Abstract
A comparison between 3-wk-old female turkeys (B.U.T. 6) and broilers (Ross 308) was performed to study the effects of species, dietary P, Ca, and phytase levels on gut mucosal phosphatase activity, myo-inositol hexakisphosphate (InsP6) degradation along the digestive tract, digestibility of P, Ca, and amino acids, and concentrations of myo-inositol in the digesta and blood. The experimental diets were corn-soybean meal-based and identical for both species. Two dietary P and Ca concentrations (CaP–: 4.1 g P/kg, 5.5 g Ca/kg and CaP+: 9.0 g P/kg, 12.0 g Ca/kg) and 2 levels of phytase supplementation (0 and 1,500 FTU/kg) were used in a 2 × 2 factorial design and fed to the animals for 7 d in their third week of age. Each diet was randomly assigned to 6 broiler and 6 turkey pens, with 10 birds each. After slaughter, blood, digesta from the crop, gizzard, duodenum, lower ileum, and mucosa from the jejunum were collected. When fed CaP– without phytase supplementation, there were no differences between species in gut mucosal phosphatase activity, prececal InsP6 disappearance, and P and Ca digestibility, indicating a similar intrinsic capacity for phytate degradation in both species. When fed CaP+ without phytase supplementation, turkeys showed higher prececal InsP6 disappearance than broilers. Phytase supplementation increased prececal InsP6 disappearance and digestibility of P and Ca in both species. However, the phytase-induced increase in prececal InsP6 disappearance was more pronounced in broilers than in turkeys, possibly due to more adequate conditions for phytase activity in the broiler crop. In broilers, phytase supplementation increased amino acid digestibility overall, whereas, in turkeys, it increased with CaP+ and decreased with CaP–. In addition, the relationship between myo-inositol concentration in the ileum and blood differed between species, indicating differences in myo-inositol metabolism. It was concluded that 3-week-old turkeys and broilers differ in nutrient digestibility and InsP degradation in some segments of the digestive tract but have similar endogenous InsP6 degradation when fed low P and Ca diets.
Key words: phytate degradation, myo-inositol, broiler, turkey, digestibility
INTRODUCTION
Most P in plant seeds is bound in the form of phytate (any salt of myo-inositol hexakisphosphate (InsP6)). Phytases and other phosphatases must hydrolyze InsP6 before InsP6-P can be absorbed in the digestive tract of animals. In non-ruminants, endogenous phytase activity is considered to be inadequate, leading to limited InsP6 degradation in the digestive tract. However, the potential of broilers to degrade InsP6 by the end of the ileum is high (64–76% prececal InsP6 disappearance) (Tamin et al., 2004; Zeller et al., 2015a; Ingelmann et al., 2019), with substantial contributions from endogenous gut mucosal phytase and phosphatases (Sommerfeld et al., 2019). However, supplementation with mineral P sources reduced the potential to degrade InsP6 (Shastak et al., 2014; Zeller et al., 2015b; Sommerfeld et al., 2018; Sommerfeld et al., 2019), and the inclusion of mineral P in poultry feed remains an industry standard. This leads to the excretion of unused phytate P, which can cause eutrophication of water bodies (Sharpley, 1999). Mineral P supplements are produced from mined phosphate rock, which is a finite resource. To reduce or avoid the inclusion of unsustainable and expensive mineral P and to reduce P excretion, a common strategy is to supplement poultry feed with exogenous phytases. These enzymes release P from InsP6 and may also increase the digestibility of amino acids (AA), and bioavailability of cations such as Zn, Fe, Mn, Ca, and Mg chelated with phytate (Rodehutscord et al., 2022).
Several studies have been conducted to better understand the process of InsP6 degradation in broiler chickens. However, such studies are scarce for turkeys. In comparative studies between turkeys and broilers, prececal InsP6 disappearance was much lower in turkeys than broilers (5.5–15.3% in turkeys vs. 63.8–76.3% in broilers) when exogenous phytase was not supplemented (Ingelmann et al., 2019). Correspondingly, upon exogenous phytase supplementation, prececal InsP6 disappearance increased more in turkeys than in broilers, although it remained lower in turkeys (Ingelmann et al., 2019; Olukosi et al., 2020). Other studies have also reported that turkeys were more responsive to phytase supplementation than broilers (Pirgozliev et al., 2007). This finding suggests the existence of fundamental differences between broiler chickens and turkeys. However, species-specific feeds were used in all studies published on this subject, which differed markedly in ingredient composition and nutrient concentrations, such as total P, InsP6-P, Ca, and crude protein. Because of the interactions between InsP6 degradation and dietary Ca and P concentrations, such differences in diet composition may confound possible species effects. In a study that used the same diet without mineral P supplementation in broiler chickens and turkeys, total tract P retention was 58% in broilers and 39% in turkeys at 4 wk of age (Rodehutscord and Dieckmann, 2005). However, InsP6 degradation was not investigated in this study.
The main objective of this study was to compare prececal InsP6 disappearance in broilers and turkeys, including the effects of dietary Ca, P, and phytase concentrations, using the same feed for both species. It was hypothesized that broilers would have a higher capability to degrade InsP6 than turkeys. Another objective was to determine the traits (gut length, pH of digestive tract contents, and jejunal mucosal phosphatase activity) that might explain potential species differences in their abilities to degrade InsP6 and utilize InsP6-P.
MATERIALS AND METHODS
Birds and Housing
The trial was conducted at the Experimental Station “Unterer Lindenhof” of the University of Hohenheim, Germany. This study was approved in accordance with the German Animal Welfare Legislation by Regierungspräsidium Tübingen, Germany (Project No. HOH 59/19 TE).
A total of 530 female Ross 308 broiler hatchlings and 530 female B.U.T. 6 turkey hatchlings were obtained from commercial hatcheries (Brüterei Süd ZN der BWE-Brüterei Weser-Ems GmbH & Co. KG, Regenstauf, Germany and Gebrüder Böcker Putenbrüterei GmbH, Wallhausen, Germany) and placed in floor pens on wood shavings (broilers in 2 pens, turkeys in 4 pens). During the 14 d post-hatch period, broilers were fed a pelleted diet that met or exceeded the supply recommendations of the Gesellschaft für Ernährungsphysiologie (1999) and contained 240 g CP/kg, 8 g P/kg, 9.6 g Ca/kg, and 12.6 MJ ME/kg, with no phytase added. Turkeys were fed a commercial granulated starter diet (Bio-PSG N10 + Ei + C, Kaisermühle Gänheim Otmar Kaiser GmbH, Arnstein-Gänheim, Germany) containing 305 g CP/kg, 10 g P/kg, 14 g Ca/kg, and 11.4 MJ ME/kg until d 8. From d 8 to 14, turkeys were fed a pelleted starter diet formulated to meet or exceed the feeding recommendations of the Gesellschaft für Ernährungsphysiologie (2004), containing 266 g CP/kg, 9 g P/kg, 14.5 g Ca/kg, and 12.3 MJ ME/kg without added phytase. On d 15, 240 birds of both species were allocated in groups of 10 to 48 perforated floor pens (1.15 m × 2.3 m for broilers and 3 m × 4 m for turkeys). The other animals remained in the pens on wood shavings for an additional 21 d, after which they underwent a similar trial reported in a companion paper (Novotny et al., 2023). Each pen was equipped with 2 feeding troughs and 1 drinker. All animals were weighed on a pen basis, and similar body weights per pen for all treatments were maintained within each species. Each pen was allocated in a randomized complete block design to 1 of 4 experimental diets, with 6 pens per diet and species, and feed and water were provided for ad libitum consumption until slaughter on d 21. The light program was 24L:0D for the first 3 d and 18L:6D from d 4 until the end of the experiment. The temperature was 34°C for the first 3 d and then gradually lowered to 26°C on d 21. The animals were inspected twice daily for abnormal behavior and overall health. No mortality was observed during the experimental period.
Experimental Diets and Treatments
The experiment was designed as a 2 × 2 × 2 factorial arrangement of treatments (2 species, 2 Ca and P levels (CaP), and 2 levels of phytase (PHY) addition). In the experimental phase, both species were fed the same experimental diets (Table 1) and were concurrently housed in the same barn in order to not confound the species comparison by differences in diet composition or environmental conditions. The experimental diets were based on corn and soybean meal and formulated to meet the supply recommendations for turkeys (Gesellschaft für Ernährungsphysiologie, 2004), except for P and Ca. The CaP+ diets were supplemented with monocalcium phosphate (MCP) and limestone to achieve P and Ca concentrations of 9.0 g/kg and 12.0 g/kg, respectively. No mineral phosphate was added to the CaP– diets, resulting in calculated P and Ca concentrations of 4.1 g/kg and 5.5 g/kg, respectively. Sand was used at the expense of MCP and limestone. In the PHY+ diets, a modified E. coli-derived 6-phytase (Quantum Blue, AB Vista, Marlborough, United Kingdom) was added at 1,500 FTU/kg. PHY– diets did not contain added phytase. All the diets contained 5 g/kg TiO2 as an indigestible marker. To achieve uniformity of all diets, ingredients except MCP, limestone, sand, and PHY were mixed in one lot. This basal lot was divided into 2 parts. One part was supplemented with MCP and limestone, and the other was supplemented with sand. Each of these mixes was split into 2 parts, and one was supplemented on top with the enzyme, while the other remained without it. The diets were pelleted without steam through a 3-mm die. Mixing and pelleting were performed at the certified feed mill of the Agricultural Experimental Station of the University of Hohenheim. The formulated concentrations of P, InsP6-P, Ca, and phytase activity were confirmed by the analyses (Table 2). The phytase activity in the PHY+ diets was lower than the calculated value but similar in both the phytase-containing diets (approximately 1,100 FTU/kg).
Table 1.
Ingredient composition and calculated nutrient concentrations of the experimental diets.
| Ingredient, g/kg | CaP–PHY– | CaP–PHY+ | CaP+PHY– | CaP+PHY+ |
|---|---|---|---|---|
| Corn | 436.8 | 436.8 | 436.8 | 436.8 |
| Soybean meal | 416.5 | 416.5 | 416.5 | 416.5 |
| Rapeseed meal | 40.1 | 40.1 | 40.1 | 40.1 |
| Soybean oil | 43.2 | 43.2 | 43.2 | 43.2 |
| L-lysine-sulfate | 7.5 | 7.5 | 7.5 | 7.5 |
| DL-methionine | 3.8 | 3.8 | 3.8 | 3.8 |
| L-threonine | 0.7 | 0.7 | 0.7 | 0.7 |
| L-valine | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline chloride | 2.0 | 2.0 | 2.0 | 2.0 |
| NaCl | 1.0 | 1.0 | 1.0 | 1.0 |
| NaHCO₃ | 4.6 | 4.6 | 4.6 | 4.6 |
| Vitamin mix1 | 2.0 | 2.0 | 2.0 | 2.0 |
| Mineral mix2 | 0.5 | 0.5 | 0.5 | 0.5 |
| Titanium dioxide | 5.0 | 5.0 | 5.0 | 5.0 |
| Limestone | 7.3 | 7.3 | 12.9 | 12.9 |
| Monocalcium phosphate | 0.0 | 0.0 | 23.1 | 23.1 |
| Sand | 28.7 | 28.7 | 0.0 | 0.0 |
| Calculated (g/kg): | ||||
| P | 4.1 | 4.1 | 9.0 | 9.0 |
| Ca | 5.5 | 5.5 | 12.0 | 12.0 |
| Crude protein | 253 | 253 | 253 | 253 |
| Phytase (FTU/kg) | 0 | 1,500 | 0 | 1,500 |
Vitamin mix (MIAVIT GmbH, Essen (Oldb.), Germany), provided per kg of complete diet: 10,000 IU vitamin A, 3,000 IU vitamin D3, 30 mg DL-α-Tocopherylacetate, 2.4 mg vitamin K3, 3 mg vitamin B1, 6 mg vitamin B2, 6 mg vitamin B6, 30 μg vitamin B12, 50 mg nicotinic acid, 14 mg pantothenic acid, 1 mg folic acid, 0.1 mg biotin.
Mineral mix (Gelamin, Gesellschaft für Tierernährung mbH, Memmingen, Germany), provided per kg of complete diet: 50 mg calcium from calcium carbonate, 80 mg manganese from manganese-(II)-oxide, 60 mg zinc from zinc-oxide, 25 mg iron from ferrous-(II)-carbonate, 7.5 mg copper from cupric-(II)-sulphate pentahydrate, 0.6 mg iodine from calcium iodate, 0.2 mg selenium from sodium selenite.
Table 2.
Analyzed composition of the experimental diets.
| Treatments1 |
||||
|---|---|---|---|---|
| Analyzed composition (g/kg2) | CaP– PHY– | CaP– PHY+ | CaP+ PHY– | CaP+ PHY+ |
| InsP6 (mmol/kg as fed) | 12.7 | 12.9 | 12.4 | 12.4 |
| InsP5 (mmol/kg as fed) | 1.6 | 1.7 | 1.6 | 1.6 |
| Myo-inositol (µmol/g) | 1.8 | 1.8 | 1.8 | 1.8 |
| InsP6-P | 2.5 | 2.4 | 2.4 | 2.4 |
| P | 4.5 | 4.5 | 9.7 | 9.9 |
| Ca | 5.7 | 5.7 | 12.2 | 12.3 |
| Crude protein | 264 | 264 | 257 | 264 |
| Arg | 17.5 | 17.9 | 17.7 | 17.8 |
| His | 7.3 | 7.4 | 7.4 | 7.5 |
| Ile | 11.6 | 12.0 | 11.8 | 11.8 |
| Leu | 22.1 | 22.5 | 22.4 | 22.5 |
| Lys | 18.9 | 19.5 | 19.2 | 19.4 |
| Met3 | 7.7 | 7.9 | 7.9 | 7.9 |
| Phe | 13.1 | 13.3 | 13.3 | 13.4 |
| Thr | 10.8 | 11.0 | 11.0 | 11.1 |
| Val | 12.9 | 13.2 | 13.0 | 13.1 |
| Phytase (FTU/kg) | < 50 | 1130 | < 50 | 1080 |
Calculated composition: CaP–, 4.1 g P/kg and 5.5 g Ca/kg; CaP+, 9.0 g P/kg and 12.0 g Ca/kg; PHY–, no supplemented phytase; PHY+: 1,500 FTU/kg supplemented phytase.
Unless stated otherwise.
Methionine determined as methionine sulfone.
Procedures and Sampling
Animals and feed were weighed on d 14 and 21 on a pen basis, and ADG, ADFI, and gain per feed (G:F) were calculated. On d 21, the feed troughs of the pens were removed 2 h before slaughter according to a fixed time schedule and returned 1 h before slaughtering to standardize the gut fill. The birds were stunned using a gas mixture consisting of 35% N2, 35% CO2, and 30% O2. Two stunned birds per pen were randomly selected and weighed. They were killed by decapitation and trunk blood was collected in tubes containing sodium fluoride. Blood samples were centrifuged for 10 min at 2,500 ×g to obtain plasma. One of the birds was eviscerated, the small intestine and ceca were spread out on a 1 cm grid, and a picture was taken to determine the length of the intestine sections later. The jejunum was dissected from the second bird, opened longitudinally, and flushed with phosphate-buffered saline. Mucosa samples were stripped off with microscopic slides, shock-frozen in liquid nitrogen, transported on dry ice to the laboratory, and stored at −80°C until further analysis. The remaining 8 stunned birds from each pen were asphyxiated using CO2. From all 10 birds in a pen, crop, gizzard, duodenum, and lower ileum, defined as the last two-thirds of the section between Meckel's diverticulum and 2 cm prior to the ileo-ceco-colonic junction, and ceca were excised. Digesta of the crop and gizzard were carefully obtained with a spatula, digesta of the duodenum was gently squeezed out, and the digesta of the ileum and ceca were flushed out using ice-cold double-distilled water. The samples of the respective sections were pooled on a pen basis and frozen at −20°C following the determination of pH values in the contents of the crop, gizzard, and duodenum (InLab Solids, Mettler-Toledo GmbH, Vienna, Austria).
Sample Preparation and Chemical Analyses
Digesta samples were freeze-dried, pulverized (PULVERISETTE 9; Fritsch GmbH, Idar-Oberstein, Germany), and stored in sealed containers at room temperature. Pulverized feed and digesta samples were analyzed for P, Ca, and Ti using inductively coupled plasma-optical emission spectrometry after wet digestion (Zeller et al., 2015a). Extraction and measurement of InsP3-6 isomers were carried out using the method of Zeller et al. (2015a) with modifications described by Sommerfeld et al. (2018) and using high-performance ion chromatography (ICS-3000 system, Dionex, Idstein, Germany). Using this methodology makes separating enantiomers impossible; therefore, we could not distinguish between the D- and L-forms. Furthermore, discrimination of isomers Ins(1,2,6)P3, Ins(1,4,5)P3, and Ins(2,4,5)P3 was not possible because of coelution; therefore, we used the term InsP3x for these InsP3 isomers of unknown proportions. Myo-inositol (MI) in the feed, digesta, and plasma samples was analyzed according to Sommerfeld et al. (2018) using a gas chromatograph/mass spectrometer (Agilent 5977A, Waldbronn, Germany) following a two-step derivatization of the samples. Phytase activity in the feed was analyzed by AB Vista Lab Service (Ystrad Mynach, Wales, UK) using a validated product-specific ELISA method. The results were calculated from a calibration curve with a known activity as determined by the Quantum Blue product analysis, and the activity was expressed as FTU/kg feed. Amino acids were analyzed according to Rodehutscord et al. (2004) using an L-8900 amino acid analyzer (VWR, Hitachi Ltd, Tokyo, Japan) following sample oxidation and acid hydrolysis.
Mucosal Phosphatase Activity Measurement
For mucosal phosphatase activity measurement, the brush-border membrane (BBM) was enriched in the mucosa samples, according to Huber et al. (2015). In brief, mucosal samples of the jejunum were ground using a mortar and pestle under liquid nitrogen and mixed with 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid/mannitol buffer (HEPES 2 mmol, mannitol 50 mmol, PMSF 25 mmol). Using a glass potter and homogenizer (homogenplus, shuett-biotec GmbH, Göttingen, Germany), samples were homogenized, and enterocytes in the mucosa sample were sheared off at the tight junctions, separating BBM from the basolateral membrane. After this step, the mucosal homogenates were mixed with 1 M MgCl2 to precipitate the basolateral membranes, which were subsequently removed by centrifugation. Precipitates containing enriched BBM after the final high-speed centrifugation were resuspended in HEPES/mannitol buffer with protease inhibitors. Aliquots (50 μL) of BBM homogenates were frozen and stored at −80°C until analysis. Protein concentrations of the BBM preparations were determined in triplicate using the Bradford assay (Bradford Reagent, 5 ×, SERVA, Heidelberg, Germany). The activity of phosphatases, including phytases, associated with BBM was measured as described by Gonzales-Uarquin et al. (2020) with modifications. Briefly, BBM preparation (equivalent to 160 µg protein) was added to a mixture of double-distilled water containing 25 µg of sodium phytate (Sirius Fine Chemicals SiChem GmbH, Bremen, Germany) and buffer (pH 5.5 buffer from a test kit (K-PHYT 05/17 assay; Megazyme International, Ireland). After 15 min of incubation at 40°C, the reaction was stopped by adding trichloric acid. A second aliquot of the BBM preparation from the same animal was incubated in the same way, but the stop reagent trichloric acid was added beforehand, thus creating a blind value. Following incubation, free phosphate (Pi) was determined photometrically (655 nm, 40°C, Infinite 200 PRO M NANO+, Tecan Trading AG, Switzerland) using the method described in the K-PHYT test kit. The released Pi was calculated by subtracting the respective blind values from the original measurement. The activity of BBM-associated phosphatase is the amount of Pi released per gram of BBM protein per minute incubation time at pH 5.5. If insufficient BBM preparation was available for the determination of a blind value, the mean of all blind values of the respective treatment was used to calculate the released Pi.
Calculations and Statistical Analysis
Prececal digestibility of P, Ca, AA, and InsP6 disappearance was calculated using the marker method and the following equation:
where y is the disappearance or digestibility of X in %; X is the concentration of InsP6, P, Ca, or AA in the feed and digesta; and TiO2 is the concentration of TiO2 in the feed and digesta.
The data were analyzed with a 3-way ANOVA using the MIXED procedure of the software package SAS (version 9.4; SAS Institute Inc., Cary, NC). Non-normally distributed data were log-transformed. The results are presented as LSmeans and pooled SEM of the untransformed data. The pen was considered the experimental unit. The following model was used.
where yijkl = response variable, µ = overall mean, αi = effect of species (fixed), βj = effect of CaP (fixed), γk = effect of PHY (fixed), (αβ)ij = interaction between species and CaP (fixed), (αγ)ik = interaction between species and PHY (fixed), (βγ)jk = interaction between CaP and PHY (fixed), (αβγ)ijk is the 3-way interaction between species, CaP, and PHY (fixed), δl = effect of block (random), and εijkl = residual error. Statistical significance was set at P < 0.05.
RESULTS
Analyses of gastrointestinal pH, mucosal enzyme activity, prececal InsP6 disappearance, and P and Ca digestibility are shown in Table 3. The pH of the crop content was higher in turkeys than in broilers. It was lower overall in CaP+ treatments than in CaP– treatments, but this effect was greater in turkeys, resulting in a species × CaP interaction (CaP+ turkey: 6.6, CaP+ broiler: 6.0, turkey: 5.7, CaP– broiler: 5.6; P < 0.001). In the gizzard content, species and CaP also significantly affected the pH value, but an interaction did not exist. Gizzard pH was lower in turkeys than in broilers (3.8 vs. 4.0, P < 0.001) and higher in CaP+ (3.9) than in CaP– (3.8, P = 0.025). The pH value of the duodenum content was higher in broilers (6.3) than in turkeys (6.1, P < 0.001) but was not affected by the other factors. The phosphatase activity in the jejunal mucosa was only affected by added phytase and was higher in PHY+ (4.7 µmol Pi/g/min) than in PHY– (3.6 µmol/g/min) (P = 0.043). Prececal InsP6 disappearance was affected by a 3-way interaction (P = 0.013). InsP6 disappearance in broilers did not differ from turkeys at CaP–PHY–, but it was higher in turkeys than in broilers (6.3% vs. −3.9%) at CaP+PHY–. In the treatments with added phytase, broilers had a higher InsP6 disappearance than turkeys, and the level was lower overall in CaP+ than in CaP–.
Table 3.
Effect of Ca and P level (CaP) and phytase supplementation (PHY) on prececal calcium (Ca) and phosphorus (P) digestibility, prececal (pc) InsP6 disappearance, jejunal endogenous mucosal phosphatase activity, and pH in the crop, gizzard, and duodenum of broilers and turkeys at 21 d of age.
| Species | Treatment1 | pH crop | pH gizzard | pH duodenum | Mucosal phosphatase activity (µmol Pi/g BBM protein/min.) | pc InsP6 disappearance (%) | pc P digestibility (%) | pc Ca digestibility (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Broiler | CaP– | PHY– | 5.8 | 4.0 | 6.3 | 3.5 | 29.3c | 41.1e | 45.5 |
| PHY+ | 6.1 | 3.9 | 6.3 | 4.8 | 77.9a | 70.8a | 57.1 | ||
| CaP+ | PHY– | 5.6 | 4.0 | 6.3 | 3.6 | −3.9e | 44.8e | 28.1 | |
| PHY+ | 5.6 | 4.1 | 6.3 | 4.6 | 62.5b | 49.8d | 27.8 | ||
| Turkey | CaP– | PHY– | 6.6 | 3.8 | 6.2 | 4.5 | 25.3c | 44.7e | 43.2 |
| PHY+ | 6.5 | 3.6 | 6.1 | 5.7 | 58.0b | 61.4b | 52.7 | ||
| CaP+ | PHY– | 5.7 | 3.8 | 6.1 | 2.9 | 6.3d | 56.2c | 39.5 | |
| PHY+ | 5.8 | 3.8 | 6.1 | 3.9 | 32.4c | 57.5bc | 39.2 | ||
| SEM | 0.07 | 0.08 | 0.04 | 0.80 | 3.35 | 1.87 | 2.51 | ||
| P-values | |||||||||
| Species | < 0.001 | < 0.001 | < 0.001 | 0.836 | < 0.001 | 0.001 | 0.003 | ||
| CaP | < 0.001 | 0.025 | 0.232 | 0.104 | < 0.001 | 0.016 | < 0.001 | ||
| PHY | 0.318 | 0.364 | 0.803 | 0.043 | < 0.001 | < 0.001 | < 0.001 | ||
| Species × CaP | < 0.001 | 0.936 | 0.341 | 0.131 | 0.673 | < 0.001 | < 0.001 | ||
| Species × PHY | 0.300 | 0.761 | 0.561 | 0.965 | < 0.001 | < 0.001 | 0.673 | ||
| CaP × PHY | 0.376 | 0.106 | 0.739 | 0.837 | 0.237 | < 0.001 | < 0.001 | ||
| Species × CaP × PHY | 0.059 | 0.598 | 0.868 | 0.989 | 0.013 | 0.020 | 0.682 | ||
Calculated composition: CaP–, 4.1 g P/kg and 5.5 g Ca/kg; CaP+, 9.0 g P/kg and 12.0 g Ca/kg; PHY–, no supplemented phytase; PHY+: 1,500 FTU/kg supplemented phytase.
Means within a column not sharing a common superscript differ (P < 0.05).
Prececal P digestibility was also significantly affected by a 3-way interaction (P = 0.020). In all PHY– treatments, prececal P digestibility was lower than in PHY+ treatments, except in turkeys fed CaP+, where phytase supplementation showed no effect. Prececal Ca digestibility was not significantly different between turkeys and broilers in CaP– treatments but was reduced by CaP+ to a greater extent in broilers than in turkeys (species × CaP: P < 0.001). Furthermore, phytase supplementation increased prececal Ca digestibility only when the CaP level was low (CaP × PHY: P < 0.001).
The InsP6 and Ins(1,2,4,5,6)P5 concentrations in crop content were significantly affected by the species × PHY interaction (P < 0.001, Table 4), indicating that phytase addition caused a reduction in the concentration of these molecules in broilers but barely in turkeys. Ins(1,2,5,6)P4 was only detected in the PHY+ treatments, and its concentration was higher in broilers than in turkeys (P < 0.001). The MI concentration in the crop content was slightly but significantly higher in turkeys than in broilers (P = 0.001).
Table 4.
Effect of Ca and P level (CaP) and phytase supplementation (PHY) on the concentrations of myo-inositol and inositol phosphates (µmol/g DM) in the crop of broilers and turkeys at 21 d of age.
| Species | Treatment1 | InsP6 | Ins(1,2,4,5,6)P5 | Ins(1,2,3,4,5)P5 | Ins(1,2,3,4,6)P5 | Ins(1,2,5,6)P4 | Myo-inositol | |
|---|---|---|---|---|---|---|---|---|
| Broiler | CaP– | PHY– | 14.0 | 1.2 | 0.7b | 0.3 | < loq2 | 2.0 |
| PHY+ | 11.6 | 0.8 | 0.6bcd | < loq | 2.4 | 2.2 | ||
| CaP+ | PHY– | 14.0 | 1.2 | 0.6cd | 0.2 | < loq | 2.0 | |
| PHY+ | 11.5 | 0.7 | 0.7a | < loq | 2.6 | 2.0 | ||
| Turkey | CaP– | PHY– | 14.4 | 1.3 | 0.6cd | 0.2 | < loq | 2.2 |
| PHY+ | 14.2 | 1.2 | 0.7bc | < loq | 0.3 | 2.2 | ||
| CaP+ | PHY– | 14.4 | 1.2 | 0.6d | 0.2 | < loq | 2.2 | |
| PHY+ | 14.3 | 1.2 | 0.7bc | 0.2 | 0.3 | 2.2 | ||
| SEM | 0.34 | 0.05 | 0.02 | 0.02 | 0.27 | 0.07 | ||
| P-values | ||||||||
| Species | < 0.001 | < 0.001 | 0.010 | 0.352 | < 0.001 | 0.001 | ||
| CaP | 1.000 | 0.313 | 0.499 | 0.071 | 0.688 | 0.136 | ||
| PHY | < 0.001 | < 0.001 | 0.002 | 1.000 | 0.136 | |||
| Species × CaP | 0.807 | 0.313 | 0.181 | 0.352 | 0.829 | 0.136 | ||
| Species × PHY | < 0.001 | < 0.001 | 1.000 | 0.136 | ||||
| CaP × PHY | 0.917 | 0.735 | 0.002 | 0.614 | ||||
| Species × CaP × PHY | 0.834 | 0.313 | 0.010 | 0.614 | ||||
Calculated composition: CaP–, 4.1 g P/kg and 5.5 g Ca/kg; CaP+, 9.0 g P/kg and 12.0 g Ca/kg; PHY–, no supplemented phytase; PHY+: 1,500 FTU/kg supplemented phytase.
loq = limit of quantification (0.3 µmol/g DM).
Means within a column not sharing a common superscript differ (P < 0.05).
The InsP6 concentration in the gizzard content was lower in turkeys than in broilers in PHY– diets (5.7 µmol/g vs. 6.8 µmol/g) and reduced to a similar level (0.8 µmol/g and 0.6 µmol/g) in PHY+ diets (species × PHY: P < 0.001) (Table 5). The InsP6 concentration was also affected by CaP x PHY interaction (P = 0.003), and it was lower in CaP+PHY– (6.0 µmol/g) than in CaP–PHY– (6.6 µmol/g). In the treatments with supplemented phytase, the InsP6 concentration was higher in CaP+ (0.8 µmol/g) than CaP– (0.5 µmol/g). Ins(1,2,5,6)P4 was detected in the gizzard content only in the PHY+ treatment. The MI concentration in the gizzard content was affected by all two-way interactions. It was increased by the addition of phytase to a greater extent in broilers than in turkeys (P = 0.006 for the interaction) in CaP– but not CaP+ (P < 0.001 for the interaction).
Table 5.
Effect of Ca and P level (CaP) and phytase supplementation (PHY) on the concentrations of myo-inositol and inositol phosphates (µmol/g DM) in the gizzard of broilers and turkeys at 21 d of age.
| Species | Treatment1 | InsP6 | Ins(1,2,4,5,6)P5 | Ins(1,2,3,4,5)P5 | Ins(1,2,5,6)P4 | InsP3x2 | Myo-inositol | |
|---|---|---|---|---|---|---|---|---|
| Broiler | CaP– | PHY– | 7.0 | 0.4 | 0.3 | n.d.3 | n.d. | 1.7 |
| PHY+ | 0.5 | n.d. | n.d. | 2.3 | < loq4 | 2.7 | ||
| CaP+ | PHY– | 6.6 | 0.5 | 0.2 | n.d. | n.d. | 1.1 | |
| PHY+ | 0.7 | n.d. | 0.1 | 3.9 | < loq | 1.2 | ||
| Turkey | CaP– | PHY– | 6.1 | 0.4 | 0.2 | n.d. | n.d. | 0.6 |
| PHY+ | 0.6 | n.d. | n.d. | 3.3 | < loq | 1.1 | ||
| CaP+ | PHY– | 5.3 | 0.4 | 0.2 | n.d. | n.d. | 0.6 | |
| PHY+ | 1.0 | n.d. | 0.3 | 3.6 | n.d. | 0.6 | ||
| SEM | 0.21 | 0.03 | 0.03 | 0.24 | 0.07 | |||
| P-values | ||||||||
| Species | 0.004 | 0.018 | 1.000 | 0.172 | < 0.001 | |||
| CaP | 0.374 | 0.771 | 0.367 | 0.001 | < 0.001 | |||
| PHY | < 0.001 | 0.546 | < 0.001 | |||||
| Species × CaP | 0.751 | 0.771 | 0.546 | 0.012 | < 0.001 | |||
| Species × PHY | < 0.001 | 0.001 | 0.006 | |||||
| CaP × PHY | 0.003 | < 0.001 | ||||||
| Species × CaP × PHY | 0.344 | 0.060 | ||||||
Calculated composition: CaP–, 4.1 g P/kg and 5.5 g Ca/kg; CaP+, 9.0 g P/kg and 12.0 g Ca/kg; PHY–, no supplemented phytase; PHY+: 1,500 FTU/kg supplemented phytase.
Ins(1,2,6)P3, Ins(1,4,5)P3, and Ins(2,4,5)P3 could not be differentiated due to co-elution and are thus referred to as InsP3x.
n.d. = not detectable (< 0.1 µmol/g DM).
loq = limit of quantification (0.3 µmol/g DM).
In the ileum, Ins(1,2,4,5,6)P5 concentrations were lowest in CaP– and unaffected by phytase or species, whereas in CaP+, concentrations where reduced by phytase in broilers but not in turkeys (P < 0.001, Table 6). Ins(1,2,3,4,5)P5 concentrations in the ileum were significantly affected by a CaP × PHY interaction (P < 0.001, Table 6) and were highest in CaP+PHY+ treatments, followed by CaP+PHY– and CaP–PHY+. CaP–PHY– was not different from CaP–PHY+ but lower than that of CaP+PHY–. The concentration of Ins(1,2,5,6)P4 in the ileum was the highest in broilers fed CaP+PHY+, and the PHY effect was lower in turkeys than in broilers (P < 0.001). InsP3x in the ileum content was only detected in CaP+PHY+ treatments, and the concentration was higher in broilers than in turkeys (P < 0.001). The MI concentration in the ileum content was affected by a three-way interaction (P < 0.001). Broilers in the PHY+ treatments had higher MI concentrations than those in the PHY– treatments, with the highest concentration in CaP–. The MI concentration in the ileum of turkeys was lower than that in broilers. The MI concentration in the blood was significantly affected by the 3-way interaction (P < 0.001). The concentration was the lowest in CaP+PHY–. It was higher when CaP was low, PHY was added, or both. At CaP–PHY+, the highest plasma MI concentration was measured in both species, with the highest value in turkeys.
Table 6.
Effect of Ca and P level (CaP) and phytase supplementation (PHY) on the concentrations of TiO2 (mg/g), myo-inositol, and inositol phosphates in the ileum (µmol/mg TiO2) and myo-inositol in blood plasma (µmol/ml) of broilers and turkeys at 21 d of age.
|
Myo-inositol |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Treatment1 | TiO2 | InsP6 | Ins(1,2,4,5,6)P5 | Ins(1,2,3,4,5)P5 | Ins(1,2,3,4,6)P5 | Ins(1,2,5,6)P4 | Ins(1,2,3,4)P4 | InsP3x2 | Ileum | Blood | |
| Broiler | CaP– | PHY– | 15.3b | 1.67c | 0.03de | 0.08 | 0.03 | n.d.3 | 0.02 | n.d. | 0.67b | 0.27c |
| PHY+ | 15.8b | 0.52e | 0.03e | 0.08 | n.d. | 0.05 | 0.01 | n.d. | 1.60a | 0.33b | ||
| CaP+ | PHY– | 16.1b | 2.45a | 0.18a | 0.12 | 0.05 | 0.04 | 0.03 | n.d. | 0.14d | 0.18e | |
| PHY+ | 17.1a | 0.89d | 0.11c | 0.29 | < loq4 | 0.66 | 0.07 | 0.37 | 0.31c | 0.26c | ||
| Turkey | CaP– | PHY– | 14.1c | 1.76c | 0.05d | 0.06 | 0.03 | n.d. | < loq | n.d. | 0.11d | 0.29c |
| PHY+ | 13.2cd | 0.99d | 0.04de | 0.11 | < loq | 0.04 | 0.01 | n.d. | 0.28c | 0.43a | ||
| CaP+ | PHY– | 12.4d | 2.21b | 0.15b | 0.10 | 0.05 | 0.02 | 0.01 | n.d. | 0.12d | 0.17e | |
| PHY+ | 14.0c | 1.60c | 0.14b | 0.29 | 0.02 | 0.24 | 0.04 | 0.09 | 0.15d | 0.21d | ||
| SEM | 0.36 | 0.084 | 0.007 | 0.014 | 0.002 | 0.024 | 0.004 | 0.024 | 0.028 | 0.011 | ||
| P-values | ||||||||||||
| Species | <0.001 | <0.001 | 0.152 | 1.000 | 0.092 | <0.001 | 0.004 | <0.001 | <0.001 | 0.055 | ||
| CaP | 0.181 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| PHY | 0.015 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Species × CaP | 0.003 | 0.688 | 0.152 | 0.475 | 0.302 | <0.001 | 0.004 | <0.001 | <0.001 | |||
| Species × PHY | 0.424 | <0.001 | 0.001 | 0.095 | <0.001 | 0.702 | <0.001 | 0.260 | ||||
| CaP × PHY | 0.003 | 0.232 | 0.002 | <0.001 | <0.001 | <0.001 | 0.014 | |||||
| Species × CaP × PHY | 0.030 | 0.012 | <0.001 | 0.422 | <0.001 | <0.001 | ||||||
Calculated composition: CaP–, 4.1 g P/kg and 5.5 g Ca/kg; CaP+, 9.0 g P/kg and 12.0 g Ca/kg; PHY–, no supplemented phytase; PHY+: 1,500 FTU/kg supplemented phytase.
Ins(1,2,6)P3, Ins(1,4,5)P3, and Ins(2,4,5)P3 could not be differentiated due to co-elution and are thus referred to as InsP3x.
n.d. = not detectable (<0.1 µmol/g DM corresponds to <0.006 µmol/mg TiO2).
loq = limit of quantification (<0.3 µmol/g DM corresponds to 0.018 µmol/mg TiO2).
Means within a column not sharing a common superscript differ (P < 0.05).
Performance traits were significantly affected by 3-way interactions (Table 7). Throughout all treatments, ADG (P < 0.001) and ADFI (P < 0.036) of broilers were higher than those of the turkeys. ADG and ADFI were lower in the CaP–PHY– treatments in each species than in the other treatments. The G:F ratio was generally higher in broilers than in turkeys. In broilers, G:F was similar in all treatments except for CaP–PHY–, which was lower. The G:F ratio in turkeys was lower in both CaP– treatments than in both CaP+ treatments (P < 0.004).
Table 7.
Effect of Ca and P level (CaP) and phytase supplementation (PHY) on average daily gain (ADG), average daily feed intake (ADFI), gain to feed ratio (G:F) from 14-21 d of age, and length of small intestine and BW of broilers and turkeys (one bird per pen) at 21 d of age.
| Species | Treatment1 | ADG2 (g/d) | ADFI (g/d) | G:F (g/g) | Small intestine length (cm) | BW of the bird (kg) | Small intestine length (cm/kg BW) | |
|---|---|---|---|---|---|---|---|---|
| Broiler | CaP– | PHY– | 66.0b | 77.0b | 0.86b | 141 | 852b | 166 |
| PHY+ | 76.8a | 84.7a | 0.91a | 142 | 1014a | 140 | ||
| CaP+ | PHY– | 77.1a | 85.5a | 0.90a | 150 | 971a | 155 | |
| PHY+ | 76.6a | 84.6a | 0.91a | 150 | 1004a | 150 | ||
| Turkey | CaP– | PHY– | 34.9d | 48.0d | 0.73d | 108 | 533d | 204 |
| PHY+ | 38.8c | 52.5c | 0.74d | 111 | 556d | 200 | ||
| CaP+ | PHY– | 39.9c | 52.6c | 0.76c | 111 | 578cd | 193 | |
| PHY+ | 40.3c | 52.5c | 0.77c | 113 | 637c | 178 | ||
| SEM | 0.76 | 0.75 | 0.006 | 3.4 | 26.7 | 7.2 | ||
| P-values | ||||||||
| Species | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| CaP | <0.001 | <0.001 | <0.001 | 0.023 | 0.004 | 0.110 | ||
| PHY | <0.001 | <0.001 | <0.001 | 0.598 | <0.001 | 0.017 | ||
| Species × CaP | 0.014 | 0.040 | 0.199 | 0.247 | 0.818 | 0.118 | ||
| Species × PHY | <0.001 | 0.200 | 0.026 | 0.749 | 0.148 | 0.519 | ||
| CaP × PHY | <0.001 | <0.001 | 0.002 | 0.872 | 0.227 | 0.583 | ||
| Species × CaP × PHY | <0.001 | 0.036 | 0.004 | 0.910 | 0.036 | 0.117 | ||
Calculated composition: CaP–, 4.1 g P/kg and 5.5 g Ca/kg; CaP+, 9.0 g P/kg and 12.0 g Ca/kg; PHY–, no supplemented phytase; PHY+: 1,500 FTU/kg supplemented phytase.
Average initial body weights per bird on d 14: 465 g (broilers) 295 g (turkeys).
Means within a column not sharing a common superscript differ (P < 0.05).
The small intestine in total was longer in broilers than in turkeys (P < 0.001, Table 7). It was longer in CaP+ than CaP– treatments (P = 0.023), irrespective of species. When expressed per kilogram of BW, the small intestine was longer in turkeys than broilers (P < 0.001), not affected by CaP level, but shorter in PHY+ than PHY– treatments (P = 0.017).
The prececal digestibility of all analyzed AA was significantly affected by 3-way interactions (P ≤ 0.035, Tables 8 and 9). In broilers, prececal AA digestibility of most AA was higher in CaP–PHY+ (Ile, Leu, Lys, Met, Phe, Val, Tyr, Ala, and Ser) or CaP+PHY+ (His, Met, Thr, Cys, Ala, and Pro) than in CaP–PHY–. Prececal digestibility of Glx and Gly was not significantly affected in broilers. In turkeys, treatments with added phytase or CaP had lower prececal digestibility values for Arg, Ile, Leu, Met, Asp, Glu, Gly, and Pro compared to the CaP–PHY– treatment. However, the turkey CaP+Phy+ treatment had higher prececal digestibility values for Arg, His, Leu, Lys, Met, Thr, Val, Cys, Tyr, Ala, Asx, Gly, Pro, and Ser compared to the turkey CaP+PHY– treatment, reaching the same level as the turkey CaP–PHY– treatment. The treatment turkey CaP+PHY– showed the lowest prececal digestibility values of all analyzed AA.
Table 8.
Effect of Ca and P level (CaP) and phytase supplementation (PHY) on prececal essential amino acid digestibility (%) of broilers and turkeys at 21 d of age.
| Treatment1 | Arg | His | Ile | Leu | Lys | Met2 | Phe | Thr | Val | |
|---|---|---|---|---|---|---|---|---|---|---|
| Broiler | ||||||||||
| CaP– | PHY– | 85.5ab | 76.6bc | 79.6b | 79.3bc | 84.8bc | 90.8bc | 80.1bc | 71.6bc | 77.9bc |
| PHY+ | 86.9a | 79.0ab | 82.6a | 82.4a | 86.7a | 92.2a | 83.1a | 74.2ab | 80.9a | |
| CaP+ | PHY– | 84.7bc | 78.0abc | 79.5b | 79.9abc | 84.6bc | 91.5ab | 80.3bc | 72.3abc | 78.3bc |
| PHY+ | 85.9ab | 79.7a | 81.3ab | 81.7ab | 85.8ab | 92.5a | 82.0ab | 74.6a | 80.1ab | |
| Turkey | ||||||||||
| CaP– | PHY– | 84.3bc | 76.3cd | 79.4b | 79.0c | 85.1abc | 91.3ab | 79.5bcd | 71.9abc | 77.5cd |
| PHY+ | 82.1de | 74.0de | 76.9c | 76.2de | 83.8cd | 89.9cd | 77.0de | 69.8cd | 75.1de | |
| CaP+ | PHY– | 80.9e | 72.5e | 75.9c | 75.0e | 82.9d | 89.1d | 75.6e | 67.6d | 73.8e |
| PHY+ | 83.2cd | 76.2cd | 78.6bc | 78.1cd | 84.8bc | 91.1abc | 78.3cde | 71.7abc | 77.1cd | |
| SEM | 0.79 | 0.97 | 1.02 | 0.98 | 0.65 | 0.69 | 0.97 | 1.05 | 1.03 | |
| P-values | ||||||||||
| Species | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | |
| CaP | 0.039 | 0.806 | 0.230 | 0.415 | 0.163 | 0.944 | 0.178 | 0.637 | 0.454 | |
| PHY | 0.140 | 0.028 | 0.063 | 0.056 | 0.031 | 0.029 | 0.064 | 0.017 | 0.036 | |
| Species × CaP | 0.765 | 0.145 | 0.851 | 0.467 | 0.955 | 0.145 | 0.498 | 0.208 | 0.621 | |
| Species × PHY | 0.216 | 0.295 | 0.086 | 0.102 | 0.174 | 0.213 | 0.087 | 0.305 | 0.137 | |
| CaP × PHY | 0.028 | 0.033 | 0.111 | 0.088 | 0.150 | 0.038 | 0.146 | 0.043 | 0.091 | |
| Species × CaP × PHY | 0.017 | 0.009 | 0.016 | 0.012 | 0.029 | 0.007 | 0.015 | 0.026 | 0.012 | |
Calculated composition: CaP–, 4.1 g P/kg and 5.5 g Ca/kg; CaP+, 9.0 g P/kg and 12.0 g Ca/kg; PHY–, no supplemented phytase; PHY+: 1,500 FTU/kg supplemented phytase.
Methionine determined as methionine sulfone.
Means within a column not sharing a common superscript differ (P < 0.05).
Table 9.
Effect of Ca and P level (CaP) and phytase supplementation (PHY) on prececal non-essential amino acid digestibility (%) of broilers and turkeys at 21 d of age.
| Treatment1 | Cys2 | Tyr | Ala | Asx3 | Glx3 | Gly | Pro | Ser | |
|---|---|---|---|---|---|---|---|---|---|
| Broiler | |||||||||
| CaP– | PHY– | 64.2b | 78.8bc | 76.3b | 73.8abc | 82.5abc | 71.4a | 77.2bc | 74.1bc |
| PHY+ | 68.1ab | 81.6a | 79.5a | 76.0a | 84.4a | 73.9a | 79.1ab | 77.0a | |
| CaP+ | PHY– | 68.4ab | 79.1bc | 77.0ab | 73.1bc | 82.0abc | 72.1a | 77.6abc | 74.4abc |
| Phy+ | 70.5a | 81.0ab | 79.1a | 75.2ab | 83.6ab | 74.0a | 79.8a | 76.6ab | |
| Turkey | |||||||||
| CaP– | PHY– | 54.1cd | 79.1bc | 77.2ab | 74.0ab | 81.3bc | 71.4a | 75.5cd | 73.6cd |
| Phy+ | 50.0de | 76.8cd | 74.4bc | 71.4cd | 78.1d | 68.7bc | 72.6ef | 71.1de | |
| CaP+ | PHY– | 46.8e | 74.9d | 73.1c | 70.1d | 77.2d | 66.7c | 70.5f | 68.5e |
| Phy+ | 55.2c | 77.7c | 76.9ab | 73.0bc | 79.8cd | 71.1ab | 74.1de | 72.7cd | |
| SEM | 1.72 | 0.94 | 1.07 | 1.01 | 0.99 | 1.12 | 0.99 | 1.05 | |
| P-values | |||||||||
| Species | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| CaP | 0.326 | 0.164 | 0.631 | 0.155 | 0.144 | 0.550 | 0.360 | 0.194 | |
| PHY | 0.027 | 0.042 | 0.030 | 0.074 | 0.248 | 0.037 | 0.073 | 0.017 | |
| Species × CaP | 0.062 | 0.211 | 0.506 | 0.718 | 0.682 | 0.262 | 0.082 | 0.214 | |
| Species × PHY | 0.717 | 0.096 | 0.144 | 0.128 | 0.110 | 0.328 | 0.197 | 0.210 | |
| CaP × PHY | 0.023 | 0.089 | 0.063 | 0.043 | 0.038 | 0.026 | 0.014 | 0.035 | |
| Species × CaP × PHY | 0.003 | 0.019 | 0.011 | 0.035 | 0.023 | 0.008 | 0.021 | 0.010 | |
Calculated composition: CaP–, 4.1 g P/kg and 5.5 g Ca/kg; CaP+, 9.0 g P/kg and 12.0 g Ca/kg; PHY–, no supplemented phytase; PHY+: 1,500 FTU/kg supplemented phytase.
Cysteine determined as cysteine sulfone.
The amide residue in the side group of asparagine and glutamine is lost during acid hydrolysis and aspartic acid and glutamic acid are formed. Hence, aspartic acid together with asparagine (Asx) and glutamic acid together with glutamine (Glx) was analyzed.
Means within a column not sharing a common superscript differ (P < 0.05).
DISCUSSION
The hypothesis that broilers exert an overall higher capability of InsP6 degradation than turkeys when fed the same feed must be rejected for 3-wk-old birds as there were no differences in jejunal mucosal phosphatase activity, prececal InsP6 disappearance, InsP3-6 concentrations in the digesta along the digestive tract, and prececal P and Ca digestibility between broilers and turkeys fed the CaP–PHY– diet. Broilers showed higher prececal InsP6 disappearance than turkeys only when phytase was supplemented. The similarities between broilers and turkeys found in the CaP–PHY– treatments indicate that 3-wk-old broilers and turkeys had the same endogenous InsP6 degradation when fed diets with low P and Ca concentrations and no phytase supplementation. These findings are inconsistent with previous comparative studies that reported higher prececal InsP6 disappearance in broilers than in turkeys (Ingelmann et al., 2019; Olukosi et al., 2020). However, in these studies, species-specific diets were compared, which differed markedly in the concentrations of nutrients such as P and Ca. In chickens, adverse effects on phytate degradation have been reported for high dietary concentrations of mineral P (Olukosi et al., 2013; Shastak et al., 2014; Walk et al., 2014; Zeller et al., 2015b; Sommerfeld et al., 2018; Siegert et al., 2019) and Ca (Tamim et al., 2004; Sommerfeld et al., 2018). Possible reasons for these adverse effects are the end-product inhibition of phytase by mineral P (Greiner et al., 1993; Zeller et al., 2015b) and precipitation and subsequent inaccessibility for the enzyme caused by chelating Ca2+ ions (Walk et al., 2012; Sommerfeld et al., 2018). There is no indication that these mechanisms do not affect turkeys. In the present study, high CaP levels markedly reduced prececal InsP6 disappearance in broilers and turkeys compared to low CaP levels, although slightly more in broilers. Hence, it is likely that in the previously mentioned comparative studies, differences in P and Ca concentrations of the feed were largely responsible for the observed differences in prececal InsP6 disappearance and not the inherent capabilities of the animals.
In broilers, Rodehutscord et al. (2012) found a slight reduction of prececal P digestibility when dietary P concentrations increased incrementally to 8.1 g/kg using MCP. Further, when dietary P concentrations increased incrementally to 7.7 g/kg DM, Rodehutscord and Dieckmann (2005) reported a reduction in the percentage of retained P in broilers and an increase in the percentage of retained P in turkeys, using the same feed for both species. Thus, the high levels of supplemented P and Ca in the present trial were likely absorbed more by turkeys than broilers, as in PHY– treatments, increased dietary Ca and P concentrations led to higher prececal P digestibility in turkeys but not in broilers. In addition, Ca digestibility in the CaP+ treatments was higher in turkeys than in broilers. This led to higher Ca and P concentrations in the ileum of broilers than in turkeys in the CaP+ treatments, whereas in CaP– treatments Ca and P concentrations in the ileum were the same for both species (data not shown). Thus, the greater effect of CaP+ on prececal InsP6 disappearance in broilers than in turkeys is assumed to be caused by the aforementioned adverse effects of higher P and Ca concentrations in the gut of broilers than in turkeys. This is assumed to have led to the significantly lower prececal InsP6 disappearance in broilers than in turkeys in the CaP+PHY– treatment. A negative value for prececal InsP6 disappearance in broilers was calculated for the CaP+PHY– treatment. Because InsP6 formation in the digestive tract is unlikely to occur, this effect was likely an artefact of the marker method. Therefore, it was assumed that, in this treatment, there was very little or no prececal InsP6 disappearance.
Sommerfeld et al. (2019) showed that up to 42% of prececal InsP6 disappearance can be attributed to endogenous mucosal phosphatase activity in broilers. In the present study, similar jejunal mucosal phosphatase activities between turkeys and broilers in the CaP–PHY– treatment coincided with similar prececal InsP6 disappearance and prececal P digestibility; however, it remains unclear to what extent the mucosal phytase activity contributed to phytate degradation, because enzymes from resident microbiota or the feed ingredients, not analyzed by the product-specific ELISA method, might have been involved. The higher mucosal phosphatase activity caused by the supplemented phytase could have been triggered by the relatively high concentrations of Ins(1,2,5,6)P4 found in the gizzard, which Walk et al. (2018) hypothesized is due to a triggering mechanism of lower InsP. Similar to the treatments without phytase supplementation, the contribution of mucosal phosphatase to phytate degradation is unclear.
As expected, supplementation with phytase increased prececal InsP6 degradation in both species. However, the effect of phytase supplementation was much greater in broilers than in turkeys. The InsP6 concentration in the crop was reduced by phytase supplementation only in broilers. When the crop was opened, we observed that feed pellets were still visible in turkeys other than broilers, whose crop content was a moist mash. Also, there was a difference in weight loss of digesta samples from the crop during freeze-drying between broilers and turkeys (broilers: 65.7%, turkeys: 36.8% weight loss, P < 0.001, data not shown). Thus, the difference in InsP6 disappearance might have been caused by the higher water content in the crop of broilers compared to turkeys, as supplemented phytase might have lacked a medium to access phytate in turkeys. The lack of an effect of phytase supplementation on InsP6 concentration in the crop of turkeys may explain the lower prececal InsP6 disappearance in turkeys compared to broilers. However, differences in prececal InsP6 disappearance were not as severe as the differences in crop InsP6 concentration might suggest. Possible explanations for this could be the following: Lower pH in the gizzard and duodenum of turkeys compared to broilers could have led to higher InsP6 solubility (Grynspan and Cheryan, 1983) and, consequently, more InsP6 degradation in these sections. In addition, according to Menezes-Blackburn et al. (2015), pH was slightly closer to the optimum of the supplemented phytase in the gizzard and duodenum of turkeys than in broilers. Furthermore, differences in retention time could not be ruled out as a reason for the species effect.
In the crop of broilers, there was a reduction in InsP6 and Ins(1,2,4,5,6)P5 concentrations when supplemented with phytase. The small variation in Ins(1,2,3,4,5)P5 concentration in the crop of all treatments indicates that the release of the first two phosphate groups from InsP6 occurred at a similar pace. Elevated Ins(1,2,5,6)P4 concentrations in the digestive tract in PHY+ treatments indicate that the supplemented phytase was limited in activity in the third degradation step. This is consistent with results from previous studies using 6-phytases in broilers (Walk et al., 2014; Zeller et al., 2015a,b; Krieg et al., 2020; Olukosi et al., 2020; Kristoffersen et al., 2021) and turkeys (Olukosi et al., 2020). However, in the terminal ileum Ins(1,2,5,6)P4 concentrations were substantially elevated only in CaP+PHY+ treatments, indicating inhibitory effects of high CaP level on mucosal phosphatase activity.
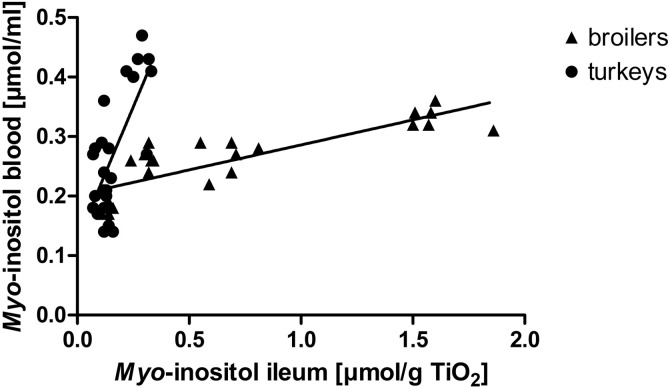
The higher MI concentrations in the ileum of broilers compared to turkeys appear to be inconsistent with similarly high prececal InsP6 disappearance and mucosal phosphatase activity, especially in the CaP–PHY– treatment. However, in the blood, MI concentrations were similar in both species, except for the treatment CaP–Phy+, where turkeys exerted a higher MI concentration than broilers, even though their prececal InsP6 disappearance was lower. In addition, a linear relationship between MI concentrations in the blood and ileum was observed in both species (Figure 1); however, the regression slope was greater in turkeys than in broilers. Thus, it is speculated that the lower concentration of MI in the ileum of turkeys was caused by a higher uptake rate, a different uptake location in the small intestine, a different turnover rate in the enterocytes, or a different transport rate from the blood to organs of turkeys. A concentration-dependent increase in active MI transport in the small intestine of broilers has been previously discussed (Walk et al., 2018), but the authors are unaware of comparable information on turkeys.
Figure 1.
Myo-inositol concentrations in ileum digesta (per g TiO2) and blood of broilers and turkeys fed the experimental diets at 21 d of age. Linear regression broilers: y = 0.08 x + 0.20, r2 = 0.69. Linear regression turkeys: y = 0.89 x + 0.13, r2 = 0.53.
P digestibility increased with phytase supplementation following an increase in InsP6 degradation. In addition, Ca digestibility increased with phytase supplementation, possibly due to fewer Ca2+ ions forming complexes with phytate and a higher metabolic Ca demand when more P is absorbed and retained in bones.
As reviewed by Rodehutscord et al. (2022), phytase supplementation increased AA digestibility in some, but not all, studies. In the present study, AA digestibility in broilers was significantly increased in 9 out of 17 analyzed AA, but only when the CaP level was low. This indicates that AA digestibility in broilers was affected by prececal InsP6 disappearance and unknown factors linked to dietary P and Ca concentrations. In turkeys, the CaP level and supplemented phytase effects were considerably different, as an increase in prececal AA digestibility due to phytase supplementation was only seen when the CaP level was high (14 out of 17 analyzed AA). In contrast, when the CaP level was low, phytase supplementation decreased prececal AA digestibility in 8 out of 17 analyzed AA. This might have been related to different digesta passage rate owing to differences in small intestine lengths between PHY+ and PHY– (Table 7). In CaP+ treatments such effect might have been compensated by higher absolute length of the small intestine. In any case, differences indicate that prececal AA digestibility is affected differently by P, Ca, and InsP6 concentrations in turkeys and broilers.
As 2 species were compared that differ markedly in their maturation rates (Zuidhof et al., 2014; Tůmová et al., 2020), it is necessary to evaluate whether observed differences in phytate degradation and nutrient digestibility can be attributed to species effects or if effects of different physiological development were involved. Thus, a second trial was conducted with 6 wk-old turkeys and broilers, receiving similar dietary treatments as in the present trial (Novotny et al., 2023). Many findings of the present study were confirmed in 6 wk-old birds. However, it was found that age affected turkeys and broilers differently regarding endogenous mucosal phosphatase activity and prececal InsP6 disappearance, both increasing with age in turkeys. This resulted in higher prececal InsP6 disappearance in 6 wk-old turkeys than 6 wk-old broilers, when no phytase was supplemented. As such, higher mucosal phosphatase activity coincided with higher prececal InsP6 disappearance. Comparisons are being discussed in more detail in the companion paper (Novotny et al., 2023).
In conclusion, there was no difference in the endogenous InsP6 degradation capabilities between 3-wk-old broilers and turkeys. Previously reported differences between species were possibly owing to differences in diet composition. Furthermore, the effect of phytase supplementation on prececal InsP6 disappearance was greater in broilers than in turkeys, which might be linked to better crop conditions. Also, the uptake and metabolism of MI, as well as the effects of phytase supplementation on AA digestibility, deserve further research, as apparent species differences in these traits require elucidation. Further research is also necessary to clarify the relevance of age of the birds for the studied traits.
Acknowledgments
ACKNOWLEDGMENTS
This work was funded by Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 328017493/GRK 2366 (Sino-German International Research Training Group AMAIZE-P).
Disclosures
The authors declare no conflicts of interest.
REFERENCES
- Gesellschaft für Ernährungsphysiologie . DLG-Verlag; Frankfurt am Main, Germany: 1999. Empfehlungen zur Energie-und Nährstoffversorgung der Legehennen und Masthühner (Broiler) [Google Scholar]
- Gesellschaft für Ernährungsphysiologie Empfehlungen zur Energie- und Nährstoffversorgung der Mastputen. Proc. Soc. Nutr. Physiol. 2004;13:199–233. [Google Scholar]
- Gonzalez-Uarquin F., Molano E., Heinrich F., Sommerfeld V., Rodehutscord M., Huber K. Research Note: Jejunum phosphatases and systemic myo-inositol in broiler chickens fed without or with supplemented phytase. Poult. Sci. 2020;99:5972–5976. doi: 10.1016/j.psj.2020.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner R., Konietzny U., Jany K.D. Purification and characterization of two phytases from Escherichia coli. Arch. Biochem. Biophys. 1993;303:107–113. doi: 10.1006/abbi.1993.1261. [DOI] [PubMed] [Google Scholar]
- Grynspan F., Cheryan M. Calcium phytate: Effect of ph and molar ratio on in vitro solubility. J. Am. Oil Chem. Soc. 1983;60:1761–1764. [Google Scholar]
- Huber K., Zeller E., Rodehutscord M. Modulation of small intestinal phosphate transporter by dietary supplements of mineral phosphorus and phytase in broilers. Poult. Sci. 2015;94:1009–1017. doi: 10.3382/ps/pev065. [DOI] [PubMed] [Google Scholar]
- Ingelmann C.-J., Witzig M., Möhring J., Schollenberger M., Kühn I., Rodehutscord M. Phytate degradation and phosphorus digestibility in broilers and turkeys fed different corn sources with or without added phytase. Poult. Sci. 2019;98:912–922. doi: 10.3382/ps/pey438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg J., Siegert W., Berghaus D., Bock J., Feuerstein D., Rodehutscord M. Phytase supplementation effects on amino acid digestibility depend on the protein source in the diet but are not related to InsP6 degradation in broiler chickens. Poult. Sci. 2020;99:3251–3265. doi: 10.1016/j.psj.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoffersen S., Itani K., Benzertiha A., Kierończyk B., Kjos N.P., Svihus B. Effect of crop retention time and acidification of the feed on phytase efficacy in broiler chickens. Br. Poult. Sci. 2021;62:443–451. doi: 10.1080/00071668.2020.1870661. [DOI] [PubMed] [Google Scholar]
- Menezes-Blackburn D., Gabler S., Greiner R. Performance of seven commercial phytases in an in vitro simulation of poultry digestive tract. J. Agr. Food Chem. 2015;63:6142–6149. doi: 10.1021/acs.jafc.5b01996. [DOI] [PubMed] [Google Scholar]
- Novotny M., Sommerfeld V., Krieg J., Kühn I., Huber K., Rodehutscord M. Mucosal phosphatase activity, phytate degradation, and mineral digestibility in 6-week-old turkeys and broilers at different dietary levels of phosphorus and phytase and comparison with 3-week-old animals. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olukosi O.A., Kong C., Fru-Nji F., Ajuwon K.M., Adeola O. Assessment of a bacterial 6-phytase in the diets of broiler chickens. Poult. Sci. 2013;92:2101–2108. doi: 10.3382/ps.2012-03005. [DOI] [PubMed] [Google Scholar]
- Olukosi O.A., González-Ortiz G., Whitfield H., Bedford M.R. Comparative aspects of phytase and xylanase effects on performance, mineral digestibility, and ileal phytate degradation in broilers and turkeys. Poult. Sci. 2020;99:1528–1539. doi: 10.1016/j.psj.2019.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirgozliev V., Oduguwa O., Acamovic T., Bedford M.R. Diets containing Escherichia coli-derived phytase on young chickens and turkeys: effects on performance, metabolizable energy, endogenous secretions, and intestinal morphology. Poult. Sci. 2007;86:705–713. doi: 10.1093/ps/86.4.705. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Kapocius M., Timmler R., Dieckmann A. Linear regression approach to study amino acid digestibility in broiler chickens. Br. Poult. Sci. 2004:85–92. doi: 10.1080/00071660410001668905. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Dieckmann A. Comparative studies with three-week-old chickens, turkeys, ducks, and quails on the response in phosphorus utilization to a supplementation of monobasic calcium phosphate. Poult. Sci. 2005;84:1252–1260. doi: 10.1093/ps/84.8.1252. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Dieckmann A., Witzig M., Shastak Y. A note on sampling digesta from the ileum of broilers in phosphorus digestibility studies. Poult. Sci. 2012;91:965–971. doi: 10.3382/ps.2011-01943. [DOI] [PubMed] [Google Scholar]
- Rodehutscord M., Sommerfeld V., Kühn I., Bedford M.R. In: Enzymes in Farm Animal Nutrition. 3rd ed. Bedford M., et al., editors. Wallinford; United Kingdom: 2022. Phytases: potential and limits of phytate destruction in the digestive tract of pigs and poultry; pp. 124–152. [DOI] [Google Scholar]
- Sharpley A. Agricultural phosphorus, water quality, and poultry production: are they compatible? Poult. Sci. 1999;78:660–673. doi: 10.1093/ps/78.5.660. [DOI] [PubMed] [Google Scholar]
- Shastak Y., Zeller E., Witzig M., Schollenberger M., Rodehutscord M. Effects of the composition of the basal diet on the evaluation of mineral phosphorus sources and interactions with phytate hydrolysis in broilers. Poult. Sci. 2014;93:2548–2559. doi: 10.3382/ps.2014-03961. [DOI] [PubMed] [Google Scholar]
- Siegert W., Zuber T., Sommerfeld V., Krieg J., Feuerstein D., Kurrle U., Rodehutscord M. Prececal amino acid digestibility and phytate degradation in broiler chickens when using different oilseed meals, phytase and protease supplements in the feed. Poult. Sci. 2019;98:5700–5713. doi: 10.3382/ps/pez355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld V., Schollenberger M., Kühn I., Rodehutscord M. Interactive effects of phosphorus, calcium, and phytase supplements on products of phytate degradation in the digestive tract of broiler chickens. Poult. Sci. 2018;97:1177–1188. doi: 10.3382/ps/pex404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld V., van Kessel A.G., Classen H.L., Schollenberger M., Kühn I., Rodehutscord M. Phytate degradation in gnotobiotic broiler chickens and effects of dietary supplements of phosphorus, calcium, and phytase. Poult. Sci. 2019;98:5562–5570. doi: 10.3382/ps/pez309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamim N.M., Angel R., Christman M. Influence of dietary calcium and phytase on phytate phosphorus hydrolysis in broiler chickens. Poult. Sci. 2004;83:1358–1367. doi: 10.1093/ps/83.8.1358. [DOI] [PubMed] [Google Scholar]
- Tůmová E., Gous R.M., Chodová D., Ketta M. Differences in growth and carcass composition of growing male and female turkeys. Czech J. Anim. Sci. 2020;65:330–336. [Google Scholar]
- Walk C.L., Bedford M.R., McElroy A.P. Influence of limestone and phytase on broiler performance, gastrointestinal pH, and apparent ileal nutrient digestibility. Poult. Sci. 2012;91:1371–1378. doi: 10.3382/ps.2011-01928. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Santos T.T., Bedford M.R. Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poult. Sci. 2014;93:1172–1177. doi: 10.3382/ps.2013-03571. [DOI] [PubMed] [Google Scholar]
- Walk C.L., Bedford M.R., Olukosi O.A. Effect of phytase on growth performance, phytate degradation and gene expression of myo-inositol transporters in the small intestine, liver and kidney of 21 day old broilers. Poult. Sci. 2018;97:1155–1162. doi: 10.3382/ps/pex392. [DOI] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Kühn I., Rodehutscord M. Hydrolysis of phytate and formation of inositol phosphate isomers without or with supplemented phytases in different segments of the digestive tract of broilers. J. Nutr. Sci. 2015;4:e1. doi: 10.1017/jns.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E., Schollenberger M., Witzig M., Shastak Y., Kühn I., Hoelzle L.E., Rodehutscord M. Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poult. Sci. 2015;94:1018–1029. doi: 10.3382/ps/pev087. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]