetic, and hygiene product use.
The example of vinblastine production using biotechnological approaches involves the refactoring into a microbial cell factory, the very long biosynthetic pathway (2). A key step in this biotechnological approach enables the intermediary strictosidine to be produced by feeding into the pathway tryptamine and the iridoid secologanin. This step can now be facilitated by the work of ref. 5 giving options as to how to achieve secologanin production substantially more economically than in the current work (2). Thus, based on choices enabled by identifying diverse enzymes for similar processes from species diversity beyond the original provider organisms as offered here (5), it may be possible for the biotechnological production of drugs to make available more sophisticated chemistry than current extraction of living material or semisynthetic production with only some involvement of heterologous expression for the synthesis genes. For the management of pests, away from the use of broad-spectrum eradicant pesticides and their rapid development of resistance, pheromones are now being realized as more sustainable alternatives, e.g., the leading pesticide manufacturer FMC’s takeover for $200 million of BioPhero (10). FMC is thereby developing the production of nonisoprenoidal insect pheromones in commercial competition with conventional pesticides by expressing genes for insect sex pheromone biosynthesis in engineered yeasts for fermenter production (11). However, the next wider goal will be to do similarly for isoprenoidal pheromones and other signal chemicals (semiochemicals) which can again benefit from the work here (5) with the choice of iridoid synthesis genes across kingdoms and other taxonomic divisions (Fig. 1). Evidence is already available for the value of chemoenzymatic production of the early stages of isoprenoid production benefitting from promiscuous kinase utilization, from diverse organisms and primary metabolism, in producing the essential precursors, e.g., GPP (geranyl diphosphate) and higher homologs (12). However, to exploit similarly the example of the iridoid phromones, for the downstream biochemical elaboration, there can be a tactical choice from the enzyme genes and expression patterns now discovered (5) with an option for synthetic genes and nonnatural isoprenoid semiochemicals already, in principle, demonstrated for development by synthetic biology (13).
Based on choices enabled by identifying diverse enzymes for similar processes from species diversity beyond the original provider organisms as offered here, it may be possible for the biotechnological production of drugs to make available more sophisticated chemistry than current extraction of living material or semi-synthetic production with only some involvement of heterologous expression for the synthesis genes.
Acknowledgments
J.A.P. thanks the School of Chemistry, Cardiff University and acknowledges the funding by the BBSRC (BB/R019681/1 and BB/V003933/1).
Author contributions
J.A.P. wrote the paper.
Competing interests
The author declares no competing interest.
Footnotes
See companion article, “Biosynthesis of iridoid sex pheromones in aphids,” 10.1073/pnas.2211254119.
References
- 1.Li C., et al. , Single-cell multi-omics enabled discovery of alkaloid biosynthetic pathway genes in the medical plant. Catharanthus roseus, bioXriv [Preprint] (2022). 10.1101/2022.07.04.498697 (Accessed 9 October 2022). [DOI]
- 2.Zhang J., et al. , A microbial supply chain for production of the anti-cancer drug vinblastine. Nature 609, 341–347 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson G. W., et al. , Identification of an aphid sex pheromone. Nature 325, 614–616 (1987). [Google Scholar]
- 4.Dawson G. W., et al. , The aphid sex pheromone. Pure Appl. Chem. 61, 555–558 (1989). [Google Scholar]
- 5.Koellner T. G., et al. , Biosynthesis of iridoid sex pheromones in aphids. Proc. Natl. Acad. Sci. U.S.A. 119, e2211254119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickett J. A., Allemann R. K., Birkett M. A., The semiochemistry of aphids. Nat. Prod. Rep. 30, 1277–1283 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Campbell C. A. M., et al. , Sex attractant pheromone of damson-hop aphid Phorodon humuli (Homoptera, Aphididae). J. Chem. Ecol. 16, 3455–3465 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Dewhirst S. Y., et al. , Dolichodial: A new aphid sex pheromone component? J. Chem. Ecol. 34, 1575–1583 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Burse A., et al. , Always being well prepared for defense: The production of deterrents by 585 juvenile Chrysomelina beetles (Chrysomelidae). Phytochemistry 70, 1899–1909 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Ellis J., FMC buys BioPhero for $200m to boost biologicals portfolio, hints at further acquisitions. AgFunder Newsletters & Research, 30 June 2022.
- 11.Petkevicius K., Wenning L., Kildegaard K. R., Sinkwitz C., Smedegaard R., Biosynthesis of insect sex pheromone precursors via engineered β-oxidation in yeast. FEMS Yeast Res. 22, foac041 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson L. A., Dunbabin A., Benton J. C. R., Mart R. J., Allemann R. K., Modular chemoenzymatic synthesis of terpenes and their analogues. Angew. Chem. Int. Ed. Engl. 59, 8486–8490 (2020), ( 10.1002/anie.202001744). [DOI] [PubMed] [Google Scholar]
- 13.Touchet S., et al. , Novel olfactory ligands via terpene synthases. Chem. Commun. 51, 7550–7553 (2015). [DOI] [PubMed] [Google Scholar]