Abstract
This literature review evaluates the absorption of methionine (Met) sources such as 2-hydroxy-4-methylthiobutyric acid (HMTBa), its calcium salts (HMTBa-Ca), and DL-methionine (DL-Met) by focusing on the state of knowledge regarding the absorption mechanism, experimental methodology, and factors affecting their absorption. The 2 Met sources differ in mechanism and site of absorption due to differences in their chemical characteristics and enzymatic conversion. This review addresses diffusion- and transport-mediated absorption systems for amino acids and carboxylic compounds, best elucidated by in vitro, ex vivo, and in vivo experimental models. Opportunities and limitations in the use of radioisotopes to depict absorption sites as well as host and microbial metabolism are described. Physiological and environmental conditions that lead to changes in gut absorptive capacity and the impact of Met source absorption are also evaluated. This review concludes that any comparison between HMTBa and DL-Met should consider their different behaviors during the absorption phase. Hence, the chemical characteristics of these 2 molecules entail different absorption sites and mechanisms, from passive absorption in the case of HMTBa and HMTBa-Ca to active transporters for DL-Met, HMTBa, and HMTBa-Ca. In addition, the different conversion modes of these 2 molecules further differentiate their absorption modes. Considering these important differences, it is easier to understand the apparent divergence between the conclusions of existing publications. When comparing these 2 molecules, it is recommended to properly adapt to the conditions under which the absorption of Met sources is evaluated.
Keywords: DL-Methionine, Hydroxy-analog of methionine, 2-Hydroxy-4-methylthiobutyric acid, Absorption, Methodology
1. Introduction
Numerous studies have been published over the past decades comparing the bioefficacy of different sources of methionine (Met), such as 2-hydroxy-4-methylthiobutyric acid (HMTBa), its calcium salts (HMTBa-Ca), and DL-methionine (DL-Met). However, these studies have provided conflicting results. Herein, the authors have extensively and critically analyzed publications related to the absorption of 2 Met sources to L-Met. This critical review provides an understanding of diverging studies and provides insights into how further evaluation should be conducted when the bioefficacy of the 2 molecules is discussed. This review aims to evaluate whether there is any scientific evidence relating the transport/absorption mechanisms to the fundamental nature of Met (i.e., L-Met, DL-Met, HMTBa) that can explain possible differences in relative efficacy between sources. Literature searches on the Web of Science, Scopus, CAB Abstracts, and Google Scholar databases were conducted using combinations of terms, covering the different names of sources, including their Chemical Abstracts Service numbers (e.g., L-Met, HMTBa, MHA, Met, DL-Met), different species, with a focus on monogastric animals (e.g., poultry and pigs), and with no time limit, allowing the coverage of a period from 1937 to 2019. The details and sequence of the search are provided in the Supplementary Information.
2. Absorption of different met sources in the gastrointestinal tract
Practical evidence from animal studies highlights that Met has a higher fractional absorption rate than other proteogenic amino acids (Webb, 1990). Met sources used in animal production also include Met precursors available as free acids, such as HMTBa or HMTBa-Ca. HMTBa exhibits chemical resemblance to other organic acids (pKa 3.53), particularly lactic acid, before being converted into L-Met (Dibner and Buttin, 2002). HMTBa absorption is similar to that of many organic acids and lactic acid (Dibner and Buttin, 2002; Martin-Venegas et al., 2007) and may not follow the same absorption site or mechanism in a biological system as Met. Previous reports have been based on the erroneous presumption that the small intestine is the major site of absorption for HMTBa, presumably this is where Met and other amino acids are absorbed (Brandsch and Brandsch, 2003). This assumption has certain limitations, resulting in misleading results in the literature.
To further understand this, Richards et al. (2005) demonstrated that HMTBa is absorbed along the entire gastrointestinal tract (GIT) of birds. This study reinforced the notion that most dietary HMTBa is absorbed in the upper GIT compartments (crop, proventriculus, and gizzard) in an acidic environment. They reported that approximately 85% of HMTBa was absorbed before reaching the gizzard and that 99.6% of its absorption was completed upon arrival in the ileum. Diffusion is a major contributor to organic acid uptake, which occurs at an accelerated rate when the pH is low, and most acids are undissociated and lipophilic (Naupert and Rommel, 1975; von Engelhardt et al., 1989; Walter and Gutknecht, 1984). The experiments reported in this review (Richards et al., 2005; Dibner et al., 1988; Knight and Dibner, 1984) demonstrate that HMTBa is efficiently absorbed in the small intestine and provide valuable insights. However, more attention must be given to the upper GIT, where most HMTBa absorption occurs.
Jendza et al. (2011) reported clear differences in the relative disappearance sites of DL-Met and HMTBa in pigs. They reported that HMTBa was not detectable in digestive samples from the duodenum and terminal ileum collected from any pig, suggesting the complete disappearance of HMTBa by the end of the duodenum. Lobley et al. (2006) infused 1-13C-HMTBa into the abomasum of sheep for 6 h and noted higher concentrations of HMTBa in the abomasum, duodenum, and jejunum than in the proximal or distal portions of the GIT. These findings demonstrate that HMTBa absorption is not restricted to the small intestine, thereby suggesting that HMTBa is absorbed earlier in the GIT.
These studies show that the absorption of HMTBa and D-, L-, and DL-Met does not follow the same route. This should be properly considered when comparing the bioefficacies of both sources.
2.1. Transport systems for methionine
The literature contains many potential systems for the transport of amino acids. However, procedural differences among publications have created confusion. There is also considerable overlap in substrate specificity among amino acid transporters (Webb, 1990). Two sets of nomenclature (solute carriers [SLCs] and systems) have been used to study the transport of amino acids and peptides in intestinal cells (Bröer and Fairweather, 2019; Mastrototaro et al., 2016). The more recent nomenclature is based on similarities between transporter gene sequences (SLCs) and has been developed to describe mammalian genomes systematically. The numbering of SLC families (SLC1, SLC3, SLC6, SLC7, SLC16, SLC36, SLC38, and SLC43) and their members are largely based on their order of discovery. However, an older nomenclature is still actively used and is based on the functional characterization of amino acid transport activities in cells, called “systems” (n = 17). This nomenclature was implemented according to the amino acid preference, namely, system L (leucine) for large hydrophobic neutral amino acids; system A (alanine) for small and polar neutral amino acids; system alanine-serine-cysteine transporter (ASCT) for alanine, serine, and cysteine; system N for asparagine, histidine, and glutamine; system T (tryptophan) for aromatic amino acids; the iminoglycine system for proline, hydroxyproline, and glycine; and the β-amino acid system for β-amino acids, including taurine.
2.1.1. Sodium-dependent transport system
Met is largely transported across the apical membrane by the low-affinity Na+-dependent system B0AT1 (SLC6A19) with a Na+:amino acid (1:1) ratio. Amino acids with longer side chains (L-Met, L-phenylalanine, and L-leucine) have a relatively higher affinity (Michaelis constant [Km] = 1.5 to 4 mM) with this system (Bröer, 2008). ATB0,+ (SLC6A14) is the second apical Met uptake system in the intestine. This transport system is Na+- and Cl−-dependent, with a ratio of Na+:Cl−:amino acid = 2:1:1 (Munck et al., 1995; Sloan and Mager, 1999). ATB0,+ has been detected in many species in different parts of the GIT, such as in the human stomach and colon, rat stomach (Sloan and Mager, 1999; Hatanaka et al., 2004; Kirchhoff et al., 2006), and rabbit ileum (Miyamoto et al., 1989, 1990; Munck, 1985). ATB0,+ is the main apical uptake transport system for L-Met in Caco-2 cells (Chen et al., 1994), but Nickel et al. (2009) attributed the primary uptake to b0,+AT. ATB0,+ is absent in porcine small intestine (Munck et al., 1995, 2000). Utsunomiya-Tate et al. (1996) reported ASCT, a third apical uptake system for the transport of neutral amino acids that derives its name from a very narrow substrate affinity: alanine, serine, and cysteine. However, its intestinal isoform ASCT2 can transport L-threonine and L-glutamine with affinities (Km approximately 20 μM) similar to those of alanine, serine, and cysteine. ASCT2 can also participate in the apical uptake of L-Met but with a much lower affinity (Km approximately 300 μM) than the amino acids mentioned above (Utsunomiya-Tate et al., 1996; Verma and Kansal, 1993). Kobayashi et al. (2012) concluded that ASCT2 transports D-Met with much better efficiency than L-Met. The IMINO system (SLC6A20), often termed the sodium/imino-acid transporter (SIT1), is known to have a specific affinity for L-proline, and L–OH–proline can be regarded as a fourth apical uptake system for Met (Bröer, 2008). Nickel et al. (2009) explained that the IMINO system is a low-affinity transporter for L-Met (Km = 6.9 mM).
2.2. Sodium-independent transport system
Met can also be transported by Na+-independent systems, for example, b0,+ (Bröer, 2008; Wells and Hediger, 1992). Nonetheless, this transport is stimulated in the presence of Na+ (Bröer, 2008). The light chain b0,+AT (SLC3A1) requires heavy chain rBAT (SLC7A9) to work at the apical membrane (Reig et al., 2002) and has been functionally identified in the small intestine of pigs (Munck et al., 2000). Previous studies (Wells and Hediger, 1992; Bertran et al., 1993) identified high affinity for cysteine uptake across the apical membrane in exchange for neutral amino acids (Km approximately 50 μM), including Met, which appears to be the most important function of this protein. b0,+ AT/rBAT (Wells and Hediger, 1992) transports Met with reasonably good affinity in rats (Km = 71 μM) (Palacín, 1994) and humans (Km = 130 μM) (Nickel et al., 2009). Moreover, Nickel et al. (2009) noted that b0,+ AT/rBAT is the main L-Met influx carrier in Caco-2 cells. Bröer (2008) suggested that under physiological conditions with amino acid mixtures, the uptake of neutral amino acids via b0,+ AT/rBAT is negligible. Conversely, for postprandial Met accumulation in enterocytes, b0,+ AT/rBAT may be a preferential carrier for Met efflux. This hypothesis is supported by studies where L-Met and other neutral amino acids stimulated the absorption of cationic amino acids when added from the luminal side (Webb, 1990; Munck and Schultz, 1969; Reiser and Christiansen, 1971).
2.3. Basolateral transporter systems
System “L-amino acid transporters (LATs)” was identified as the primary basolateral influx mechanism for L-Met in Caco-2 cells (Chen et al., 1994). It has 4 different molecular isoforms (LAT1–LAT4), among which LAT1 (SLC7A5) and LAT2 (SLC7A8) are amino acid exchangers that require 4F2hc (SLC3A2) for their operational and transport functions (Bröer, 2008). Both LAT1 and LAT2 have been found in the rat jejunum, ileum, and colon membrane fractions, and LAT1 is most prominent in the colon (Fraga et al., 2005). Isoform 4F2hc/LAT1 primarily transports large neutral amino acids with high affinity (Km = 10 to 50 μM), including L-Met (Km = 20 μM) (Kanai et al., 1998; Yanagida et al., 2001). D-Met is equally accepted by the transporter (Kanai et al., 1998; Yanagida et al., 2001).
In contrast, isoform 4F2hc/LAT2 is expressed in the basolateral membrane of the murine stomach and intestine (Dave et al., 2004; Rossier et al., 1999), and all neutral amino acids, except proline, serve as substrates (Bröer, 2008; Wagner et al., 2001). Substrate amino acids, including L-Met transported by 4F2hc/LAT2, exhibited relatively lower affinity (Km = 30 to 300 μM, L-Met Km = 204 μM) compared to 4F2hc/LAT1, with D-Met not being transported (Rossier et al., 1999; Segawa et al., 1999).
The main distinguishing feature between the transport mechanisms of 4F2hc/LAT1 and 4F2hc/LAT2 is their pH dependence (Fraga et al., 2002). The latter is stimulated by low external pH (Segawa et al., 1999), while the transport of amino acids by LAT1 is pH-independent (Prasad et al., 1999). Bröer (2008) suggested that 4F2hc/LAT1 and 4F2hc/LAT2 did not contribute to the net flux of amino acids across the basolateral membrane. Instead, they contributed only to the exchange of abundant amino acids for less abundant amino acids.
The recent identification of 4F2hc/LAT4 has solved the long-standing issue of a uniporter system that can ensure the net transport of amino acids across the basolateral membrane into the bloodstream. This system, which is Na-, Cl-, and pH-independent, has a very narrow substrate base, including L-Met (Bodoy et al., 2005). Therefore, 4F2hc/LAT4 plays a vital role in absorbing amino acids, particularly Met. Prior studies with Caco-2 cells have demonstrated that the basolateral efflux of Met is the rate-limiting step and that 4F2hc/LAT4 regulates this process (Chen et al., 1994).
L-Met can also be transported across the basolateral membrane of enterocytes through 4F2hc/y+LAT1 (SLC3A2/SLC7A7), which shares homology with the yeast Met permease MUP1 (Torrents et al., 1998, 1999). This transporter is electroneutral and exchanges cationic amino acids for large neutral amino acids, including L-Met, with Na+ cotransport. Generally, Na+ accompanies the transport of neutral amino acids (Kanai et al., 2000). Therefore, the Na+ gradient directed from the extracellular to the intracellular direction can result in an influx of neutral amino acids coupled with an efflux of cationic amino acids against a 5-fold gradient (Bröer, 2008). Therefore, 4F2hc/y+LAT1 could facilitate the reinflux and retention of L-Met in epithelial cells and trigger L-Met metabolism in enterocytes. Similarly, the 4F2hc/y+LAT2 (SLC3A2/SLC7A6) isoform has also been speculated to be an intestinal Met transporter (Zhang et al., 2015). It shares similar transport characteristics with those of 4F2hc/y+LAT1, but a much narrower substrate base, limited to L-arginine (Km = 120 μM), L-lysine, L-leucine (Km = 236 μM), L-glutamine (Km = 295 μM), L-histidine and L-Met (Bröer et al., 2000).
Lerner and Taylor (1967) used a prolonged fasting period and long uptake periods in chickens but could not establish a clear differentiation between apical and basolateral uptake. They hypothesized a common uptake mechanism preferring L-Met over D-Met (Km = 4.1 mM vs. 10.0 mM). The Ki values to inhibit the other stereoisomer were 4.5 mM for D-Met inhibition of L-Met uptake and 14.1 mM for D-Met inhibition of L-Met uptake (Lerner and Taylor, 1967). Later, Maenz and Engele-Schaan (1996) validated the Ki for D-Met inhibition of L-Met uptake using chicken brush border membrane vesicles (BBMVs). Nevertheless, a smaller Km (137 μM) value for apical L-Met uptake was noted, indicating both Na+-dependent and Na+-independent components. Brachet et al. (1987) identified 2 different apical uptake pathways for L-Met with very similar Km values (1.7 and 1.4 mM) and noncompetitive inhibition by D-Met in rats. They further noted that D-Met has a higher Km value (11.7 mM) and can be inhibited by L-Met (Brachet et al., 1987). These results could negate the participation of the IMINO transporter in D-Met uptake and attributed to Na+-dependent B0AT1 as the most likely D-Met carrier (Brachet et al., 1987). Conversely, Zheng et al. (1994) reported similar Km values for L-Met (1.34 mM) and D-Met transport (1.79 mM) in Caco-2 cells, which were comparable to those determined in perfused rat intestines.
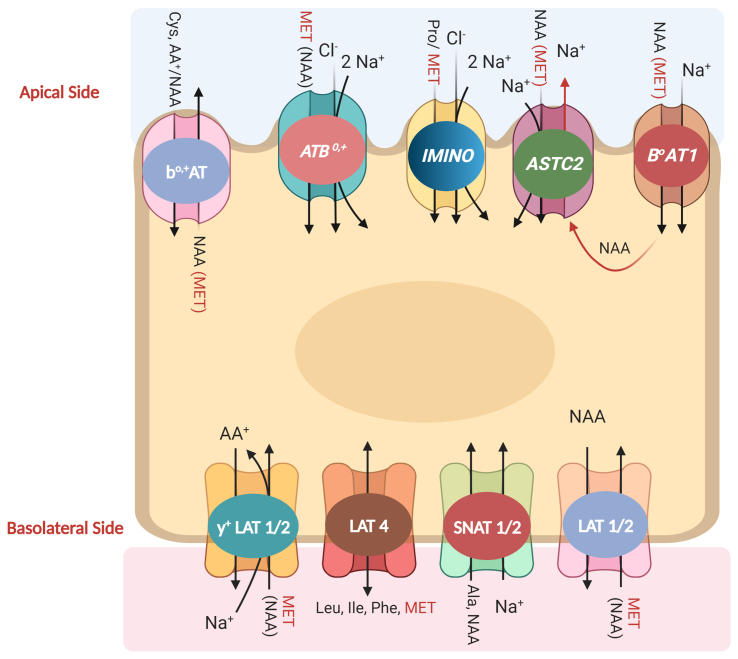
Kobayashi et al. (2012) claimed that ASCT2 has a higher affinity for D-Met than for L-Met, while 4F2hc/LAT2 transports L-Met only and not D-Met (Rossier et al., 1999; Segawa et al., 1999). However, some reports suggest that 4F2hc/LAT1 may transport both L-Met and D-Met with a higher affinity than 4F2hc/LAT2 (Kanai et al., 1998; Yanagida et al., 2001). Therefore, the stereoselectivity of intestinal Met uptake studies is far from conclusive. Fig. 1 provides a hypothetical representation of the different influx and efflux transporters involved in the transport of Met at the enterocyte level.
Fig. 1.
Representation of different influx and efflux transporters involved in the transport of methionine (Met) at an enterocyte (hypothesis). MET = Met; NAA = neutral amino acid; ASTC2 = alanine-serine-cysteine transporter-2; LAT = L-type amino acid transporters; SNAT = sodium-coupled neutral amino acid transporters; Leu = leucine; Ile = isoleucine; Phe = phenylalanine; Ala = alanine (Created with BioRender.com).
2.4. Transport systems for HMTBa
It is well-known that HMTBa is not solely transported through diffusion. Carrier-mediated transport also contributes significantly to its total uptake. This phenomenon is evident in reports where HMTBa is transported across the apical membrane of chicken enterocytes by diffusion and an H+-dependent carrier-mediated transporter (Maenz and Engele-Schaan, 1996; Martin-Venegas et al., 2006a).
In their study, ter Kuile and Cook (1994) reported findings on carrier-mediated transport. It is tempting to extrapolate that the uptake of a substrate by facilitated diffusion is only effective when the subsequent metabolism of the test compound maintains a low intracellular concentration of the original substrate. According to ter Kuile and Cook (1994), a clear understanding of the basic principles of carrier-mediated transport of a substance can help to define an appropriate model for transport studies. Carrier-mediated transport involves the following 3 important steps:
-
1)
An initial rapid uptake driven by a concentration difference between the inside and outside of the cell.
-
2)
A steady increase in the amount of the substance incorporated into macromolecules, such as protein, due to the conversion of the substance into a metabolite and can be termed the “trapping step”. This situation occurs most often when a substance is metabolizable into another form.
-
3)
The uptake is stabilized when the efflux of end products and the uptake are balanced due to the saturation of the internal pools (ter Kuile and Cook, 1994).
However, the relative contribution of each step depended on the range of concentrations used in the measurements. In the lower concentration range, the kinetics of the transporters make the dominant contribution, while the metabolic step contributes to the maintenance of transport in the higher concentration range (ter Kuile and Cook, 1994). The mutual influence of the trapping step and facilitated diffusion process indicates that trapping of the substrate by a high-affinity enzymatic reaction can give the entire process the properties of active transport (ter Kuile and Cook, 1994). This signifies that facilitated diffusion is particularly effective for the uptake of rapidly converted substrates because low internal concentrations resulted in high net transport.
It is widely known that monocarboxylate transporters (MCTs) belong to the SLC16 gene family and mediate the cotransport of monocarboxylic substrates with H+ and H+:substrate stoichiometry ratio is 1:1, suggesting an electroneutral transport process (Ganapathy et al., 2008). Additionally, the second class of MCTs has been identified, comprising at least 2 members (SLC5A8 and SLC5A12). SLC5A12 is differentially expressed in colonic and intestinal epithelial cells (Gopal et al., 2007; Iwanaga et al., 2006). This localization is consistent with its role in the cellular uptake of short-chain fatty acids. Ganapathy et al. (2008) screened a mouse kidney cDNA library and isolated a closely related cDNA that encodes a transporter protein highly homologous to SLC5A8. This transporter, known as SLC5A12, belongs to the same family as SLC5A8 and is primarily expressed in the kidneys and intestinal tract. The substrate selectivity of this transporter is similar to that of SLC5A8 but with a lower affinity.
The expression pattern of SLC5A12 in the intestinal tract is still under investigation; however, its lack of expression in the colon has been widely accepted.
Martin-Venegas et al. (2007) characterized HMTBa uptake across the Caco-2 cell apical membrane and noted the benefit of lower apical pH (5.5 vs. 7.4) on HMTBa molecule uptake, suggesting an H+-dependent process similar to that of MCT1. Na+/H+ exchangers (NHEs) comprise a family of highly related proteins that mediate the electroneutral 1:1 exchange of intracellular H+ for extracellular Na+ across the membrane. Therefore, HMTBa uptake exhibits partial Na+ dependence, reflecting the participation of NHE3 in the chicken small intestine (Martin-Venegas et al., 2008).
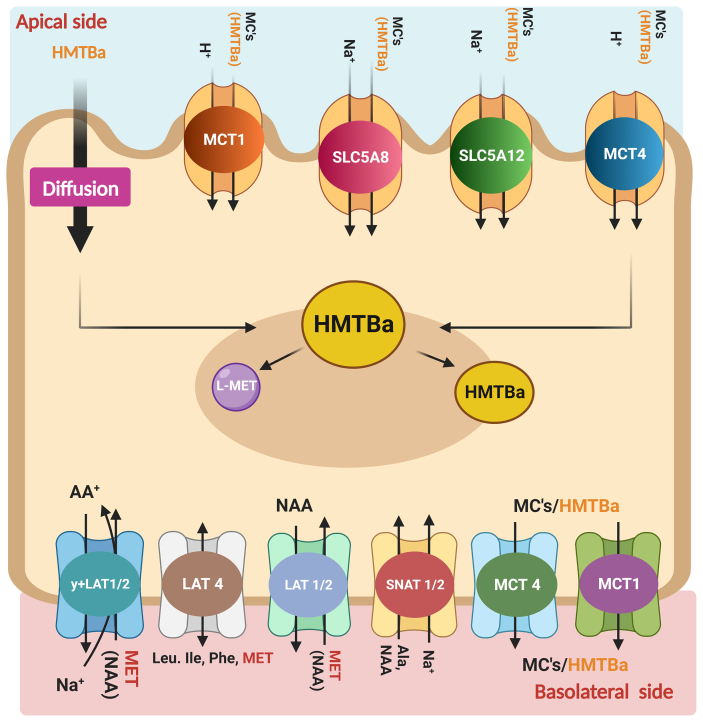
Generally, MCTs demonstrate a low affinity for their substrates, and the values for Km are in the millimolar range. Both Na+-coupled electrogenic carriers (SLC5A8 and SLC5A12) effectively transport monocarboxylic substrates with varying affinities, and their role in HMTBa transportation cannot be ruled out. Most recently, To et al. (2020) suggested the presence of a distinct transport mechanism for HMTBa based on their segmental localization in the trout gut. They argued that the apical influx and basolateral efflux of HMTBa presumably occur via Na+-dependent and H+-independent carrier-mediated transporters in the upper gut (pyloric ceca and midgut). In the hindgut, apical influx and basolateral efflux of HMTBa are Na+-dependent (medium affinity compared to the upper gut) and H+-dependent carrier-mediated transporters. The Km values of 0.5 to 1.1 mM at high (0.2 to 20 mM) and 7 to 10 μM at low substrate concentrations (0 to 150 μM) in the upper gut were closely correlated with low-affinity sodium-dependent MCT2 (SMCT2) and high-affinity SMCT1, respectively. Arguably, Caco-2 cells may not account for the putative contribution of SLC5A12 to HMTBa transport, as these cells are of colon origin, and SLC5A12 is not expressed in the colon. Therefore, it is tempting to explore the possible contribution of these transporters in future studies on HMTBa transport. Fig. 2 shows the different influx and efflux transporters involved in the transport of HMTBa and Met at the enterocyte level.
Fig. 2.
Representation of different influx and efflux transporters involved in transport of HMTBa and methionine at an enterocyte (hypothesis). MC = microcystin; HMTBA = 2-hydroxy-4-methylthiobutyric acid; MCT = monocarboxylate transporter; MET = methionine; NAA = neutral amino acid; SLC = solute carrier; LAT = L-type amino acid transporters; SNAT = sodium-coupled neutral amino acid transporters; Leu = leucine; Ile = isoleucine; Phe = phenylalanine; Ala = alanine (Created with BioRender.com).
Brachet and Puigserver (1987) used BBMV and everted rings of the rat jejunum to study the absorption of HMTBa. The initial uptake rates of both L- and D-Met were 6.8 and 1.5 times higher than that of HMTBa, respectively, under Na+ gradient conditions. Uptake of HMTBa into rat jejunal rings was the sum of the saturable Michaelian component and diffusion, suggesting that HMTBa is mediated by an Na+-independent carrier system associated with L-lactate transport and differs from the L-Met pathway. In another study, Brachet and Puigserver (1989) found that the transport systems for HMTBa are different from those for DL-Met, both quantitatively and qualitatively. This carrier system is Na+-independent, nonstereospecific, and electroneutral. They further added that this may indicate slow absorption of HMTBa from the chicken intestine, but many contributing factors, such as luminal surface pH, microclimate along the intestine (Cummings, 1981; Lucas et al., 1978), and absorptive capacity of the hindgut (Dibner et al., 1988), could not be excluded before reaching a definitive conclusion. In this latter study, differences in the absorption of Met from various Met sources must be considered with some caution due to the limitations of the model used. BBMV is an efficient model to assess Met uptake because the carrier systems can pump Met against the concentration gradient in the vesicles, whereas this is not the case for HMTBa, which goes primarily through passive transport that cannot counteract HMTBa accumulation in vesicles. Therefore, the BBMV model is not suitable for transport studies of HMTBa. Specifically, 1) there is no blood flow to carry away HMTBa once it has been absorbed. Therefore, the concentration gradient that drives diffusion is quickly disrupted; 2) the vesicles likely lack the enzymes to convert HMTBa to Met, which further disrupts the concentration gradient; and 3) the pH in these experiments (7.4) (Brachet and Puigserver, 1989; Brachet and Puigserver, 1989) is typically substantially greater than that in luminal microclimates (pH approximately 6) (Brachet and Puigserver, 1989; Richards et al., 2005). A high pH significantly hinders HMTBa absorption by diffusion (Maenz and Engele-Schaan, 1996). Knight and Dibner (1984) measured the in vitro uptake of HMTBa and L-Met in the duodenum, jejunum, and ileum using radiolabeled materials. Their findings demonstrated that low uptake levels occurred for both compounds in the duodenum, with increasing levels of L-Met (but not HMTBa) uptake in the jejunum and ileum. However, there has been a general disagreement in the literature regarding the intestinal site of absorption for Met sources. Some studies have demonstrated rapid uptake of L-Met in the proximal part of the jejunum (Lerner and Kratzer, 1976), while another report (Hudson et al., 1971) found higher in vitro uptake of L-Met in the ileum and the slowest uptake in the upper jejunum. The contrasting results between these 2 studies are difficult to explain, but the age (mature chicken vs. young chicks) and strain (Barred Plymouth Rock × Rhode Island Red vs. Hubbard Cross Hubbard) of the birds could be reasons for these differences (Lerner and Kratzer, 1976). Despite clear differences in the site of absorption and mechanism for L-Met and HMTBa, the 2 compounds were absorbed at similar rates, especially at low concentrations. Moreover, HMTBa is rapidly converted to Met following absorption and subsequently incorporated into proteins at the intestinal level (Knight and Dibner, 1984). This is likely to assist normal intestinal blood flow in maintaining a favorable gradient for HMTBa absorption.
It is imperative to emphasize the choice of the intestinal model to characterize the transport systems for different Met sources. Conflicting results have been obtained depending on the selection of the intestinal model. Dibner et al. (1992) used a different model (everted intestinal slices) and suggested that HMTBa is primarily absorbed through diffusion compared to the energy and Na+-dependent transportation for DL-Met. This mode of transportation assumes greater importance during heat stress conditions, wherein HMTBa is absorbed more effectively. This study also showed that the everted gut sac model measures active transport and is also highly efficient in considering the movement of molecules via diffusion. Hence, the comparison between HMTBa and DL-Met is improved when using this model.
3. Factors affecting the relative efficiency of transport systems for methionine sources
As explained earlier, different methodologies have been used to study the transport systems for methionine sources. In this regard, choosing an appropriate experimental model for Met transport studies can invariably be considered the most important factor affecting the outcome. In experimental models that are not appropriate for evaluating diffusion-mediated systems (Brachet and Puigserver, 1987, 1989), the HMTBa uptake values are usually lower than those of Met. In contrast, when experimental models were appropriate for evaluating diffusion-mediated systems (Richards et al., 2005), the HMTBa uptake values were similar to or higher than those of Met. A critical analysis of different models is presented in the following sections.
3.1. Radioactive position in the molecule
The use of radioisotopes has been instrumental in unraveling metabolic fate and increasing our understanding of test compounds in animal experiments (Dalvie, 2000). Carbon-14 (14C) is among the most commonly used radionucleotides due to its synthetic versatility, safety relative to other radioisotopes, and favorable nuclear properties dictating optimal half-life and specific activity (McCarthy, 2000). However, hydrogen-3 (3H) has also been used instead of or in addition to 14C to obtain in vitro (Guroff et al., 1967; Kler et al., 1992; Linnet, 2004) and in vivo (Ehlhardt et al., 1998; Koller-Lucae et al., 1999; Prakash et al., 1997; Rosenborg et al., 1999; Gray et al., 1994) biotransformation data. Low cost, ease of preparation, and rapid turnaround of 3H incorporation (Saljoughian and Williams, 2000) are desirable attributes in situations where specific metabolism-related question(s) during substance discovery or development should be addressed. Many studies have suggested that 14C atoms can be detached from the substructure during metabolism if their position corresponds to a metabolically labile site (Chasseaud and Fry, 1974; Hawkins et al., 1977; Larsson and Lund, 1981). However, passive chemical exchange into nonradiolabeled compounds and/or metabolites has never been reported. On the other hand, the intrinsic properties of the hydrogen atom can lead to 3H exchange within a molecule under varying pH values of aqueous physiological ambiance by chemical (e.g., α-H exchange during keto-enol tautomerization) or metabolic means (Lewis et al., 1988). In such situations, 3H exchange ultimately forms tritiated water (HTO), which is a marker of 3H loss. Therefore, it could result in both the absence of radiotracer capabilities from the test compound and/or its metabolites and misleading biotransformation data (Shaffer et al., 2006). Using 3H-labeled compounds in biological systems brings a predominant risk linked to the chemical and metabolic instability of the 3H atom itself at its specific site within the test molecule (Shaffer et al., 2006).
This raises a proposition because, in some studies, 3H radiolabeling has been correlated with higher 3H residual activity in HMTBa-fed pigs with preferential utilization by resident bacteria. The authors concluded poorer efficacy of HMTBa compared to DL-Met (Malik et al., 2009). Nevertheless, in a subsequent report, 3H labeling of Met was found to have a different metabolic fate. Cells demonstrated a differential preference for Met when labeled with 3H or 14C. Lipid synthesis (phosphatidylcholine) is the predominant pathway for L-(methyl-3H)-Met (Kuang et al., 2014). From this perspective, a higher residual 3H activity could be linked to the desquamation of epithelial membranes with retained 3H activity. Alternatively, high 3H activity in tissues of the proximal intestinal part in DL-Met-fed pigs indicated higher first-pass metabolism of Met, which under normal conditions accounted for approximately 35% and could deviate substantially on refeeding after an extended fasting period, which was the case in this experiment. While HMTBa effectively bypassed this step and became available for protein synthesis, the higher residual 3H activity observed in HMTBa-fed conventional piglets was due to continuous endogenous losses of intestines harboring a normal gut microbiota. However, in monoassociated pigs, intestinal development did not follow the same trajectory, and regressed intestinal mass can be expected in such animals. Logically, endogenous losses will not be of the same magnitude in both classes of pigs that support variable microbiota. This could be considered a crucial factor in the findings of Malik et al. (2009), as the authors were only observing the resident 3H activity rather than the complete product to which this radioisotope belonged. Hegedus et al. (1993) established that at least 3 Lactobacillus species can efficiently utilize Met and not HMTBa in the gut, further strengthening the idea that higher 3H residual activity is not an indication of bacterial utilization of HMTBa. In the past, Esteve-Garcia and Austic (1993) suggested that in any experiment involving administrative radioactive HMTBa to the animals, there is a substantial portion of radioactive material that is not absorbed or cleared efficiently by the kidneys, and it could be expected that losses in excreta would be substantial and may appear independent of the route of administration.
Therefore, in 3H experiments involving either in vivo or in vitro samples, there is a clear possibility of HTO being detected radiographically within the solvent front. This could potentially result in erroneous identification as a highly polar (and possibly significant) metabolite. In addition, 3H recovery could confound the ultimate findings from biofluids undergoing organic extraction; low organic-phase 3H levels may be ascribed to highly polar metabolites, HTO, or both (Shaffer et al., 2006). For total radioactivity PK analyses, 3H exchange causes similar data interpretation complexities due to tritium's 3-component exponential function biological half-life, which ranges from 10 to 300 d in humans (Robertson, 2013). If 3H exchange occurs, the total radioactivity measured using the area under the curve is useless because some of the radioactivity can be attributed to HTO and not necessarily to substance-related material (Kim et al., 2004). There are certain prerequisites to ensure for 3H compounds to be effectively used in metabolic studies. The first is strategic selection of the most chemically and metabolically stable molecular site for 3H incorporation. The second involves the empirical confirmation of all samples by quantifying the HTO via lyophilization and determination of the true inertness of the 3H atom (Shaffer et al., 2006).
3.2. Effects of the physiological state of the animal
Saroka and Combs (1986) reported that L-Met was the major excreted form of Met in roosters dosed with either L-Met or HMTBa. Roosters were fasted for 24 h before infusion of radiolabeled substances. Rostagno and Barbosa (1995) followed an identical fasting period in adult cockerels. It is widely accepted that a long fasting period changes the normal body metabolism of experimental birds, resulting in a negative energy balance. Moreover, the immediate response of the small intestine to fasting is the reduction in absorptive surface area, which decreases the total absorptive capacity; conversely, it reduces the body's energy and nutrient demand required for gut maintenance (Ferraris and Carey, 2000). Therefore, nutrient supplementation immediately after refeeding postfasting cannot be equated with normal metabolic processes. Many reports on rats and chickens have demonstrated that intestinal absorption of essential and nonessential amino acids, primarily or in part through a Na+-dependent pathway, increases with fasting and malnutrition (Ferraris and Carey, 2000; Muñíz et al., 1993). Enterocytes likely utilize a major portion of L-Met during first-pass metabolism (Stoll et al., 1998). Oxygen consumption and protein synthesis increased in fasted or restricted caloric-fed rats (Muñíz et al., 1993; Merry et al., 1992). As a result, there was uncoupling of the influx and efflux of amino acids in enterocytes under fasting conditions. Enterocytes increase their utilization of absorbed amino acids (Marciani et al., 1987) or, in other words, high first-pass metabolism. HMTBa may bypass this first-pass metabolism and become available for protein synthesis. In addition, adult roosters may support limited protein accretion, and most of the absorbed nutrients could be utilized for maintenance. Therefore, it can be imagined that birds under steady-state conditions will exhibit better performance than those under fasting conditions.
3.3. Selection of intestinal models
Amino acid transport at the cellular level is often studied independently of metabolism. However, it is widely accepted that individual cells may take up certain amino acids and release others (Wellner and Meister, 1981), so the question of “real” transport through the cells becomes far more complicated than examining it in a reconstituted system. Intestinal models do not faithfully replicate in vivo processes per se but are still considered extremely useful tools for obtaining valuable information about transport mechanisms. Therefore, the model selected should be based on the physiological path that the tested product is predicted to follow to provide the necessary milieu that corresponds with in vivo conditions. BBMVs (Brachet and Puigserver, 1987, 1989; Maenz and Engele-Schaan, 1996; Soriano-García et al., 1998), everted gut sacs (Dibner et al., 1992; Richards et al., 2005), ligated intestinal segments (Knight and Dibner, 1984), and the Caco-2 cell model (Martin-Venegas et al., 2007, 2008, 2011, 2014) are some of the commonly used models for Met transport studies. Variable results were obtained in these studies, and intestinal model selection appears to be the leading cause of these conflicting outcomes.
For the evaluation of apical transporters, BBMVs have been extensively used to determine the kinetic attributes of different compounds. The accuracy of the results depends on many factors, including but not limited to the purity of BBMV, and is usually validated by marker enzymes, such as aminopeptidase N and different disaccharidases (maltase and sucrase). However, marker enzymes may not be present in a single membrane. The yield, recovery, and enrichment can be easily misinterpreted when an appreciable amount of marker enzymatic activity is present in membranes other than the one under study. A second separation step should follow the measurement of marker enzymes, for example, free-flow electrophoresis after differential and/or density gradient centrifugation, or vice versa, or differential and/or gradient centrifugation in combination with phase partitioning (Murer and Gmaj, 1986). Likewise, magnesium precipitation in calcium-free media yields better BBMVs with considerably reduced leak permeability. The choice of calcium or magnesium precipitation induced some differences in the ion fluxes. Calcium precipitation leads to electro-diffusional coupling of H+ and Na+, whereas magnesium precipitation limits this electro-diffusional coupling and favors the Na/H electroneutral exchange of these ions (Sabolic and Bruckhardt, 1984).
Brachet and Puigserver (1989) used BBMVs prepared with calcium precipitation. These vesicles contained some Na+/K+ ATPase activity, although it was 2.5-fold lower than that of the parent homogenate, indicating some cross-contamination. Various studies measuring the transport kinetics of Met sources did not follow similar temperature conditions. Some authors used 25 °C (Brachet and Puigserver, 1987, 1989), whereas others (Maenz and Engele-Schaan, 1996; Soriano-García et al., 1998) used 37 °C for uptake experiments. Temperature variation could affect the overall results. Moreover, it has been suggested that BBMVs should be incubated at 37 °C to avoid changes in cationic and neutral amino acid transport activity at lower incubation temperatures (Furesz et al., 1995; Maenz and Engele-Schaan, 1996). Maenz and Engele-Schaan (1996) reported that the imposition of an internally directed H+ gradient (pHout = 5.5; pHin = 7.5) led to substantial uptake of 3H-L-HMTBa across BBMVs. This demonstrates the influence of pH on HMTBa transport. Therefore, it could be inferred that the influence of the acidic microclimate at the brush borders of enterocytes in vivo on the transport kinetics of HMTBa cannot be ignored. Uptake experiments should be performed under physiological conditions if possible. For instance, the temperature and pH should be kept the same as those of the species for which the in vitro investigation is being performed.
In addition, BBMVs may be a suitable model for obtaining the transport kinetics of compounds transported by Na+-dependent active transport systems, although the electrochemical potential could potentially favor the transport values of Na+-dependent pathways (Lücke et al., 1978). Therefore, higher uptake values for DL-Met and L-Met compared to HMTBa (Brachet and Puigserver, 1987, 1989) using BBMVs are questionable. Moreover, the absence of metabolism in BBMVs (Murer and Gmaj, 1986) could underestimate the HMTBa transport values that undergo oxidation and dehydrogenation, followed by transamination. Experimental evidence for BBMVs is far from conclusive due to the limitations of the model system in studying diffusion. Therefore, the BBMV model represents a simple way to mimic intestinal transport studies that only replicates active transport, not carrier-mediated transport, or diffusion. Studies on the transport of fructose across BBMVs face similar criticism, indicating the inapplicability of BBMVs to accurately measure the transport of molecules whose movement predominantly depends on their conversion into another metabolic moiety, ensuring continuous transport across the membrane (Lücke et al., 1978). HMTBa is converted into L-Met and cysteine in the cell, maintaining the chemical concentration difference and unabated cross-membrane transport.
Wilson and Wiseman (1954) first introduced the in vitro everted gut sac model. Modifications and improvements have been made to increase the viability of tissues and maintain an intact mucosal epithelium that mimics in vivo conditions. An improved everted gut sac model can be used as an ex vivo tool to study the mechanisms and kinetics of absorption (Barthe et al., 1998; Ugolev et al., 1980). The everted gut sac model has been extensively explored to perform pharmacokinetic investigations, such as substance absorption, metabolism or pro-drug conversion in GIT segments, efflux transport, multidrug resistance, drug interactions, and the impact of efflux transport modulators on the absorption of substances. The advantages of this model are its relatively large surface area available for absorption and the presence of a mucus layer. In addition, slices from different intestinal sections retain region-specific molecular and functional differences and have been used to compare transport kinetics across intestinal sections.
Dibner et al. (1992) and Richards et al. (2005) used everted gut sacs from the jejunum and ileum of broilers to compare and validate the transport kinetics of DL-Met and HMTBa, respectively. Their findings for HMTBa transport kinetics differed from those of the BBMV studies. The everted gut sac provides a near-complete in vivo milieu for amino acids and amino acid precursors, such as HMTBa.
Nevertheless, tissue viability is a limiting parameter in the everted gut-sac experiments. The recommended experimental duration is approximately 2 h based on tissue viability and metabolic activity of the intestine under physiological conditions (Barthe et al., 1998). None of the above-mentioned studies included data beyond 2 h. Richards et al. (2005) periodically evaluated the structural integrity of sacs. Hence, it is reasonable to assume that observations in these experiments were obtained using an everted ring whose physiological architecture was intact. The presence of muscularis mucosa, which is not typically removed from everted gut sac preparations, is another disadvantage of this approach. The muscularis mucosa may evoke an underestimation of the transport of compounds with a tendency to bind to muscle cells. However, this has not been further evaluated in the literature.
Cultured Caco-2 cells from human colon adenocarcinomas can differentiate into enterocyte-like cells. Despite their colorectal origin, differentiated Caco-2 cells express many of the morpho-functional features of mature small intestinal enterocytes, including columnar polarization, apical microvilli, tight junctions, carrier-mediated transport systems, and apical brush border membranes endowed with organotypic hydrolases (Picariello et al., 2016). Monolayers of confluent Caco-2 cells grown on porous membranes (e.g., Transwell inserts) have been proposed by Hidalgo et al. (1989) and are largely used as a transport model system for the small intestinal epithelium (Artursson et al., 2001). Additionally, a good correlation between passive transport across Caco-2 monolayers and the availability of the tested nutrients in humans has been observed. Martin-Venegas et al. (2011) successfully demonstrated that Caco-2 cells express L-hydroxy acid oxidase and D-hydroxy acid dehydrogenase, enzymes that are important for converting HMTBa into L-Met.
Caco-2 monolayers are impermeable to hydrophilic molecules and do not exhibit paracellular transport, which restricts their reliability as a model for compounds that are transported by the paracellular pathway (Behrens et al., 2001; Gamboa and Leong, 2013). The Caco-2 monolayer has other drawbacks, such as the lack of a mucous layer in the intestinal wall and a lack of some intestinal metabolic enzymes, as it only corresponds to epithelial cells in the intestinal epithelium. In contrast, many other cell types are present in the intestine, including mucosal cells and microfold cells. This does not preclude the potential of Caco-2 cells for an accurate study of transport kinetics, especially for amino acids. However, cocultures could be an important addition to the Caco-2 monolayer culture that may better simulate the multicellular intestinal epithelium (Gamboa and Leong, 2013).
Based on a critical appraisal of the intestinal models found in the literature, the Caco-2 model seems to comply better with in vivo conditions than other methods. Importantly, it represents metabolically active cells, a prerequisite for HMTBa transport studies. From these studies, it can be concluded that selecting an intestinal model is key to obtaining realistic results for transport kinetics, considering the physiology of the tissue, metabolism of the compound, and representativeness of the test method of animal physiology.
4. Oligomer hydrolysis and utilization
The manufactured forms of HMTBa are aqueous solutions that consist of an equilibrium mixture of monomer (65% to 70%), dimer (15% to 20%), and trimers (3%) (Dibner, 2003). The manufactured form of HMTBa-Ca is a powder containing 100% monomers. The monomer/oligomer equilibrium is dynamic, with the continuous formation and hydrolysis of dimers and trimers. No energy is required for these reactions; however, the equilibrium is affected by the matrix. Once HMTBa is added to the feed, the equilibrium shifts to monomer formation (Bruyer and Vanbelle, 1990a). The percentage of oligomers is highest in the liquid solution, decreases in the feed, and further decreases after ingestion (Dibner, 2003).
Various methodologies have been used to evaluate the efficacy of HMTBa oligomers for L-Met. Multiple in vitro (Koban and Koberstein, 1984) and in vivo (Boebel and Baker, 1982; van Weerden et al., 1992) studies have concluded that utilization of dimers and trimers is inferior to that of monomers. Koban and Koberstein (1984) evaluated the in vitro hydrolysis of dimer and trimer HMTBa in dilute hydrochloric acid solution and concluded that the rate constants obtained corresponded to the half-lives of 1.8 and 1.6 d for dimers and trimers, respectively, suggesting that the dimers and trimers were not biologically available. This in vitro study lacked enzymes that are available in vivo. Bruyer and Vanbelle (1990b) demonstrated that the ester bonds between HMTBa molecules were the same as those found in triglycerides, closely resembled those occurring in lactic acid, and underwent homologous intermolecular esterification. Pancreatin and intestinal digestive enzymes readily break down polymers of HMTBa (Lawson and Ivey, 1986). Lawson and Ivey (1986) used 2 methods to assess the hydrolysis of HMTBa dimers. In Exp. 1, simulated intestinal fluid was used, and in Exp. 2, in vitro sections of the chick small intestine (both systems contained enzymes) were used. They demonstrated that the rapid hydrolysis of dimers was enzyme-mediated by experiments in which heat denaturation resulted in a total loss of hydrolytic activity.
Another important consideration in evaluating oligomer efficacy is the preparation of the polymer fraction. Previous work (van Weerden et al., 1992) employed polymers isolated using a lab-synthesized solvent extraction procedure. The isolated fraction of polymers contained 95% polymers and 5% monomers. The authors acknowledged that the recovery rates of both the free acid and polymer compounds tested were unsatisfactory, and they indicated a difference between the calculated and actual analyses of the HMTBa treatments. In addition, the actual composition (percentage of dimers, trimers, and higher oligomers) of the polymer preparation was not presented; it is important to compare the composition of monomers/dimers/trimers and larger oligomers to that of HMTBa, as produced today, to confirm their relevance. Boebel and Baker (1982) also compared the efficacy of HMTBa in polymer form in broilers using a freeze-dried preparation. The composition of this preparation was 21% monomers, 35% dimers, 30% trimers, 12% higher oligomers, and 2% H2O. The simple removal of water resulted in the formation of much larger oligomers. These larger oligomers are formed when the product is freeze-dried (Boebel and Baker, 1982) or concentrated via solvent evaporation (van Weerden et al., 1992). Both Boebel and Baker (1982) and van Weerden et al. (1992) found lower performance with HMTBa than with DL-Met, but the oligomer tested was not representative of the oligomers present in HMTBa, as produced today; therefore, these findings may not translate under practical conditions. Martin-Venegas et al. (2006b) used everted intestinal sac and in vivo jejunum perfusion to compare HMTBa-ammonium salt containing 99% monomers and HMTBa (mixture of monomer and polymers). The authors found no differences between the HMTBa products, demonstrating that nonmonomeric forms are hydrolyzed in intestinal sections and are not a limiting factor for HMTBa absorption.
5. Physiological factors affecting HMTBa vs. DL-methionine absorption
Animals experiencing heat stress partition blood to the periphery to maximize radiant heat dissipation, which is supported by vasoconstriction of the GIT (Pearce et al., 2013). Consequently, reduced blood flow leads to hypoxia in the intestinal epithelium, which compromises intestinal integrity and is associated with inflammation (Pearce et al., 2013). Inflammation and hypoxia exert detrimental effects on intestinal tight junction proteins (Pearce et al., 2013; Yi et al., 2020) and alter nutrient digestibility and absorption across the intestinal epithelium.
Oxidative stress damages lipids, proteins, carbohydrates, and DNA through the generation of reactive oxygen species. As glutathione (GSH) is an important biomarker of antioxidant defense against oxidative stress, measurement of GSH, in addition to cysteine, taurine, and reactive oxygen species metabolites, can be used to differentiate efficacy between HMTBa and DL-Met, especially under environmental challenges, such as disease and oxidative stress (e.g., high, or low environmental temperatures, nutrient imbalances, etc.) (Swennen et al., 2011; Willemsen et al., 2011; Yodseranee and Bunchasak, 2012). Using ex vivo chicken everted intestinal sacs, Martin-Venegas et al. (2006a) demonstrated increased cysteine and taurine levels with HMTBa than with DL-Met. A later study by Martin-Venegas et al. (2013), using an in vitro model of intestinal inflammation in Caco-2 cells treated with H2O2 or TNF-α, found greater concentrations of reduced GSH (rGSH) and taurine but, more importantly, confirmed greater gut protection with HMTBa compared to DL-Met in the presence of oxidative stress or cytokine challenge.
It was reported that HMTBa supplementation has been shown to partially prevent the growth-depressing effects of heat stress (Knight et al., 1994) and alleviate oxidative damage caused by heat stress in broiler chickens (Willemsen et al., 2011). According to Dibner et al. (1992) and Knight et al. (1994), the uptake of DL-Met into intestinal epithelial cells is reduced in heat-stressed birds. Greater absorption occurred for HMTBa via diffusion when the absorption capacity of the villi was compromised. In vitro studies by Dibner et al. (1992) and Knight et al. (1994) demonstrated that diffusion of DL-HMTBa increased, whereas D-Met active transport decreased during heat stress conditions.
6. Methionine sources and gut microbiota interaction
It is widely known that microflora from the hind gut can compete with the host for nutrients, such as amino acids. Numerous studies have compared the utilization of HMTBa relative to DL-Met and concluded that these 2 molecules have similar bioefficacies, ranging from 80% to 100% (Dilger and Baker, 2008). Some studies (Drew et al., 2003; Malik et al., 2009) have concluded that intestinal microbial degradation explains the lower HMTBa absorption than DL-Met. Conventional vs. germ-free chickens were fasted overnight and then fed diets containing 1.11 × 107 L-[methyl3H]-HMTBa or L-[methyl3H]-Met Bq/kg of feed for 3 h, euthanized, and the intestinal tracts were removed. The residual activity of 3H was also measured (Drew et al., 2003). Residual radiolabels were lower in germ-free (4.7%) vs. conventional birds (10.2%) in birds fed HMTBa, but no differences in radiolabels were observed between germ-free (3.0%) and conventional (3.7%) birds fed L-Met. The growth of the animals was also unchanged. However, these studies have some experimental limitations. First, the 2 sources were supplemented on an equal weight basis, not equimolar. If these authors concluded that HMTBa results in higher microbial degradation than Met, then growth performance should have been different. Second, the authors used radioactive L-HMTBa and L-Met instead of DL. DL-Met absorption is much slower than that of L-Met (Brachet and Puigserver, 1989), leading to an increased opportunity for microbial uptake and degradation of Met, and comparison of the DL forms for both products has greater relevance to practice. Third, 3H exchange within a molecule can readily occur; therefore, quantification of tritiated water to determine the inertness of the 3H atom needs to be confirmed, and this was not addressed. Higher residual 3H activity may not indicate bacterial utilization (see Section 3.1) and thus does not necessarily equate to lower utilization. Hence, the conclusions of these studies can be criticized and are contrary to other findings in the literature.
Malik et al. (2009) also evaluated the effect of microbiota on Met utilization in conventional and mono-associated pigs (e.g., not germ-free). Bacterial contamination resulted in a single bacterial population (Gram-negative in some pigs, Gram-positive in others). In the Malik et al. (2009) document, the following elements impair the conclusions: 1) No indication of the specific activity of each molecule to compare incorporation of radiolabel; 2) Details are given in the Materials and Methods section for the 3H Methyl Met and 3H Methyl L-HMTBa, which resulted in 2 labeled products at 99%. At such concentrations of HMTBa, deprived of water, there should be a high oligomerization level for HMTBa that has not been assessed. It should also be pointed out that in this trial, radiolabeling was performed on the methyl group of the Met or HMTBa compounds. When the L-[methyl-3H] of these 2 molecules go through the sulfur amino acid metabolism Met → SAM → S-adenosyl-homocysteine (SAH), the methyl group is lost from the original molecule. This means that in the first steps of sulfur amino acid metabolism, the radiolabeled portion follows a metabolic pathway other than the sulfur amino acid pathway (one-carbon pathway). Therefore, the residual activity observed in this study may be due not only to Met retention in the GIT but also to endogenous loss from the host. The difference observed between microbiological status may not be due to an increase in the residual activity in the HMTBa group but due to a higher (or at least different) amount of residual activity in the L-Met group between microbiological conditions.
Therefore, the author's hypothesis that intestinal microbial degradation is higher for HMTBa is not well substantiated based on the criticisms of both trials and is contrary to other findings in the literature. Han et al. (1990) compared the growth responses to crop intubation and intraperitoneal injection in chicks fed DL-Met or HMTBa. These authors found no significant difference in absorption between DL-Met and HMTBa or crop intubation and intraperitoneal injection for either Met source, indicating that the intestinal absorption of HMTBa is as efficient as that of DL-Met. Since intraperitoneal injection bypasses intestinal absorption, any inefficiency in intestinal absorption of HMTBa should have been reflected by lower utilization of crop intubation than intraperitoneal injection. Hence, these findings suggest complete HMTBa absorption in the intestine. Both methodologies indicated nonsignificant differences between HMTBa and DL-Met utilization and demonstrated that HMTBa utilization is not limited by intestinal absorption.
Esteve-Garcia and Austic (1993) also studied the absorption of radiolabeled HMTBa in broilers and concluded that the performance of birds fed HMTBa would not be limited by inefficient intestinal absorption.
7. Conclusion
A thorough assessment of the literature in this review demonstrates that the physicochemical characteristics of HMTBa and DL-Met are distinct, leading to unique behaviors with respect to their absorption and metabolism. Therefore, it is important to select methods that consider these aspects when comparing the absorption of these 2 molecules. The same evaluation method may not be adequate to compare the absorption of the 2 molecules, leading to contradictory results. The contradictory results in the literature can be explained by the use of inappropriate methodologies, either for DL-Met or HMTBa.
Author contributions
Philippe Becquet, Mercedes Vazquez-Anon, Yves Mercier, Dolores Batonon-Alavo, Frances Yan: Writing – Review & Editing. Karen Wedekind: Writing – Original Draft, Writing – Review & Editing. Tahir Mahmood: Writing – Original Draft, Writing – Review & Editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors are employees of association and corporate companies producing and placing on the market methionine sources, such as DL-methionine, HMTBa and HMTBa-Ca. The corresponding author is the General Manager of the International Methionine Analogue Association (IMAA asbl).
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Artursson P., Palm K., Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv. 2001;22:67–84. doi: 10.1016/s0169-409x(00)00128-9. [DOI] [PubMed] [Google Scholar]
- Barthe L., Woodley J.F., Kenworthy S., Houin G. An improved everted gut sac as a simple and accurate technique to measure paracellular transport across the small intestine. Eur J Drug Metab Pharmacokinet. 1998;23:313–323. doi: 10.1007/BF03189357. [DOI] [PubMed] [Google Scholar]
- Behrens I., Stenberg P., Artursson P., Kissel T. Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells. Pharm Res. 2001;18:1138–1145. doi: 10.1023/a:1010974909998. [DOI] [PubMed] [Google Scholar]
- Bertran J., Werner A., Chillarón J., et al. Expression cloning of a human renal cDNA that induces high affinity transport of L-cystine shared with dibasic amino acids in xenopus oocytes. J Biol Chem. 1993;268:14842–14849. [PubMed] [Google Scholar]
- Bodoy S., Martín L., Zorzano A., Palacín M., Estévez R., Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002–12011. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- Boebel K.P., Baker D.H. Efficacy of the calcium and free acid forms of methionine hydroxy analogue for chicks. Poultry Sci. 1982;61:1167–1175. [Google Scholar]
- Brachet P., Alvarado F., Puigserver A. Kinetic evidence for separate systems in transport of D- and L-methionine by rat small intestine. Am J Physiol Gastrointest Liver Physiol. 1987;252:G320–G324. doi: 10.1152/ajpgi.1987.252.3.G320. [DOI] [PubMed] [Google Scholar]
- Brachet P., Puigserver A. Transport of methionine hydroxy analog across the brush border membrane of rat jejunum. J Nutr. 1987;117:1241–1246. doi: 10.1093/jn/117.7.1241. [DOI] [PubMed] [Google Scholar]
- Brachet P., Puigserver A. Na+-independent and nonstereospecific transport of 2-hydroxy 4-methylthiobutanoic acid by brush border membrane vesicles from chick small intestine. Comp Biochem Physiol Part B: Comp Biochem. 1989;94:157–163. doi: 10.1016/0305-0491(89)90027-8. [DOI] [PubMed] [Google Scholar]
- Brandsch M., Brandsch C. Progress in research on energy and protein metabolism. European Federation of Animal Science Proceedings. Vol. 109. Rostock-Warnemünde; Germany: 2003. Intestinal transport of amino acids, peptides, and proteins; pp. 667–680. [Google Scholar]
- Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- Bröer S., Fairweather S.J. Amino acid transport across the mammalian intestine. Compr Physiol. 2019;9(1):363–373. doi: 10.1002/cphy.c170041. [DOI] [PubMed] [Google Scholar]
- Bröer A., Wagner C.A., Lang F., Bröer S. The heterodimeric amino acid transporter 4F2hc/y+LAT2 mediates arginine efflux in exchange with glutamine. Biochem J. 2000;349(3):787–795. doi: 10.1042/bj3490787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyer D.C., Vanbelle M. Unité de Biochimie de la Nutrition; Louvain-la-Neuve: 1990. Estimation of bioavailable methionine hydroxy analogue free acid dimer for poultry and pigs. [Google Scholar]
- Bruyer D.C., Vanbelle M. Efficacité comparée pour la croissance du poussin de différentes sources de methionine. Ann zootech. 1990;39:45–51. [Google Scholar]
- Chasseaud L.F., Fry B.J. Metabolic and pharmacokinetic study of bucloxic acid. II. Pharmacokinetic study in the baboon. Arzneim-Forsch. 1974;24(9A):1390–1397. [PubMed] [Google Scholar]
- Chen J., Zhu Y., Hu M. Mechanisms and kinetics of uptake and efflux of L-methionine in an intestinal epithelial model (Caco-2) J Nutr. 1994;124:1907–1916. doi: 10.1093/jn/124.10.1907. [DOI] [PubMed] [Google Scholar]
- Cummings J.H. Short chain fatty acids in the human colon. Gut. 1981;22:763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave M.H., Schulz N., Zecevic M., Wagner C.A., Verrey F. Expression of heteromeric amino acid transporters along the murine intestine. J Physiol. 2004;558:597–610. doi: 10.1113/jphysiol.2004.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvie D. Recent Advances in the applications of radioisotopes in drug metabolism, toxicology, and pharmacokinetics. CPD. 2000;6:1009–1028. doi: 10.2174/1381612003399941. [DOI] [PubMed] [Google Scholar]
- Dibner J.J. Review of the metabolism of 2-hydroxy-4-(methylthio)butanoic acid. World’s Poultry Sci J. 2003;59:99–110. [Google Scholar]
- Dibner J.J., Atwell C.A., Ivey F.J. Effect of heat stress on 2-hydroxy-4-(methylthio)butanoic acid and DL-methionine absorption measured in vitro. Poultry Sci. 1992;71:1900–1910. doi: 10.3382/ps.0711900. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Buttin P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J Appl Poultry Res. 2002;11:453–463. [Google Scholar]
- Dibner J.J., Knight C.D., Swick R.A., Ivey F.J. Absorption of 14C-2-hydroxy-4-(methylthio)butanoic acid (Alimet-«) from the hindgut of the broiler chick. Poultry Sci. 1988;67:1314–1321. doi: 10.3382/ps.0671314. [DOI] [PubMed] [Google Scholar]
- Dilger R.N., Baker D.H. Cyst(e)ine imbalance and its effect on methionine precursor utilization in chicks. J Anim Sci. 2008;86:1832–1840. doi: 10.2527/jas.2007-0712. [DOI] [PubMed] [Google Scholar]
- Drew M.D., Van Kessel A.G., Maenz D.D. Absorption of methionine and 2-hydroxy-4-(methylthio) butanoic acid in conventional and germ-free chickens. Poultry Sci. 2003;82:1149–1153. doi: 10.1093/ps/82.7.1149. [DOI] [PubMed] [Google Scholar]
- Ehlhardt W.J., Woodland J.M., Baughman T.M., et al. Liquid chromatography/nuclear magnetic resonance spectroscopy and liquid chromatography/mass spectrometry identification of novel metabolites of the multidrug resistance modulator LY335979 in rat bile and human liver microsomal incubations. Drug Metab Dispos. 1998;26:42–51. [PubMed] [Google Scholar]
- Esteve-Garcia E., Austic R.E. Intestinal absorption, and renal excretion of dietary methionine sources by the growing chicken. J Nutr Biochem. 1993;4:576–587. [Google Scholar]
- Ferraris R.P., Carey H.V. Intestinal transport during fasting and malnutrition. Annu Rev Nutr. 2000;20:195–219. doi: 10.1146/annurev.nutr.20.1.195. [DOI] [PubMed] [Google Scholar]
- Fraga S., Pinho M.J., Soares-da-Silva P. Expression of LAT1 and LAT2 amino acid transporters in human and rat intestinal epithelial cells. Amino Acids. 2005;29:229–233. doi: 10.1007/s00726-005-0221-x. [DOI] [PubMed] [Google Scholar]
- Fraga S., Serrão M.P., Soares-da-Silva P. L-type amino acid transporters in two intestinal epithelial cell lines function as exchangers with neutral amino acids. J Nutr. 2002;132:733–738. doi: 10.1093/jn/132.4.733. [DOI] [PubMed] [Google Scholar]
- Furesz T.C., Moe A.J., Smith C.H. Lysine uptake by human placental microvillous membrane. Comparison of system y+ with basal membrane. Am J Physiol, Cell Physiol. 1995;268:C755–C761. doi: 10.1152/ajpcell.1995.268.3.C755. [DOI] [PubMed] [Google Scholar]
- Gamboa J.M., Leong K.W. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv Drug Delivery. 2013;65:800–810. doi: 10.1016/j.addr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy V., Thangaraju M., Gopal E., et al. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10:193–199. doi: 10.1208/s12248-008-9022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal E., Miyauchi S., Martin P.M., Ananth S., Roon P., Smith S.B., Ganapathy V. Transport of nicotinate and structurally related compounds by human SMCT1 (SLC5A8) and its relevance to drug transport in the mammalian intestinal tract. Pharm Res. 2007;24:575–584. doi: 10.1007/s11095-006-9176-1. [DOI] [PubMed] [Google Scholar]
- Gray A., Wilkinson D.J., Seddon H. In: Synthesis and applications of isotopically labelled compounds. Pleiss U., Voges R., editors. ume 7. John Wiley & Sons, Ltd.; West Sussex, UK: 1994. The use of tritium radiolabelled compounds in the investigation of the DMPK properties of new pharmaceuticals; pp. 496–499. [Google Scholar]
- Guroff G., Renson J., Udenfriend S., Daly J.W., Jerina D.M., Witkop B. Hydroxylation-Induced Migration. The NIH shift: recent experiments reveal an unexpected and general result of enzymatic hydroxylation of aromatic compounds. Science. 1967;157:1524–1530. doi: 10.1126/science.157.3796.1524. [DOI] [PubMed] [Google Scholar]
- Han Y., Castanon F., Parsons C.M., Baker D.H. Absorption and bioavailability of DL-methionine hydroxy analog compared to DL-methionine. Poultry Sci. 1990;69:281–287. doi: 10.3382/ps.0690281. [DOI] [PubMed] [Google Scholar]
- Hatanaka T., Haramura M., Fei Y.J., Miyauchi S., et al. Transport of amino acid-based prodrugs by the Na+- and Cl–-coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Therapeut. 2004;308:1138–1147. doi: 10.1124/jpet.103.057109. [DOI] [PubMed] [Google Scholar]
- Hawkins D.R., Weston K.T., Chasseaud L.F., Franklin E.R. Fate of methoprene (isopropyl (2E,4E)-11-methoxy-3,7,11-trimethyl-2,4-dodecadienoate) in rats. J Agric Food Chem. 1977;25:398–403. doi: 10.1021/jf60210a005. [DOI] [PubMed] [Google Scholar]
- Hegedus M., Andrasofszky E., Brydl E., Veresegyhazy T., Tams J. Microbiological activity of methionine derivatives. Magyar Allattenyésztök Lapja. 1993;48:527–532. [Google Scholar]
- Hidalgo I.J., Raub T.J., Borchardt R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–749. [PubMed] [Google Scholar]
- Hudson D.A., Levin R.J., Smyth D.H. Physiology and biochemistry of the domestic fowl (bell DJ and freeman BM. Academic Press; New York: 1971. Absorption from the alimentary tract; pp. 52–71. [Google Scholar]
- Iwanaga T., Takebe K., Kato I., Karaki S., Kuwahara A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed Res. 2006;27:243–254. doi: 10.2220/biomedres.27.243. [DOI] [PubMed] [Google Scholar]
- Jendza J.A., Geraert P.A., Ragland D., Adeola O. The site of intestinal disappearance of DL-methionine and methionine hydroxy analog differs in pigs. J Anim Sci. 2011;89:1385–1391. doi: 10.2527/jas.2010-3235. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Fukasawa Y., Cha S.H., et al. Transport properties of a system y+L neutral and basic amino acid transporter. Insights into the mechanisms of substrate recognition. J Biol Chem. 2000;275:20787–20793. doi: 10.1074/jbc.M000634200. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Segawa H., Miyamoto K., Uchino H., Takeda E., Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Kim H., Prelusky D., Wang L., Hesk D., Palamanda J., Nomeir A.A. Importance of radiochemical analysis of biological fluids before and after lyophilization from animals dosed with [ˆ 3H]-labeled compounds in drug discovery. Am Pharmaceut Rev. 2004;7:44–48. [Google Scholar]
- Kirchhoff P., Dave M.H., Remy C., et al. An amino acid transporter involved in gastric acid secretion. Pflueg Arch Eur J Physiol. 2006;451:738–748. doi: 10.1007/s00424-005-1507-2. [DOI] [PubMed] [Google Scholar]
- Kler R.S., Sherratt H.S.A., Turnbull D.M. The measurement of mitochondrial β-oxidation by release of 3H2O from [9,10-3H]-hexadecanoate. Application to skeletal muscle and the use of inhibitors as models of metabolic disease. Biochem Med Metab Biol. 1992;47:145–156. doi: 10.1016/0885-4505(92)90018-t. [DOI] [PubMed] [Google Scholar]
- Knight C.D., Dibner J.J. Comparative absorption of 2-hydroxy-4-(methylthio)-butanoic acid and L-methionine in the broiler chick. J Nutr. 1984;114:2179–2186. doi: 10.1093/jn/114.11.2179. [DOI] [PubMed] [Google Scholar]
- Knight C.D., Wuelling C.W., Atwell C.A., Dibner J.J. Effect of intermittent periods of high environmental temperature on broiler performance responses to sources of methionine activity. Poultry Sci. 1994;73:627–639. doi: 10.3382/ps.0730627. [DOI] [PubMed] [Google Scholar]
- Koban H.G., Koberstein E. Kinetics of hydrolysis of dimeric and trimeric methionine hydroxy analogue free acid under physiological conditions of pH and temperature. J Agric Food Chem. 1984;32:393–396. [Google Scholar]
- Kobayashi M., Hashimoto F., Ohe K., et al. Transport mechanism of (11)C-labeled L- and D-methionine in human-derived tumor cells. Nucl Med Biol. 2012;39:1213–1218. doi: 10.1016/j.nucmedbio.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Koller-Lucae S.K.M., Suter M.J.-F., Rentsch K.M., Schott H., Schwendener R.A. Metabolism of the new liposomal anticancer drug N4-octadecyl-1-β-D-arabinofuranosylcytosine in mice. Drug Metab Dispos. 1999;27:342–350. [PubMed] [Google Scholar]
- Kuang Y., Wang F., Corn D.J., Tian H., Lee Z. Metabolism of radiolabeled methionine in hepatocellular carcinoma. Mol Imag Biol. 2014;16:44–52. doi: 10.1007/s11307-013-0678-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H., Lund J. Metabolism of femoxetine. Acta Pharmacol Toxicol. 1981;48:424–432. doi: 10.1111/j.1600-0773.1981.tb01642.x. [DOI] [PubMed] [Google Scholar]
- Lawson C.Q., Ivey F.J. Hydrolysis of 2-hydroxy-4-(methylthio)butanoic acid in two model systems. Poultry Sci. 1986;65:1749–1753. doi: 10.3382/ps.0651749. [DOI] [PubMed] [Google Scholar]
- Lerner J., Kratzer F.H. A comparison of intestinal amino acid absorption in various avian and mammalian species. Comp Biochem Physiol Part A Mol. 1976;53:123–127. doi: 10.1016/s0300-9629(76)80023-0. [DOI] [PubMed] [Google Scholar]
- Lerner J., Taylor M.W. A Common step in the intestinal absorption mechanisms of D- and L-methionine. Biochim Biophys Acta Biomembr. 1967;135:991–999. [PubMed] [Google Scholar]
- Lewis C.J., Havler M.E., Humphrey M.J., Lloyd-Jones J.G., McCleavy M.A., Muir N.C., Waltham K. The pharmacokinetics and metabolism of idazoxan in the rat. Xenobiotic. 1988;18:519–532. doi: 10.3109/00498258809041689. [DOI] [PubMed] [Google Scholar]
- Linnet K. In vitro microsomal metabolism of imipramine under conditions mimicking the in vivo steady-state situation. Hum Psychopharm Clin. 2004;19:31–36. doi: 10.1002/hup.550. [DOI] [PubMed] [Google Scholar]
- Lobley G.E., Wester T.J., Holtrop G., Dibner J.J., Parker D.S., Vazquez-Anon M. Absorption and digestive tract metabolism of 2-hydroxy-4-methylthiobutanoic acid in lambs. J Dairy Sci. 2006;89:3508–3521. doi: 10.3168/jds.S0022-0302(06)72391-8. [DOI] [PubMed] [Google Scholar]
- Lucas M.L., Cooper B.T., Lei F.H., Johnson I.T., Holmes G.K., Blair J.A., Cooke W.T. Acid microclimate in coeliac and Crohn's disease. A model for folate malabsorption. Gut. 1978;19:735–742. doi: 10.1136/gut.19.8.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lücke H., Berner W., Menge H., Murer H. Sugar transport by brush border membrane vesicles isolated from human small intestine. Pflügers Archiv. 1978;373:243–248. doi: 10.1007/BF00580831. [DOI] [PubMed] [Google Scholar]
- Maenz D.D., Engele-Schaan C.M. Methionine and 2-hydroxy-4-methylthiobutanoic acid are transported by distinct Na(+)-dependent and H(+)-dependent systems in the brush border membrane of the chick intestinal epithelium. J Nutr. 1996;126:529–536. doi: 10.1093/jn/126.2.529. [DOI] [PubMed] [Google Scholar]
- Malik G., Hoehler D., Rademacher M., Drew M.D., van Kessel A.G. Apparent absorption of methionine and 2-hydroxy-4-methylthiobutanoic acid from gastrointestinal tract of conventional and gnotobiotic pigs. Animal. 2009;3:1378–1386. doi: 10.1017/S1751731109990267. [DOI] [PubMed] [Google Scholar]
- Marciani P., Lindi C., Faelli A., Esposito G. Effects of semistarvation on transintestinal d-glucose transport and d-glucose uptake in brush border and basolateral membranes of rat enterocytes. Pflügers Archiv. 1987;408:220–223. doi: 10.1007/BF02181462. [DOI] [PubMed] [Google Scholar]
- Martin-Venegas R., Brufau M.T., Manas-Cano O., Mercier Y., Nonis M.K., Ferrer R. Monocarboxylate transporter 1 is up-regulated in caco-2 cells by the methionine precursor DL-2-hydroxy-(4-methylthio)butanoic acid. Vet J. 2014;202:555–560. doi: 10.1016/j.tvjl.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Martin-Venegas R., Brufau M.T., Mercier Y., Geraert P.A., Ferrer R. Intestinal cell conversion of DL-2-hydroxy-(4-methylthio) butanoic acid in vitro: dietary up-regulation by this methionine precursor. Br J Nutr. 2011;106:350–356. doi: 10.1017/S0007114511000183. [DOI] [PubMed] [Google Scholar]
- Martin-Venegas R., Geraert P.A., Ferrer R. Conversion of the methionine hydroxy analogue 2-hydroxy-4-(methylthio)butanoic acid to sulfur -containing amino acids in the chicken small intestine. Poult.Sci. 2006;85:1932–1938. doi: 10.1093/ps/85.11.1932. [DOI] [PubMed] [Google Scholar]
- Martin-Venegas R., Geraert P.A., Ferrer R. Partial Na+ Dependence of DL-2-hydroxy-4-(methylthio)butanoic acid uptake in the chicken small intestine. Poultry Sci. 2008;87:1392–1394. doi: 10.3382/ps.2007-00218. [DOI] [PubMed] [Google Scholar]
- Martin-Venegas R., Rodriguez-Lagunas M.J., Geraert P.-A., Ferrer R. Monocarboxylate transporter 1 mediates DL-2-Hydroxy-(4-methylthio)butanoic acid transport across the apical membrane of Caco-2 cell monolayers. J Nutr. 2007;137:49–54. doi: 10.1093/jn/137.1.49. [DOI] [PubMed] [Google Scholar]
- Martin-Venegas R., Soriano-Garcia J.F., Vinardell M.P., Géraert P.A., Ferrer R. Oligomers are not the limiting factor in the absorption of DL-2-hydroxy-4-(methylthio)butanoic acid in the chicken small intestine. Poultry Sci. 2006;85:56–63. doi: 10.1093/ps/85.1.56. [DOI] [PubMed] [Google Scholar]
- Mastrototaro L., Sponder G., Saremi B., Aschenback J.R. Gastrointestinal methionine shuttle: priority handling of precious goods. IUBMB Life. 2016;68:924–936. doi: 10.1002/iub.1571. [DOI] [PubMed] [Google Scholar]
- McCarthy K. Recent advances in the design and synthesis of carbon-14 labelled pharmaceuticals from small molecule precursors. CPD. 2000;6:1057–1083. doi: 10.2174/1381612003400029. [DOI] [PubMed] [Google Scholar]
- Merry B.J., Lewis S.E.M., Goldspink D.F. The influence of age and chronic restricted feeding on protein synthesis in the small intestine of the rat. Exp Gerontol. 1992;27:191–200. doi: 10.1016/0531-5565(92)90043-y. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Nakamura H., Hoshi T., Ganapathy V., Leibach F.H. Uphill transport of beta-alanine in intestinal brush-border membrane vesicles. Am J Physiol, Cell Physiol. 1990;259(3):G372–G379. doi: 10.1152/ajpgi.1990.259.3.G372. 25953°/G372–G379. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Tiruppathi C., Ganapathy V., Leibach F.H. Active transport of taurine in rabbit jejunal brush-border membrane vesicles. Am J Physiol, Cell Physiol. 1989;257:G65–G72. doi: 10.1152/ajpgi.1989.257.1.G65. [DOI] [PubMed] [Google Scholar]
- Munck B.G. Transport of neutral and cationic amino acids across the brush-border membrane of the rabbit ileum. J Membr Biol. 1985;83:1–13. doi: 10.1007/BF01868733. [DOI] [PubMed] [Google Scholar]
- Munck B.G., Schultz S.G. Interactions between leucine and lysine transport in rabbit ileum. Biochim Biophys Acta Biomembr. 1969;183:182–193. doi: 10.1016/0005-2736(69)90142-4. [DOI] [PubMed] [Google Scholar]
- Munck L.K., Grøndahl M.L., Skadhauge E. Beta-amino acid transport in pig small intestine in vitro by a high-affinity, chloride-dependent carrier. Biochim Biophys Acta Biomembr. 1995;1238:49–56. doi: 10.1016/0005-2736(95)00107-e. [DOI] [PubMed] [Google Scholar]
- Munck L.K., Grøndahl M.L., Thorboll J.E., Skadhauge E., Munck B.G. Transport of neutral, cationic and anionic amino acids by systems B, b(o,+), X(AG), and ASC in swine small intestine. Comp Biochem Physiol Part A Mol Integr Physiol. 2000;126:527–537. doi: 10.1016/s1095-6433(00)00227-0. [DOI] [PubMed] [Google Scholar]
- Muñíz R., Burguillo L., del Castillo J.R. Effect of starvation on neutral amino acid transport in isolated small-intestinal cells from Guinea pigs. Pflügers Archiv. 1993;423–423:59–66. doi: 10.1007/BF00374961. [DOI] [PubMed] [Google Scholar]
- Murer H., Gmaj P. Transport studies in plasma membrane vesicles isolated from renal cortex. Kidney Int. 1986;30(2):171–186. doi: 10.1038/ki.1986.169. [DOI] [PubMed] [Google Scholar]
- Naupert C., Rommel K. Absorption of short and medium chain fatty acids in the jejunum of the rat. Z Klin Chem Klin Biochem. 1975;13:553–562. doi: 10.1515/cclm.1975.13.12.553. [DOI] [PubMed] [Google Scholar]
- Nickel A., Kottra G., Schmidt G., Danier J., Hofmann T., Daniel H. Characteristics of transport of selenoamino acids by epithelial amino acid transporters. Chem Biol Interact. 2009;177:234–241. doi: 10.1016/j.cbi.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Palacín M. A new family of proteins (rBAT and 4F2hc) involved in cationic and zwitterionic amino acid transport. A tale of two proteins in search of a transport function. J Exp Biol. 1994;196:123–137. doi: 10.1242/jeb.196.1.123. [DOI] [PubMed] [Google Scholar]
- Pearce S.C., Mani V., Boddicker R.L., et al. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picariello G., Ferranti P., Addeo F. Use of brush border membrane vesicles to simulate the human intestinal digestion. Food Res Int. 2016;88:327–335. [Google Scholar]
- Prakash C., Gummerus J., Wilner K. Metabolism and excretion of a new antipsychotic drug, Ziprasidone, in humans. Drug Metab Dispos. 1997;25 863–000. [PubMed] [Google Scholar]
- Prasad P.D., Wang H., Huang W., Kekuda R., Rajan D.P., Leibach F.H., Ganapathy V. Human LAT1, a subunit of system L amino acid transporter. Molecular cloning and transport function. Biochem Biophys Res Commun. 1999;255:283–288. doi: 10.1006/bbrc.1999.0206. [DOI] [PubMed] [Google Scholar]
- Reig N., Chillarón J., Bartoccioni P., et al. The light subunit of system b(o,+) is fully functional in the absence of the heavy subunit. EMBO J. 2002;21:4906–4914. doi: 10.1093/emboj/cdf500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser S., Christiansen P.A. Stimulation of basic amino acid uptake by certain neutral amino acids in isolated intestinal epithelial cells. Biochim Biophys Acta. 1971;241:102–113. doi: 10.1016/0005-2736(71)90308-7. [DOI] [PubMed] [Google Scholar]
- Richards J.D., Atwell C.A., Vazquez-Anon M., Dibner J.J. Comparative in vitro and in vivo absorption of 2-hydroxy-4(methylthio) butanoic acid and methionine in the broiler chicken. Poultry Sci. 2005;84:1397–1405. doi: 10.1093/ps/84.9.1397. [DOI] [PubMed] [Google Scholar]
- Robertson J.S. In: Tritium turnover rates in mammals in Tritium. Moghissi A.A., Carter M.W., editors. Messenger Graphics; Phoenix, Arizona: 2013. pp. 314–321. [Google Scholar]
- Rosenborg J., Larsson P., Tegnér K., Hallström G. Mass balance and metabolism of [3H] formoterol in healthy men after combined i.v. and oral administration–mimicking inhalation. Drug Metab Dispos D. 1999;27:1104–1116. [PubMed] [Google Scholar]
- Rossier G., Meier C., Bauch C., Summa V., Sordat B., Verrey F., Kühn L.C. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J Biol Chem. 1999;274:34948–34954. doi: 10.1074/jbc.274.49.34948. [DOI] [PubMed] [Google Scholar]
- Rostagno H.S., Barbosa W.A. Biological efficacy and absorption of DL-methionine hydroxy analog free acid compared to DL-methionine in chickens as affected by heat stress. Br Poultry Sci. 1995;36:303–312. doi: 10.1080/00071669508417777. [DOI] [PubMed] [Google Scholar]
- Sabolic I., Burckhardt Gas. Effect of the preparation method on Na+-H+ exchange and ions permeabilities rat renal brush border membranes. Biochim Biophys Acta Biomembr. 1984;772:140–148. doi: 10.1016/0005-2736(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Saljoughian M., Williams P.G. Recent developments in tritium incorporation for radiotracer studies. Curr Pharmaceut Des. 2000;6:1029–1056. doi: 10.2174/1381612003399969. [DOI] [PubMed] [Google Scholar]
- Saroka J.M., Combs G.F. Studies of the renal excretion of the hydroxyl analogue of methionine by the chick. Poultry Sci. 1986;65:1375–1382. doi: 10.3382/ps.0651375. [DOI] [PubMed] [Google Scholar]
- Segawa H., Fukasawa Y., Miyamoto K.-I., Takeda E., Endou H., Kanai Y. Identification and functional characterization of a Na + -independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- Shaffer C.L., Gunduz M., Thornburgh B.A., Fate G.D. Using a tritiated compound to elucidate its preclinical metabolic and excretory pathways in vivo. Exploring tritium exchange risk. Drug Metab Dispos. 2006;34:1615–1623. doi: 10.1124/dmd.106.010934. [DOI] [PubMed] [Google Scholar]
- Sloan J.L., Mager S. Cloning and functional expression of a human Na(+) and Cl(-)-dependent neutral and cationic amino acid transporter B(0+) J Biol Chem. 1999;274:23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- Soriano-García J.F., Torras-Llort M., Ferrer R., Moretó M. Multiple pathways for L-methionine transport in brush-border membrane vesicles from chicken jejunum. J Physiol. 1998;509:527–539. doi: 10.1111/j.1469-7793.1998.527bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll B., Henry J., Reeds P.J., Hung Y., Jahoor F., Burrin D.G. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J Nutr. 1998;128:606–614. doi: 10.1093/jn/128.3.606. [DOI] [PubMed] [Google Scholar]
- Swennen Q., Geraert P., Mercier Y., Everaert N., Stinckens A., Willemsen H., Li Y., Decuypere E., Buyse J. Effects of dietary protein content and 2-hydroxy-(4-methylthio) butanoic acid or DL-methionine supplementation on performance and oxidative status of broiler chickens. Br J Nutr. 2011;106:1845–1854. doi: 10.1017/S0007114511002558. [DOI] [PubMed] [Google Scholar]
- ter Kuile B.H., Cook M. The kinetics of facilitated diffusion followed by enzymatic conversion of the substrate. Biochim Biophys Acta. 1994;1193:235–239. doi: 10.1016/0005-2736(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Torrents D., Estévez R., Pineda M., et al. Identification and characterization of a membrane protein (y+L Amino Acid transporter 1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J Biol Chem. 1998;273:32437–32445. doi: 10.1074/jbc.273.49.32437. [DOI] [PubMed] [Google Scholar]
- To V.P.T.H., Subramaniam M., Masagounder K., Loewen M.E. Characterization of the segmental transport mechanisms of DL-methionine hydroxy analogue along the intestinal tract of rainbow trout with an additional comparison to DL-methionine. Comp Biochem Physiol Part A Mol Integr Physiol. 2020;249 doi: 10.1016/j.cbpa.2020.110776. [DOI] [PubMed] [Google Scholar]
- Torrents D., Mykkänen J., Pineda M., et al. Identification of SLC7A7, encoding y+LAT-1, as the lysinuric protein intolerance gene. Nat Genet. 1999;21:293–296. doi: 10.1038/6809. [DOI] [PubMed] [Google Scholar]
- Ugolev A.M., Bagiian A.A., Ekkert L.G. New method of studying membrane hydrolysis and transport as well as metabolic processes in the small intestine in vitro (everted small intestinal sac with bilateral oxygenation) Fiziol Zh. 1980;66:1674–1677. [PubMed] [Google Scholar]
- Utsunomiya-Tate N., Endou H., Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- van Weerden E.J., Schutte J.B., Bertram H.L. Utilization of the polymers of methionine hydroxy analogue free acid (MHA-FA) in broiler chicks. Archiv Geflügelkunde. 1992;56:63–68. [Google Scholar]
- Verma N., Kansal V.K. Characterisation of the routes of methionine transport in mouse mammary glands. Indian J Med Res. 1993;98:297–304. [PubMed] [Google Scholar]
- von Engelhardt W., Rönnau K., Rechkemmer G., Sakata T. Absorption of short-chain fatty acids and their role in the hindgut of monogastric animals. Anim Feed Sci Technol. 1989;23:43–53. [Google Scholar]
- Wagner C.A., Lang F., Bröer S. Function and structure of heterodimeric amino acid transporters. Am J Physiol, Cell Physiol. 2001;281:C1077–C1093. doi: 10.1152/ajpcell.2001.281.4.C1077. [DOI] [PubMed] [Google Scholar]
- Walter A., Gutknecht J. Monocarboxylic acid permeation through lipid bilayer membranes. J Membr Biol. 1984;77:255–264. doi: 10.1007/BF01870573. [DOI] [PubMed] [Google Scholar]
- Webb J. Intestinal absorption of protein hydrolysis products: a review. J Anim Sci. 1990;68:3011–3022. doi: 10.2527/1990.6893011x. [DOI] [PubMed] [Google Scholar]
- Wellner D., Meister A. A Survey of inborn errors of amino acid metabolism and transport in man. Annu Rev Biochem. 1981;50:911–968. doi: 10.1146/annurev.bi.50.070181.004403. [DOI] [PubMed] [Google Scholar]
- Wells R.G., Hediger M.A. Cloning of a rat kidney cDNA that stimulates dibasic and neutral amino acid transport and has sequence similarity to glucosidases. Proc Natl Acad Sci USA. 1992;89:5596–5600. doi: 10.1073/pnas.89.12.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen H., Swennen Q., Everaert N., Geraert P.A., Mercier Y., Stinckens A., Decuypere E., Buyse J. Effects of dietary supplementation of methionine and its hydroxy analog DL-2- hydroxy-4-(methylthio)butanoic acid on growth performance, plasma hormone levels, and the redox status of broiler chickens exposed to high temperatures. Poultry Sci. 2011;90:2311–2320. doi: 10.3382/ps.2011-01353. [DOI] [PubMed] [Google Scholar]
- Wilson T.H., Wiseman G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol. 1954;123:116–125. doi: 10.1113/jphysiol.1954.sp005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida O., Kanai Y., Chairoungdua A., et al. Human L-type amino acid transporter 1 (LAT1). Characterization of function and expression in tumor cell lines. Biochim Biophys Acta Biomembr. 2001;1514:291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- Yi H., Xiong Y., Wu Q., Wang M., Liu S., Jiang Z., Wang L. Effects of dietary supplementation with l-arginine on the intestinal barrier function in finishing pigs with heat stress. J Anim Physiol Anim Nutr. 2020;104:1134–1143. doi: 10.1111/jpn.13277. [DOI] [PubMed] [Google Scholar]
- Yodseranee R., Bunchasak C. Effects of dietary methionine source on productive performance, blood chemical, and hematological profiles in broiler chickens under tropical conditions. Trop Anim Health Prod. 2012;44:1957–1963. doi: 10.1007/s11250-012-0164-7. [DOI] [PubMed] [Google Scholar]
- Zhang S., Wong E.A., Gilbert E.R. Bioavailability of different dietary supplemental methionine sources in animals. Gene. 2015;7:478–490. doi: 10.2741/E744. [DOI] [PubMed] [Google Scholar]
- Zheng L., Chen J., Zhu Y., Yang H., Elmquist W., Hu M. Comparison of the transport characteristics of D- and L-methionine in a human intestinal epithelial model (Caco-2) and in a perfused rat intestinal model. Pharm Res. 1994;11:1771–1776. doi: 10.1023/a:1018923618747. [DOI] [PubMed] [Google Scholar]