Introduction
Bullous pemphigoid (BP) is a chronic autoimmune blistering disorder defined by autoantibody formation to hemidesmosomal proteins in the basement membrane zone of the skin. This disease presents most commonly in elderly individuals as tense bullae on erythematous or normal skin, often affecting the trunk and extremities and accompanied by significant pruritus.1 Prompt therapeutic intervention allows for healing of eroded lesions and a reduction in morbidity/mortality (eg, secondary infections).1 Depending on the amount of cutaneous involvement, topical or systemic corticosteroids are traditionally used as the first-line treatment, and systemic immunosuppressive drugs, such as methotrexate or mycophenolate mofetil, are deployed as second-line agents in those with extensive or persistent disease.2 Janus kinase (JAK) inhibitors are currently used to treat conditions, such as rheumatoid arthritis and atopic dermatitis (AD), and have future potential in the management of other inflammatory skin conditions. However, the use of these medications in autoimmune bullous diseases has not been widely studied. We present a case of an 81-year-old woman with BP, not fully responsive to a course of prednisone, who achieved complete disease resolution with upadacitinib treatment.
Case report
An 81-year-old woman presented to our dermatology rapid access clinic with a 4-month history of an intensely pruritic rash. The rash initially started as urticarial plaques on the trunk and spread to involve the extensor surfaces of the arms and anterior aspects of the thighs. Tense bullae developed overlying the erythematous plaques as well as on normal adjacent skin. She endorsed the presence of painful oral lesions but no ocular or gastrointestinal symptoms. Her medical history was significant for hypertension, dyslipidemia, osteoarthritis, and endometriosis. She was taking aspirin, amlodipine, perindopril, and rosuvastatin at the time of rash development, and these were all longstanding drugs.
A punch biopsy of a bulla on the anterior aspect of the thigh that had been completed by the family physician before consultation revealed an inflammatory process showing subepidermal blister with many eosinophils, histologically compatible with BP. She was started on prednisone 50 mg daily by her family physician and had been on prednisone for 1 month at the time of presentation to the clinic. Prednisone resulted in some improvement in itch; however, she still had quite extensive cutaneous involvement (Fig 1). A perilesional biopsy taken for direct immunofluorescence demonstrated linear IgG and C3 at the dermoepidermal junction, confirming the diagnosis of BP. She had an elevated white count with neutrophilic predominance, but her complete blood cell count was otherwise normal. Serum Immunoglobulin E was 2887, and she had a normal C-reactive protein. Our institute does not offer BP180/BP230 enzyme-linked immunosorbent assay testing.
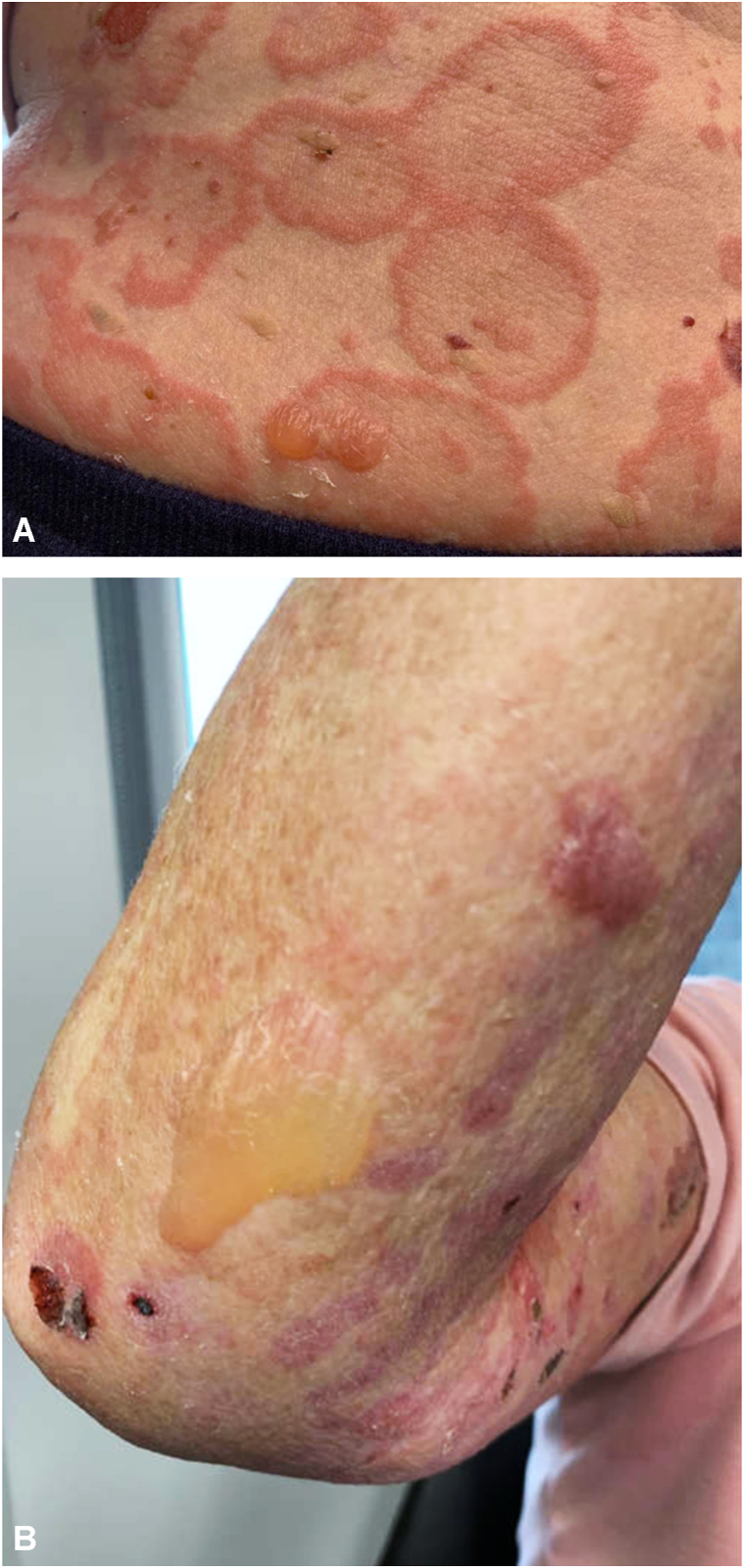
Fig 1.
Bullous pemphigoid lesions after 1 month of systemic corticosteroids. A, Numerous tense bullae and hemorrhagic erosions on a background of coalescing annular urticarial plaques on the right lateral flank and abdomen. B, Large intact bulla on right extensor forearm.
The patient indicated that the itch associated with the BP was having the biggest impact on her life. Her dermatologic history was further explored at this point, and she did endorse pruritic eczematous-like lesions before BP onset. Because of its rapid itch relief and clinical benefit in AD, a decision was ultimately made to start upadacitinib 15 mg by mouth daily, and she was tapered off her prednisone over 20 days.
At the 2-month follow-up visit, she had been off prednisone for over a month and was only taking upadacitinib. She experienced complete resolution of disease. She did not have any new blister formation since starting upadacitinib, and her itch had completely resolved (Fig 2). Further follow-up 3 months later (5 months of upadacitinib), showed continued efficacy of the treatment with further healing of the skin and complete resolution of the disease and no recurrence or flares of the disease. Thus far, the patient has tolerated the medication well with no noted adverse events.
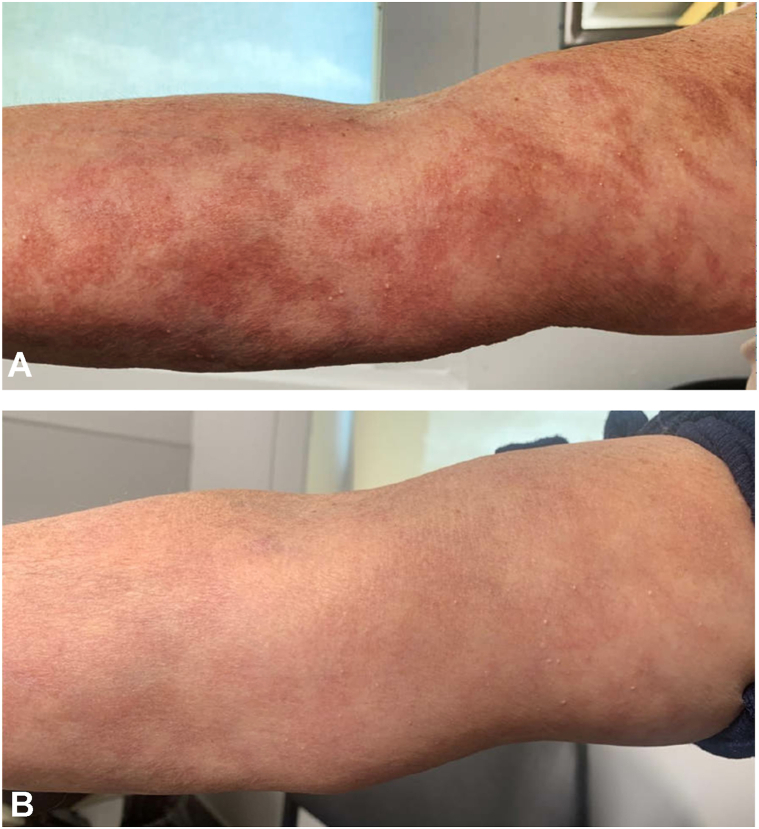
Fig 2.
Bullous pemphigoid response to upadacitinib. A, Right arm 2 months after initiation of upadacitinib showing mild erythema, no erosions, no bullae, and no urticarial lesions. B, Right arm 5 months after initiation of upadacitinib showing postinflammatory hyperpigmentation and no bullae.
Discussion
Upadacitinib is a second-generation JAK inhibitor, selective for the JAK1 enzyme. JAK enzymes phosphorylate signal transducers and activators of transcription (STAT) to regulate gene transcription in immune cells (ie, JAK/STAT pathway).3 The inhibition of these enzymes prevents inflammatory cytokines from utilizing the JAK/STAT pathway to augment immune cell function.3 Upadacitinib was initially approved for use in severe rheumatoid arthritis and is now being utilized in severe AD with more rapid and improved resolution of disease in comparison with dupilumab, an interleukin 4 (IL-4)/IL-13 inhibitor.4 A greater range of inflammatory markers that use JAK1 to signal (eg, IL-4, IL-13, IL-22, and IL-31) and are involved in AD pathogenesis are perturbed by JAK1 inhibition.4
Although the inflammatory signaling involved in BP pathogenesis is not fully known, T-helper type 2 cytokines, such as IL-4 and IL-13, do seem to play a role.5 An increased number of cells that produce these cytokines have been found in the bullae of patients with BP,5 and there is now an increasing amount of clinical data that support the use of dupilumab in BP.6 More widespread cytokine perturbation via JAK inhibition is beneficial when disease pathogenesis is complex and not well understood,7 and because our patient had a history of pruritic eczematous lesions before BP onset, this provided an opportunity to treat both conditions and assess the efficacy of upadacitinib for BP.
The use of JAK inhibition in BP has also been reported by Xiao et al,8 who successfully treated a patient with psoriasis and BP with the JAK1/JAK2 inhibitor, baricitinib. Elevated expressions of JAK/STAT proteins have been found in lesions of patients with BP and dermatitis herpetiformis,9 and Xiao et al8 postulate that the disruption of the JAK/STAT signaling pathway may dampen the autoimmune response in BP, with subsequent reduction in development of bullae. Baricitinib has also been documented to halt the progression of a related disorder, mucous membrane pemphigoid, in a patient who failed other systemic agents.10
As more is understood about the immunopathogenesis of BP, this provides more potential targets for treatment. There is a need for improved, safer medications for this condition because it so often affects elderly patients with multiple other comorbidities. Further study on the use of JAK inhibitors, such as upadacitinib, will be of value to potentially broaden the therapeutic options for this condition.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Miyamoto D., Santi C.G., Aoki V., Maruta C.W. Bullous pemphigoid. An Bras Dermatol. 2019;94(2):133–146. doi: 10.1590/abd1806-4841.20199007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalid S.N., Khan Z.A., Ali M.H., Almas T., Khedro T., Nagarajan V.R. A blistering new era for bullous pemphigoid: a scoping review of current therapies, ongoing clinical trials, and future directions. Ann Med Surg (Lond) 2021;70 doi: 10.1016/j.amsu.2021.102799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padda I.S., Bhatt R., Parmar M. StatPearls. StatPearls; 2022. Upadacitinib. [Google Scholar]
- 4.Blauvelt A., Teixeira H.D., Simpson E.L., et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055. doi: 10.1001/jamadermatol.2021.3023. Published correction appears in JAMA Dermatol. 2022;158(2):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teraki Y., Hotta T., Shiohara T. Skin-homing interleukin-4 and -13-producing cells contribute to bullous pemphigoid: remission of disease is associated with increased frequency of interleukin-10-producing cells. J Invest Dermatol. 2001;117(5):1097–1102. doi: 10.1046/j.0022-202x.2001.01505.x. [DOI] [PubMed] [Google Scholar]
- 6.Abdat R., Waldman R.A., de Bedout V., et al. Dupilumab as a novel therapy for bullous pemphigoid: a multicenter case series. J Am Acad Dermatol. 2020;83(1):46–52. doi: 10.1016/j.jaad.2020.01.089. [DOI] [PubMed] [Google Scholar]
- 7.James H., Paley G.L., Brasington R., Custer P.L., Margolis T.P., Paley M.A. Tofacitinib for refractory ocular mucous membrane pemphigoid. Am J Ophthalmol Case Rep. 2021;22 doi: 10.1016/j.ajoc.2021.101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao Y., Xiang H., Li W. Concurrent bullous pemphigoid and plaque psoriasis successfully treated with Janus kinase inhibitor baricitinib. Dermatol Ther. 2022;35(10) doi: 10.1111/dth.15754. [DOI] [PubMed] [Google Scholar]
- 9.Juczynska K., Wozniacka A., Waszczykowska E., et al. Expression of the JAK/STAT signaling pathway in bullous pemphigoid and dermatitis herpetiformis. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/6716419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarny S., Hucke M., El-Shabrawi Y. Treatment of mucous membrane pemphigoid with Janus kinase inhibitor baricitinib. JAMA Ophthalmol. 2018;136(12):1420–1422. doi: 10.1001/jamaophthalmol.2018.3789. [DOI] [PubMed] [Google Scholar]