Significance
The control scheme for length-dependent activation (LDA), a fundamental, striated muscle property where submaximal contraction is improved at longer compared to shorter lengths, is poorly understood. We demonstrate that 50% titin cleavage in passive sarcomeres attenuates all measured structural markers of LDA for thick (e.g., myosin) and thin (e.g., actin, troponin) filaments, as well as a functionally required thick-thin filament bridge in the A-band. These culminate to attenuate LDA in contracting fibers. The identified regulatory role of titin to LDA structural features is critical to the future study of many myo- and cardiomyopathies, of which LDA impairment is a hallmark.
Keywords: elasticity, X-ray diffraction, mouse, ultrastructure, length-dependent activation
Abstract
Skeletal muscle force production is increased at longer compared to shorter muscle lengths because of length-dependent priming of thick filament proteins in the contractile unit before contraction. Using small-angle X-ray diffraction in combination with a mouse model that specifically cleaves the stretch-sensitive titin protein, we found that titin cleavage diminished the length-dependent priming of the thick filament. Strikingly, a titin-sensitive, length-dependent priming was also present in thin filaments, which seems only possible via bridge proteins between thick and thin filaments in resting muscle, potentially myosin-binding protein C. We further show that these bridges can be forcibly ruptured via high-speed stretches. Our results advance a paradigm shift to the fundamental regulation of length-dependent priming, with titin as the key driver.
Since the contemporary theory of muscle contraction was proposed (1), research into force generation has been overwhelmingly centered around the actomyosin motor and regulatory proteins of the thick and thin myofilaments within the contractile units known as the sarcomeres. Maximum isometric tension is a function of the thick and thin filament overlap, where optimal force is produced at maximal filament (i.e., cross bridge) overlap, and decreases as the overlap decreases (2). However, at submaximal activation levels, tension is a product of the filament overlap and Ca2+ sensitivity, in such a way that the maximum tension is produced at suboptimal thick–thin filament overlap (3, 4). One theory to explain this phenomenon is that the sarcomeric motor complex is not merely “on” or “off” but exists within a range of possible activation states that give the muscle maximal flexibility to tune contraction for demand. As a sarcomere passively lengthens—e.g., in a passive antagonist muscle—thick and thin filament proteins are reoriented in such a way that “primes” them for contraction, functionally lowering the calcium threshold needed to engage them, resulting in increased force production. Thus, at comparable calcium levels, contraction force is greater at longer compared to shorter sarcomere length (SL) (5, 6). These processes are summarized under the term “length-dependent activation” (LDA). The mechanism behind LDA is incompletely understood, but it has been shown to involve both thin and thick filament-associated structures and the giant sarcomeric protein titin (5, 7–9). To understand these regulatory mechanisms, some researchers have studied the molecular alterations to myofilaments in diseased or experimentally modified muscles using small-angle X-ray diffraction (10–12), which leverages the semicrystalline order of sarcomeric proteins to visualize their structures ex-vivo (Fig. 1). However, the underlying length sensor that drives LDA is ill-defined, although the giant sarcomeric protein titin is implicated (13, 14).
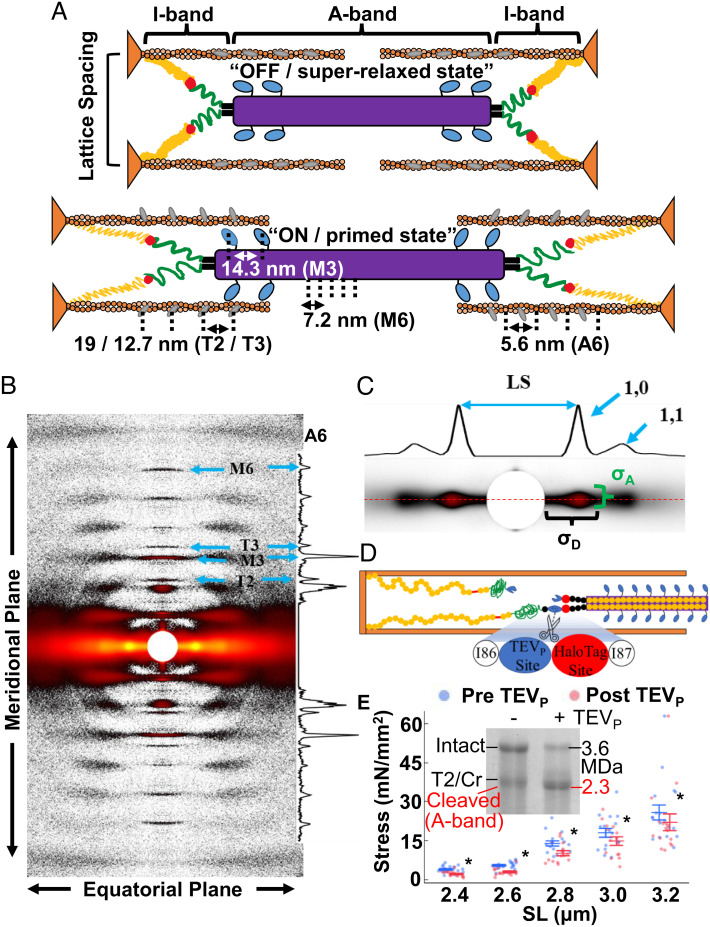
Fig. 1.
X-ray diffraction of skeletal fibers. (A) The passive sarcomeres at short (Top) and long (Bottom) lengths are made up of organized repeating structures (approximate spacing and IDs indicated) including the thick filament backbone (purple), myosin heads (light blue), thin filament backbone (orange), and troponin complex (gray). Thick and thin filaments are attached in the I-band by titin, which is made up of the proximal Ig (yellow), N2A (red), PEVK (green), and distal Ig regions (black). From short to long lengths, proteins reorient from “OFF / superrelaxed state” to “ON / primed state”. Note that spacing values are indicative of the respective spacing parameter assessed. (B) Representative X-ray image of fibers (heatmap gradient) with reflections labeled with the ID shown in (A). (C) The main equatorial-plane reflections. 2D intensity projections are shown and critical features marked. (D) TC muscle has a TEV protease (TEVP) recognition site built into I-band titin, allowing for quick and targeted titin cleavage. HaloTag site can, e.g., be used for imaging purposes. (E) Genetically heterozygous TC psoas fibers before (blue) and after (red) TEVP treatment, where ~50% titin cleavage decreased force across SL. Inset: Coomassie-stained titin gel of Het TC fibers before and after TEVP treatment. Cr = Cronos. *P < 0.05 after titin cleavage. Data throughout reported as mean ± SEM.
Titin extends from the sarcomeric Z-disk to the M-band, maintaining connections to the thick filaments in the A-band and thin filaments at and near the Z-disk, while being extensible in most of the I-band (9, 15). In this organization (Fig. 1A), sarcomere stretch also extends I-band titin, and the associated increase in titin-based force correlates to the movement in myofilament structures toward the primed state (14), potentially explaining how LDA is triggered (13). As supporting evidence, a more compliant titin spring expressed in genetically modified rodents attenuated the length-dependent response in cardiac (7) and skeletal muscles (16). While informative, the evaluation of muscle with titin mutations is cumbersome because titinopathic fibers often present secondary disease states, potentially by affecting titin’s other signalosome functions (17–19), making it nearly impossible to separate the immediate mechanical effect of titin on myofilaments from the long-term consequences of the titinopathy.
To circumvent these methodological limitations, we engineered a mouse model (titin cleavage model, TC) that presents no myopathy yet provides the controlled ability to decrease titin-based forces via a targeted cleavage of an inserted cleavage site in the titin spring activated by tobacco etch virus protease (TEVP) (20). This presents a unique opportunity to track mechanical and structural changes to the sarcomere before and after a reduction of titin-based force within the same sample (21). Here, we have used the TC model to measure changes in sarcomeric protein periodicities by X-ray diffraction induced by targeted titin cleavage. Our results demonstrate that a 50% titin cleavage in muscle fibers prepared from the heterozygous (Het) TC mouse (50% mutant titin, 50% normal titin) significantly blunts the length-dependent priming of myofilament structures across physiological operating lengths (2.4–3.2 µm SL). Surprisingly, thin filament proteins are also sensitive to titin-based forces—a mechanism that only seems possible if directed through a thick–thin filament bridge in the A-band. Furthermore, a faster stretch velocity blunts stretch-dependent priming in a way that suggests titin and A-band bridge structures are velocity sensitive. Our data conclusively demonstrate the importance of titin-based forces to length-dependent priming in passive sarcomeres.
Results and Discussion
Titin Promotes Myofilament Lattice Order That Improves Force Production and Transmission.
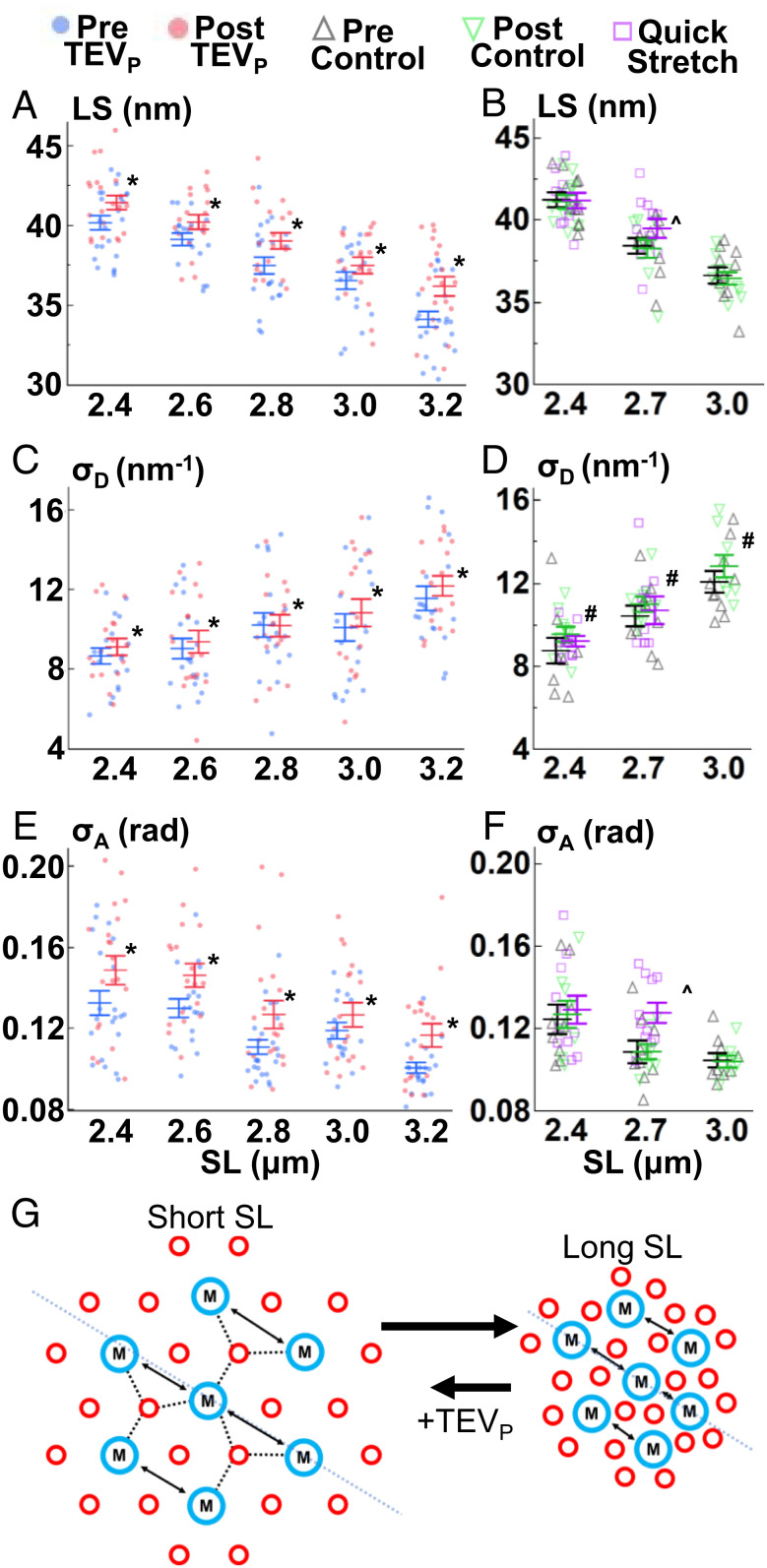
The equatorial 1,0 X-ray reflections offer insights into sarcomeric lattice organization and alignment (Fig. 1 B and C), notably the inter-thick filament lattice spacing (LS), the spacing between 1,0 lattice planes. Theoretically, increasing LS decreases force production and blunts LDA by affecting the kinetics of the myosin head–thin filament complex (22). In passive muscle, along with cytosol osmotic pressure, titin regulates LS and is a primary connector of the lattice filaments, providing both longitudinal and lateral titin-based forces; sarcomere stretch extends titin, increasing titin-based forces that pull filaments together and shrink the lattice (23). We studied this relationship using permeabilized TC psoas fibers (Fig. 1D), where ~50% titin cleavage by TEVP (confirmed via Coomassie-stained titin gels, Fig. 1E) reduced passive force from 2.4 μm to 3.2 μm SL (P = 0.001, Fig. 1E and SI Appendix, Table S1), similar to our previous report (21). As described previously (21, 24, 25), extrasarcomeric structures also contribute to passive force, especially at lengths >2.8 μm SL, reducing the impact of titin cleavage on total passive force. Generally, stretching sarcomeres decreased LS, while titin cleavage shifted the entire relationship toward larger LS (P < 0.0001, Fig. 2A and SI Appendix, Table S1). Control experiments (solution exchange only, no TEVP) indicated no significant pre to post differences (P = 0.65, Fig. 2B and SI Appendix, Table S2). This relationship is in line with cardiac muscle after unspecific titin degradation by trypsin digestion (26). Also of note, LS heterogeneity, quantified using the fitted width parameter of the 1,0 reflections (27) along the equatorial plane (σD, Figs. 1C and 2C), increased with SL (P < 0.0001, Fig. 2B and SI Appendix, Table S1), an important detail for muscle modelers who are attempting to represent the three-dimensional radial and longitudinal forces that define LS in the sarcomere (28). σD increases with both titin cleavage (P = 0.008, Fig. 2C and SI Appendix, Table S1) and control experiments (P = 0.02, Fig. 2D and SI Appendix, Table S2) with a similar magnitude (protocol effect P = 0.91, SI Appendix, Fig. S1 and Table S3), suggesting that general relaxation of the fiber over time also increases LS heterogeneity, with titin-based forces not strongly associated. Along with LS heterogeneity, SL heterogeneity has also been shown to increase at longer SLs (29–31), which could potentially explain LS heterogeneity changes. The most surprising feature in our data was the significant cleavage-induced increase in LS at 2.4 μm SL, where titin-based forces are estimated to be very low (24)—yet cleavage still swelled the lattice by 3.38 ± 0.70% of the initial values. The geometry of titin between the thick and thin filaments provides both longitudinal and radial titin-based forces, with the radial component being very small. For example, the titin–thin filament angle would be 2.65° at 2.4 μm SL with 46 nm LS, which equates to a radial component of only 4.62% of the total titin force. With (longitudinal) titin-based force only ~1 pN/titin at 2.4 μm SL (24), radial force per titin would be ~0.036 pN, which is small (~1% of a single myosin head power stroke) but apparently sufficient for an effect on LS.
Fig. 2.
Lattice changes with SL, titin cleavage, and velocity. LS (A and B), σD (C and D), and σA (E and F) were recorded before (blue) and after (red) titin cleavage (A, C, and E), before (black) and after (green) a control solution, and after a relatively quicker stretch (magenta) (B, D, and F). Details in main text. (G) Cartoon summary of the thick (blue) and thin (red) filament lattice (cross-section of sarcomeres) after stretch, and after TEVP treatment. *P < 0.05 from pre to post values. ^P < 0.05 between the control (pre) and quick stretch experiments. #P < 0.05 between pre and post control values. Also see SI Appendix, Tables S1, S2, S7, and S8.
The register of the thick filaments when a sarcomere is viewed perpendicularly to its long axis is an important consideration for force production, as it affects myosin–actin interactions and/or transmission of myosin-generated force out of the A-band. The interthick filament orientation and order can be quantified via the parameter angle σ (σA, Figs. 1D and 2 E and F, blue line in Fig. 2G), a quantification of the angular width of the 1,0 reflections in the meridional plane. σA has been used to quantify the disruption of inter-thick filament orientation in cardiomyopathies (10). Now, we demonstrate that σA decreased with sarcomere lengthening in skeletal muscle fibers, improving inter-thick filament order, while titin cleavage maintained this trend but at a generally increased σA, suggesting less order (P < 0.0001, Fig. 2E and SI Appendix, Table S1). Control experiments indicated no pre to post trial change in σA (P = 0.98, Fig. 2F and SI Appendix, Table S2), supporting a titin-based effect. Each half-thick filament has six titins that run along the thick filament, forming an oligopolymer “end filament” at the tip of the thick filament, and then fans out in a hexagonal array toward the six surrounding thin filaments (32). The 12 titins per whole thick filament are responsible for centering the thick filament within the A-band (33), and it makes mechanical sense that as titin-based forces increase with increasing SL, the centering of thick filaments would be more rigid and in register with the surrounding thick filaments. In comparison, after 50% titin cleavage, where (on average) three of the six titin filaments are cleaved from each half-thick filament, there is an overall disruption to the force balance and level of force applied to each thick filament. This leads to increasing σA and potentially accounts for some of the impaired force production observed and visible A-band misalignment in transmission electron micrographs of treated Het TC fibers (21).
Thick and Thin Filament Periodicities Are Sensitive to Titin-Based Forces.
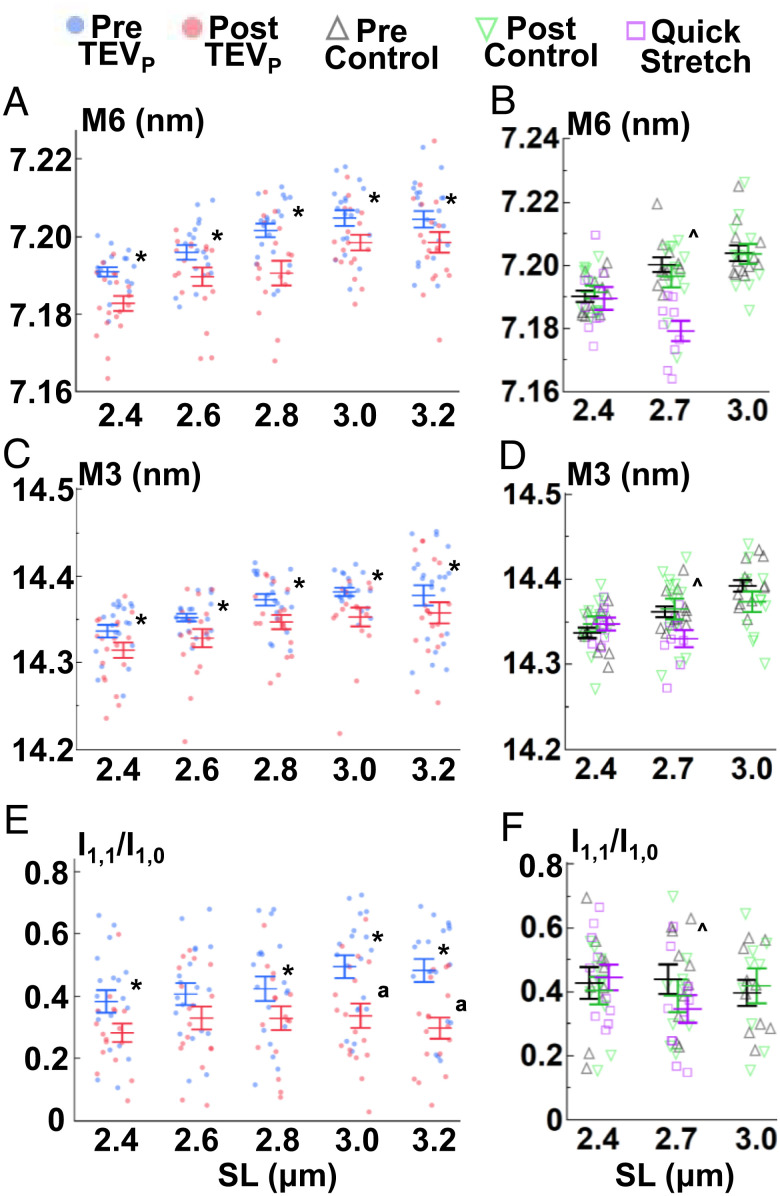
Myosin-based meridional reflections (Fig. 1 A and B) provide critical information concerning the thick filament backbone (M6 spacing) and myosin head orientation (M3 spacing), potentially important to LDA (Reviewed by ref. 34). The mechanosensing hypothesis (35) poses that the activation state of the thick filaments is not merely “on” or “off” but exists in an “unprimed” state (OFF, assumed to be in the biochemically defined super relaxed state) or “primed” (ON) state either incapable or capable of interaction with the thin filament, respectively, regardless of the presence of Ca2+, although these ideas are quickly evolving (13). Thus, molecular events that shift more myosin heads toward ON states will improve force production. Structurally, myosin heads move from a position lying against helical tracks on the thick filament to a position extended away from the thick filament backbone (36). This radial extension is associated with increased M3 spacing. At constant lengths, ON states can be achieved by phosphorylation of myosin-binding protein C (MyBPC; 37) and/or the myosin regulatory light chain (38). During stretch, titin-based passive forces increase and stretch thick filaments (14), ultimately leading to a transition of the myosin heads into an ON state (7). We found supporting evidence for the stretch effect at increased SL in treated and control experiments showing increased M6 and M3 spacings (P < 0.001, Fig. 3 A–D and SI Appendix, Tables S4 and S5) until plateauing >2.8 μm SL (Tukey’s honestly significantly difference (HSD) analysis). Furthermore, we demonstrate that titin-based forces are important to this property, as 50% titin cleavage led to a decrease in M6 and M3 spacing (P < 0.0001, Fig. 3 A and C and SI Appendix, Table S4), with no similar effect in control experiments for M6 (P = 0.78, Fig. 3B and SI Appendix, Table S5) or M3 (P = 0.75, Fig. 3D and SI Appendix, Table S5) spacing. Moreover, we ran a multivariant analysis that compared the differences after titin cleavage from all the collected X-ray features (SI Appendix, Table S6) and found a strong positive correlation between M3 and M6 spacing differences after titin cleavage (Spearman’s ρ = 0.43, P < 0.0001; SI Appendix, Table S6). The most direct explanation would be a relationship between thick filament stretch and (potentially) all proteins associated with the myosin head position (e.g., MyBPC).
Fig. 3.
Thick filament changes with SL, titin cleavage, and velocity. M6 spacings (A and B), M3 spacings (C and D), and I1,1/I1,0 (E and F) were recorded as explained in Fig. 2. Details in main text. *P < 0.05 from pre to post values. ^P < 0.05 between quick stretch and control (pre) data. ªP < 0.05 similar effect magnitude at those SLs. Also see SI Appendix, Tables S1, S2, S4, S5, S7, and S8.
As a final separate estimate of the OFF / ON transition, we calculated the ratio of the equatorial 1,0 and 1,1 reflections (i.e., the equatorial intensity ratio (IR), I1,1/I1,0), where increasing I1,1/I1,0 indicates a shift of mass toward the thin filament (39). Indeed, our data demonstrate that 50% titin cleavage reduced I1,1/I1,0 at all SLs, indicating an increase in the proportion of OFF myosin heads but this was most evident at the longest SLs (treatment × SL interaction P = 0.038, Tukey’s HSD analysis, Fig. 3E and SI Appendix, Fig. S1). These effects on I1,1/I1,0 were not present in control experiments (P = 0.36, Fig. 3F and SI Appendix, Table S2). Interpretation of changes of I1,1/I1,0 across SLs requires some caution because decreasing thick and thin filament overlap with increasing SL, as well as changes to the thick filament order (see σA), also impacts I1,1/I1,0. In support of our findings, intact cardiac preparations with a compliant titin (i.e., reduced titin-based force) also present evidence that reduced titin-based forces attenuate the stretch-dependent movement of the myosin heads away from the thick filament backbone (7). Considering previous and current data, we posit that increasing SL in passively stretched sarcomeres increases titin-based forces, which strain the thick filament leading to a shift of myosins from the OFF to the ON state, a primary indication of LDA.
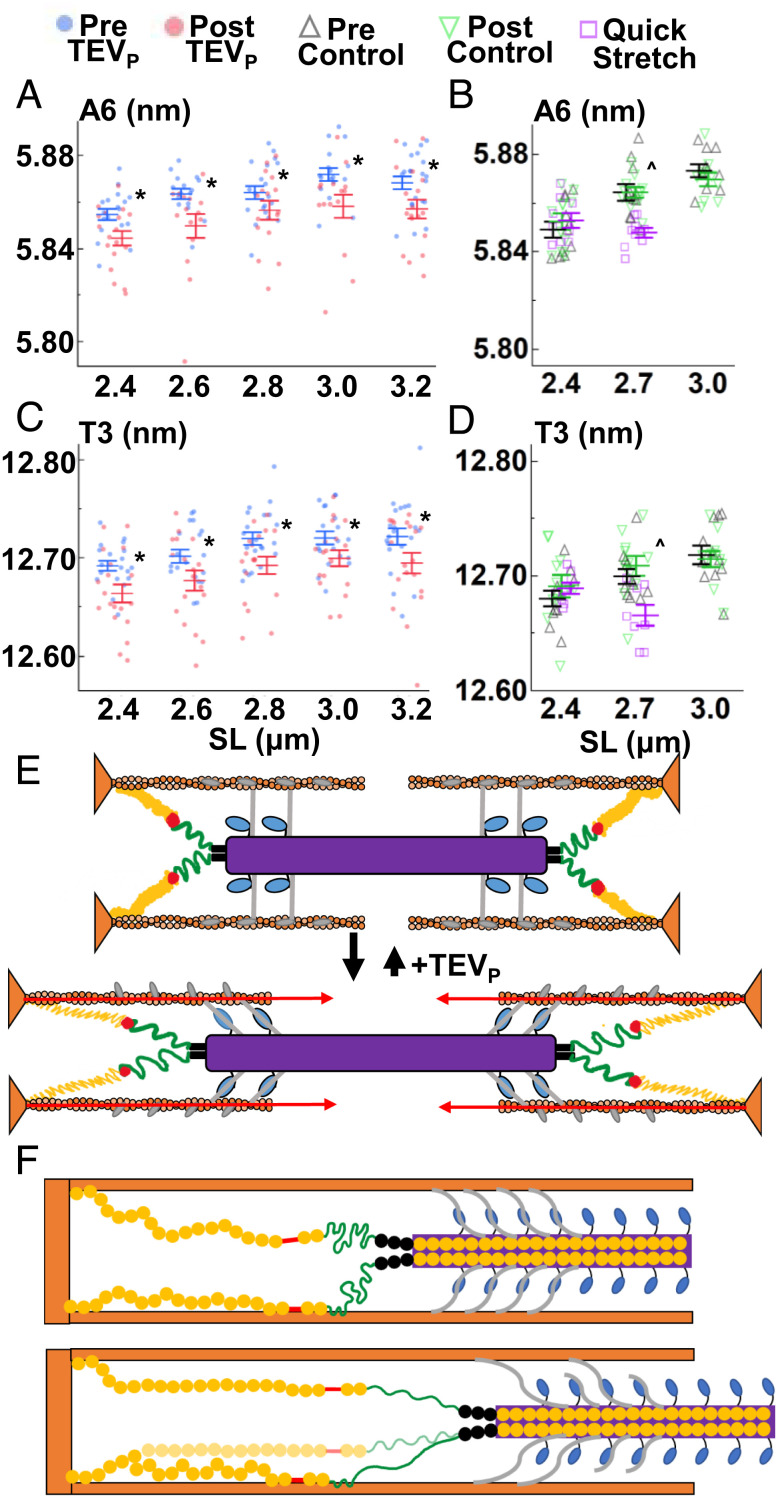
Not only thick filament proteins are affected by stretch, but also thin filament proteins. X-ray reflections associated with the thin filament length (A6 reflection, Fig. 1) and troponin periodicity (T3 reflection, Fig. 1) increased spacing with passive sarcomere stretch in mammalian cardiac muscle (7), as well as insect flight muscle (40). Further, others have found relationships between the thin filament state and Ca2+ sensitivity in cardiac muscle (41, 42). We now report that thin filament elongation is observed in mouse skeletal fibers upon fiber stretch in treatment and control experiments (A6 spacing, P < 0.0001, Fig. 4 A and B and SI Appendix, Tables S4 and S5) along with an increase in the periodicity of the troponin complex (T3 spacing, P < 0.0001, Fig. 4 C and D and SI Appendix, Tables S4 and S5). Strikingly, titin cleavage led to an overall shortening of the A6 and T3 spacings (P < 0.0001, Fig. 2 A, C, and D and SI Appendix, Table S4) that was not present in control experiments (T3 spacing, P = 0.19; A6 spacing, P = 0.60; Fig. 4 B and D and SI Appendix, Table S5). Indeed, even the lower-order (less resolved) T2 periodicity showed a titin cleavage effect (T2 spacing P < 0.0001, SI Appendix, Fig. S2 and Table S4), providing robust evidence that titin cleavage leads to a reduction of strain in the thin filaments, summarized in Fig. 4E. To date, a physiological role for this length- and titin-dependent property has not, to our knowledge, been demonstrated. A previous X-ray study (43) found that, during isometric contraction, thin filament length increased linearly with increasing active tension, suggesting a passive elastic response; however, the implications of these small length changes on contractile properties (e.g., Ca2+ sensitivity, active force production) are not known.
Fig. 4.
Thin filament changes with SL, titin cleavage, and velocity. A6 (A and B), spacings and T3 spacings (C and D) were recorded as explained in Fig. 2. (E) Cartoon summary of the sarcomere at short and long lengths, with proposed A-band bridges added. The ON state is reduced at long lengths after titin cleavage. (F) Sarcomere stretch from short (Top) to long (Bottom) extends the entire I-band titin producing relatively low titin-based forces, but evidence indicates that titin extension can be modified by titin–thin filament interactions around the N2A/PEVK region, functionally shortening the free titin to the stiffer PEVK region, leading to relatively larger titin-based forces after stretch. Higher velocities can be expected to detach the interaction, reducing titin-based forces. *P < 0.05 from pre to post values. ^P < 0.05 between quick stretch and control (pre) data. Also see SI Appendix, Tables S1, S2, S4, S5, S7, and S8.
Thin–Thick Filament Bridges Likely Occur in Passive A-bands.
How can titin affect the thin filament? Titin’s orientation in the sarcomere is not conducive to thin filament stretch in the A-band, as its known interaction points (apart from anchorage within the Z-disk) are either in the I-band close to the Z-disk, via relatively weak interactions in the PEVK region (44), or potentially tethered at the N2A region by cardiac ankyrin repeat protein, CARP (45). No interaction of titin to thin filaments is known in the A-band, where titin is bound to the thick filament backbone. The simplest explanation is that there are bridges between thick and thin filaments in the A-band, where connection points would pull on most of the thin filament. Some insect flight muscles have troponin bridges that reach across to the thick filament (40) but to our knowledge, no such phenomenon has yet been observed in mammals, although they may just not be resolved due to technical limitations (13). The thick filament-associated protein, MyBPC, directly interacts with titin and myosin and also seems to form bridges to the thin filament via interaction with actin (7, 46, 47). MyBPC–thin filament connections were previously observed in skeletal muscle using electron tomography (46) and mechanically deduced in cardiac muscle using a unique cut-and-paste transgenic mouse model that can remove and replace the thin-filament binding N-terminal region of MyBPC (47). Furthermore, X-ray evidence from cardiac muscle also supports the presence of a thick–thin filament bridge (7). A plausible hypothesis is that thick–thin filament bridges are altered by titin-based stretch of the thick filament––a stretch-sensing mechanism. A measure of supporting evidence for a thick–thin filament bridge in passive muscle is provided by a multivariate analysis of our data, where the titin cleavage-dependent spacing changes of thin filament T2, T3, and A6 reflections were highly correlated to thick filament M3 and M6 changes (SI Appendix, Table S5), with the T3 vs. M6 changes producing the strongest positive correlation (Spearman’s ρ = 0.47, P < 0.0001, SI Appendix, Table S5). Another interesting relationship is that the stretch effect plateaus >2.8 μm SL, a point where the MyBPC closest to the M-band would lose overlap with actin and so the putative bridges would be lost; the first MyBPC overlap ends with ~2.76 μm SL based on a thin filament length of 1.2 μm and MyBPC data from ref. 48. MyBPC will stretch somewhat while producing force, as it is also composed of serially linked globular domains similar to titin (49). However, the bridges would eventually detach at some unknown force level (Fig. 4F). To estimate the magnitude of forces exerted by these potential bridges, we approximated the force on each thin filament, based on the thin filament stretch (A6 spacing) vs. force relationships (43). In our data, the average change of A6 spacing from 2.4 to 3.2 μm SL suggests an increase of ~60 pN longitudinal force per thin filament. With ~27 MyBPCs potentially attached to a thin filament from the three surrounding thick filaments, each MyBPC would hold ~2.2 pN of longitudinal force, which seems reasonable. However, more specific experiments need to be done, potentially with a genetically altered MyBPC that allows for controlled removal and reattachment of the N-terminal region, the putative thin filament-interacting site (50).
If such thick–thin bridges existed in the A-band, regardless of what makes the bridge, it should be possible to forcibly rupture them without long-term damage to the muscle, as would be needed over the 300–800 nm (half I-band) range that sarcomeres operate. To test this, we used a stretch-hold protocol where we stretched fibers at a much greater stretch velocity than described above for the previous experiments (~0.3 vs. ~0.0067 μm SL s−1) from 2.4 to 2.7 μm SL, a moderately short SL range where passive stretch-related damage is negligible, maintaining all possible MyBPCs in the overlap region. In comparison to control data at those lengths (prevalues vs. quick stretch; Figs. 2–4), all parameters except for σD (P = 0.65, Fig. 2D and SI Appendix, Tables S7 and S8) produced a significant experiment type × SL interaction effect (P < 0.05, SI Appendix, Tables S7 and S8). Specifically, values were similar between experiments at 2.4 μm SL, but diverged at 2.7 μm SL, with no statistically detectable stretch response for σA, I11/I10, M3, and T3, while M6 and T3 both showed reversed effects (i.e., lower spacing distances, Tukey’s HSD analysis, Fig. 3 and SI Appendix, Tables S7 and S8). LS showed the expected trend albeit attenuated slightly longer after quick stretch vs. the slow stretch of the control experiments (Fig. 2B and SI Appendix, Tables S7 and S8). In summary, compared to the slower stretch-hold protocol, the quicker stretch-hold protocol produced either smaller or negligible markers of LDA / sarcomere “priming.”
Another possibility, which could work in parallel with A-band bridges, is a stretch velocity-dependent effect on titin-based passive forces (Fig. 4F). A likely mechanism is based on the fact that distal I-band titin (N2A / PEVK regions) can stick to the thin filament (21, 44, 51) and can even be tethered together via proteins like CARP (45), although its expression in healthy skeletal muscle is very low (52). These interactions shorten the free length of I-band titin to only the more distal parts, in such a way that a stretch would produce more titin-based forces than if there were no titin–thin filament interactions (and so relatively larger priming effect). Faster stretches acting on viscoelastic titin would produce more force and so the detachment of titins from thin filaments would be greater compared to slower stretch velocities, reducing titin-based forces and blunting the priming effect. However, even at slow velocities, titin-based forces will grow, especially >2.8 um SL, leading to increasing probability of titin detachment, which may, along with A-band bridge detachment, explain our results that priming effects level off >2.8 μm SL.
The structural evidence provided here indicates that titin cleavage shifts the activation state of sarcomeres toward the “OFF” state, which is predicted to also decrease Ca2+ sensitivity during contraction (7, 13, 53). To test this prediction, we evaluated Ca2+ sensitivity at a short (2.4 μm) and long (3.0 μm) SL before and after titin cleavage (SI Appendix, Fig. S3 and Table S10). We found an increase in Ca2+ sensitivity at the longer compared to shorter lengths for both pre- (2.4 μm SL: 6.04 ± 0.03 pCa50; 3.0 μm SL: 6.14 ± 0.02 pCa50) and post- (2.4 μm SL 5.97 ± 0.03 pCa50; 3.0 μm SL 6.02 ± 0.02 pCa50) titin cleavage fibers (ANOVA SL effect P = 0.047); however, the post-treatment fibers were generally shifted toward less Ca2+ sensitivity (ANOVA treatment effect P < 0.0001). Therefore, decreasing titin-based forces maintained LDA but shifted the whole relationship toward a less Ca2+-sensitive state. These findings corroborate our structural X-ray evidence where titin cleavage generally blunted, but did not ablate, length-dependent effects across SLs, suggesting a relationship between the two. Furthermore, a previous study in cardiac muscle with varying titin stiffness also found similar changes to Ca2+ sensitivity (7).
Limitations.
Permeabilized fiber bundles were used in this study as a requirement for TEVP incubation; a method to specifically and quickly insert TEVP into fibers in vivo is not yet available. Permeabilized fiber bundles, instead of single fibers, were required to collect higher-quality X-ray reflections, as more sample volume leads to higher diffracted intensities that are more readily analyzed. Membrane-intact preparations can provide extra structural details about myosin head orientation, such as from myosin layer lines 1 and 4 (54), but in order to efficiently cut titin, we used permeabilized muscle fibers. While we previously took great pains to make sure TEVP was pure and specific (20, 21), some unseen nontargeted cleavage is theoretically possible. TEVP cocktail contamination with other proteases was unlikely, as it was commercially produced with quality measurements provided.
Conclusions
Our results demonstrate that I-band titin-based force pulls on the thick filaments in passively stretched sarcomeres and modifies them in a way consistent with sarcomere priming, with a proportion of myosin heads moving from the OFF to ON (primed) conformation. Thin filaments are also titin-force sensitive, which only seems mechanically possible if there are thick–thin filament bridges in the A-band, potentially MyBPC, that provide some force against the thin filament and are sensitive to changes in titin force—a paradigm shift of the mechanism underlying length-dependent priming. Impairment to priming effects is a hallmark of striated myopathies, and this control theory provides new targets for potential therapeutic interventions.
Materials and Methods
Animal Model and Muscle Preparation.
Animal procedures were performed according to the guidelines of the local animal care and use committee and approved by the local authorities (LANUV NRW, 81-02.04.2019.A472). HaloTag-TEV (titin cleavage, TC, (20)) mice were bred and housed at the University Clinic Muenster. Genotyping was conducted by PCR in duplicate using custom primers: 5′cgtggtggcttatcttctagc3′, 5′ctgttggttcatgcatctcc3′, as previously described (21). Genetically heterozygous adult TC mice (age range, 1 – 6 mo) were humanly euthanized and psoas muscle immediately extracted for long-term storage and permeabilization (“skinned) at −20°C using standard glycerol techniques (1:1 rigor: glycerol; rigor contains (mM) KCl (100), MgCl2 (2), ethyleneglycol- bis(β-aminoethyl)-N,N,N′,N′-tetraacetic acid (EGTA,5), Tris (10), dideoxythymidin (DTT, 1), protease inhibitors [Complete, Roche Diagnostics, Mannheim, Germany], pH 7.0). Samples were shipped to the BioCAT facility on ice for all experimental tests and stored at −20°C until used. On the day of experiments, psoas muscles were removed from the storage solution and vigorously washed in relaxing solution (composition (in mM): potassium propionate (45.3), N,N-Bis(2-hydroxyethyl)-2-aminoethanesulfonic acid BES (40); EGTA (10), MgCl2 (6.3), Na-ATP (6.1), DTT (10), protease inhibitors (complete), pH 7.0)). Bundles containing 15-30 fibers (3-6 mm long) were carefully excised and kept in physiological register by tying silk suture knots (sizing 6-0 or 4-0) at the distal and proximal ends of the bundle. The samples were then immediately transferred to the experimental chamber (see below). Post experiments, a subset of fibers were used to check for titin cleavage using Coomassie-stained titin gels, as explained previously (21).
Small-Angle X-Ray Diffraction and Fiber Mechanics Apparatus.
X-ray diffraction patterns were collected using the small-angle instrument on the BioCAT beamline 18ID at the Advanced Photon Source, Argonne National Laboratory (55). The X-ray beam (0.103 nm wavelength) was focused to ~0.06 × 0.15 mm at the detector plane. X-ray exposures were set at 1 s with an incident flux of ~3 × 1012 photons per second. The sample to detector distance was set between 3.0 and 3.5 m, and the X-ray fiber diffraction patterns were collected with a downstream CCD-based X-ray detector (Mar 165, Rayonix Inc, Evanston IL, USA). An inline camera built into the system allowed for initial alignment with the X-ray beam and continuous sample visualization during the experiment. Prepared fiber bundles were attached longitudinally to a force transducer (402A, Aurora Scientific, Aurora, Canada) and motor (322C, Aurora Scientific, Aurora, Canada) and placed into a bath of relaxing solution at 24°C. Force and length data were collected at 1,000 Hz using a 600A: Real-Time Muscle Data Acquisition and Analysis System (Aurora Scientific, Aurora, Canada). SL was measured via laser diffraction using a 4-mW Helium–Neon laser. Force baseline was set at slack length. After this initial setup, fiber length changes were accomplished through computer control of the motor. The mechanical rig was supported on a custom-designed motorized platform that allowed placement of muscle into the X-ray flight path and small movements to target X-ray exposure during experiments. Using the inline camera of the X-ray apparatus, the platform was moved to target the beam at different locations along the length of the sample. To limit X-ray exposure of any one part of the preparation, no part of the sample was exposed more than once. Fiber diameter was measured using the inline camera, and physiological cross-sectional area was calculated at initial fiber length, with the assumption that the sample was a uniform cylinder longitudinally.
Experimental Protocols and Analysis.
The experimental approach captured X-ray images in samples at several SLs across the in vivo physiological operating range (56), followed by TEVP incubation (100 units acTEV™ [Thermo Fischer Scientific, Waltham, MA, USA] in 300 μl relaxing solution for 20 min), and then the imaging protocol was repeated. Samples were run through one of the two protocols, either stretches from 2.4 μm SL to 2.8 μm SL to 3.2 um SL or 2.6 μm SL to 3.0 μm SL, at 0.0067 μm SL s−1 (1 min stretches) with a 1-min hold phase to allow for stress relaxation. X-ray images were collected at the end of each hold phase. Two separate stretch protocols were necessary to ensure a reasonable number of images could be collected on each sample before degradation from X-ray exposure. Before experiments began, all samples were conditioned at their initial fiber length via repeated sinusoidal oscillations (20 Hz, ± 5% initial fiber length for 30 s) until steady-state forces were consistent, typically 3–5 runs. A control dataset was also collected with the same protocol as above, except the incubation buffer was only relaxing solution. Furthermore, a quicker stretch protocol was also performed, where fibers were stretched from 2.4 to 2.7 μm SL over ~1 s, and measurements were taken at each SL after at least 1 min rest. Finally, length dependence of Ca2+ sensitivity was assessed by performing force–pCa curves at SLs of 2.4 (short) and 3.0 (long) μm using standard protocols (57). Each sample was assessed at either 2.4 or 3.0 μm SL. Steady-state active force was assessed at pCa’s: 9.0 (relaxing solution), 6.6, 6.3, 6.0, 5.7, 4.2 (supramaximal [Ca2+]). Force was normalized to the maximum force at pCa 4.2. A four-parameter Hill equation was fit to the normalized force–pCa data (58) to calculate pCa50 (pCa at 50% maximum force; a measure of Ca2+ sensitivity).
X-Ray Image Analysis.
X-ray images were analyzed using the MuscleX open-source data reduction package (59). The “Scanning Diffraction” routine was used to measure the angular divergence of the 1,0 equatorial reflection. The routine obtains 2D and 1D radially integrated intensities of the equatorial intensities, and then fits Gaussian functions over the diffraction peaks to calculate the standard deviation (width σ) intensity distribution pattern. In this process, the routine obtains the integrated intensity of each equatorial reflection as a function of the integration angle. Gaussian profiles are fit to the projected peak intensities to find the standard deviation of the orientation angle (angle σ; σA) as a measure of the angular divergence of the angle that the sarcomeres in the myofibrils make to the long axis of the preparation. σA is used as a proxy for interthick filament ordering (10). The “Equator” routine of Muscle X was used to calculate the I1,1 / I1,0 IR, LS between thick filaments, and σD, a measure of the variability in thick filament LS (a proxy for lattice ordering). Meridional (T2, M3, T3, M6) and off-meridional reflections (A6) were collected using the MuscleX routines “Diffraction Centroids” and “Projection Traces, respectively.” Every image provides intensities of different quality, which lead to various levels of Gaussian fit errors for each intensity modeled, which increases the variation in spacings in the dataset. To limit these effects, fit errors >10% were discarded. Positions of X-ray reflections on the diffraction patterns in pixels were converted to sample periodicities in nm using the 100-diffraction ring of silver behenate at d001 = 5.8380 nm. Note: The intensity values of each reflection are not discussed in the text but are presented in SI Appendix, Table S9 for completeness.
Statistics.
Statistical analysis was conducted using JMP Pro (V14, SAS Institute, Cary, NC, USA). Significance level was always set at α = 0.05. Response variables included LS, IR, σD, σA, T2, M3, T3, M6, and A6. We first built a repeated-measures analysis of variance (ANOVA) design with fixed effects SL (2.4, 2.6, 2.8, 3.0, 3.2 μm SL), treatment (pre /post TEVP incubation), SL × treatment interaction term, and a random (repeated-measures) effect of individual. Data were best Box-Cox transformed to meet assumptions of normality and homoscedasticity when necessary, which were assessed by residual analysis, Shapiro–Wilk’s test for normality, and Levene’s test for unequal variance. Significant main effects were subject to Tukey’s highly significant difference (HSD) multiple comparison procedures to assess differences between factor levels. These data are indicated in graphs via the so-called connecting letters, where factor levels sharing a common letter are not significantly different from each other. We next conducted a multivariate analysis to explore the correlation among all the recorded features from the X-ray images. To do this, we used the multivariate analysis routine from JMP and conducted a nonparametric correlation (ranked sum approach) to the non-transformed datasets and measured Spearman’s rho (ρ). Spearman’s ρ statistic is similar to Pearson’s correlation but with ranked sums, with negative and positive correlations, and larger values indicating a stronger relationship. We also provide the corresponding p-value to indicate the significance level of the correlation. From statistically significant correlations, we ran a follow-up analysis of covariance (ANCOVA) test with the two correlated parameters of interest as response or covariate, and the fixed main effect of treatment. Similar analyses were run for control and quick stretch experiments. All data were presented as mean ± SEM.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Acknowledgments
We thank the BioCAT beamline support staff at the APS for their steadfast commitment to our project, Johanna Freundt for animal handling and genotyping, and Anna Good for critical text and artistic editing. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE - AC02 - 06CH11357, and further NIH support. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute of General Medical Sciences or the National Institutes of Health. A.L.H. received funding from German Research Foundation grant 454867250; W.A.L. received funding from German Research Foundation grant SFB1002, A08; W.A.L. received funding from IZKF Münster Li1/029/20; and T.I. received funding from National Institutes of Health P41 GM103622 and P30 GM138395.
Author contributions
A.L.H., W.M., T.I., and W.A.L. designed research; A.L.H., N.M., P.E.R., D.N., H.G., and M.K. performed research; W.M. contributed new reagents/analytic tools; A.L.H., N.M., D.N., and M.K. analyzed data; A.L.H. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. R.J.S. is a guest editor invited by the Editorial Board.
Contributor Information
Anthony L. Hessel, Email: anthony.hessel@uni-muenster.de.
Wolfgang A. Linke, Email: wlinke@uni-muenster.de.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Huxley A. F., Simmons R. M., Proposed mechanism of force generation in striated muscle. Nature 233, 533–538 (1971). [DOI] [PubMed] [Google Scholar]
- 2.Gordon A. M., Huxley A. F., Julian F. J., The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 184, 170–192 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt N. C., Williams C. D., Can strain dependent inhibition of cross-bridge binding explain shifts in optimum muscle length? Integr. Comp. Biol. 58, 174–185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martyn D. A., Gordon A. M., Length and myofilament spacing-dependent changes in calcium sensitivity of skeletal fibres: Effects of pH and ionic strength. J. Muscle. Res. Cell Motil. 9, 428–445 (1988). [DOI] [PubMed] [Google Scholar]
- 5.de Tombe P. P., et al. , Myofilament length dependent activation. J. Mol. Cell Cardiol. 48, 851–858 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ter Keurs H. E., Rijnsburger W. H., van Heuningen R., Nagelsmit M. J., Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ. Res. 46, 703–714 (1980). [DOI] [PubMed] [Google Scholar]
- 7.Ait-Mou Y., et al. , Titin strain contributes to the Frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins. Proc. Natl. Acad. Sci. U.S.A. 113, 2306–2311 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irving T. C., Craig R., Getting into the thick (and thin) of it. J. Gen. Physiol. 151, 610–613 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linke W. A., Titin gene and protein functions in passive and active muscle. Annu. Rev. Physiol. 80, 389–411 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Ma W., et al. , Myofibril orientation as a metric for characterizing heart disease. Biophys. J. 121, 565–574 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwamoto H., Oiwa K., Suzuki T., Fujisawa T., States of thin filament regulatory proteins as revealed by combined cross-linking/X-ray diffraction techniques. J. Mol. Biol. 317, 707–720 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Iwamoto H., Synchrotron radiation X-ray diffraction studies on muscle: Past, present, and future. Biophys. Rev. 11, 547–558 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma W., Irving T. C., Small angle x-ray diffraction as a tool for structural characterization of muscle disease. Int. J. Mol. Sci. 23, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irving T., et al. , Thick-filament strain and interfilament spacing in passive muscle: Effect of titin-based passive tension. Biophys. J. 100, 1499–1508 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fürst D. O., Osborn M., Nave R., Weber K., The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: A map of ten nonrepetitive epitopes starting at the Z line extends close to the M line. J. Cell Biol. 106, 1563–1572 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mateja R. D., Greaser M. L., de Tombe P. P., Impact of titin isoform on length dependent activation and cross-bridge cycling kinetics in rat skeletal muscle. Biochim. Biophys. Acta 1833, 804–811 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishikawa K., Lindstedt S. L., Hessel A., Mishra D., N2A titin: Signaling hub and mechanical switch in skeletal muscle. Int. J. Mol. Sci. 21, 3974 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swist S., et al. , Maintenance of sarcomeric integrity in adult muscle cells crucially depends on Z-disc anchored titin. Nat. Commun. 11, 4479 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radke M. H., et al. , Deleting full length titin versus the titin m-band region leads to differential mechanosignaling and cardiac phenotypes. Circulation 139, 1813–1827 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivas-Pardo J. A., et al. , A HaloTag-TEV genetic cassette for mechanical phenotyping of proteins from tissues. Nat. Commun. 11, 2060 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y., et al. , Graded titin cleavage progressively reduces tension and uncovers the source of A-band stability in contracting muscle. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams C. D., Salcedo M. K., Irving T. C., Regnier M., Daniel T. L., The length-tension curve in muscle depends on lattice spacing. Proc. Biol. Sci. 280, 20130697 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irving T. C., Konhilas J., Perry D., Fischetti R., de Tombe P. P., Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. Am. J. Physiol. Heart Circ. Physiol. 279, H2568–H2573 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Prado L. G., et al. , Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J. Gen. Physiol. 126, 461–480 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hettige P., Mishra D., Granzier H., Nishikawa K., Gage M. J., Contributions of titin and collagen to passive stress in muscles from mdm mice with a small deletion in titin’s molecular spring. Int. J. Mol. Sci. 23, 8858 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuda N., Wu Y., Farman G., Irving T. C., Granzier H., Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle. Pflugers. Arch. 449, 449–457 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Irving T. C., Millman B. M., Changes in thick filament structure during compression of the filament lattice in relaxed frog sartorius muscle. J. Muscle Res. Cell Motil. 10, 385–394 (1989). [DOI] [PubMed] [Google Scholar]
- 28.Mijailovich S. M., et al. , Three-dimensional stochastic model of actin-myosin binding in the sarcomere lattice. J. Gen. Physiol. 148, 459–488 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moo E. K., Leonard T. R., Herzog W., In vivo sarcomere lengths become more non-uniform upon activation in intact whole muscle. Front. Physiol. 8, 1015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobirumaki-Shimozawa F., et al. , Nano-imaging of the beating mouse heart in vivo: Importance of sarcomere dynamics, as opposed to sarcomere length per se, in the regulation of cardiac function. J. Gen. Physiol. 147, 53–62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobirumaki-Shimozawa F., et al. , Synchrony of sarcomeric movement regulates left ventricular pump function in the in vivo beating mouse heart. J. Gen. Physiol. 153, e202012860 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liversage A. D., Holmes D., Knight P. J., Tskhovrebova L., Trinick J., Titin and the sarcomere symmetry paradox. J. Mol. Biol. 305, 401–409 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Horowits R., Kempner E. S., Bisher M. E., Podolsky R. J., A physiological role for titin and nebulin in skeletal muscle. Nature 323, 160–164 (1986). [DOI] [PubMed] [Google Scholar]
- 34.Irving M., Regulation of contraction by the thick filaments in skeletal muscle. Biophys. J. 113, 2579–2594 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linari M., et al. , Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature 528, 276–279 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Oshima K., et al. , Axial dispositions and conformations of myosin crossbridges along thick filaments in relaxed and contracting states of vertebrate striated muscles by X-ray fiber diffraction. J. Mol. Biol. 367, 275–301 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Mamidi R., Gresham K. S., Stelzer J. E., Length-dependent changes in contractile dynamics are blunted due to cardiac myosin binding protein-C ablation. Front. Physiol. 5, 461 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan C.-C., et al. , Constitutive phosphorylation of cardiac myosin regulatory light chain prevents development of hypertrophic cardiomyopathy in mice. Proc. Natl. Acad. Sci. U.S.A. 112, E4138–146 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Millman B. M., The filament lattice of striated muscle. Physiol. Rev. 78, 359–391 (1998). [DOI] [PubMed] [Google Scholar]
- 40.Perz-Edwards R. J., et al. , X-ray diffraction evidence for myosin-troponin connections and tropomyosin movement during stretch activation of insect flight muscle. Proc. Natl. Acad. Sci. U.S.A. 108, 120–125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terui T., et al. , Troponin and titin coordinately regulate length-dependent activation in skinned porcine ventricular muscle. J. Gen. Physiol. 131, 275–283 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terui T., et al. , Regulatory mechanism of length-dependent activation in skinned porcine ventricular muscle: Role of thin filament cooperative activation in the Frank-Starling relation. J. Gen. Physiol. 136, 469–482 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiss B., et al. , Nebulin stiffens the thin filament and augments cross-bridge interaction in skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 115, 10369–10374 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linke W. A., et al. , PEVK domain of titin: An entropic spring with actin-binding properties. J. Struct. Biol. 137, 194–205 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Zhou T., et al. , Molecular characterisation of titin N2A and its binding of CARP reveals a titin/actin cross-linking mechanism. J. Mol. Biol. 433, 166901 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luther P. K., et al. , Direct visualization of myosin-binding protein C bridging myosin and actin filaments in intact muscle. Proc. Natl. Acad. Sci. U.S.A. 108, 11423–11428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris S. P., Making waves: A proposed new role for myosin-binding protein C in regulating oscillatory contractions in vertebrate striated muscle. J. Gen. Physiol. 153, e202012729 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett P., Rees M., Gautel M., The axial alignment of titin on the muscle thick filament supports its role as a molecular ruler. J. Mol. Biol. 432, 4815–4829 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karsai A., Kellermayer M. S. Z., Harris S. P., Mechanical unfolding of cardiac myosin binding protein-C by atomic force microscopy. Biophys. J. 101, 1968–1977 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Napierski N. C., et al. , A novel “cut and paste” method for in situ replacement of cMyBP-C reveals a new role for cMyBP-C in the regulation of contractile oscillations. Circ. Res. 126, 737–749 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raynaud F., Astier C., Benyamin Y., Evidence for a direct but sequential binding of titin to tropomyosin and actin filaments. Biochim. Biophys. Acta 1700, 171–178 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Wette S. G., Smith H. K., Lamb G. D., Murphy R. M., Characterization of muscle ankyrin repeat proteins in human skeletal muscle. Am. J. Physiol. Cell Physiol. 313, C327–C339 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma W., et al. , The super-relaxed state and length dependent activation in porcine myocardium. Circ. Res. 129, 617–630 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma W., Gong H., Irving T., Myosin head configurations in resting and contracting murine skeletal muscle. Int. J. Mol. Sci. 19, 2643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma W., Irving T. C., X-ray diffraction of intact murine skeletal muscle as a tool for studying the structural basis of muscle disease. J. Vis. Exp. 149, 10.3791/59559 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Llewellyn M. E., Barretto R. P. J., Delp S. L., Schnitzer M. J., Minimally invasive high-speed imaging of sarcomere contractile dynamics in mice and humans. Nature 454, 784–788 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hessel A. L., Joumaa V., Eck S., Herzog W., Nishikawa K. C., Optimal length, calcium sensitivity and twitch characteristics of skeletal muscles from mdm mice with a deletion in N2A titin. J. Exp. Biol. 222, jeb200840 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Walker J. S., Li X., Buttrick P. M., Analysing force-pCa curves. J. Muscle Res. Cell Motil. 31, 59–69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiratrakanvong J., et al. , MuscleX: Software Suite for Diffraction X-Ray Imaging (BioCAT, Argonne National Labs, 2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Data Availability Statement
All study data are included in the article and/or SI Appendix.