Abstract
Background: The B vitamins can potentially help prevent migraine. This study was designed to examine the effects of supplementation with thiamine (B1), pyridoxine (B6), cobalamin (B12), folic acid (B9), and a combination of these vitamins on women with episodic migraine (EM).
Methods: This study was a double-blind, placebo-controlled, randomized, clinical trial conducted on 120 women with EM. The participants were divided into the 6 groups of B1 (n = 20), B6 (n = 20), B12 (n = 20), B9 (n = 20), vitamin B complex (n = 20), and placebo (n = 20). Subjects received 1 capsule daily for 12 weeks. As part of the baseline and post-intervention phases, paper-based headache diaries were used to record the number of abortive drugs consumed and the frequency of headache attacks, and the Migraine Disability Assessment Questionnaire (MIDAS) was used to assess migraine disability.
Results: A 16-week study on women with EM revealed that the mean changes in the frequency of headache attacks decreased significantly in all vitamin groups in comparison with the placebo group (P < 0.001). In contrast to the placebo, there was also a significant improvement in the migraine disability score in each vitamin group (P < 0.001). The 12-week supplementation with vitamins B9, B1, B6, B12, and B complex also brought on a significant decrease in the use of abortive drugs compared to the placebo group (P = 0.032).
Conclusion: The results of this study showed that B1, B6, B12, and B9, and a combination of these vitamins could be effective as an adjuvant in treatment and prophylaxis of EM. Further large trials with long-term follow-ups will be required to confirm our results.
Key Words: Thiamine, Pyridoxine, Vitamin B12, Folic Acid, Disability Evaluation, Headache, Migraine Disorders, Vitamin B Complex
Introduction
The prevalence of migraine is highest among people aged 25-55 years, who are considered as part of the economically active workforce in each country. It is a persistent neurological disorder that significantly affects the functional activity of patients with severe headache attacks which is the second most common cause of disability-related years, according to the Global Burden of Disease Study of Neurological Diseases 2016. 1 Based on the International Headache Classification (ICHD) III criteria, migraine is classified into episodic and chronic forms. The episodic migraine (EM) is characterized by 0-14 headache days per month, while chronic migraine (CM) is characterized by 15 or more headache days per month for at least 3 months, with the presentation of symptoms of migraine at least 8 times per month. 2
The prevalence of migraine in the world population and among Iranian people is estimated at 15% (14-16%) and 14% (12-17%), respectively.3,4 Migraine is 2 to 3 times more prevalent in women than in men, and causes more burden of disease and has higher severity in women.5
Migraine headaches are usually recurrent with moderate to high severity and last 4 to 72 hours. Migraine-related symptoms include sensitivity to light, sound, or smell, and vomiting and nausea.6 It is worth noting that comorbidities of migraine such as neck pain, stress, and depression make it one of the most debilitating disorders in the world.7
Although the pathophysiological causes of migraine attacks are still unknown, the onset of migraine attacks is thought to be due to the interaction of endogenous and exogenous stimuli. Vascular dysfunction, cortical spreading depression (CSD), neurogenic inflammation, and activation of the trigeminovascular system (TVS) lead to an attack of migraine. As a result of stimulation of nociceptive fibers originating from trigeminal ganglion calcitonin gene-related peptide (CGRP), substance P (SP) and vasoactive intestinal peptide (VIP) are released which, along with other inflammatory mediators, cause vasodilation, neurogenic inflammation and nociceptive transmission. These mediators initiate pain signals along the trigeminovascular pathway.8-10 Methylene tetrahydrofolate reductase (MTHFR) gene mutations, low serotonin levels, hyperhomocysteinemia, nitric oxide (NO) release, energy deficit, and mitochondrial dysfunction are among the most important factors that predispose a person to migraine headache. Based on the comorbidity of migraine and thrombotic disorders, the possible role of homocysteine (Hcy) in the development of coagulation in the cerebrovascular system is plausible. Several factors can lead to elevated homocysteine concentration. In particular, a polymorphism in the 5,10-methylene tetrahydrofolate reductase (MTHFR) gene has been described, which is responsible for the defective production of the enzyme and the increase in Hcy levels, and is also associated with migraine.11 Hcy is a highly reactive amino acid that causes endothelial damage by impairing the release of NO.
Migraine may be induced and maintained due to endothelial dysfunction.12 In addition to vascular dysfunction, in the metabolic context, the possibility exists that Hcy plays a role in the pathogenesis of migraine by causing mitochondrial dysfunction and oxidative stress. According to migraine metabolic theory, impaired cerebral homeostasis (including energy reserve deficiency due to mitochondrial dysfunction) may activate the TVS and facilitate the generation of migraine headache.13
Pain relief and restoration of function are the goals of acute treatments of migraine, while prophylactic treatments are designed to reduce the frequency, severity, duration of attacks, anxiety, stress, disability symptoms, and the acute drugs use.7,14 Current treatments are not always effective and are often expensive or have side effects.15 Therefore, it is worthwhile to combine or replace current treatments with safe, effective, and inexpensive alternatives for the successful management of migraine. Of the prophylactic treatments, we can mention the intervention with nutritional supplements (magnesium, coenzyme Q10, a-lipoic, vitamins B2, B3, B12, and D), which has already attracted much attention in the prevention of migraine and other types of headache.16
Vitamin B supplements are known for their established therapeutic role in neurological diseases.17 The clinical use of B1, B6, and B12 has been proposed for controlling chronic pain, reducing pain severity, and disability.16,17 It seems that these vitamins, as an important part of the diet, can affect migraine characteristics. Studies have suggested that B6, B9, and B12 can reduce the severity and frequency of migraine attacks.18-20 However, to the best of our knowledge, no randomized controlled trials have been conducted on the effect of thiamine on migraine.
Therefore, there are very few researches on the effect of B vitamins (such as B1, B6, B9, and B12) and the results are controversial. Accordingly, in the present study, the effects of each supplement such as B1, B6, B9, and B12 on EM were assessed separately and in combination. No studies have been performed through this method on EM. It seems that this study is the first one to examine the effects of these vitamins on reducing the use of abortive drugs. Consequently, we designed a double-blind, placebo-controlled, randomized trial to determine whether using different vitamin B supplements can reduce the frequency of headaches, migraine-related disability, and the use of abortive drugs.
Materials and Methods
Participants: In the present study, 120 women with EM were recruited from November 2019 to March 2021. The participants were selected from among patients who were referred to a tertiary private headache clinic in Sina Hospital, Tehran, Iran, with a diagnosis by an expert neurologist and headache specialist. The study inclusion criteria included having a body mass index (BMI) of 18.5-30 kg/m2, age of 18-50 years, migraine diagnosis according to the International Classification of Headache Disorders, 3rd edition (ICHD3), and EM with 3 or more attacks per month, and suffering from migraine for a minimum of 6 months before the study. Patients who were known to have gastrointestinal disorders (malabsorption, IBD, etc.), diabetes, liver disorders (cirrhosis, hepatitis, etc.), kidney disorders, congestive heart failure, hypertension, cancer, and coronavirus disease were excluded from the study. Other exclusion criteria were unwillingness to take part in the study, taking vitamin B supplements in the 3 months before the study, pregnancy and lactation, taking antipsychotic drugs, smoking, changing the type of prophylactic drug or its dosage, or starting a prophylactic drug in less than 3 months before the study, and not taking more than 10% of the supplements given in the study presented as the intervention.
Design and intervention of the study: This study was a randomized, double-blind, placebo-controlled, clinical trial. Our study period lasted 16 weeks, including 12 weeks of treatment after a 4-week baseline period without intervention for measuring headache features. Ethical approval was obtained from Tehran University of Medical Sciences in Tehran, Iran, with certificate No.IR.TUMS.NI.REC.1398.029. The research has also been registered with the Iranian Center for Clinical Trials (No. IRCT20190921044828N1, accessible at www.irct.ir).
Patients with EM were selected according to the study inclusion criteria from a tertiary private headache clinic in Sina Hospital. The aims, methods of intervention, and the period of the study were described to the patients, and written informed consent was obtained from all participants. Subjects were randomly allocated to 6 groups using a random allocation rule procedure.21 After calculating the total sample size, we randomly chose a subset of the sample to assign to A (folic acid), B (vitamin B1), C (vitamin B6), D (vitamin B12), E (placebo), and F (a combination of vitamins B1, B12, B6, and folic acid) codes. For a total sample size of 120, 20 cards were inserted in an envelope for each code and were taken out randomly without replacement, then, the sequences were recorded. After randomization, people were assigned to 1 of the groups of vitamins B1, B6, B12, folic acid and vitamin B complex, and placebo. In order to maintain ‘blinding’, the medicine bottles were coded by an individual not involved in the study and assigned to the patients. Participants, and clinical and laboratory staff were kept ‘blind’ until the end of data analyses.
Each participant received 1 bottle per month containing 30 capsules and was instructed to take 1 capsule daily for 12 weeks. The dosage of the vitamin in the B1, B6, B12, and folic acid groups were 300 mg, 80 mg, 500 μg, and 2 mg, respectively. The vitamin B complex supplement contained all of the stated doses of B1, B6, B12, and folic acid. Placebo capsules consisting of methylcellulose and similar in weight, size, and color to the vitamin capsules were prepared. Participants were contacted by telephone calls every 2 weeks to check their compliance with the intervention and were asked to inform the research staff immediately if they had any adverse reactions to the treatment. The participants were asked to continue to take their regular medications without any change, but to let the researcher know if at any point the type or dose of their medications were changed by their physician. All patients were instructed to call the researcher if they had any questions. Study participants were excluded if they had changed their current migraine treatment. Patients were asked to return all used bottles of capsules. Any remaining capsules were counted to further evaluate their adherence to the study protocol, and if more than 10% of the capsules remained, the participant was considered to be non-compliant and was consequently excluded from the study.
Data collection: All information including age, Years of headache onset, number and type of medications, and food triggers were collected at baseline. Using a dial column medical scale (755, Seca GmbH & Co., Hamburg, Germany) and standard stadiometer, body weight and height were measured to the nearest 0.5 kg and 0.1 cm, respectively. BMI is computed as weight in kilograms divided by height in square meters. Tape measures were used to measure the circumference of the waist at the iliac crest and the circumference of the hips at the site of the greatest circumference between the waist and thighs. Dietary intake was evaluated through 24-hour recall for 3 days (2 regular weekdays and 1 day of the weekend).
Headache diaries: Headache characteristics, including the frequency and number of headache attacks per month, and the number of abortive drugs consumed per month, were recorded by participants in a paper-based 30-day headache diary. This diary is designed by the lead researcher, Prof. M.T.22 The first diary was completed a month before beginning the intervention. Then, the participants were asked to fill out their headache diaries during the trial. The instructions for completing the paper-based diary were given by researchers and if there was any question or problem about the completion of the headache diaries, the participants could get in touch with the researchers.
Migraine Disability Assessment Questionnaire: An assessment of migraine-related disability was conducted at the start and end of the intervention using the Migraine Disability Assessment Questionnaire (MIDAS). There are 7 questions in the questionnaire that assess the impact headaches have had on work, home, social, and leisure activities over the last 3 months. Questions 1 through 5 are related to migraine-related impairment of performance.23,24 This questionnaire has been validated in Iran by Zandifar et al.25
Non-parametric variables are expressed as median (25th, 75th percentiles) while parametric variables are expressed as mean (± standard deviation). Categorical variables are expressed as frequency (percentage). The Kolmogorov-Smirnov test was used to evaluate the normality of the distribution. One-way analysis of variance (ANOVA) or the Kruskal-Wallis test was used for comparisons among groups for continuous data, and the chi-square test was applied to categorical data. In order to identify significant differences within groups prior to and following the intervention, paired t-test and Wilcoxon test were used for parametric and non-parametric variables, respectively.
Analysis of covariance (ANCOVA) was used to eliminate the effects of confounders, including triggers and analgesics, between groups. Changes in the means of the headache characteristics are presented in the figures. R statistical software was used for post hoc analysis of non-parametric tests. Statistical significance was determined by P-value < 0.05 for differences between and within the studied groups. Data were analyzed using the Statistical Package for Social Sciences software (version 19, SPSS Inc., Chicago, IL, USA). All of the 24-hour recall questionnaires were analyzed using Nutritionist IV software.
Results
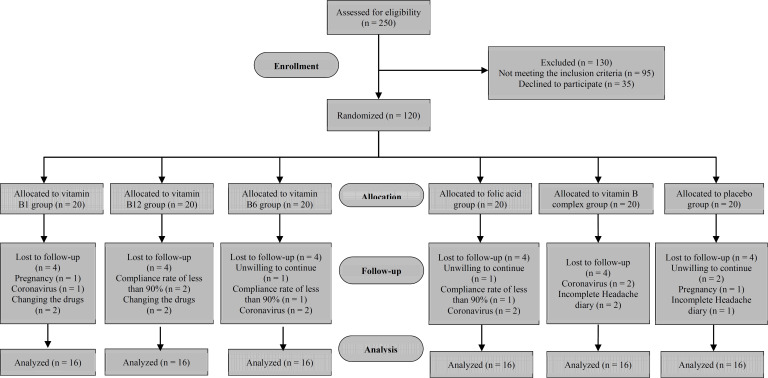
Patient characteristics: Based on the study sample size, 20 patients were allocated to each group. At the 16-week follow-up, 4 patients had withdrawn from each group due to unwillingness to continue, not completing the headache dairies, changes in the dosage and type of migraine prophylactic drugs, pregnancy, coronavirus disease, and compliance rate of less than 90% (Figure 1).
Figure 1.
Flowchart of studied participants
The final analysis, therefore, included 16 patients in the intervention groups and the placebo group. No significant differences were found between groups for any demographic and anthropometric variables, and headache onset years (Tables 1 and 2).
Table 1.
Baseline demographic characteristics, food triggers, and clinical data of episodic migraine patients enrolled in a trial of vitamin B9, B1, B6, B12, and B complex vs. placebo supplementation
| Variable | B9 | B1 | B6 | B12 | Placebo | Vitamin B complex | P * |
|---|---|---|---|---|---|---|---|
| Age (mean ± SD) | 38.00 ± 9.12 | 37.56 ± 7.90 | 35.69 ± 7.85 | 35.63 ± 9.52 | 34.81 ± 7.82 | 35.50 ± 5.56 | 0.849 |
| Years of headache onset (mean ± SD) | 14.62 ± 11.89 | 11.65 ± 8.10 | 11.34 ± 9.13 | 12.53 ± 9.37 | 10.00 ± 7.89 | 13.43 ± 8.61 | 0.777 |
| Food triggers [n (%)] | 5 (31.3) | 6 (37.5) | 6 (37.5) | 12 (75.0) | 5 (31.3) | 11 (68.8) | 0.029 |
SD: Standard deviation
Using either one-way ANOVA for continuous variables or Chi square for categorical variables
Table 2.
Anthropometric characteristics and physical activity of episodic migraine patients enrolled in a trial of vitamin B9, B1, B6, B12, and B complex vs. placebo supplementation
| Variable | Group | Before | After | P * |
|---|---|---|---|---|
| Weight (kg) | B9 | 60.70 ± 6.58 | 60.75 ± 7.49 | 0.893 |
| B1 | 61.19 ± 8.94 | 60.42 ± 6.53 | 0.064 | |
| B6 | 67.24 ± 5.11 | 65.50 ± 7.79 | 0.557 | |
| B12 | 61.68 ± 12.46 | 61.63 ± 12.41 | 0.884 | |
| Placebo | 65.09 ± 12.91 | 62.88 ± 9.49 | 0.933 | |
| Vitamin B complex | 64.06 ± 9.31 | 64.20 ± 9.02 | 0.809 | |
| P** | 0.062 | 0.566 | ||
| BMI (kg/m2) | B9 | 22.96 ± 2.59 | 22.90± 2.76 | 0.646 |
| B1 | 23.08 ± 2.56 | 23.53± 2.93 | 0.042 | |
| B6 | 24.56 ± 2.93 | 24.52± 2.76 | 0.743 | |
| B12 | 23.57 ± 4.35 | 23.57± 4.44 | 0.984 | |
| Placebo | 24.02 ± 4.23 | 24.05± 4.28 | 0.872 | |
| Vitamin B complex | 23.86 ± 3.80 | 23.90± 3.66 | 0.833 | |
| P | 0.797 | 0.859 | ||
| Waist circumference (cm) | B9 | 83.34 ± 9.71 | 83.18 ± 10.37 | 0.641 |
| B1 | 80.96 ± 7.31 | 81.28 ± 8.02 | 0.435 | |
| B6 | 86.25 ± 9.32 | 85.53 ± 9.07 | 0.150 | |
| B12 | 78.75 ± 11.26 | 78.46 ± 11.46 | 0.543 | |
| Placebo | 84.31 ± 11.38 | 84.53 ± 11.83 | 0.575 | |
| Vitamin B complex | 85.28 ± 10.76 | 85.96 ± 11.50 | 0.395 | |
| P | 0.291 | 0.318 | ||
| Hip circumference (cm) | B9 | 102.43 ± 9.23 | 102.34 ± 9.55 | 0.688 |
| B1 | 100.33 ± 5.09 | 101.10 ± 5.14 | 0.005 | |
| B6 | 105.93 ± 7.78 | 106.21 ± 7.91 | 0.357 | |
| B12 | 99.93 ± 10.05 | 100.09 ± 9.96 | 0.580 | |
| Placebo | 104.18 ± 9.15 | 102.66 ± 6.28 | 0.890 | |
| Vitamin B complex | 106.25 ± 8.35 | 106.15 ± 8.35 | 0.694 | |
| P | 0.158 | 0.171 | ||
| IPAQ (MET-m/d) | B9 | 790.06 ± 514.49 | 934.50 ± 680.28 | 0.365 |
| B1 | 1030.00 ± 985.13 | 915.86 ± 867.98 | 0.608 | |
| B6 | 703.06 ± 521.98 | 628.00 ± 504.30 | 0.415 | |
| B12 | 1054.87 ± 699.42 | 1068.93 ± 884.11 | 0.914 | |
| Placebo | 578.75 ± 418.62 | 704.37 ± 557.30 | 0.181 | |
| Vitamin B complex | 682.62 ± 350.02 | 773.25 ± 407.23 | 0.243 | |
| P | 0.237 | 0.451 |
Data are presented as mean ± standard deviation (SD).
P-value < 0.05 was considered significant.
BMI: body mass index; IPAQ: International physical activity questionnaires
Paired t-test was used to compare before-after tests.
One-way ANOVA was used to compare groups.
There were also no significant differences between groups in terms of the baseline recorded characteristics of drug consumption (Table 3), except for the consumption of analgesics (P = 0.030).
Table 3.
Consumption of medications in the studied groups at baseline
| Variable | B9 | B1 | B6 | B12 | Placebo |
Vitamin B
complex |
P * |
|---|---|---|---|---|---|---|---|
| NSAIDS | 14 (87.5) | 15 (93.8) | 12 (75.0) | 13 (81.3) | 13 (81.3) | 12 (75.0) | 0.764 |
| Other analgesics | 7 (43.8) | 11 (68.8) | 4 (25.0) | 4 (25) | 7 (43.8) | 11 (68.8) | 0.030 |
| Triptans | 6 (37.5) | 4 (25.0) | 6 (37.5) | 7 (43.8) | 5 (31.3) | 4 (25.0) | 0.840 |
| Propranolol | 1 (6.3) | 1(6.3) | 5 (31.3) | 3 (18.8) | 2 (12.5) | 3 (18.8) | 0.453 |
| TCAs | 2 (12.5) | 0 (0) | 5 (31.3) | 3 (18.8) | 3 (18.8) | 3 (18.8) | 0.268 |
| SNRIs | 0 (0) | 0 (0) | 2 (12.5) | 0 (0) | 1 (6.3) | 0 (0) | 0.427 |
| SSRIs | 2 (12.5) | 1 (6.3) | 1 (6.3) | 0 (0) | 3 (18.8) | 1 (6.3) | 0.667 |
| Topiramate | 2 (12.5) | 2 (12.5) | 0 (0) | 0 (0) | 2 (12.5) | 0 (0) | 0.221 |
| Sodium valproate/valproic acid.divalpro |
0 (0) | 1 (6.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | > 0.999 |
P value < 0.05 was considered significant.
Data are presented as n (%).
NSAIDS: Non-steroidal anti-inflammatory drugs; TCAs: Tricyclic antidepressants; SNRIs: Serotonin-norepinephrine reuptake inhibitors; SSRIs: Selective serotonin reuptake inhibitors
P-values for categorical variables were calculated using Fisher's exact test, except triptans were calculated using chi-square test.
Table 4 shows the mean daily dietary intakes of vitamin B supplements before the intervention, on the sixth week, and upon completing the intervention.
Table 4.
Dietary intake of vitamins B in episodic migraine patients enrolled in a trial of vitamin B9, B1, B6, B12, and B complex vs. placebo supplementation at three times
| Variable | Group | Before | Week 6 | After | P * |
|---|---|---|---|---|---|
| Riboflavin (mg) | B9 | 1.19 ± 0.37 | 1.23 ± 0.33 | 1.22 ± 0.34 | 0.877 |
| B1 | 1.19 ± 0.33 | 1.14 ± 0.34 | 1.13 ± 0.26 | 0.754 | |
| B6 | 1.34 ± 0.38# | 1.15 ± 0.36#,¥ | 1.28 ± 0.32¥ | 0.029 | |
| B12 | 1.06 ± 0.27 | 1.06 ± 0.31 | 1.02 ± 0.33 | 0.837 | |
| Placebo | 1.15 ± 0.30 | 1.08 ± 0.30 | 1.08 ± 0.28 | 0.308 | |
| Vitamin B complex | 1.25 ± 0.47 | 1.11 ± 0.37 | 1.11 ± 0.27 | 0.209 | |
| P** | 0.354 | 0.763 | 0.180 | ||
| Pyridoxine (mg) | B9 | 1.15 ± 0.36 | 0.94 ± 0.17 | 1.09 ± 0.38 | 0.149 |
| B1 | 1.23 ± 0.41 | 1.22 ± 0.38 | 1.12 ± 0.30 | 0.646 | |
| B6 | 1.20 ± 0.32 | 1.08 ± 0.20 | 1.07 ± 0.21 | 0.159 | |
| B12 | 1.01 ± 0.20 | 1.03 ± 0.34 | 1.11 ± 0.38 | 0.640 | |
| Placebo | 1.00 ± 0.31 | 1.05 ± 0.32 | 1.20 ± 0.56 | 0.280 | |
| Vitamin B complex | 1.33 ± 0.63 | 1.19 ± 0.37 | 1.10 ± 0.45 | 0.406 | |
| P-value | 0.156 | 0.060 | 0.956 | ||
| Cobalamin (µg) | B9 | 1.86 ± 0.80 | 2.00 ± 0.64 | 2.11 ± 0.96 | 0.429 |
| B1 | 2.33 ± 1.70 | 1.75 ± 0.75€ | 2.05 ± 0.79 | 0.231 | |
| B6 | 2.57 ± 1.10 | 2.61 ± 0.99€ | 2.41 ± 0.88 | 0.670 | |
| B12 | 1.85 ± 1.00 | 1.81 ± 0.72 | 1.97 ± 1.04 | 0.624 | |
| Placebo | 2.59 ± 1.34# | 1.86 ± 0.88 | 1.81 ± 0.76# | 0.048 | |
| Vitamin B complex | 2.40 ± 1.16 | 1.83 ± 0.61 | 3.28 ± 3.17 | 0.210 | |
| P | 0.331 | 0.029 | 0.312 | ||
| Folate (µg) | B9 | 175.85 ± 88.46 | 221.32 ± 132.68 | 153.40 ± 50.36 | 0.231 |
| B1 | 194.64 ± 72.22 | 176.36 ± 84.52# | 137.80 ± 67.48# | 0.030 | |
| B6 | 185.49 ± 74.88# | 129.68 ± 46.75 | 137.30 ± 34.84# | 0.040 | |
| B12 | 180.63 ± 81.44 | 161.98 ± 104.83 | 119.36 ± 44.90 | 0.095 | |
| Placebo | 148.52 ± 45.17 | 143.19 ± 71.80 | 145.92 ± 63.02 | 0.358 | |
| Vitamin B complex | 190.94 ± 94.82 | 159.25 ± 84.06 | 161.62 ± 103.24 | 0.390 | |
| P | 0.634 | 0.128 | 0.540 | ||
| Thiamine (mg) | B9 | 1.13 ± 0.18 | 1.21 ± 0.31 | 1.10 ± 0.34 | 0.231 |
| B1 | 1.25 ± 0.47 | 1.11 ± 0.26 | 1.16 ± 0.36 | 0.363 | |
| B6 | 1.05 ± 0.18 | 1.05 ± 0.26 | 1.07 ± 0.25 | 0.831 | |
| B12 | 1.28 ± 0.23 | 1.25 ± 0.25 | 1.28 ± 0.22 | 0.823 | |
| Placebo | 1.13 ± 0.32 | 1.07 ± 0.22 | 1.00 ± 0.25 | 0.136 | |
| Vitamin B complex | 1.03 ± 0.32 | 1.05 ± 0.26 | 1.00 ± 0.29 | 0.406 | |
| P | 0.069 | 0.131 | 0.082 | ||
| Niacin (mg) | B9 | 14.83 ± 4.14 | 15.09 ± 3.93 | 14.12 ± 4.69 | 0.611 |
| B1 | 17.43 ± 6.57 | 18.21 ± 7.56 | 17.98 ± 4.93 | 0.847 | |
| B6 | 16.55 ± 4.83 | 16.26 ± 2.65 | 16.13 ± 3.58 | 0.905 | |
| B12 | 16.73 ± 3.82 | 17.45 ± 3.49 | 17.32 ± 5.32 | 0.741 | |
| Placebo | 14.73 ± 3.82 | 15.96 ± 5.17 | 15.88 ± 4.02 | 0.224 | |
| Vitamin B complex | 15.63 ± 3.97 | 16.33 ± 4.00# | 13.68 ± 4.70# | 0.038 | |
| P | 0.488 | 0.553 | 0.060 | ||
| Pantothenic acid (mg) | B9 | 3.35 ± 0.94# | 4.02 ± 1.16# | 3.72 ± 0.89 | 0.041 |
| B1 | 3.49 ± 1.35 | 3.32 ± 1.13 | 3.39 ± 0.91 | 0.755 | |
| B6 | 4.00 ± 1.36 | 3.71 ± 0.87 | 3.96 ± 0.89 | 0.450 | |
| B12 | 3.54 ± 0.63 | 3.35 ± 0.83 | 3.53 ± 1.07 | 0.529 | |
| Placebo | 3.62 ± 0.91 | 3.62 ± 0.65 | 3.43 ± 0.73 | 0.492 | |
| Vitamin B complex | 3.61 ± 1.14 | 3.71 ±1.01 | 3.37 ± 0.84 | 0.690 | |
| P | 0.669 | 0.323 | 0.389 |
P value < 0.05 was considered significant.
Data are presented as mean ± standard deviation (SD).
Within group P-values (Repeated-measures analysis),
Between group P-values (One-way ANOVA),
Significant differences between each time and two other times
Significant differences between each vitamin group and other groups calculated by post-hoc test
Variations in migraine characteristics
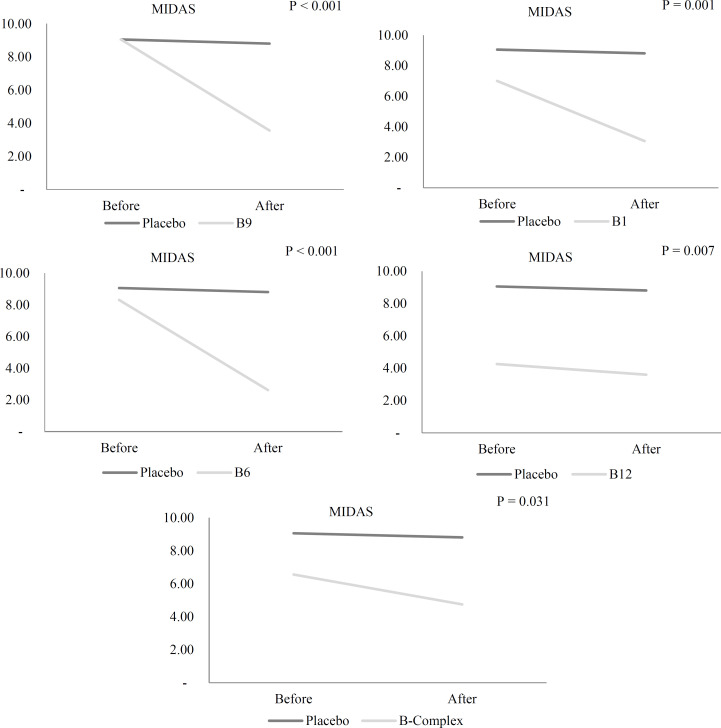
Migraine disability: Migraine disability reduced significantly in patients with EM in the vitamin B1 (P = 0.002), B12 (P = 0.003), B6 (P < 0.001), B9 (P < 0.001), and vitamin B complex (P = 0.007) groups, while the placebo group did not experience any significant changes. After adjusting for confounders including food triggers, analgesics and mean migraine disability at baseline in ANCOVA models, for confounders including food triggers, analgesics and mean migraine disability at baseline, migraine disability significantly reduced in EM patients in the vitamin B1, B12, B6, B9, and vitamin B complex groups compared to the placebo group (P < 0.001) (Table 5) (Figure 2).
Table 5.
Changes in migraine characteristics and the use of abortive drugs before and after supplementation with vitamin B9, B1, B6, B12, and B complex or placebo in episodic migraine patients
| Variable | B9 | B1 | B6 | B12 | Placebo |
Vitamin B
complex |
P |
Adjusted
P $ |
|
|---|---|---|---|---|---|---|---|---|---|
| MIDAS | Before | 9.06 ± 3.92 | 7.00 ± 5.27 | 8.31 ± 6.10 | 4.26 ± 3.32 | 9.06 ± 5.96 | 6.56 ± 6.30 | 0.015* | |
| After | 3.56 ± 2.85 | 3.06 ± 3.76 | 2.62 ± 2.77 | 3.06 ± 3.58 | 8.81 ± 7.53 | 4.75 ± 4.72 | 0.087* | < 0.001 | |
| Difference | -5.50 2.73 | -3.53 3.48 | -5.68 4.58 | -1.66 1.79 | -0.25 6.98 | -1.81 2.34 | < 0.001* | ||
| P** | < 0.001 | 0.002 | < 0.001 | 0.003 | 0.888 | 0.007 | |||
| Frequency of attacks (attacks per month) |
Before | 6.18 ± 4.08 | 6.56 ± 3.55 | 5.37 ± 1.85 | 6.50 ± 2.33 | 5.81 ± 2.88 | 6.68 ± 3.91 | 0.720# | |
| 4.50 (3.25, 7.00) | 5.00 (4.00, 8.50) | 5.00 (4.00,6.75) | 6.00 (4.25, 8.00) | 5.00 (4.00, 7.75) | 5.00 (4.25, 9.00) | ||||
| After | 4.25 ± 2.84 | 4.43 ± 2.75 | 3.75 ± 1.87 | 3.68 ± 2.18 | 6.62 ± 3.77 | 5.12 ± 2.91 | 0.037* | < 0.001 | |
| 4.00 (2.00, 6.00) | 4.00 (2.00, 6.75) | 3.50 (2.25,5.00) | 3.50 (2.00, 4.75) | 5.50 (4.25, 8.00) | 4.00 (2.25, 7.75) | ||||
| Difference | -2.00 (-2.00, -1.00) | -2.00 (-4.75, 0.75) | -1.50 (-3.00,-0.25) | -3.00 (-4.00, -2.00) | 0 (-0.75, 2.75) | -2.00 (-4.75, 0) | 0.006# | ||
| P** | 0.003¥ | 0.017¥ | < 0.001 | < 0.001 | 0.265¥ | 0.090 | |||
| Number of abortive drugs |
Before | 6.87 5.97 | 7.20 6.69 | 5.06 3.33 | 7.75 4.80 | 5.14 3.84 | 7.00 4.09 | 0.554* | |
| After | 4.43 3.38 | 4.42 3.29 | 3.62 2.65 | 4.43 3.32 | 7.06 5.09 | 5.12 2.96 | 0.120* | 0.032 | |
| Difference | -1.00 (-4.25, 0) | -1.00 (-4.00, 1.50) | -0.50 (-3.75, 0.75) | -3.50 (-5.50, -1.00) | 0.50 (-1.00, 2.25) | -2.00 (-3.00, -1.00) | 0.014# | ||
| P** | 0.049 | 0.146 | 0.023 | 0.001 | 0.192 | 0.025 |
All values are Mean ± SD except frequency of attacks and the mean change in number of abortive drugs in the form of median (25th, 75th percentiles)
MIDAS: Migraine Disability Assessment Questionnaire
Obtained using the ANOVA test for comparison of data among the intervention and placebo groups.
Obtained using the Kruskal-Wallis for comparison of data among the intervention and placebo groups.
Obtained using ANCOVA adjusted for the baseline values, analgesics, and food triggers among the intervention and placebo groups.
Obtained using the paired samples t-test for comparison of data between the beginning and end of the study.
Obtained using the Wilcoxon test for comparison of data between the beginning and end of the study.
Figure 2.
Comparison of Migraine Disability Assessment Questionnaire (MIDAS) scores in episodic migraineurs before and after the 12-week supplementation with vitamin B1, B6, B12, B9, and B complex, and placebo after considering analgesics, food triggers, and baseline MIDAS scores in ANCOVA model by LSD post-hoc test
No significant difference was observed in terms of the effectiveness of supplements between various B vitamin groups in the pairwise comparisons.
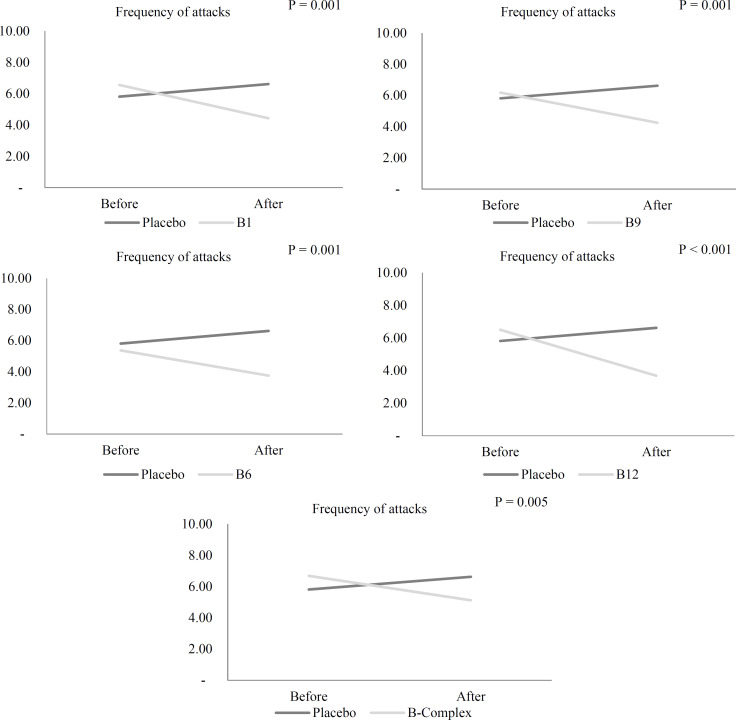
Migraine attack frequency: As shown in table 5, the frequency of headache attacks in the B9 (P = 0.003), B1 (P = 0.017), B6 (P < 0.001), and B12 (P < 0.001) groups decreased significantly during the 12-week intervention, but no significant decrease was observed in the B complex group. In the placebo group, the increase in attack frequency was not significant. Supplementation with vitamin B9, B1, B12, B6, and vitamin B complex led to a significant reduction in attack frequency after 12 weeks compared with the placebo group, after considering food triggers, analgesics, and the mean attack frequency at baseline as confounders in the ANCOVA model (P < 0.001) (Table 5) (Figure 3). No significant difference was observed in the effectiveness of supplements between various B vitamin groups in the pairwise comparisons.
Figure 3.
Comparison of frequency of headache attacks in episodic migraineurs before and after the 12-week supplementation with vitamin B1, B6, B12, B9, and B complex and placebo after considering analgesics, food triggers, and means frequency of headache attacks at baseline in ANCOVA model by LSD post-hoc test
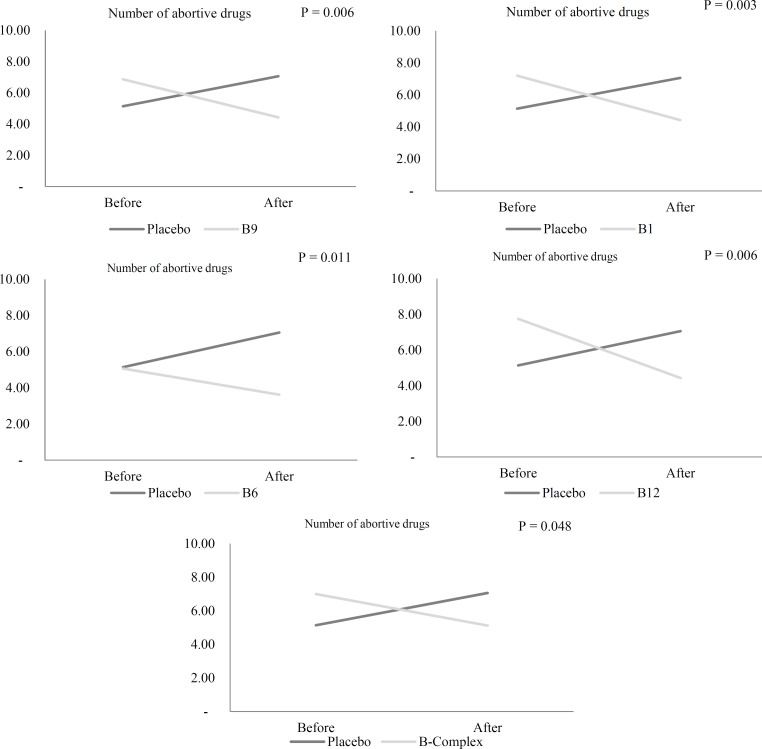
The number of abortive drugs: The consumption of abortive drugs by participants in the vitamin B12 (P = 0.025), B6 (P = 0.023), B9 (P = 0.049), and vitamin B complex groups (P = 0.002) reduced significantly compared to baseline, while placebo and vitamin B1 recipients did not experience any significant changes in abortive drugs consumption (Table 5).
After consideration of food triggers, analgesics, and the number of abortive drugs at baseline as confounders in the ANCOVA model, supplementation with vitamin B1, B12, B6, B9, and vitamin B complex lowered the consumption of abortive drugs as opposed to placebo after 12 weeks (P = 0.032) (Table 5) (Figure 4). No significant difference was observed in terms of the effectiveness of supplements between various vitamin B groups in the pairwise comparisons.
Figure 4.
Comparison of number of abortive drugs in episodic migraineurs before and after the 12-week supplementation with vitamin B1, B6, B12, B9, and B complex and placebo after considering analgesics, food triggers and means number of abortive drugs at baseline in ANCOVA model by LSD post-hoc test
Discussion
This randomized, placebo-controlled, double-blind study investigated the effects of supplementing with vitamins B1, B6, B12, folic acid, and a combination of these vitamins on EM characteristics. This 16-week study on women with EM showed that supplementation with vitamin B9, B1, B6, B12, and a combination of these vitamins resulted in a significant decrease in the frequency of headache attacks and number of abortive drugs consumed, and improved the migraine disability score compared with the placebo group. Significant reductions were also observed in the MIDAS score, frequency of headache attacks, and number of abortive drugs within groups compared to baseline. Hence, both the separate and synergistic positive effects of vitamins B1, B12, B6, and B9 are indicated by the improvement of these variables.
There are a few studies on vitamin B supplementation as treatment for migraine; however, our findings are in accordance with some previous studies in which vitamin B1, B6, B12, and folic acid supplementation was used and resulted in the improvement of migraine characteristics and quality of life (QOL). Menon et al. showed that 6-month supplementation with tablets containing 2 mg of folic acid, 25 mg of vitamin B6, and 400 g of vitamin B12 in migraine patients reduced the percentage of high migraine disability from 60% to 30%, while the frequency of headache did not decrease from a median of 2 for either the vitamin (P = 0.46) or placebo (P = 0.147) groups after 6 months.20 Lea et al. provided evidence that folic acid along with vitamin B6 and B12 supplements in doses similar to that in the study by Menon et al. improved QOL and frequency of headache attacks associated with MTHFRC677T genotype in migraineurs.18 Moreover, Askari et al. found that taking folic acid (5 mg) with pyridoxine (80 mg) was associated with a remarkable reduction in the frequency of headache attacks.24
Only one case report conducted by Prakash et al. has examined the effect of vitamin B1 on migraine. In this study, 2 patients with chronic migraine were reported. Both patients had low blood thiamine levels. Intravenous thiamine supplementation (500 mg three times daily) improved the frequency of exacerbations (or severe headache attacks).25
The therapeutic benefits of high doses of B vitamins have been demonstrated in painful disorders. Research has confirmed the antiallodynic, antinociceptive, and antihyperalgesic effects of vitamin B12, B1, and B6 on the severity of pain and disability in spite of no typical symptoms of nutritional deficiency.17,26 Considering the antinociceptive activity of the B vitamins, some studies have shown the beneficial effects of vitamin B1, B12, B6, and folic acid on migraine characteristics.19,20,24,25
The positive impacts of vitamin B1, B6, B12, and folic acid on migraine can be due to several possible mechanisms. Some studies have shown that B vitamins, specifically vitamins B1 and B12, increase the synthesis and secretion of serotonin in several areas of the brain. Likewise, suppression of serotonin metabolism following vitamin B deficiency may indicate an association between serotonin and B-induced analgesia.17,27 Migraine is pathophysiologically and therapeutically affected by serotonin. A low serotonin level is thought to contribute to migraine attacks. One of the goals of migraine treatment is to regulate serotonin levels to amplify its signals, which leads to pain relief through the vasoconstriction of blood vessels and inhibition of the release of peptides such as SP and CGRP.8
Moreover, B vitamins can reduce pain associated with inflammatory conditions.28 It has been shown that vitamins B1, B12, and B6 can suppress P2X3 and transient receptor potential vanilloid type 1 (TRPV1) expression and the increased levels of interleukin 1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and nerve growth factor (NGF). High levels of inflammatory mediators and sensitization of TRP channels are observed in migraine. Sensitization of these TRP channels releases CGRP and NO at the trigeminal terminals, which in turn causes cranial vasodilatation, trigeminal sensitivity, and pain.29,30 Moreover, NO, by binding to complexes I, III, and cytochrome c-oxidase, inhibits the mitochondrial respiratory chain and, in turn, may lead to its dysfunction and oxidative stress, which contributes to the pathogenesis of migraine.31,32 NO is produced from the amino acid L-Arginine by NO synthase (NOS). It is known as an intercellular signaling molecule and neurotransmitter in the pain pathways which playing a key role in the pathophysiology of migraine.33 Based on the hypothesis that NO removal can be beneficial in migraine, van der Kuy et al. conducted an open-label trial and assessed the effect of hydroxocobalamin on migraine prevention.34 Intranasal administration of hydroxocobalamin reduced the number of migraine attacks in 53% of patients. Based on this, it is emphasized that vitamin B12 has a radical scavenging role against NO.35 Rajanayagam et al. indicated that hydroxocobalamin (10-30µM) produced concentration-dependent reductions of the relaxant action of NO in rat aortic rings. It is suggested that hydroxocobalamin sequesters NO by forming nitrosocobalamin.36 A theory of mitochondrial dysfunction and decreased energy status is one of the hypotheses related to the thiamine-migraine association. Thiamine acts as a cofactor for various enzymes in the Krebs cycle and the pentose phosphate pathway. So thiamine is essential for the human body to use carbohydrates and produce mitochondrial adenosine triphosphate (ATP). These roles described how low intracellular thiamine levels can lead to acute energy failure, a propensity to oxidative stress, and mitochondrial abnormalities. Any deficiency and improper functioning of this vitamin can lead to the accumulation of different intermediate products from different pathways. These toxic mediators can have serious effects on the nervous system.4,34,37
Another mechanism of action to explain the effect of B vitamins such as B6, B12, and folic acid in the treatment of migraine is the reduction of homocysteine levels. Polymorphisms in genes 5, 10-methylene tetrahydrofolate reductase (MTHFR) and nicotinamide N-methyltransferase (NNMT) may be associated with the risk of migraine. Defective or deficient production of these enzymes leads to hyperhomocysteinemia. Therefore, an increase in homocysteine may also play a role in migraine.11 Hyperhomocysteinemia could contribute to neuronal overstimulation, central nervous system (CNS) excitability, and activation of the trigeminovascular system in migraine patients.31 Vitamin B6, folic acid, and vitamin B12 are required for Hcy metabolism. Therefore, supplementation with these vitamins may reduce Hcy levels.13 Di Rosa et al. reported a reduction in migraine attacks after folic acid supplementation in a group of children with migraine associated with hyperhomocysteinemia and MTHFR polymorphism.38
However, as opposed to our results, Menon et al. indicated that vitamin supplementation with folic acid (1 mg) in combination with vitamin B6 (25 mg) and B12 (400 μg) did not significantly improve high migraine disability and migraine characteristics in patients who suffered from migraine with aura at the end of the 6-month intervention in comparison to the placebo group.39 The dosages we used in our study might be a reason for this difference in findings. In our study, the supplements contained 2 mg of folic acid, 80 mg of B6, and 500 μg of B12 for the intervention. By reviewing previous studies, we considered the most effective doses to address the limitations of those studies.40
Moreover, we examined the effects of vitamins both separately and in combination. Another reason for the different outcomes could be the lack of separate consideration of the effects of vitamins on episodic and chronic migraine. In this regard, the study by Sadeghi et al. is worth mentioning.41 While the dosage of pyridoxine supplement and duration of the intervention in their study were similar to ours, the frequency of attacks did not significantly decrease.42 Therefore, vitamins B1, B6, B9, and B12 may only be effective on EM. In the current study, we performed interventions on patients with EM. Treatment of EM is critical because it can progress to chronic migraine and increase migraine disability.43,44
The strengths of our study include its prospective, double-blind, placebo-controlled design, its use of a safe dose of vitamin B1, B6, B12, and folic acid, and a combination of these vitamins to treat episodic migraineurs for the first time, its use of an expert neurologist’s (headache specialist) diagnosis, and validation of the EM diagnosis according to the ICHD criteria (ICHDIII). A limitation of our study was that we did not assess markers that are involved in migraine pathophysiology. In addition, because the study was undertaken in the coronavirus pandemic, the drop-out rate (about 25%) was quite high. Furthermore, it is important to clarify the impact of MTHFR gene polymorphism in order to study the effect of supplementing with vitamin B6, B12, and folic acid on migraine characteristics and migraine-related disability. The higher initial MIDAS score in the placebo group compared to the B12, B complex, and B1 groups could be considered as a limitation; however, we adjusted the baseline value of MIDAS in ANCOVA as a confounding factor.
Conclusion
The results of the current research indicated that the vitamin B group could be effective and well-tolerated supplements for improving migraine headaches and disability levels in episodic migraineurs. For the confirmation of our research findings and a better understanding of how the vitamin B group affects migraine pathogenesis, randomized controlled trials with a large sample size and an extended follow-up period would be beneficial. Additionally, conducting the same research on chronic migraineurs could be a recommendation for future studies.
Acknowledgments
We would like to thank the participants of the present study. In particular, we extend our gratitude to Ms. Falahati, Ms. Shahrokni, and the staff of the National Nutrition and Food Technology Research Institute and Iranian Center of Neurological Research for their kind cooperation. The authors would like to thank the statistics consultants of the Research Development Center of Sina Hospital for their technical assistance. This study was conducted as part of a PHD thesis in Shahid Beheshti University of Medical Sciences.
Notes:
How to cite this article: Nematgorgani S, Razeghi-Jahromi S, Jafari E, Togha M, Rafiee P, Ghorbani Z, et al. B vitamins and their combination could reduce migraine headaches: A randomized double-blind controlled trial. Curr J Neurol 2022; 21(2): 105-18.
Conflict of Interests
The authors declare no conflict of interest in this study.
References
- 1.GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459–80. doi: 10.1016/S1474-4422(18)30499-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farhadi Z, Alidoost S, Behzadifar M, Mohammadibakhsh R, Khodadadi N, Sepehrian R, et al. The prevalence of migraine in Iran: A systematic review and meta-analysis. Iran Red Crescent Med J. 2016;18(10):e40061. doi: 10.5812/ircmj.40061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gazerani P, Cairns BE. Sex-specific pharmacotherapy for migraine: A narrative review. Front Neurosci. 2020;14:222. doi: 10.3389/fnins.2020.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kursun O, Yemisci M, van den Maagdenberg AMJM, Karatas H. Migraine and neuroinflammation: The inflammasome perspective. J Headache Pain. 2021;22(1):55. doi: 10.1186/s10194-021-01271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jawed S, Ali W, Yaqoob U, Shah S, Uddin SMM, Haq A. Effect of migraine headache on productivity of patients according to migraine disability assessment score: A cross-sectional study. Pain Ther. 2019;8(2):233–8. doi: 10.1007/s40122-019-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan J, Asoom LIA, Sunni AA, Rafique N, Latif R, Saif SA, et al. Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed Pharmacother. 2021;139:111557. doi: 10.1016/j.biopha.2021.111557. [DOI] [PubMed] [Google Scholar]
- 9.Ji Y, Rizk A, Voulalas P, Aljohani H, Akerman S, Dussor G, et al. Sex differences in the expression of calcitonin gene-related peptide receptor components in the spinal trigeminal nucleus. Neurobiol Pain. 2019;6:100031. doi: 10.1016/j.ynpai.2019.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghorbani Z, Togha M, Rafiee P, Ahmadi ZS, Rasekh MR, Djalali M, et al. Vitamin D3 might improve headache characteristics and protect against inflammation in migraine: A randomized clinical trial. Neurol Sci. 2020;41(5):1183–92. doi: 10.1007/s10072-019-04220-8. [DOI] [PubMed] [Google Scholar]
- 11.Liampas I, Siokas V, Mentis AA, Aloizou AM, Dastamani M, Tsouris Z, et al. Serum homocysteine, pyridoxine, folate, and vitamin B12 levels in migraine: Systematic review and meta-analysis. Headache. 2020;60(8):1508–34. doi: 10.1111/head.13892. [DOI] [PubMed] [Google Scholar]
- 12.Aydin H, Bucak IH, Geyik M. Vitamin B12 and folic acid levels in pediatric migraine patients. Acta Neurol Belg. 2021;121(6):1741–4. doi: 10.1007/s13760-020-01491-3. [DOI] [PubMed] [Google Scholar]
- 13.Liampas IN, Siokas V, Aloizou AM, Tsouris Z, Dastamani M, Aslanidou P, et al. Pyridoxine, folate and cobalamin for migraine: A systematic review. Acta Neurol Scand. 2020;142(2):108–20. doi: 10.1111/ane.13251. [DOI] [PubMed] [Google Scholar]
- 14.Buse DC, Armand CE, Charleston L, Reed ML, Fanning KM, Adams AM, et al. Barriers to care in episodic and chronic migraine: Results from the Chronic Migraine Epidemiology and Outcomes Study. Headache. 2021;61(4):628–41. doi: 10.1111/head.14103. [DOI] [PubMed] [Google Scholar]
- 15.Antonaci F, Ghiotto N, Wu S, Pucci E, Costa A. Recent advances in migraine therapy. Springerplus. 2016;5:637. doi: 10.1186/s40064-016-2211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nattagh-Eshtivani E, Sani MA, Dahri M, Ghalichi F, Ghavami A, Arjang P, et al. The role of nutrients in the pathogenesis and treatment of migraine headaches: Review. Biomed Pharmacother. 2018;102:317–25. doi: 10.1016/j.biopha.2018.03.059. [DOI] [PubMed] [Google Scholar]
- 17.Reyes-Garcia G, Caram-Salas NL, Medina-Santillan R, Granados-Soto V. Oral administration of B vitamins increases the antiallodynic effect of gabapentin in the rat. Proc West Pharmacol Soc. 2004;47:76–9. [PubMed] [Google Scholar]
- 18.Lea R, Colson N, Quinlan S, Macmillan J, Griffiths L. The effects of vitamin supplementation and MTHFR (C677T) genotype on homocysteine-lowering and migraine disability. Pharmacogenet Genomics. 2009;19(6):422–8. doi: 10.1097/FPC.0b013e32832af5a3. [DOI] [PubMed] [Google Scholar]
- 19.Togha M, Rahmanzadeh R, Nematgorgani S, Yari Z, Razeghi Jahromi S, et al. Suppression of menstrual-related migraine attack severity using pyridoxine, thiamine, and cyanocobalamin: A quasi-experimental within-subject design. Arch Neurosci. 2020;7(3):e93103. [Google Scholar]
- 20.Menon S, Lea RA, Roy B, Hanna M, Wee S, Haupt LM, et al. Genotypes of the MTHFR C677T and MTRR A66G genes act independently to reduce migraine disability in response to vitamin supplementation. Pharmacogenet Genomics. 2012;22(10):741–9. doi: 10.1097/FPC.0b013e3283576b6b. [DOI] [PubMed] [Google Scholar]
- 21.Faraji H, Paknahad Z, Chitsaz A. dietary intake of thiamine in migraine patients and healthy subjects: A case-control study. Clin Nutr Res. 2018;7(1):40–7. doi: 10.7762/cnr.2018.7.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonio C, Massimo T, Gianpaolo Z, Immacolata PM, Erika T. Oral high-dose thiamine improves the symptoms of chronic cluster headache. Case Rep Neurol Med. 2018;2018:3901619. doi: 10.1155/2018/3901619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammady M, Janani L. Randomization in randomized clinical trials: From theory to practice. Hayat. 2016;22(2):102–14. [Google Scholar]
- 24.Razeghi JS, Abolhasani M, Ghorbani Z, Sadre-Jahani S, Alizadeh Z, Talebpour M, et al. Bariatric surgery promising in migraine control: A controlled trial on weight loss and its effect on migraine headache. Obes Surg. 2018;28(1):87–96. doi: 10.1007/s11695-017-2793-4. [DOI] [PubMed] [Google Scholar]
- 25.Zandifar A, Asgari F, Haghdoost F, Masjedi SS, Manouchehri N, Banihashemi M, et al. Reliability and validity of the migraine disability assessment scale among migraine and tension type headache in Iranian patients. Biomed Res Int. 2014;2014:978064. doi: 10.1155/2014/978064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Askari G, Nasiri M, Mozaffari-Khosravi H, Rezaie M, Bagheri-Bidakhavidi M, Sadeghi O. The effects of folic acid and pyridoxine supplementation on characteristics of migraine attacks in migraine patients with aura: A double-blind, randomized placebo-controlled, clinical trial. Nutrition. 2017;38:74–9. doi: 10.1016/j.nut.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Prakash S, Kumar SA, Rathore C. Chronic migraine responding to intravenous thiamine: a report of two cases. Headache. 2016;56(7):1204–9. doi: 10.1111/head.12838. [DOI] [PubMed] [Google Scholar]
- 28.Mauro GL, Martorana U, Cataldo P, Brancato G, Letizia G. Vitamin B12 in low back pain: A randomised, double-blind, placebo-controlled study. Eur Rev Med Pharmacol Sci. 2000;4(3):53–8. [PubMed] [Google Scholar]
- 29.Fu QG, Carstens E, Stelzer B, Zimmermann M. B vitamins suppress spinal dorsal horn nociceptive neurons in the cat. Neurosci Lett. 1988;95(1-3):192–7. doi: 10.1016/0304-3940(88)90655-6. [DOI] [PubMed] [Google Scholar]
- 30.Franca DS, Souza AL, Almeida KR, Dolabella SS, Martinelli C, Coelho MM. B vitamins induce an antinociceptive effect in the acetic acid and formaldehyde models of nociception in mice. Eur J Pharmacol. 2001;421(3):157–64. doi: 10.1016/s0014-2999(01)01038-x. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Ke Z, Luo J. Thiamine deficiency and neurodegeneration: the interplay among oxidative stress, endoplasmic reticulum stress, and autophagy. Mol Neurobiol. 2017;54(7):5440–8. doi: 10.1007/s12035-016-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Artero-Morales M, Gonzalez-Rodriguez S, Ferrer-Montiel A. TRP channels as potential targets for sex-related differences in migraine pain. Front Mol Biosci. 2018;5:73. doi: 10.3389/fmolb.2018.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bianchi A, Salomone S, Caraci F, Pizza V, Bernardini R, D'Amato CC. Role of magnesium, coenzyme Q10, riboflavin, and vitamin B12 in migraine prophylaxis. Vitam Horm. 2004;69:297–312. doi: 10.1016/S0083-6729(04)69011-X. [DOI] [PubMed] [Google Scholar]
- 34.van der Kuy PH, Merkus FW, Lohman JJ, ter Berg JW, Hooymans PM. Hydroxocobalamin, a nitric oxide scavenger, in the prophylaxis of migraine: An open, pilot study. Cephalalgia. 2002;22(7):513–9. doi: 10.1046/j.1468-2982.2002.00412.x. [DOI] [PubMed] [Google Scholar]
- 35.Togha M, Razeghi JS, Ghorbani Z, Ghaemi A, Rafiee P. An investigation of oxidant/antioxidant balance in patients with migraine: A case-control study. BMC Neurol. 2019;19(1):323. doi: 10.1186/s12883-019-1555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taheri P, Mohammadi F, Nazeri M, Zarei MR, Chamani G, Esfahlani MA, et al. Nitric oxide role in anxiety-like behavior, memory and cognitive impairments in animal model of chronic migraine. Heliyon. 2020;6(12):e05654. doi: 10.1016/j.heliyon.2020.e05654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajanayagam MA, Li CG, Rand MJ. Differential effects of hydroxocobalamin on NO-mediated relaxations in rat aorta and anococcygeus muscle. Br J Pharmacol. 1993;108(1):3–5. doi: 10.1111/j.1476-5381.1993.tb13429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Rosa G, Attina S, Spano M, Ingegneri G, Sgro DL, Pustorino G, et al. Efficacy of folic acid in children with migraine, hyperhomocysteinemia and MTHFR polymorphisms. Headache. 2007;47(9):1342–4. doi: 10.1111/j.1526-4610.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- 39.Bager P, Hvas CL, Rud CL, Dahlerup JF. Randomised clinical trial: High-dose oral thiamine versus placebo for chronic fatigue in patients with quiescent inflammatory bowel disease. Aliment Pharmacol Ther. 2021;53(1):79–86. doi: 10.1111/apt.16166. [DOI] [PubMed] [Google Scholar]
- 40.Wadekar A, Hepat S, Giri A, Acharya S. Wernicke's encephalopathy with normal neuroimaging -suspect and treat -a case report. J Evol Med Dent Sci. 2021;10:2021. [Google Scholar]
- 41.Sadeghi O, Nasiri M, Maghsoudi Z, Pahlavani N, Rezaie M, Askari G. Effects of pyridoxine supplementation on severity, frequency and duration of migraine attacks in migraine patients with aura: A double-blind randomized clinical trial study in Iran. Iran J Neurol. 2015;14(2):74–80. [PMC free article] [PubMed] [Google Scholar]
- 42.Menon S, Nasir B, Avgan N, Ghassabian S, Oliver C, Lea R, et al. The effect of 1 mg folic acid supplementation on clinical outcomes in female migraine with aura patients. J Headache Pain. 2016;17(1):60. doi: 10.1186/s10194-016-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nematgorgani S, Gholi Z, Razeghi Jahromi S, Togha M, Karimzadeh F. The effect of vitamins b on improving the symptoms of migraine: An overview. Neurosci J Shefaye Khatam. 2020;8(2):119–29. [In Persian] [Google Scholar]
- 44.Dumanlidag S, Milanlioglu A. Comparison of static and dynamic balance measurements among chronic and episodic migraine patients. Arq Neuropsiquiatr. 2021;79(5):399–406. doi: 10.1590/0004-282X-ANP-2020-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]