Abstract
Cell-mediated immunity by Th1-type CD4+ T cells is the predominant host defense mechanism against mucosal candidiasis. However, studies using an estrogen-dependent murine model of vaginal candidiasis have demonstrated little to no change in resident vaginal T cells during infection and no systemic T-cell infiltration despite the presence of Candida-specific systemic Th1-type responses in infected mice. The present study was designed to further investigate these observations by characterizing T-cell activation and cell adhesion molecule expression during primary and secondary C. albicans vaginal infections. While flow cytometry analysis of activation markers showed some evidence for activation of CD3+ draining lymph node and/or vaginal lymphocytes during both primary and secondary vaginal Candida infection, CD3+ cells expressing the homing receptors and integrins α4β7, αM290β7, and α4β1 in draining lymph nodes of mice with primary and secondary infections were reduced compared to results for uninfected mice. At the local level, few vaginal lymphocytes expressed integrins, with only minor changes observed during both primary and secondary infections. On the other hand, immunohistochemical analysis of vaginal cell adhesion molecule expression showed increases in mucosal addressin cell adhesion molecule 1 and vascular cell adhesion molecule 1 expression during both primary and secondary infections. Altogether, these data suggest that although the vaginal tissue is permissive to cellular infiltration during a vaginal Candida infection, the reduced numbers of systemic cells expressing the reciprocal cellular adhesion molecules may preempt cellular infiltration, thereby limiting Candida-specific T-cell responses against infection.
Vulvovaginal candidiasis (VVC) is a common mucosal infection that affects an estimated 75% of women at least once during their reproductive years (17). Candida albicans, a commensal fungal organism of the gastrointestinal and female genitourinary tracts, is the causative agent in 85 to 90% of all VVC cases (33). Although most women experience infrequent episodes of VVC, 5 to 10% of otherwise healthy women without any recognizable predisposing factors (i.e., pregnancy, use of oral contraceptives, uncontrolled diabetes mellitus, and antibiotic usage) suffer from recurrent VVC (RVVC) (more than three episodes per annum) (34). An undefined immune deficiency or dysfunction is postulated to be responsible for RVVC (12, 33, 37). Although T-helper1 (Th1)-type cell-mediated immunity (CMI) is the predominant host defense mechanism against mucosal C. albicans infections (3, 28, 29), several clinical studies have demonstrated that most women experience RVVC despite normal levels of Candida-specific systemic Th1-type CMI (8, 14, 23, 35).
Experimental studies with mice have shown that although a C. albicans vaginal infection stimulates systemic Th1-type CMI (9), preinduced Candida-specific systemic CMI was not protective against the vaginal infection (11). In contrast, partial protection against a secondary vaginal challenge with C. albicans was achieved following the spontaneous resolution of a primary challenge in the absence of estrogen, suggestive of a modest, locally acquired immune response (7). Evaluation of local immunity, however, showed no change in the percentage or composition of vaginal T cells in response to vaginal infection (6) and no evidence for systemic T-cell infiltration into the vagina despite increased chemokine production, most notably that of monocyte chemoattractant protein 1 (MCP-1) (30). Moreover, high vaginal levels of transforming growth factor beta (TGF-β), a potent down-regulatory cytokine (21, 22), were found in naïve mice and in mice during experimental vaginal candidiasis together with the absence or low levels of other Th1/Th2 cytokines (36). Clinically, no appreciable differences in Th1/Th2 cytokine profiles were found in cervicovaginal lavage fluid of women with RVVC compared to that of control women without a history of RVVC (5). Together, these data suggested that some form of immunoregulation is acting at the vaginal and/or draining lymph nodes that precludes a more profound local CMI response and/or systemic T-cell infiltration in response to C. albicans vaginal infections.
The vaginal mucosa lacks a specialized area of lymphoid tissue, thus limiting the localization of antigen presentation (25, 26). Therefore, local immune responses must depend on the activated clonal expansion of resident T cells dispersed throughout the vagina in response to vaginal pathogens and/or the trafficking of systemic effector cells into the vaginal mucosa. T-cell migration or recirculation into inflamed tissues requires the regulated and sequential adhesion of T-cell homing receptors (i.e., selectins and integrins) on activated cells to their complementary ligands expressed primarily on tissue endothelium at the site of antigen deposition (31). The purpose of this study was to examine putative manifestations of immunoregulation by evaluating vaginal and systemic T-cell activation and homing receptors during primary and secondary C. albicans vaginal infections together with vaginal endothelial cell adhesion molecule expression.
MATERIALS AND METHODS
Mice.
Female CBA/J (H-2k) mice, 8 to 10 weeks of age (purchased from the National Cancer Institute, Frederick, Md.), were used throughout these studies. All animals were housed and handled according to institutionally recommended guidelines.
Antibodies.
For flow cytometry experiments, phycoerythrin-, biotin-, and fluorescein isothiocyanate-conjugated antibodies specific for CD3, CD11a (LFA-1), interleukin 2 receptor alpha (CD25), CD29 (integrin β1), CD49d (integrin α4), CD62L (L-selectin), CD69 (H1.2F3), CD103 (M290), and integrin β7 were purchased from BD PharMingen Corp., San Diego, Calif. Biotin-conjugated antibodies were identified with Cy-Chrome-conjugated streptavidin (BD PharMingen). Fluorochrome-conjugated isotype control antibodies included hamster immunoglobulin G (IgG), hamster IgM, rat IgG2a, and rat IgM (BD PharMingen). Antibodies used for immunohistochemistry included purified anti-vascular cell adhesion molecule 1 (anti-VCAM-1) antibody (MVCAM.A), anti-mucosal addressin cell adhesion molecule 1 (anti-MAdCAM-1) antibody (MECA-367), and anti-intercellular adhesion molecule 1 (anti-ICAM-1) antibody (3E2) (BD PharMingen). The purified isotype control antibodies included rat IgG and hamster IgG (BD PharMingen).
Yeast isolate.
A laboratory-cultivated clinical isolate of C. albicans (strain 3153A) was used to initiate vaginitis. The yeast isolate was grown to stationary phase in 1% Phytone-peptone medium (Becton Dickinson, Cockeysville, Md.) supplemented with 0.1% glucose for 16 to 18 h at 25°C in a shaking water bath. The culture was then washed twice with phosphate-buffered saline (PBS) and quantified using trypan blue dye exclusion.
Primary and secondary vaginal infections.
Primary and secondary C. albicans vaginal infections were initiated as previously described (7). Briefly, mice that were to experience a secondary vaginal infection received an initial inoculum of 5 × 105 viable C. albicans 3153A blastospores in 20 μl of PBS intravaginally in the absence of exogenous estrogen and were allowed 4 weeks to resolve the infection. At the conclusion of the fourth week, vaginal lavages were performed to confirm the resolution of infection. Those mice with negative lavage cultures received a subcutaneous injection of 0.02 mg of β-estradiol 17-valerate (estrogen) (Sigma, St. Louis, Mo.) in 100 μl of sesame oil (Sigma), followed 72 h later by a vaginal inoculation with 5 × 104 viable C. albicans blastospores in a volume of 20 μl of PBS. Controls included mice treated with estrogen and inoculated with C. albicans (primary infection) or given PBS alone (uninfected). On days 2, 4, 10 (mice with primary and secondary infections), and 17 (mice with primary infection cell-only) postinoculation, separate groups of mice were sacrificed, vaginal lavages were conducted using 100 μl of sterile PBS, and the fluid was cultured at 1:10 serial dilutions on Sabouraud-dextrose agar plates (Beckton Dickinson) supplemented with gentamicin (Sigma) as previously described (10). CFU were enumerated after incubation at 35°C for 48 h.
Isolation of lymphoid cells.
Vaginal and lymph node cells (LNC) were isolated and processed as previously described (10, 13). For vaginal lymphocytes (VL), mice vaginae were excised, the cervix was removed, and the tissue was minced using a sterile scalpel in complete tissue culture medium consisting of RPMI 1640 medium supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), l-glutamine (2 mM), 2-mercaptoethanol (5 × 10−5 M), sodium pyruvate (2 mM), HEPES buffer (HEPES; 20 mM), and 5% heat-inactivated fetal bovine serum (FBS) (Life Technologies, Grand Island, N.Y.). The tissues were then digested in complete tissue culture medium with 0.25% collagenase type IV (Sigma) for 45 min at 37°C in a shaking water bath with intermittent (15 s) Stomacher (Tekmar, Cincinnati, Ohio) homogenization every 15 min. After dissociation, the resultant suspension was filtered through sterile nylon mesh of various pore sizes (40 and 20 μm). The lymphoid cell-enriched cells then were collected by centrifugation (800 × g) for 10 min, washed twice in Hanks balanced salt solution (HBSS), and enumerated by trypan blue dye exclusion. For LNC, mesenteric, inguinal and lumbar lymph nodes or lumbar lymph nodes alone were collected and prepared as single-cell suspensions as previously described (10, 13). Briefly, LNC were made into single-cell suspensions by passage through a sterile mesh screen. The cells were then washed and resuspended in HBSS, and the concentration and viability were determined using trypan blue dye exclusion.
T-cell stimulation in vitro.
Mesenteric, inguinal, and lumbar LNC were isolated from naïve mice and processed into single-cell suspensions as previously described (10, 13). LNC (2 × 106 cells/ml) were cultured in complete tissue culture medium alone or supplemented with concanavalin A (ConA) (2 μg/ml) (Sigma) at 37°C in an atmosphere of 7.5% CO2 for 24 h. A portion of the cells (2 × 106 cells/ml) with or without ConA was removed from culture and cultured in the presence of 0.25% collagenase type IV (Sigma) for 45 min to evaluate the effects of collagenase treatment on cell surface molecule expression. Following the final culture the cells were washed in HBSS, enumerated using trypan blue dye exclusion, and subjected to flow cytometry for activation marker or homing receptor expression.
Flow cytometry.
Standard methodology was employed for the direct and indirect immunofluorescence of vaginal cells and LNC. Briefly, 105 VL or 106 LNC were pelleted into the wells of a 96-well U-bottom tissue culture cluster (Corning, Inc., Corning, N.Y.) by centrifugation (250 × g). The cells were then incubated for 30 min on ice with an optimal concentration of fluorochrome-conjugated antibodies (between 0.06 and 0.25 μg/106 cells) in various combinations to allow for dual- or triple-staining experiments in a volume of 100 μl of PBS supplemented with 2% heat-inactivated FBS (PBS-FBS). Following incubation, the cells were washed with PBS-FBS. Biotinylated samples were then resuspended in 100 μl of PBS-FBS containing an optimal concentration of Cy-Chrome-conjugated streptavidin (BD PharMingen) for 30 min on ice. All other samples were resuspended in 100 μl of PBS-FBS and incubated for 30 min on ice. After incubation, the cells were washed with PBS-FBS and fixed in 400 μl of Poly/LEM Fixative (Polysciences, Inc., Warrington, Pa.) diluted 1:4 in PBS-FBS. Cells incubated with either PBS-FBS alone or fluorochrome-conjugated isotype control antibodies were used to determine background fluorescence. The samples were analyzed using software on an Epochs Elite flow cytometer (Coulter, Inc., Miami, Fla.). Dead cells were excluded on the basis of forward angle and 90° light scatter. For data analyses, 5,000 events (cells) were evaluated from a predominantly leukocytic population identified by backgating from CD3+-stained cells and using isotype-specific antibody staining as a negative control. Compensation for each fluorochrome was determined by parallel single-color analysis of cells labeled with each fluorochrome-conjugated antibody.
Immunohistochemistry.
Vaginal tissue was excised (with the cervix attached) using aseptic technique and embedded in optimum-cutting-temperature medium (Sakura Finetek U.S.A., Inc., Torrance, Calif.) within Tissue-Tek cryomolds (Miles, Inc., Elkhart, Ind.). The vaginal tissue was placed into the cryomolds in an orientation that allowed for cross-sectional cutting. Tissue sections were cut (5 to 10 μm), fixed in acetone (3 min), and then washed in PBS for 5 min. Sections were then incubated (1 h) with purified rat anti-mouse VCAM-1, MAdCAM-1, or purified hamster anti-mouse ICAM-1 antibody (10 μg) at room temperature. Control slides were incubated with purified rat IgG or hamster IgG (10 μg) to observe any nonspecific staining. Following incubation, the washed slides were incubated with biotinylated rabbit anti-rat or hamster IgG antibody (10 μg) for 1 h at room temperature. Washed slides then were incubated with avidin-biotin-peroxidase (Vector Laboratories, Burlingame, Calif.) (30 min), washed, and incubated with the substrate 3-amino-9-ethylcarbaxole (Vector Laboratories). Slides were counterstained with hematoxylin (Fisher Diagnostics, Fair Lawn, N.J.) and preserved using Crystal mount (Biomeda Corp., Foster City, Calif.) aqueous mounting solution.
Statistical analysis.
The unpaired Student t test and/or one-way analysis of variance with the Bonferroni post hoc test for multiple comparisons was used to detect significant differences. Significant differences were defined as P < 0.05.
RESULTS
T-cell activation during experimental vaginal candidiasis.
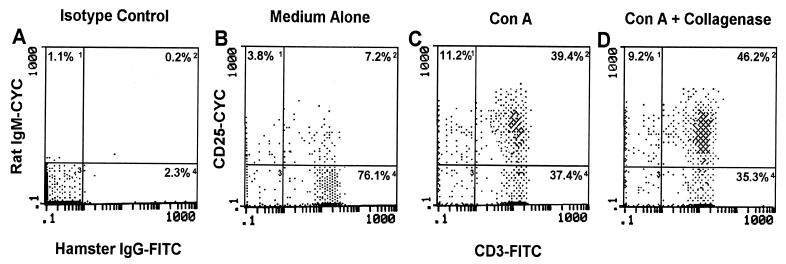
In order to eventually evaluate the activation status (indicated by CD25 expression) of LNC and VL during infection, it was first important to confirm their activation potential and any putative effects that collagenase (required for vaginal cell isolation) may have on cell surface CD25 expression. For this, LNC were stimulated for 24 h in complete tissue culture medium with or without ConA. After 24 h, the cells were cultured an additional 45 min with or without collagenase and CD25 expression was evaluated by flow cytometry. As illustrated in Fig. 1, ConA stimulation led to a nearly sixfold increase in the percentage of CD3+ LNC expressing CD25 in comparison to those cultured in medium alone. In addition, collagenase treatment was shown to have little to no effect on the percentage of stimulated CD3+ LNC expressing CD25. Likewise, collagenase had no affect on the percentage of unstimulated CD3+ LNC expressing CD25 (treated, −8.8%; untreated, −8.6%).
FIG. 1.
Expression of CD25 following ConA stimulation and collagenase type IV treatment. LNC from four mice were cultured alone (B) or in the presence of ConA (C) for 24 h. Following the culture period, ConA-stimulated cells were incubated with or without collagenase (D) for 45 min. At the conclusion of these treatments, the cells were dually labeled with anti-CD3 and anti-CD25 antibodies or isotype control antibodies (A) and were analyzed by flow cytometry using isotype control antibodies to set fluorescent limits (gates). Numbers within each quadrant represent the percentage of fluorescent positive cells within lymphoid cell limits. Data shown are representative of three separate experiments. FITC, fluorescein isothiocyanate; CYC, cytochrome c.
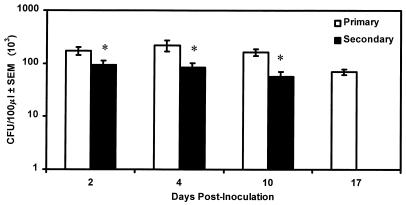
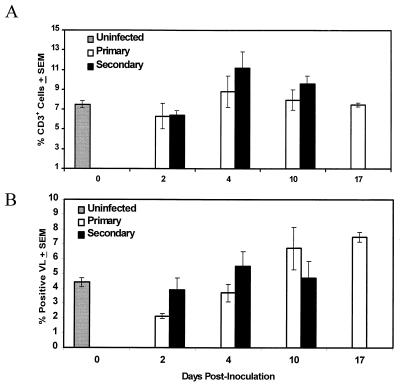
To characterize the activation status of T cells during primary and secondary C. albicans vaginal infections, lymphocytes were isolated from the draining lumbar lymph nodes and vaginae of mice on days 2, 4, 10, and 17 and days 2, 4, and 10 of primary and secondary vaginal infections, respectively, and were evaluated for the percentage of CD3+ cells expressing CD25 by flow cytometry. Vaginal fungal burden was quantified to confirm infection and partial protection. Figure 2 shows that a persistent infection was achieved in mice with primary infection and that partial protection was demonstrated in mice with secondary infection, compared to mice with primary infection (P < 0.05, 0.01, and 0.001 on days 2, 4, and 10, respectively), similar to that reported previously (7, 9). As illustrated in Fig. 3A, there was no significant difference between the percentage of CD3+ LNC expressing CD25 during a primary infection and the percentage found for uninfected mice. In contrast, the percentage of CD3+ LNC from mice with secondary infection expressing CD25 was significantly increased on days 4 and 10 postinoculation (P < 0.05) compared to that found in uninfected mice. There was no significant difference in the percentage of CD3+ cells expressing CD25 between mice with primary and secondary infections (Fig. 3A). As shown in Fig. 3B, compared to the result for uninfected mice, the percentage of VL expressing CD25 in mice with primary infection was significantly decreased on day 2 postinoculation (P < 0.05) and significantly increased on days 10 and 17 postinoculation (P < 0.05 and 0.01, respectively). In mice with secondary infection, the percentage of VL expressing CD25 was significantly higher than that in uninfected mice on day 4 postinoculation (P < 0.05). There was no significant difference in the percentage of VL expressing CD25 between mice with primary and secondary infections (Fig. 3B) at each time point. Analysis of the mean fluorescence intensity (MFI) of CD25 expression on VL and CD3+ LNC showed no differences between groups during infection (data not shown).
FIG. 2.
Vaginal fungal burden during primary or secondary experimental vaginitis. Mice received a primary vaginal inoculation and were allowed 4 weeks to resolve the infection, followed by a second inoculation in the presence of estrogen. Control mice that were previously naïve received a simultaneous primary inoculation in the presence of estrogen. C. albicans vaginal fungal burden was monitored over a 17-day period in mice with primary infection and a 10-day period in mice with secondary infection. Data are cumulative of four experiments using 10 to 13 mice per group. Separate mice were used for each time point. Asterisks indicate where significant decreases from results found in mice with primary infection were observed. SEM, standard error of the mean.
FIG. 3.
Activation marker expression on murine T cells during experimental vaginal candidiasis. Lymphocytes were isolated from the draining lumbar lymph nodes and vaginae of mice with primary and secondary infections as well as of uninfected mice. The LNC (A) and VL (B) were dually labeled with anti-CD3 and anti-CD25 antibodies and analyzed by flow cytometry. Control samples were labeled with isotype control antibodies, and gates were set from these controls. Data shown are cumulative of four experiments using 10 mice per experiment. SEM, standard error of the mean.
Lymphocyte homing receptor expression during experimental vaginal candidiasis.
To evaluate T-cell homing receptor properties during infection, we measured the expression of L-selectin, LFA-1, α4β7, αM290β7, and α4β1 by flow cytometry. L-selectin and LFA-1 are involved in the initial tethering and firm adhesion, respectively, of T cells with their reciprocal receptors on high endothelial venules prior to diapedesis. Integrins α4β7 and αM290β7 are considered important for the migration and retention, respectively, of T cells at mucosal tissues (1, 15), whereas α4β1 integrin, a peripheral homing receptor (32), has been implicated in T-cell homing to the genital tract (27). Prior to the analysis of these receptors during infection, it was first necessary to confirm our ability to detect changes in cells expressing homing receptors and again any potential effects that collagenase may have on their surface expression. For this, LNC were evaluated by flow cytometry for homing receptors immediately following sacrifice and after culture in complete tissue culture medium for 24 h with or without ConA. Following culture, cells were cultured for an additional 45 min with or without collagenase. As shown in Table 1, various levels of CD3+ LNC expressing each homing receptor were observed at the time of sacrifice (0 h). Following culture in medium alone, increases in the percentage of CD3+ cells expressing α4β7, L-selectin, and LFA-1 were detected, whereas CD3+ cells expressing αM290β7 remained relatively unchanged. A slight decrease in the percentage of CD3+ cells expressing α4β1 was also observed. Of note, LFA-1 was constitutively expressed on a high percentage of CD3+ cells (0 h). Results were not different following culture with ConA (data not shown). Nevertheless, increases in the percentage of cells expressing homing receptors were verifiable by culture. The percentage of cells expressing most homing receptors was not significantly affected by collagenase treatment (Table 1). The exception was the percentage of CD3+ LNC expressing L-selectin, which was reduced nearly 70%.
TABLE 1.
Homing receptor expression on LNC
| Marker | % of CD3+ cellsa at:
|
||
|---|---|---|---|
| 0 h | <24 h 45 min
|
||
| (with medium alone) | (with collagenase) | ||
| α4β7 | 48.8 | 69.1 | 63.3 |
| αM290β7 | 14.1 | 16.1 | 13.7 |
| α4β1 | 66.2 | 59.1 | 53.7 |
| LFA-1 | 87.5 | 97.1 | 98.5 |
| L-selectin | 65.0 | 92.2 | 19.0 |
LNC were assayed immediately following isolation (0 h), after 24 h 45 min of culture, and after 24 h 45 min of culture with the last 45 min in culture with 0.25% collagenase type IV.
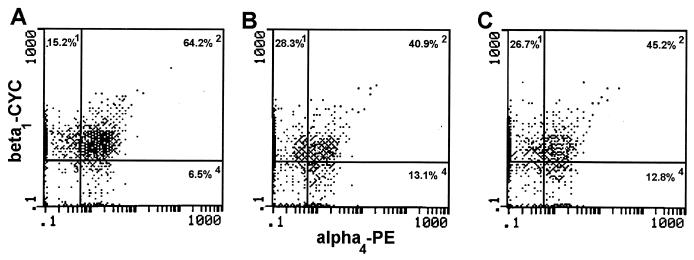
To evaluate lymphocyte homing receptors on T cells during experimental vaginitis, we evaluated homing receptors on LNC and VL during primary and secondary infections by flow cytometry. As shown in Table 2, the percentages of CD3+ LNC expressing L-selectin and LFA-1 during primary or secondary infection were not significantly different from those in uninfected mice. In contrast, fewer CD3+ LNC expressing α4β7, αM290β7, and α4β1 were found at all time points in mice with primary and secondary infections than in uninfected mice, with significant decreases found on several days during either infection. Additionally, the percentages of cells expressing αM290β7 and α4β1 were reduced in mice with secondary infection compared to those in mice with primary infection on day 2. In contrast to the reduction in the percentage of CD3+ LNC expressing homing receptors observed during infection, the MFI of homing receptors expressed on CD3+ LNC derived from mice with primary and secondary infections was similar to that of uninfected mice (data not shown). An example of the reduction in the percentage of CD3+ LNC expressing homing receptors with the same MFI during primary and secondary infections is illustrated in Fig. 4. In this representative example, the MFI of α4β1 on CD3+ LNC was virtually the same for uninfected mice as for mice with primary and secondary infections 10 days postinoculation, while the percentage of CD3+ LNC expressing α4β1 was reduced in infected mice. The percentage of VL expressing homing receptors was low in uninfected mice (Table 3) and was similarly low during both primary and secondary infections. Nevertheless, compared to uninfected mice, significant differences included an increased percentage of VL expressing LFA-1 and α4β7 on select days in mice with secondary infection, more cells expressing αM290β7 on select days during both primary and secondary infections, and fewer cells expressing α4β1 early during primary infection. Similar to homing receptor expression in the periphery, the MFI of homing receptors expressed on VL during infection was similar to that in uninfected mice (data not shown).
TABLE 2.
Cell adhesion molecule expression on draining lumbar LNC during experimental vaginitis
| Type of infection and days postinoculation | % of CD3+ cellsa expressing:
|
||||
|---|---|---|---|---|---|
| L-selectin | LFA-1 | α4β7 | αM290β7 | α4β1 | |
| No infection | 65.2 ± 3.7 | 84.3 ± 4.0 | 49.0 ± 5.5 | 18.4 ± 0.8 | 67.2 ± 4.0 |
| Primary infection | |||||
| 2 | 56 ± 8.6 | 77.6 ± 9.9 | 40.0 ± 7.8 | 18.4 ± 2.2 | 67.2 ± 5.9 |
| 4 | 72.5 ± 6.3 | 90.8 ± 4.9 | 43.2 ± 6.1 | 15.0 ± 1.4b | 59.4 ± 4.0 |
| 10 | 71.3 ± 13 | 87.1 ± 4.7 | 28.7 ± 6.0b | 13.3 ± 1.9b | 41.4 ± 6.0b |
| 17 | 64.6 ± 1.9 | 96 ± 0.5 | 21.4 ± 3.2b | 11.9 ± 0.9b | 30.8 ± 5.4b |
| Secondary infection | |||||
| 2 | 53.6 ± 8.0 | 77.5 ± 8.3 | 26.8 ± 4.3b | 11.5 ± 1.3bc | 48.9 ± 2.8bc |
| 4 | 64.7 ± 5.3 | 82 ± 7.9 | 34 ± 6.0 | 12.9 ± 1.0b | 51.3 ± 4.7b |
| 10 | 55.9 ± 18 | 86.6 ± 6.6 | 30.5 ± 9.6 | 12.2 ± 0.8b | 43.3 ± 7.7b |
Values are the mean ± standard error of the mean for three to four experiments using lymphocytes pooled from 10 mice per group.
Significantly different (P < 0.05) from results for uninfected mice.
Significantly different (P < 0.05) from results for mice with primary infections on the same day of infection.
FIG. 4.
Expression of α4β1 on CD3+ LNC during experimental vaginal candidiasis. Lymphocytes were isolated from the draining lumbar lymph nodes of uninfected mice (A), mice with primary infection (B), and mice with secondary infection (C) on day 10 postinoculation. The LNC were triply labeled with anti-CD3, anti-α4, and anti-β1 antibodies and analyzed by flow cytometry. The numbers within each histogram represent the percentage of gated CD3+ cells present within each quadrant. PE, phycoerythrin; CYC, cytochrome c.
TABLE 3.
Cell adhesion molecule expression on VL during experimental vaginal candidiasis
| Type of infection and days postinoculation | % of VLa expressing:
|
|||
|---|---|---|---|---|
| LFA-1 | α4β7 | αM290β7 | α4β1 | |
| No infection | 7.3 ± 1.6 | 1.8 ± 0.3 | 1.49 ± 0.2 | 6.1 ± 0.9 |
| Primary infection | ||||
| 2 | 4.1 ± 0.1 | 1 ± 0.2 | 1 ± 0.2 | 2.4 ± 0.4b |
| 4 | 5.1 ± 1.2 | 2.7 ± 0.6 | 2.2 ± 0.6 | 5.8 ± 1.1 |
| 10 | 8.3 ± 3.0 | 2.7 ± 0.6 | 2.7 ± 0.6b | 6.2 ± 1.1 |
| 17 | 5.4 ± 0.9 | 2.8 ± 0.7 | 4.1 ± 1.5b | 7.0 ± 2.0 |
| Secondary infection | ||||
| 2 | 5.3 ± 4.3 | 1.75 ± 0.1 | 1.5 ± 0.2 | 4.9 ± 1.3 |
| 4 | 8 ± 2.9 | 2.9 ± 0.3b | 3.0 ± 0.7b | 7.9 ± 1.4 |
| 10 | 13.2 ± 1.7b | 3.1 ± 0.5b | 3.7 ± 0.6b | 7.9 ± 3.6 |
Values are the mean ± standard error of the mean for three to four experiments using lymphocytes pooled from 10 mice per group.
Significantly different (P < 0.05) from results for uninfected mice.
Vaginal endothelial cell adhesion molecule expression during infection.
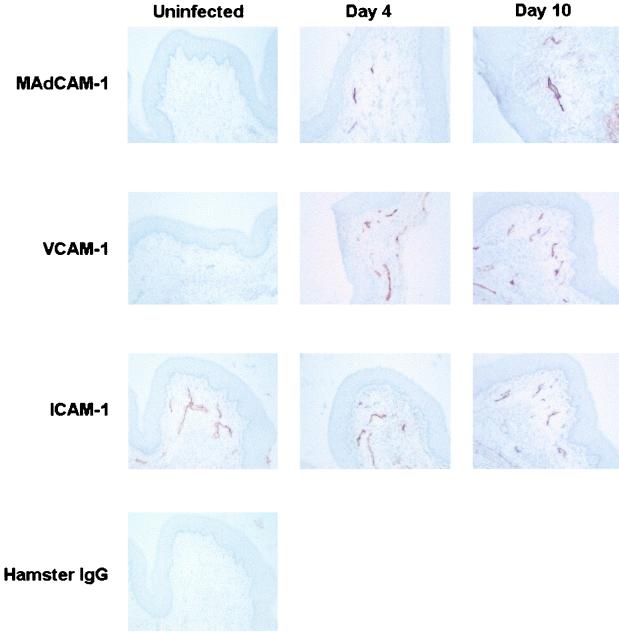
To evaluate the capacity of the vaginal endothelium to support a systemic lymphocyte infiltration, vaginal tissue sections from mice with primary and secondary infections were analyzed for their expression of MAdCAM-1, VCAM-1, and ICAM-1 on days 4 and 10 postinoculation by immunohistochemisty and were compared to sections from uninfected mice. As illustrated in Fig. 5, ICAM-1 but not MAdCAM-1 and VCAM-1 was found constitutively expressed on vaginal endothelial venules of uninfected mice. Vaginal endothelial venules were found to express MAdCAM-1 and VCAM-1 on days 4 and 10 in mice with primary infection. ICAM-1 expression remained unchanged during infection. Similar results were observed in mice with secondary infection with no differences observed between mice with primary and secondary infections (data not shown).
FIG. 5.
Expression of endothelial cell adhesion molecules at the vaginal mucosa during experimental vaginal candidiasis. Frozen vaginal tissue sections from mice with primary infection and uninfected mice were labeled with anti-MAdCAM-1 (A to C), VCAM-1 (D to F), or ICAM-1 (G to I) antibodies. Frozen sections were also labeled with isotype-matched control antibodies to observe any background staining; however, only labeling with hamster IgG (J) is shown to confirm the positive staining for ICAM-1 in uninfected mice. The figure shows representative staining for several mice from four experiments.
DISCUSSION
The present study evaluated T-cell activation and homing receptor expression during experimental vaginal candidiasis in order to better understand the lack of a substantial protective response by CMI against Candida vaginitis. To this end, studies evaluating T-cell activation in the draining lymph nodes during infection showed that despite controls that confirmed our ability to detect activation (increases in the percentage of CD3+ and CD25+ LNC following ConA stimulation), the percentage of LNC expressing CD25 remained relatively unchanged during a primary infection. This was supported by the observation of neither fewer cells expressing L-selectin nor an increased number of cells expressing LFA-1, which is normally associated with T-cell activation (18). Also, the intensity of L-selectin and LFA-1 expression on these cells did not change during infection. Although these observations suggest that T cells in the draining lymph nodes of mice with primary infection are not being activated, Candida-specific T cells clearly exist in the draining lymph nodes, based on in vitro studies that found Th1-type cytokine production in response to Candida antigens (10). This represents the second instance using this model where in vivo observations have not supported in vitro results (36), suggesting that putative in vivo immunoregulatory events may not similarly be present and/or functional ex vivo.
The decreased percentage of CD3+ cells expressing α4β7, αM290β7, and α4β1 integrins in the draining lymph nodes during a primary infection gives additional support to a putative in vivo immunoregulation and may represent a critical manifestation of such. In addition, these homing receptors may be directly involved in regulating the anti-Candida response, since the cellular changes were evident during times when an adaptive anti-Candida response would be expected (days 10 to 17). In particular, α4β7 integrin has been shown to be important for cellular infiltration into the genital tract in response to experimental Chlamydia trachomatis infections (16, 20). Thus, the absence of a vaginal cellular infiltrate in response to a primary infection may be explained by the observed reduction in T cells expressing these integrins, particularly α4β7, in the draining lymph nodes.
Locally, there was some evidence for both vaginal T-cell activation and integrin modulation during a primary infection—the increased percentage of cells expressing CD25 and αM290β7 with no demonstrable effects of collagenase treatment on receptor expression. The expression of all other homing receptors on VL evaluated during a primary infection remained unchanged. Due to an observed reduction in L-selectin expression following collagenase treatment attributable to either enzymatic cleavage or the removal or disruption of antibody-reactive epitopes, changes in L-selectin expression on VL during infection could not be evaluated. The increases in CD25 and αM290β7 were observed during a time when adaptive responses would be expected (days 10 and 17). The increased percentage of VL expressing CD25 and αM290β7, however, did not correlate with any change in the C. albicans fungal burden. Perhaps, despite an increase in the percentage of VL expressing CD25 and αM290β7, the numbers of VL were not sufficiently high enough to have an effect on the infection. In fact, despite this evidence for modest local T-cell activation, similar to a previous report by our laboratory (6), the percentage and composition of vaginal T cells remained unchanged during the experimental infection. This provides yet additional support for the concept of immunoregulation at the vaginal mucosa.
Mice given a second vaginal challenge of C. albicans following resolution of a primary infection were partially protected as reported previously (7). This is largely supported by the results of the present study showing modest increases in the percentage of activated local or systemic T cells or vaginal T cells expressing homing receptors following secondary challenge. However, there were no differences in activation markers or homing receptors between mice with secondary and primary infections, and as previously observed, the protection was not accompanied by any local T-cell changes or systemic T-cell infiltration (6). Overall, decreases in the percentage of systemic T cells expressing homing receptors during a secondary infection (compared to that found in uninfected mice) may be responsible for the lack of a T-cell infiltrate and may be an important factor in the lack of more significant protection. These data are in direct contrast to studies using a rat model of vaginal candidiasis where significant increases in resident VL expressing CD25 were evident together with significant T-cell infiltration following Candida challenge (4). Interestingly, the rats cleared their vaginal fungal burden quickly following secondary exposure, and the clearance was accompanied by significant increases in vagina-associated Th1-type cytokines. The disparate results for the two models give credibility to the immunoregulatory concept in the murine model.
Noteworthy in this study was that changes in CD3+ LNC and VL expressing CD25 and homing receptors during experimental vaginal candidiasis were shown to be associated with overall decreases in the percentage of cells expressing surface markers and not with significant changes in cell surface expression (mean fluorescence intensity). Furthermore, if these data are evaluated on the basis of absolute cell numbers, the interpretations do not change. Based on the analysis restricted to predominantly CD3+ cells, decreases in the percentage of CD3+ cells were observed during infection. However, it is difficult to ascertain from these data whether the CD3+ cells were truly reduced or if CD3− cells were increased in the evaluated population. Regardless, due to greater decreases in CD3+ cells expressing the homing receptors during infection, it can be concluded that in either case CD3+ cells expressing homing receptors were reduced. These declines in CD3+ LNC expressing homing receptors may be interpreted as either their migration out of the draining lymph nodes or apoptosis. Of the cells remaining, we presume that the majority, if Candida specific, do not have the full complement of homing receptors or appropriate signals necessary to migrate to the vaginal mucosa.
In contrast to the effects on the T cells that may preclude migration into the tissue, the vaginal endothelium had the expected increased expression of MAdCAM-1 and VCAM-1 during both primary and secondary infections. Thus, the lack of systemic T-cell infiltration during vaginal candidiasis appears associated more with reduced numbers of T cells expressing homing receptors specific for the reciprocal molecules on the vaginal endothelium than with the capacity for the vaginal endothelium to support cellular migration. This is consistent with the lack of cellular migration following an increase in a chemokine for T-cell and monocyte migration, mcl-1, during an experimental vaginal Candida infection (30).
Taken together, fewer numbers of cells expressing homing receptors and modest activation in vivo during primary and secondary vaginal Candida infections are consistent with the concept of local immunoregulation. Although the vaginal mucosa can serve as an inductive site for tolerance (2), this immunoregulation and/or tolerance appears specific to C. albicans. This is exemplified by the fact that host responses to herpes simplex virus type 2 (24) or C. trachomatis (16, 20) genital tract infections involve T-cell activation in the draining lymph nodes, followed by CD4 T-cell migration into the vaginal mucosa orchestrated by adhesive interactions between T-cell and vaginal endothelial cell adhesion molecules. In fact, a dual genital tract infection of mice with C. trachomatis and C. albicans showed complete independence of responses with no influence of the Chlamydia response on the ability of mice to clear the C. albicans infection (19). The adverse effects on the T cells rather than on the vaginal endothelium through which the cells migrate during the Candida infection support this. Perhaps the lack of a more profound response to a vaginal Candida infection (6) is due to the commensal nature of C. albicans for which immune reactivity has evolved to protect commensalism and reduce local inflammation following exposure. An important question will be whether a reversal of such putative immunoregulatory events can allow for a more profound response by these otherwise seemingly competent Candida-specific T cells in the draining lymph nodes (evidenced by Candida-specific proliferation and cytokine production in vitro [10] and delayed-type hypersensitivity in vivo [9]). Identification of immunotherapies that enhance systemic and/or local CMI in response to vaginal Candida infections will be important to the treatment of acute and recurrent C. albicans vaginal infections.
ACKNOWLEDGMENTS
This work was supported by grant AI 32556 and an accompanying minority supplement for underrepresented minorities from the National Institute of Allergy and Infectious Diseases (National Institutes of Health).
REFERENCES
- 1.Austrup F, Rebstock S, Kilshaw P J, Hamann A. Transforming growth factor-β1-induced expression of the mucosa-related integrin αE on lymphocytes is not associated with mucosa-specific homing. Eur J Immunol. 1995;25:1487–1491. doi: 10.1002/eji.1830250602. [DOI] [PubMed] [Google Scholar]
- 2.Black C A, Rohan L C, Cost M, Watkins S C, Draviam R, Alber S, Edwards R P. Vaginal mucosa serves as an inductive site for tolerance. J Immunol. 2000;165:5077–5083. doi: 10.4049/jimmunol.165.9.5077. [DOI] [PubMed] [Google Scholar]
- 3.Cenci E, Mencacci A, Spaccapelo R, Tonnetti L, Mosci P, Enssle K H, Puccetti P, Romani L, Bistoni F. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J Infect Dis. 1995;171:1279–1288. doi: 10.1093/infdis/171.5.1279. [DOI] [PubMed] [Google Scholar]
- 4.DeBernardis F, Santoni G, Boccanera M, Spreghini E, Adriani D, Morelli L, Cassone A. Local anticandidal immune responses in a rat model of vaginal infection by and protection against Candida albicans. Infect Immun. 2000;68:3297–3304. doi: 10.1128/iai.68.6.3297-3304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fidel P L, Jr, Ginsburg K A, Cutright J L, Wolf N A, Leaman D, Dunlap K, Sobel J D. Vaginal-associated immunity in women with recurrent vulvovaginal candidiasis: evidence for vaginal Th1-type responses following intravaginal challenge with Candida antigen. J Infect Dis. 1997;176:728–739. doi: 10.1086/514097. [DOI] [PubMed] [Google Scholar]
- 6.Fidel P L, Jr, Luo W, Steele C, Chabain J, Baker M, Wormley F L. Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect Immun. 1999;67:3135–3140. doi: 10.1128/iai.67.6.3135-3140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidel P L, Jr, Lynch M E, Conaway D H, Tait L, Sobel J D. Mice immunized by primary vaginal Candida albicans infection develop acquired vaginal mucosal immunity. Infect Immun. 1995;63:547–553. doi: 10.1128/iai.63.2.547-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidel P L, Jr, Lynch M E, Redondo-Lopez V, Sobel J D, Robinson R. Systemic cell-mediated immune reactivity in women with recurrent vulvovaginal candidiasis (RVVC) J Infect Dis. 1993;168:1458–1465. doi: 10.1093/infdis/168.6.1458. [DOI] [PubMed] [Google Scholar]
- 9.Fidel P L, Jr, Lynch M E, Sobel J D. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:1990–1995. doi: 10.1128/iai.61.5.1990-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fidel P L, Jr, Lynch M E, Sobel J D. Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:4202–4207. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidel P L, Jr, Lynch M E, Sobel J D. Effects of preinduced Candida-specific systemic cell-mediated immunity on experimental vaginal candidiasis. Infect Immun. 1994;62:1032–1038. doi: 10.1128/iai.62.3.1032-1038.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidel P L, Jr, Sobel J D. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335–348. doi: 10.1128/cmr.9.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fidel P L, Jr, Wolf N A, KuKuruga M A. T lymphocytes in the murine vaginal mucosa are phenotypically distinct from those in the periphery. Infect Immun. 1996;64:3793–3799. doi: 10.1128/iai.64.9.3793-3799.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong I W, McCleary P, Read S. Cellular immunity of patients with recurrent or refractory vulvovaginal moniliasis. Am J Obstet Gynecol. 1992;166:887–890. doi: 10.1016/0002-9378(92)91356-f. [DOI] [PubMed] [Google Scholar]
- 15.Hamann A, Andrew D P, Jablonski-Westrich D, Holzmann B, Butcher E C. Role of α4-integrins in lymphocytes homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 16.Hawkins R A, Rank R G, Kelly K A. Expression of mucosal homing receptor α4β7 is associated with enhanced migration to the Chlamydia-infected murine genital mucosa in vivo. Infect Immun. 2000;68:5587–5594. doi: 10.1128/iai.68.10.5587-5594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurley R, De Louvois J. Candida vaginitis. Postgrad Med J. 1979;55:645–647. doi: 10.1136/pgmj.55.647.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung T M, Dailey M O. Rapid modulation of homing receptors (gp90MEL-14) induced by activators of protein kinase C. J Immunol. 1990;144:3130–3136. [PubMed] [Google Scholar]
- 19.Kelly K A, Gray H L, Walker J C, Rank R G, Wormley F L, Jr, Fidel P L., Jr Chlamydia trachomatis infection does not enhance local cellular immunity against concurrent Candida vaginal infection. Infect Immun. 2001;69:3451–3454. doi: 10.1128/IAI.69.5.3451-3454.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly K A, Rank R G. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65:5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letterio J J, Roberts A B. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 22.Ludviksson B R, Seegers D, Resnick A S, Strober W. The effect of TGF-β on immune responses of naive versus memory CD4+ Th1/Th2 T cells. Eur J Immunol. 2000;30:2101–2111. doi: 10.1002/1521-4141(200007)30:7<2101::AID-IMMU2101>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Mendling W, Koldovsky U. Investigations by cell-mediated immunologic tests and therapeutic trials with thymopentin in vaginal mycoses. Infect Dis Obstet Gynecol. 1996;4:225–231. doi: 10.1155/S1064744996000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milligan G N, Bernstein D I. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 25.Nandi D, Allison J P. Characterization of neutrophils and T lymphocytes associated with the murine vaginal epithelium. Reg Immunol. 1993;5:332–338. [PubMed] [Google Scholar]
- 26.Parr M B, Parr E L. Langerhans cells and T lymphocyte subsets in the murine vagina and cervix. Biol Reprod. 1991;44:491–498. doi: 10.1095/biolreprod44.3.491. [DOI] [PubMed] [Google Scholar]
- 27.Perry L L, Feilzer K, Portis J L, Caldwell H D. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J Immunol. 1998;160:2905–2914. [PubMed] [Google Scholar]
- 28.Romani L, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Puccetti P, Bistoni F. CD+ subset expression in murine candidiasis. J Immunol. 1993;150:925–931. [PubMed] [Google Scholar]
- 29.Romani L, Mocci S, Bietta C, Lanfaloni L, Puccetti P, Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991;59:4647–4654. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saavedra M, Taylor B, Lukacs N W, Fidel P L., Jr Local production of chemokines during experimental vaginal candidiasis. Infect Immun. 1999;67:5820–5829. doi: 10.1128/iai.67.11.5820-5826.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmi M, Jalkanen S. How do lymphocytes know where to go: current concepts and enigmas of lymphocyte homing. Adv Immunol. 1997;64:139–218. doi: 10.1016/s0065-2776(08)60889-5. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu Y, Rose D M, Ginsberg M H. Integrins in the immune system. Adv Immunol. 1999;72:325–380. doi: 10.1016/s0065-2776(08)60024-3. [DOI] [PubMed] [Google Scholar]
- 33.Sobel J D. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann N Y Acad Sci. 1988;544:547–557. doi: 10.1111/j.1749-6632.1988.tb40450.x. [DOI] [PubMed] [Google Scholar]
- 34.Sobel J D. Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis. 1992;14:S148–S153. doi: 10.1093/clinids/14.supplement_1.s148. [DOI] [PubMed] [Google Scholar]
- 35.Syverson R A, Buckley H, Gibian J, Ryan J J M. Cellular and humoral immune status in women with chronic Candida vaginitis. Am J Obstet Gynecol. 1979;134:624–627. doi: 10.1016/0002-9378(79)90641-0. [DOI] [PubMed] [Google Scholar]
- 36.Taylor B N, Saavedra M, Fidel P L., Jr Local Th1/Th2 cytokine production during experimental vaginal candidiasis. J Med Mycol. 2000;38:419–431. doi: 10.1080/mmy.38.6.419.431. [DOI] [PubMed] [Google Scholar]
- 37.Witkin S S. Immunology of recurrent vaginitis. Am J Reprod Immunol Microbiol. 1987;15:34–37. doi: 10.1111/j.1600-0897.1987.tb00147.x. [DOI] [PubMed] [Google Scholar]