Abstract
Helicobacter pylori induces cell death by apoptosis. However, the apoptosis-inducing factor is still unknown. The virulence factor vacuolating cytotoxin A (VacA) is a potential candidate, and thus its role in apoptosis induction was investigated in the human gastric epithelial cell line AGS. The supernatant from the vacA wild-type strain P12 was able to induce apoptotic cell death, whereas the supernatant from its isogenic mutant strain P14 could not. That VacA was indeed the apoptosis-inducing factor was demonstrated further by substantial reduction of apoptosis upon treatment of AGS cells with a supernatant specifically depleted of native VacA. Furthermore, a recombinant VacA produced in Escherichia coli was also able to induce apoptosis in AGS cells but failed to induce cellular vacuolation. These findings demonstrate that the vacuolating cytototoxin of H. pylori is a bacterial factor capable of inducing apoptosis in gastric epithelial cells.
Helicobacter pylori is a gram-negative, spiral-shaped, microaerophilic bacterium that plays a major role in the development of chronic gastritis, peptic ulcer, and gastric cancer (24, 31, 33). H. pylori is adapted to colonize the human stomach (24). It causes inflammation and epithelial cell damage (11), including cytoplasmic vacuolation and induction of apoptosis (40). Different virulence factors of H. pylori, including urease, lipopolysaccharides (LPS), adhesins, the cytotoxin-associated gene A product (CagA), and vacuolating cytotoxin A (VacA), have been described (for a review, see reference 5). About 50% of all isolated H. pylori strains express a functional vacuolating cytotoxin (3). The vacuolating cytotoxin is a major virulence factor in the pathogenesis of H. pylori-related diseases (38). The monomeric 87-kDa protein results from cleavage of a 139-kDa precursor protein (7). In bacterial cultures the monomers show the tendency to form a high-molecular-mass complex, consisting of 12 monomers with a molecular mass of about 1,000 kDa (8). VacA is suggested to exert its cytotoxic activity after internalization by epithelial cells (14).
Cell death can be executed via different mechanisms. One way is the apoptotic pathway described by Kerr et al. (18). Apoptosis is characterized by morphological and biochemical changes such as membrane blebbing, chromatin condensation, and DNA fragmentation (18). Recently, it has been shown that apoptosis is involved in H. pylori-induced epithelial cell damage (20, 23, 28, 34, 40, 46). It has been demonstrated that H. pylori-induced apoptosis is mediated via the CD95 receptor and ligand system and that this pathway is activated both from CD95 ligand (CD95L)-expressing lymphocytes and from CD95L-expressing gastric epithelial cells (40). CD95 receptor activation leads to oligomerization of CD95 receptors and to formation of a death-inducing signaling complex (19, 25, 29, 45). The death signal is transmitted via the proteolytic activation of a cascade of different caspases (for a review, see reference 36). Inoculation with H. pylori increases CD95L expression in epithelial cells in vitro and in the gastric epithelium in vivo (40). Nevertheless, it remains unclear which bacterial factor participates in the induction of epithelial apoptosis. Ultrafiltration of an apoptosis-inducing, cytotoxic H. pylori strain supernatant revealed that the apoptosis-inducing factor has a molecular mass above 300 kDa (D. Kuck et al., unpublished observation). Therefore, it was supposed that the cytotoxin VacA could be the candidate protein leading to the induction of apoptosis in gastric epithelial cells. Previous studies from Manetti et al. suggested that recombinant VacA lacks any cytotoxic activity (22). We hypothesized, however, that this lack of cytotoxic activity in their study was due to the purification of the recombinant protein under denaturing conditions. In the present study a recombinant protein was expressed and purified under native conditions, and it was able to induce apoptosis in the human gastric epithelial cell line AGS. To confirm the results obtained with recombinant VacA, the cytotoxic H. pylori strain P12, which expresses a functional cytotoxin, and its isogenic mutant strain P14, which possesses an inactivated vacA gene, were evaluated for their apoptosis-inducing properties. It was demonstrated that the supernatant of the cytotoxic strain P12 induces apoptosis, unlike the isogenic vacA mutant strain P14. We conclude that both recombinant and native VacA cytotoxins of H. pylori induce apoptosis in gastric epithelial cells.
MATERIALS AND METHODS
Bacterial culture.
The following strains were used: H. pylori 60190 (ATCC 49503), a wild-type, cytotoxic, cagA-positive strain with the vacA genotype s1a/m1 (39); P12, a cytotoxic, cagA-positive strain with the vacA genotype s1/m1; and its isogenic mutant strain P14, which was created by transposon insertion mutagenesis (the last two were kindly provided by R. Haas, Munich, Germany). The mutagenesis of the 3′ region of the vacA gene was carried out via transformation of strain P12 with the plasmid pTn-73 (41). All strains were minimally passaged. A preculture was grown with shaking at 100 rpm in brucella broth containing 10% fetal calf serum (FCS) under microaerophilic conditions (10% CO2, 5% O2 and 85% N2) at 37°C. H. pylori medium (70% RPMI 1640, 10% FCS, 10% brain heart infusion, 10% brucella broth, 1% l-glutamine) was inoculated with the preculture, and the bacteria were cultivated for 2 to 4 days to an optical density at 600 nm of 0.5. The cultures were centrifuged at 5,000 × g, and the supernatants were removed and passed through a 0.2-μm filter. For VacA concentration, the supernatant was separated in a stirring cell by ultrafiltration (Centricon; Millipore, Eschborn, Germany) using a cellulose membrane (cutoff, 100 kDa; Millipore) and stored at −70°C. The supernatants were reduced 10-fold in volume by ultrafiltration.
Cell culture and cell vacuolation assay.
AGS cells were cultured in Ham's F-12 medium (PAA Laboratories, Martinsried, Germany) containing 10% FCS and 1% penicillin-streptomycin (PAA Laboratories). For the cell vacuolation assay, the cells were incubated with culture supernatants of H. pylori for up to 24 h at 37°C in chamber slides (Lab-Tek; Nunc, Naperville, Ill.). To detect the vacuoles, cells were stained with 0.05% neutral red solution for 5 min, washed twice with phosphate-buffered saline (PBS), and analyzed by light microscopy immediately after washing.
Detection of apoptosis in AGS cells. (i) FACScan analysis.
Apoptosis in AGS cells, detected by the appearance of a typical sub-G1 fraction of fragmented nuclei, was assessed by FACScan analysis carried out in a FACScan flow cytometer (Becton Dickinson, Heidelberg, Germany). Cells floating in the culture medium were collected by centrifugation at 100 × g. Adherent cells were harvested by incubation with 1% trypsin for 3 min. The cells were washed with PBS and fixed in 70% ethanol for less than 1 h at −20°C. After fixation, the cells were washed with PBS and stained with propidium iodide (50 μg/ml in PBS) (Sigma-Aldrich, Taufkirchen, Germany) according to the method of Nicoletti et al. (30). A minimum of 10,000 cells per sample was measured. Acquisition and analysis were conducted using Cell Quest software (Becton Dickinson).
(ii) DAPI staining and annexin V-propidium iodide staining.
To identify apoptotic nuclei, DAPI (4′,6′-diamidino-2-phenylindole) (Roche-Boehringer, Mannheim, Germany) staining was performed according to the manufacturer's protocol. An annexin V-Fluos staining kit (Roche-Boehringer) was used to detect early stages of apoptosis, as represented by phosphatidylinositol flip to the outer membrane. The cells were washed with PBS and stained according to the manufacturer's protocol. Slides were mounted with Permafluor mounting medium (Immunotech, Marseille, France) and viewed under a fluorescence microscope (Axiophot Olympus).
Preparation of anti-VacA antibodies, immunoprecipitation, and Western blot analysis. (i) Preparation of rabbit anti-VacA antibodies.
A DNA fragment spanning nucleotides 2317 to 2952 of the vacA gene of H. pylori strain NCTC 11638 (GenBank no. U07145) was generated by PCR using genomic DNA from H. pylori as template DNA. The DNA fragment was subsequently inserted into a modified pET8c vector for overexpression of the His-tagged fusion protein in Escherichia coli, and the bacteria were cultured at 37°C. After lysis the crude lysate was flash frozen in liquid nitrogen and thawed at 37°C. The lysate was treated with RNase at a final concentration of 5 μg/ml and with DNase at a final concentration of 5 μg/ml. The mixture was finally mixed with PBS-equilibrated ProBond resin (4:1; Invitrogen, Groningen, The Netherlands). The proteins were then eluted with PBS by column chromatography. Cleavage of the His tag was performed with enterokinase (1 U/10 μg of protein; Invitrogen). The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the corresponding band was cut out, solved in PBS, and precipitated with acetone. Finally, the protein was resuspended in PBS and sterile filtered. Anti-VacA antibodies were then obtained by immunization of rabbits.
(ii) Immunoprecipitation.
For immunoprecipitation of VacA, 100 μl of anti-VacA rabbit antibodies or 100 μl of control serum (preimmune rabbit serum) was incubated with 100 μl of protein A solution at 4°C on a tumbler overnight. After coupling of antibodies to protein A, the solutions were centrifuged at 3,000 × g and washed twice with H. pylori medium. The antibody-protein A pellets were resuspended in 1 ml of concentrated H. pylori 60190 supernatant. The bacterial supernatant was concentrated by ultrafiltration using a cutoff of >100 kDa. Depletion of VacA with anti-VacA was carried out for 4 h at 4°C on a tumbler. After centrifugation at 3,000 × g for 3 min, the depleted supernatants were removed, sterile filtered, and stored at −70°C. The antibody-protein A pellets were washed twice with medium and then resuspended in 100 μl of SDS sample buffer.
(iii) Western blot analysis.
For protein detection, 50 μl containing supernatant and antibody-protein A pellets was loaded onto an SDS–12% polyacrylamide gel (or an SDS–15% polyacrylamide gel for detection of recombinant VacA). The proteins were transferred to a nitrocellulose membrane and washed for 20 min in washing buffer (PBS with 0.1% Tween 20). Blocking was carried out in I-Block (0.2 g of I-Block in 100 ml of washing buffer; Tropix, Perkin-Elmer, Ueberlingen, Germany) for 1 h. The anti-VacA rabbit serum was diluted 1:10,000 in washing buffer and I-Block (1:1) and incubated overnight at 4°C. After washing, the blot was incubated for 15 min with secondary antibody (alkaline phosphatase-conjugated goat anti-rabbit; Tropix) at a dilution of 1:25,000 in washing buffer. The blot was washed for 30 min and incubated in assay buffer (9.4 ml of diethanolamine, 1 mM MgCl2 [pH 10.0]) for 30 min. The blot was then incubated with Nitroblock II (1.5 ml of Nitroblock in 30 ml of assay buffer; Tropix) for 10 min, and finally, the blot was incubated for 5 min with CDP-Star (1:200 in assay buffer; Tropix) and then exposed to Hyperfilm ECL film (Amersham, Ueberlingen, Germany).
Cloning of vacA.
For cloning of the vacA gene from strain NCTC 11638, PCR was carried out with the forward primer 5′ TTTGAGGCCTTTTTCACAACCGTGATCATTCCAGCC 3′ and the reverse primer 5′ TTTCTGCAGCTATTACGAAATTTTAGAGCCATTAGCTGTTTTGCT 3′. A DNA fragment spanning nucleotides 504 to 2892 of the vacA gene was generated by PCR using genomic DNA from H. pylori as the template. The PCR product encodes amino acids 34 to 821. The first 33 amino acids of the vacA gene product resemble bacterial signal peptides and have been deleted.
The E. coli strain TOP10 (Invitrogen) was used for subcloning. The PCR fragment was subsequently inserted in the pBADHisA plasmid (Invitrogen) for overexpression. The recombinant protein had a molecular mass of 86 kDa (796 residues) without the His tag. Double-strand sequencing was carried out by the dideoxy sequencing method. Sequence alignment was carried out with the BLASTN database software tool (1).
Expression and purification of recombinant VacA.
E. coli LMG 194 bacteria were transformed by incubation with 2 μg of DNA, and ampicillin-resistant clones were selected. For expression, a 30-ml volume was precultured overnight in RM base medium (Invitrogen) with 0.2% glucose and 100 μg of ampicillin/ml. An overnight culture with 1 liter of RM base medium containing 0.2% glucose, 100 μg of ampicillin/ml, and 25 μM dithiothreitol (Sigma-Aldrich) was inoculated at 37°C on a shaker to an optical density at 600 nm of 0.4. Protein expression was then induced with 0.0002% arabinose for 5 h. The culture was centrifuged for 30 min at 5,000 × g, and the cells were resuspended in PBS (pH 7.2). Egg white lysozyme was added to give a final concentration of 100 μg/ml, and the suspension was incubated for 15 min on ice. Sonication was performed with three 10-s bursts at a medium intensity while holding the suspension on ice. The crude lysate was flash frozen in liquid nitrogen and thawed at 37°C. The cells were then lysed by three successive freeze-thaw cycles. The lysate was treated with RNase at a final concentration of 5 μg/ml for 15 min at 30°C and with DNase at a final concentration of 5 μg/ml. Insoluble debris was removed by centrifugation at 3,000 × g for 15 min. The supernatant was mixed with PBS-equilibrated ProBond resin (4:1) (Invitrogen) and incubated on a shaker for 1 h on ice. After binding, the mixture was loaded on a column and washed with 5 volumes of PBS (pH 6.0). Elution was performed with 2 volumes of PBS (pH 4.0). The eluate was concentrated using an Amicon-10 ultrafiltration unit. Protein concentration was determined according to the method of Bradford (4).
Statistical analysis.
Comparison of the data was performed using the Mann-Whitney U test.
RESULTS
Cell vacuolation assay.
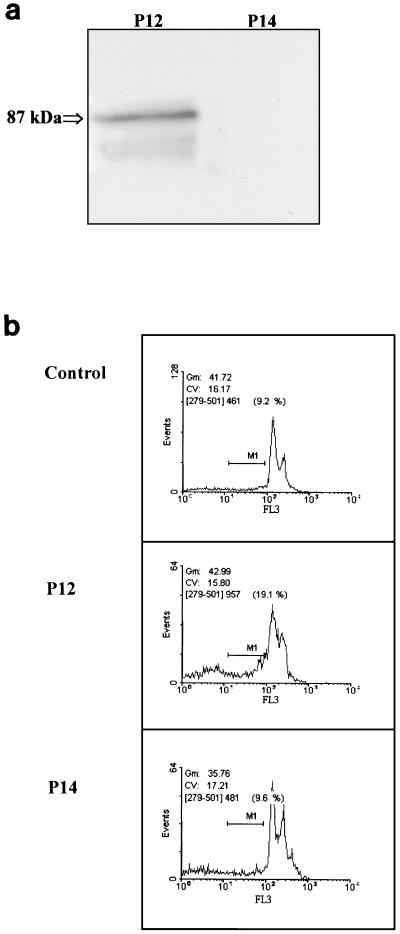
AGS cells were treated for 48 h with 250 μl of the concentrated supernatants/ml obtained from H. pylori vacA wild-type strain P12 and the isogenic vacA mutant strain P14. In AGS cells treated with P12 supernatant, 80% of all cells showed cellular vacuoles. In contrast, the supernatant from P14 failed to induce the formation of vacuoles (data not shown). The presence of VacA in the supernatants was confirmed by Western blotting with anti-VacA antibodies (Fig. 1a). Only the wild-type strain P12 showed a VacA signal in the range of 90 kDa by Western blotting, demonstrating that the formation of vacuoles was indeed induced by VacA.
FIG. 1.
(a) Western blot analysis of VacA. Fifty-microliter samples of concentrated supernatant from H. pylori P12 and P14 were subjected to SDS-PAGE and immunoblotted with anti-VacA antibodies. Native VacA appeared (at approximately 87 kDa) only with the supernatant of strain P12. (b) FACScan analysis of AGS cells. AGS cells were treated for 48 h with 250 μl of supernatant of H. pylori strains P12 and P14/ml. The abscissa displays the counts and the ordinate displays the fluorescence on a logarithmic scale. Apoptotic cells appear as a sub-G1 peak measured through the marker region (M1). Treatment with concentrated supernatant of the vacA wild-type strain P12 increased the amount of apoptotic cells compared to untreated AGS cells or AGS cells treated with the isogenic vacA mutant strain P14.
Induction of apoptosis by H. pylori strains P12 and P14.
Apoptosis was determined by FACScan analysis and DAPI staining. Apoptosis induction in AGS cells was observed at a minimum volume of about 100 to 150 μl of supernatant of strain P12. AGS cells incubated with 250 μl of concentrated supernatant/ml from the wild-type strain P12 for 48 h revealed in several FACScan analyses a distinct increase in cell death from 7.6% ± 1.5% (mean ± standard deviation) in untreated cells to about 20.1% ± 5.9% (P = 0.05) in treated cells. In contrast, the concentrated supernatant from the vacA mutant strain P14 showed no increase in cell death (8.6% ± 1.1%, P = 0.7) after 48 h of incubation compared with untreated AGS cells (Fig. 1b).
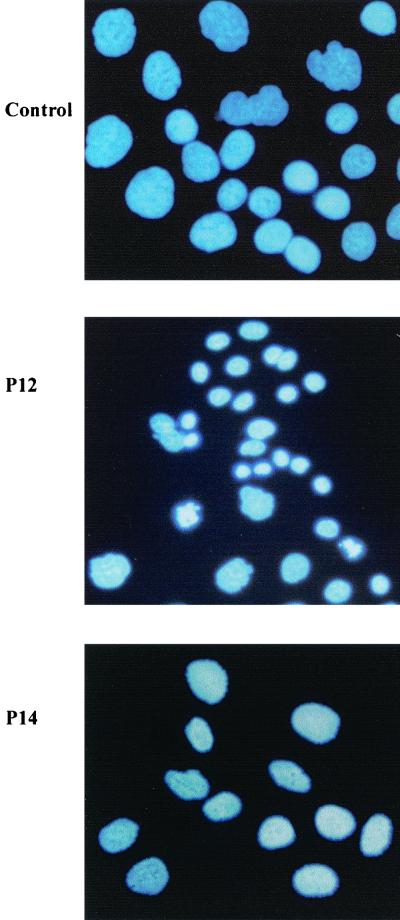
Comparable results were obtained by DAPI staining of AGS cells. After 48 h of incubation with 250 μl of the concentrated supernatants from P12 and P14/ml, only incubation with supernatant from strain P12 led to the typical apoptosis hallmark of chromatin condensation. Untreated cells and cells treated with P14 supernatant presented both unchanged, normal nuclei without signs of apoptosis (Fig. 2).
FIG. 2.
DAPI staining of AGS. AGS cells were incubated for 48 h with 250 μl of supernatants from strains P12 and P14/ml. Nuclei are stained blue. Incubation with supernatant of the vacA wild-type strain P12 increased the amount of apoptotic cells, which are characterized by chromatin condensation with brighter fluorescence. In contrast, the supernatant from its isogenic vacA mutant strain P14 showed only normal, noncondensed nuclei. Magnification, ×400.
Immunoprecipitation of VacA.
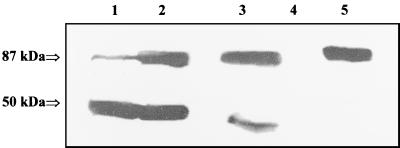
To demonstrate that VacA is the apoptosis-inducing factor, the VacA protein was removed from the concentrated supernatant of H. pylori 60190 by immunoprecipitation with anti-VacA antibodies. Successful depletion was confirmed by Western blotting (Fig. 3) and by a cell vacuolation assay (not shown). Treatment of the H. pylori 60190 supernatant with anti-VacA antibodies led to a substantial removal of VacA from the supernatant. Control treatment with control serum (preimmune serum) revealed only a minor, nonspecific depletion of VacA from the supernatant.
FIG. 3.
Western blot analysis of immunodepleted and concentrated supernatants of H. pylori 60190. The antibody (anti-VacA antibodies or control serum)-protein A pellets and the depleted supernatants were subjected to SDS–12% PAGE and immunoblotted with anti-VacA antibodies. Lane 1, precipitated pellets with control serum; lane 2, precipitated pellets with anti-VacA antibodies; lane 3, supernatant depleted with control serum; lane 4, supernatant depleted with anti-VacA antibodies; lane 5, native H. pylori 60190 supernatant. The 87-kDa band represents VacA, and the 50-kDa band represents the heavy chain of Igs. Incubation with anti-VacA antibodies coupled to protein A substantially removed VacA from the H. pylori 60190 supernatant (lanes 2 and 4). Precipitation with control antibodies demonstrated only a slight precipitation of VacA (lanes 1 and 3).
Complete depletion of VacA was also confirmed in vivo by a cell vacuolation assay. AGS cells were treated for 24 h with 100 μl of supernatant/ml. The native H. pylori 60190 supernatant led to an extensive cellular vacuolation, whereas the vacuolation substantially decreased when AGS cells were incubated with VacA-depleted supernatant. Cells treated with control serum-depleted supernatant also showed only slight vacuole formation.
FACScan analysis of AGS cells treated with VacA-depleted supernatant.
To investigate further the role of VacA in the induction of apoptosis, AGS cells were treated for 24 h with 100 μl of concentrated native and VacA-depleted supernatant/ml. The mean percentage of apoptotic cells was assessed. The percentage of apoptotic cells in untreated cells was 4.9% ± 3.2%. In cells treated with H. pylori 60190 supernatant, 26.0% ± 6.3% of cells underwent apoptosis (P = 0.008) compared to 13.4% ± 6.0% (P = 0.05) of cells treated with VacA-depleted supernatant and 23.5% ± 6.6% (P = 0.008) of cells treated with control serum-depleted supernatant. There was a significant difference in apoptosis rates between cells treated with H. pylori 60190 supernatant and cells treated with anti-VacA (P = 0.008). A trend to significance was obtained for the differences between apoptosis rates of anti-VacA-treated cells (13.4% ± 6.0%) and cells treated with control serum-depleted supernatant (23.5% ± 6.6%, P = 0.095).
Recombinant VacA induces apoptosis in AGS cells.
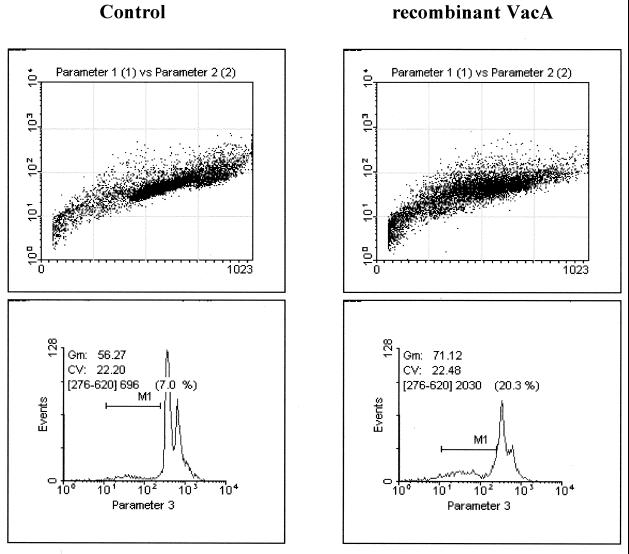
To confirm the apoptosis-inducing activity of recombinant VacA, we expressed the recombinant cytotoxin in E. coli LMG 194 using the pBADHis system. Recombinant VacA was analyzed by Coomassie staining (data not shown) and by Western blotting with anti-VacA antibodies (Fig. 4). The recombinant protein, composed of an 86-kDa VacA part corresponding to the sequence of the native soluble VacA and an N-terminal 3-kDa histidine tag, showed the expected molecular mass of about 90 kDa by Western blot analysis. Double-strand sequencing of the plasmid revealed three point mutations in the amino terminus-coding region when compared with the native vacA sequence of strain NCTC 11638. The mutations led to exchanges of residues 68 (Tyr→His), 149 (Ala→Thr), and 231 (Tyr→His). To detect apoptosis, AGS cells were incubated with 100 μg of recombinant VacA/ml for 60 h. FACScan analysis demonstrated a distinct increase in apoptotic cell death in the range of 19.9% ± 2.1% in treated cells compared to 8.2% ± 2.5% (P = 0.05) in untreated cells (Fig. 5). Additionally, after 60 h of incubation, a remarkable increase of apoptotic, condensed nuclei was observed by DAPI staining (Fig. 6). Similar findings were obtained with annexin V-propidium iodide staining, which showed an increase in apoptotic cells (Fig. 6). The minimum concentration of recombinant VacA for apoptosis induction was in the range of 50 to 100 μg/ml. However, recombinant VacA failed to induce cellular vacuolation (data not shown).
FIG. 4.
Western blot analysis of recombinant VacA. Twenty-five micrograms of recombinant protein and 50 μl of concentrated H. pylori 60190 supernatant were subjected to SDS–15% PAGE and immunoblotted with anti-VacA antibodies. Native VacA appeared at nearly 87 kDa, and the recombinant VacA appeared at 90 kDa.
FIG. 5.
FACScan analysis of AGS cells. AGS cells were incubated for 60 h with 100 μg of recombinant VacA/ml. The abscissa displays the counts, and the ordinate displays the fluorescence on a logarithmic scale. Apoptotic cells appear as a sub-G1 peak, which was statistically acquired through the marker region (M1). Incubation with recombinant VacA increases apoptotic cell death (right).
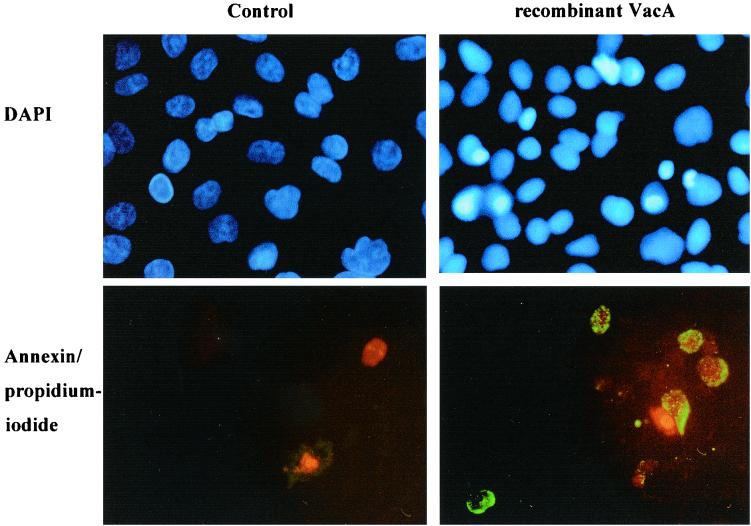
FIG. 6.
DAPI staining and annexin V-propidium iodide staining of AGS cells. AGS cells were treated for 60 h with 100 μg of recombinant VacA/ml. DAPI-stained nuclei are blue. Annexin V-stained cells appear green, which characterizes the early stage of the apoptotic process. Propidium iodide labels the necrotic cells red. After incubation with recombinant VacA, DAPI-stained nuclei showed apototic, condensed chromatin (top right) and annexin V-propidium iodide-stained cells showed signs of early apoptotic stages (bottom right). Untreated cells demonstrated normal nuclei with DAPI staining (top left) and no apoptosis induction with annexin V-propidium iodide staining (bottom left). Magnification, ×400.
DISCUSSION
Apoptosis plays a major role in the pathogenic action of H. pylori (20, 23, 28, 34, 40, 46). The CD95 receptor and ligand system was previously identified as a mediator of apoptosis induction in gastric epithelial cells in vitro and in patients with H. pylori-induced chronic gastritis in vivo (40). However, it was still unclear which bacterial factors of H. pylori are involved in apoptosis induction. The results of the present study demonstrate that VacA is at least one of the H. pylori factors capable of apoptosis induction.
First, it was shown by FACScan analysis and DAPI staining that the supernatant of the cytotoxic wild-type H. pylori strain P12 induces apoptosis in AGS cells. In contrast, the supernatant from its isogenic vacA mutant strain P14 has lost its apoptosis-inducing activity. Further confirmation was provided by immunodepletion of native VacA with anti-VacA antibodies from the supernatant of H. pylori 60190. Quantitative removal of VacA from the supernatant after immunoprecipitation was confirmed by cell vacuolation assay and by Western blotting with anti-VacA antibodies. Depletion of VacA led to a substantial reduction of vacuole formation. Analysis of AGS cells treated with VacA-depleted supernatant demonstrated a reduced induction of apoptosis, compared to the apoptosis induction by native, nondepleted H. pylori 60190 supernatant. Control immunoglobulin (Ig)-depleted supernatant showed only a slight reduction of apoptotic activity due to unspecific binding of VacA to Ig. These results strongly indicate that VacA is a bacterial factor capable of inducing apoptosis.
In contrast to the present results, apoptosis could not be induced with lower concentrations of VacA (10 μg/ml) in a recent study (35). This may be due to larger amounts of VacA used in the present study, with estimated VacA concentrations of about 200 μg/ml in 10-fold-concentrated supernatant.
To further confirm the role of VacA in the induction of apoptosis, recombinant VacA was generated using an expression system consisting of the vector pBADHis and the E. coli strain LMG 194 (15, 16). Using this system, a recombinant protein that induces apoptosis in the human gastric epithelial cell line AGS was expressed and purified under native conditions. FACScan analysis of AGS cells after incubation with recombinant VacA revealed a substantial induction of apoptosis. In addition, the apoptosis-inducing activity of recombinant VacA was confirmed by DAPI staining and annexin V-propidium iodide staining of AGS cells incubated with recombinant VacA. The recombinant protein in this system was not, however, able to induce vacuole formation. Double-strand sequencing of the plasmid revealed three mutations in the amino terminus coding region. These amino acid exchanges may be responsible for incomplete protein folding during expression, causing the protein to lose its vacuolating activity. Recently, it has been shown that the vacuolating activity of VacA may be confined to the amino-terminal domain of VacA (9). Previous attempts to express a recombinant VacA resulted in a protein which completely lacked any biological activity (22). This may be due to inclusion bodies formed during protein overexpression. The presence of inclusion bodies requires the protein to be purified under denaturating conditions and subsequently refolded. To avoid incomplete or incorrect folding, an expression system for VacA which allows the generation of the protein without overexpression was used (15, 16). A functional active recombinant VacA with apoptosis-inducing properties could be generated using this system.
However, VacA might exert apoptosis induction and vacuolating cytotoxicity through different domains and by different mechanisms, which could explain the presence of induction of apoptosis and the lack of vacuolating activity. The mechanism of cell vacuolation by VacA has been partly characterized. It has been hypothesized that VacA assembles at an acidic pH into a hexameric anion-selective channel in planar phospholipid bilayers (8, 10, 17, 21). After binding to the cell surface, the toxin translocates into the cytosol, where it may affect a key molecule involved in the control of membrane trafficking of late endosomes and lysosomes. VacA can assemble to an ion channel in the vacuolar membrane, which at least in part is responsible for vacuolar swelling (26, 27). In contrast, the way VacA induces apoptosis is still unclear. A proposed mechanism of apoptosis induction by VacA is the activation of the CD95 receptor and ligand system (40). Microinjection of DNA encoding VacA-GFP (green fluorescent protein) into HEP-2 cells induced apoptotic cell death in a very recent study (13). It was further shown that the N-terminal fragment of VacA (p34) induces cytochrome c release and apoptosis (13).
The results, however, indicate that bacterial factors of H. pylori other than VacA may be involved. Immunodepletion of VacA did not result in total disappearance of the apoptosis-inducing capacity from the supernatant as was expected based on the results of Western blotting and cell vacuolation assays. This may also be explained by differences in the limits of detection of these methods, and several other factors, such as LPS (37), urease (20), and the picB product (35), have been suggested to contribute to H. pylori-mediated apoptosis. Isolated LPS from a cytotoxic H. pylori strain increased the rate of apoptosis throughout the gastric epithilium in rats (37). In addition, a significant correlation between urease activity and the level of apoptosis in the mucosa of infected patients with active ulcer disease has been reported (20). H. pylori protease and lipase degrade gastric mucus and disrupt the phospholipid layer of the cells, which may lead to apoptotic cell death (43). Another factor contributing to apoptosis induction could be CagA. Recently, several investigators have shown that CagA is delivered into epithelial cells by the cag type IV secretion system and is subsequently phosphorylated (2, 32, 44). The majority of analyzed cagA+ strains induced enhanced apoptosis when compared to cagA-negative strains (35). Other investigators could not confirm these findings (42). The presence of other possible apoptosis-inducing factors might, in part, explain why some investigators could not find a significant difference in apoptosis induction between VacA-positive and VacA-negative strains (46).
This study provides evidence that the cytotoxin VacA is a bacterial factor capable of inducing apoptosis in AGS cells. It was shown for the first time that both native and recombinant VacA have apoptosis-inducing activity. VacA-producing H. pylori strains are found in the vast majority of patients with peptic ulcer disease (PUD) (6, 12). VacA has been shown to be directly involved in mucosal damage in PUD (43). This damage might be attributed to the vacuolating as well as to the apoptosis-inducing activity of VacA. Therefore, elucidation of VacA as an apoptosis-inducing factor might offer a better understanding of the pathogenesis of H. pylori-related diseases such as PUD.
ACKNOWLEDGMENTS
We gratefully acknowledge the generous assistance of M. Müller-Schilling, G. M. Hänsch, and W. P. Hosch.
This work was supported in part by a grant from Astra GmbH, Wedel, Germany.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang H Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahi M, Azuma T, Ito S, Suto H, Nagai Y, Tsubokawa M, Tohyama Y, Maeda S, Omata M, Suzuki T, Sasakawa G. Helicbacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atherton J C, Cao P, Peek R M, Tummuru M K R, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 6.Cover T L, Cao P, Lind C D, Tham K T, Blaser M J. Correlation between vacuolating cytotoxin production by Helicobacter pylori isolates in vitro and in vivo. Infect Immun. 1993;61:5008–5012. doi: 10.1128/iai.61.12.5008-5012.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cover T L, Blaser M J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 8.Cover T L, Hanson P I, Heuser J E. Acid-induced dissociation of VacA, the Helicobacter pylori vacuolating cytotoxin, reveals its pattern of assembly. J Cell Biol. 1997;138:759–769. doi: 10.1083/jcb.138.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bernard M, Burroni D, Papini E, Rappuoli R, Telford J, Montecucco C. Identification of the Helicobacter pylori VacA toxin domain active in the cell cytosol. Infect Immun. 1998;66:6014–6016. doi: 10.1128/iai.66.12.6014-6016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bernard M, Papini E, De Filippis V, Gottardi E, Telford J L, Manetti R, Fontana A, Rappuoli R, Montecucco C. Low pH activates the vacuolating cytotoxin of Helicobacter pylori, which becomes acid and pepsin resistant. J Cell Biol. 1995;270:23937–23940. doi: 10.1074/jbc.270.41.23937. [DOI] [PubMed] [Google Scholar]
- 11.Dixon M F, Genta R M, Yardley J H, Correa P the Participants in the International Workshop on the Histopathology of Gastritis, Houston 1994. Classification and grading of gastritis. The updated Sydney System. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Figura N, Guglielmetti A P, Rossolini A, Barberi A, Cusi G, Musmanno R A, Russi M, Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989;27:225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galmiche A, Rassow J, Doye A, Cagnol S, Chambard J C, Contamin S, de Thillot V, Just I, Ricci V, Solcia E, Van Obberghen E, Boquet P. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 2000;19:6361–6370. doi: 10.1093/emboj/19.23.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garner J A, Cover T L. Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect Immun. 1996;64:4197–4203. doi: 10.1128/iai.64.10.4197-4203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzman L-M, Barondess J J, Beckwith J. FtsL, an essential cytoplasmic membrane protein involved in cell division in Escherichia coli. J Bacteriol. 1992;174:7716–7728. [PMC free article] [PubMed] [Google Scholar]
- 17.Iwamoto H, Czajkowsky D M, Cover T L, Szabo G, Shao Z. VacA from Helicobacter pylori: a hexameric chlorid channel. FEBS Lett. 1999;450:101–104. doi: 10.1016/s0014-5793(99)00474-3. [DOI] [PubMed] [Google Scholar]
- 18.Kerr J F, Wyllie A H, Currie A R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kischkel F C, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer P H, Peter M E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins from a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohda K, Tanaka K, Aiba Y, Yasuda M, Miwa T, Koga Y. Role of apoptosis induced by Helicobacter pylori infection in the development of duodenal ulcer. Gut. 1999;44:456–462. doi: 10.1136/gut.44.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lupetti P, Heuser J E, Manetti R, Massari P, Lanzavecchia S, Bellon P L, Dallai R, Rappuoli R, Telford J L. Oligomeric and subunit structure of the Helicobacter pylori vacuolating cytotoxin. J Cell Biol. 1996;133:801–807. doi: 10.1083/jcb.133.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manetti R, Massari P, Burroni D, de Bernard M, Marchini A, Olivieri R, Papini E, Montecucco C, Rappuoli R, Telford J L. Helicobacter pylori cytotoxin: importance of native conformation for induction of neutralizing antibodies. Infect Immun. 1995;63:4476–4480. doi: 10.1128/iai.63.11.4476-4480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannick E E, Bravo L E, Zarama G, Realpe J L, Zhang X-J, Ruiz B, Fontham E T H, Mera R, Miller M J S, Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- 24.Marshall B J, Warren J R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;i:1311–1314. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 25.Medema J P, Scaffidi C, Kischkel F C, Shevchenko A, Mann M, Krammer P H, Peter M E. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montecucco C. Protein toxins and membrane transport. Curr Opin Cell Biol. 1998;10:530–536. doi: 10.1016/s0955-0674(98)80069-0. [DOI] [PubMed] [Google Scholar]
- 27.Montecucco C. Molecular and cellular activities of Helicobacter pylori pathogenic factors. FEBS Lett. 1999;452:16–21. doi: 10.1016/s0014-5793(99)00652-3. [DOI] [PubMed] [Google Scholar]
- 28.Moss S F, Calam J, Agarwal B, Wang S, Holt P R. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagata S. Apoptosis by death factors. Cell. 1997;68:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 30.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardiet C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 31.NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 32.Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 33.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orenteich N, Sibley R. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1231. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 34.Peek R M, Moss S F, Tham K T. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial cell proliferation from apoptosis. J Natl Cancer Inst. 1997;89:863–868. doi: 10.1093/jnci/89.12.863. [DOI] [PubMed] [Google Scholar]
- 35.Peek R M, Jr, Blaser M J, Mays D J, Forsyth M H, Cover T L, Song S Y, Krishna U, Pietenpol J A. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 1999;59:6124–6131. [PubMed] [Google Scholar]
- 36.Peter M E, Krammer P H. Mechanisms of CD95(APO-1/Fas)-mediated apoptosis. Curr Opin Immunol. 1998;10:545–551. doi: 10.1016/s0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- 37.Piotrowski J, Skrodzka D, Slomiany A, Slomiany B L. Helicobacter pylori lipopolysaccharide induces gastric epithelial cells apoptosis. Biochem Mol Biol Int. 1996;40:597–602. doi: 10.1080/15216549600201183. [DOI] [PubMed] [Google Scholar]
- 38.Rudi J, Rudy A, Maiwald M, Kuck D, Sieg A, Stremmel W. Direct determination of Helicobacter pylori vacA genotypes and cagA gene in gastric biopsies and relationship to gastrointestinal diseases. Am J Gastroenterol. 1999;946:1525–1531. doi: 10.1111/j.1572-0241.1999.1138_a.x. [DOI] [PubMed] [Google Scholar]
- 39.Rudi J, Kolb C, Maiwald M, Kuck D, Sieg A, Galle P R, Stremmel W. Diversity of Helicobacter pylori vacA and cagA genes and relationship to VacA and CagA protein expression, cytotoxin production, and associated diseases. J Clin Microbiol. 1998;364:944–948. doi: 10.1128/jcm.36.4.944-948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudi J, Kuck D, Strand S, von Herbay A, Mariani S M, Krammer P H, Galle P R, Stremmel W. Involvement of the CD95 (APO-1/Fas) receptor and ligand system in Helicobacter pylori induced gastric epithelial apoptosis. J Clin Investig. 1998;1028:1506–1514. doi: 10.1172/JCI2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt W, Haas R. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol Microbiol. 1994;12:307–319. doi: 10.1111/j.1365-2958.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 42.Shirin H, Sordillo E M, Oh S H, Yamamoto H, Delohery T, Weinstein I B, Moss S F. Helicobacter pylori inhibits the G1 to S transition in AGS gastric epithelial cells. Cancer Res. 1999;59:2277–2281. [PubMed] [Google Scholar]
- 43.Smoot D T. How does Helicobacter pylori cause mucosal damage? Direct mechanisms. Gastroenterology. 1997;113(Suppl.):31–34. doi: 10.1016/s0016-5085(97)80008-x. [DOI] [PubMed] [Google Scholar]
- 44.Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trauth B C, Klas C, Peters A M J, Matzku P, Möller P, Falk W, Debatin K M, Krammer P H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 46.Wagner S, Beil W, Westermann J, Logan R P, Trautwein C, Bleck J S, Manns M P. Regulation of gastric epithelial cell growth by Helicobacter pylori: evidence for a major role of apoptosis. Gastroenterology. 1997;113:1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]