Abstract
Background
This study aimed to investigate the association between the extent and severity of coronary atherosclerosis, epicardial adipose tissue (EAT) accumulation, and left ventricular (LV) global longitudinal strain (GLS) in patients with preserved LV ejection fraction (LVEF) and without LV regional wall motion abnormalities.
Methods
This study included 169 preserved LVEF patients without LV wall motion abnormalities who underwent coronary computed tomography (CT) angiography for the assessment of suspected coronary artery disease (CAD). The segment stenosis score (SSS) and segment involvement score (SIS) were calculated to evaluate CAD extent. The EAT volume was defined as CT attenuation values ranging from −250 to −30 HU within the pericardial sac. LVGLS was measured using echocardiography to assess subclinical LV dysfunction.
Results
All patients had preserved LVEF of ≥50%, and the mean LVGLS was −18.7% (−20.5% to −16.9%). Mean SSS and SIS were 2.0 (0–5) and 4.0 (0–36), respectively, while mean EAT volume was 116.1 mL (22.9–282.5 mL). Multivariate analysis using linear regression model demonstrated that LVEF (β, −17.0; 95% CI, −20.9 − -13.1), LV mass index (β, 0.03; 95% CI, 0.01–0.06), and EAT volume (β, 0.010; 95% CI, 0.0020–0.0195) were independently associated with LVGLS; however, obstructive CAD was not. The multivariate models demonstrated that SSS (Î, 0.12; 95% CI, 0.05–0.18) and SIS (Î, 0.27; 95% CI, 0.10–0.44) were correlated with deterioration of LVGLS, independent of other parameters.
Conclusion
This study demonstrates that EAT volume and CAD extent are associated with the deterioration of LVGLS in this population.
Keywords: Coronary atherosclerosis, Epicardial adipose tissue, Coronary computed tomography angiography, Heart failure, Longitudinal strain
1. Introduction
Coronary artery disease (CAD) is a progressive disease that contributes to the development of acute coronary syndrome (ACS) and heart failure [1]. CAD is the most common cause of heart failure, both with preserved and reduced ejection fractions, in the US adult population [2], [3], [4]. The importance of coronary atherosclerosis in relevant underlying conditions, such as left ventricular (LV) dysfunction, is increasingly being recognized [5], [6]. CAD is a risk factor for incident heart failure with preserved ejection fraction (HFpEF) after adjusting for common cardiovascular comorbidities, and this risk is partially explained by left ventricular diastolic dysfunction [7]. Left ventricular global longitudinal strain (LVGLS), assessed using echocardiography, is a sensitive marker of subclinical LV dysfunction which precedes the deterioration of LV function and heart failure [8], [9]. Furthermore, the presence of regional asynergy can cause deterioration of the LVGLS, which also allows the assessment of LV wall motion abnormalities [10]. Nevertheless, there is limited knowledge on the relationship between CAD extent and severity and subclinical LV dysfunction in preserved LVEF patients without LV wall motion abnormalities (see Fig. 1).
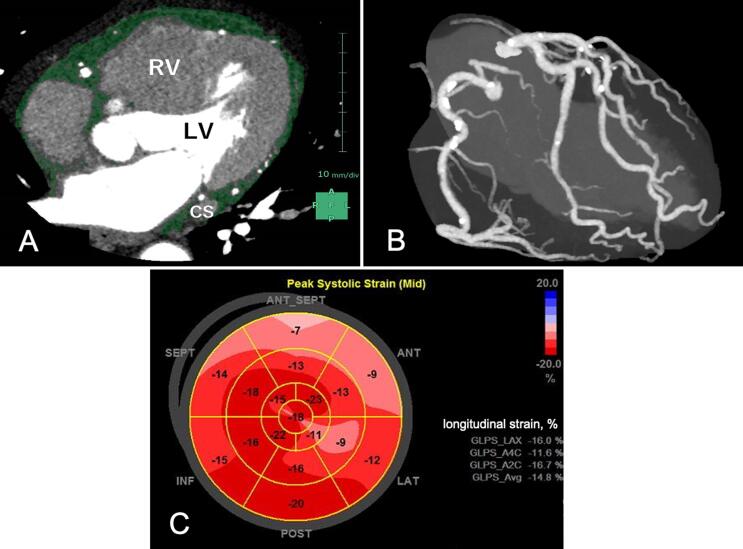
Fig. 1.
A representative case of increased EAT volume with coronary atherosclerosis and impaired LVGLS. A 61-year male with obstructive CAD and increased EAT volume (A) and CAD extent (B) showing impaired LVGLS (C). (A) EAT volume, 194 mL; (B) nonobstructive CAD with a CACS of 440.6 Agatston units; (C) impaired LVGLS, −14.8%; LVGLS was calculated by averaging the negative peak strain from 18 ventricular segments from the apical 4-chamber, 2-chamber, and long-axis views.
Coronary computed tomography angiography (CCTA) is widely used in clinical practice as a first-line test for symptomatic or asymptomatic patients at risk of obstructive CAD, allowing noninvasive assessment of CAD severity and extent [11]. In addition, CCTA enables the quantification of epicardial adipose tissue (EAT) volume, which is closely related to the development of CAD, atrial fibrillation, and heart failure, with or without preserved LV ejection fraction (LVEF) [12], [13], [14], [15], [16]. Recent clinical studies have demonstrated that EAT volume is associated with CAD severity and coronary microvascular dysfunction (CMD) [17]. These findings suggest that the EAT may play a pivotal role in the development of subclinical LV dysfunction in patients with CAD. In this CCTA study, we aimed to investigate the association between structural features, including CAD severity and extent, and EAT volume with LVGLS in patients with preserved LVEF without LV wall motion abnormalities.
2. Methods
2.1. Study population
The ethics committee of Kashibaseiki Hospital approved the study protocol (2021-F), and each patient provided written informed consent before the CCTA examination, which was conducted in compliance with the protocol and the Declaration of Helsinki. This study was a retrospective analysis from our database, consisting of patients with suspected CAD who underwent CCTA examination between April 2017 and December 2019. We identified consecutive symptomatic patients who underwent CCTA and two-dimensional transthoracic Doppler echocardiography (TTDE) (n = 402). The exclusion criteria for this study were as follows: (1) ACS (n = 19), (2) previous history of myocardial infarction (n = 10) or coronary revascularization (n = 79), (3) LVEF < 50% or abnormal LV wall motion abnormality (n = 105), (4) atrial fibrillation (n = 15), and (5) poor image quality (n = 5). The final study population included 169 patients with preserved LVEF but without focal LV wall motion abnormalities or known CAD.
2.2. Echocardiography and global longitudinal strain
TTDE examination was performed before CCTA examination in all study patients. All TTDE examinations were performed in a standard manner by experienced cardiac echo sonographers using the Vivid S70 (General Electric, Milwaukee, WI, USA) under continuous ECG monitoring [18]. LVEF was calculated using apical 2-chamber and 4-chamber views. Mitral inflow was assessed using pulse-wave Doppler to measure the early (E) and late (A) peak velocities. Early diastolic mitral annular velocity (e′) was also measured using tissue Doppler imaging in the septal wall, providing the ratio of E to e′ (E/e′).
Measurements of the longitudinal strain at the peak systolic phase were obtained using grayscale images recorded in the apical 4-chamber, 2-chamber, and long-axis views. The LVGLS was analyzed offline using a dedicated software. For the accurate evaluation of LVGLS, sonographers ensured that TTDE images clearly detected the endocardial border throughout the cardiac cycle. The LV endocardial border was manually traced, and the software algorithm automatically divided the LV apical view into six segments for speckle tracking throughout the cardiac cycle. The LVGLS was obtained by averaging all segmental strain values from the apical 4-chamber, 2-chamber, and long-axis views [9]. All echocardiographic measurements were performed based on the recommendations of the American Society of Echocardiography by two independent cardiologists (Y.K. and K.H.) who were blinded to the patient demographics or CCTA results.
2.3. CCTA image acquisition and analysis
All CCTA examinations were performed using 320-raw multidetector computed tomography (Aquilion ONE/NATURE Edition; Canon Medical Systems, Inc., Japan) [19]. The scan parameters were detector collimation of 0.5 × 320 mm, gantry rotation time of 0.35 sec, tube voltage of 120 kV, and tube current of 130–600 mA. An electrocardiogram-triggered prospective gating method was used for CCTA imaging.
The Agatston method was used to assess coronary artery calcium score (CACS) at a fixed thickness of 3 mm [20]. A bolus tracking method was used for image acquisition, in which a nonionic contrast medium of 270 mg I/kg was administered using a power injector. A region of interest (ROI) was placed in the ascending aorta at the bronchial bifurcation level. When the computed tomography (CT) value of the ROI exceeded 150 Hounsfield units (HU), ECG-synchronized scans were performed within a single breath-hold. Images were reconstructed for coronary artery analysis with a cross-sectional thickness of 0.5 mm and reconstruction increment of 0.25 mm.
All CCTA image analyses were performed using a dedicated software (VINCENT, Fujifilm Inc., Tokyo, Japan). CACS was categorized into four categories as follows: 0, 1–99, 100–399, and >400 Agatston units [21]. Coronary luminal stenosis was reported based on a 16-segment AHA model by 2 observers (K.O. and H.I.). To evaluate the extent and severity of CAD, segment stenosis score (SSS) and segment involvement score (SIS) were calculated [20]. Briefly, using 16 segments, we measured the percentage of luminal stenosis in images with plaques on CPR short-axis images and classified luminal stenosis as grade 1 to grade 5. The SIS score ranged from 0 to 16 points, whereas the SSS ranged from 0 to 80 points. Obstructive CAD was defined as the presence of coronary plaques with ≥50% luminal stenosis of one or more major epicardial vessels and/or ≥50% luminal stenosis of the left main coronary segment. Non-obstructive CAD was defined as patients with <50% luminal stenosis of the epicardial coronary arteries. Otherwise, the patients were categorized as not having CAD.
2.4. EAT volume measurement
EAT volume was measured from contrast-enhanced CT images using Synapse Vincent software (Fujifilm Inc.) [19]. To measure EAT volume, we extracted multiple equidistant axial planes according to the size of each heart. The upper limit of the slice was at the bifurcation of the pulmonary artery trunk and the lower slice contained any structure of the heart. In each plane, the software automatically detected a smooth, closed pericardial contour as the region of interest, where the software automatically identified adipose tissue with CT attenuation values ranging from −250 to −30 HU within the pericardial sac. The EAT volume was calculated as the sum of the EAT areas.
2.5. Statistical analysis
Statistical analysis was performed using R software package (version 3.2.1; R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were reported as counts (percentage), and continuous variables were reported as mean (SD) or median (interquartile range, IQR). Statistical significance was assessed using the chi-square test, Fisher’s exact test, Student’s t-test, Kruskal-Wallis test, or Mann-Whitney U test, as appropriate. A one-way analysis of variance (ANOVA) was used for multiple group comparisons. Correlations between variables were analyzed using Pearson’s correlation test. Univariate and multivariate linear regression analyses were performed to investigate the association between echocardiographic and CCTA findings and the LVGLS. After adjusting for age, LVEF, LV mass index, and EAT volume, the parameters included in the models were obstructive CAD, CACS, SIS, and SSS. The CACS was log-transformed for the analysis. Statistical significance was set at a 2-sided P value of < 0.05.
3. Results
All patients had preserved LVEF of ≥ 50%, but without LV regional wall motion abnormalities. Table 1 shows the characteristics of all the study patients (n = 169). The mean age of the study patients was 67 ± 13.6 years and 59% were male. Approximately 70% of the patients had hypertension and dyslipidemia and 25% had diabetes mellitus.
Table 1.
Baseline characteristics.
| n = 169 | |
|---|---|
| Age, n (%) | 67 (53–75) |
| Male, n (%) | 100 (59%) |
| Smoking, n (%) | 36 (21%) |
| Body mass index, kg/m2 | 23.2 (21.4–25.6) |
| Systolic blood pressure, mmHg | 139 (127–155) |
| Diastolic blood pressure, mmHg | 78 (70–89) |
| Heart rate, bpm | 73 (66–82) |
| Comorbidity | |
| Hypertension, n (%) | 121 (72%) |
| Diabetes, n (%) | 42 (25%) |
| Dyslipidemia, n (%) | 120 (71%) |
| Medication at index CCTA examination | |
| Calcium channel blocker, n (%) | 58 (34%) |
| ACE inhibitor or ARB, n (%) | 38 (23%) |
| β-blocker, n (%) | 7 (4.1%) |
| Statin, n (%) | 44 (26%) |
| Laboratory tests | |
| Hemoglobin, g/dl | 13.9 ± 2.3 |
| eGFR, ml/min/1.73 m2 | 50.6 ± 20.8 |
| hs-CRP, g/dl | 0.03 ± 0.68 |
| hs-Troponin T, pg/ml | 2.0 (1.0–4.8) |
| LDL-cholesterol, mg/dl | 123 (100–141) |
| HDL-cholesterol, mg/dl | 65 (51–73) |
| Triglyceride, mg/dl | 134 (81–161) |
| Values are presented mean ± standard deviation, or median (inter-quartile range), or n (%). ACE; angiotensin convert enzyme, ARB; angiotensin type1 receptor blocker, eGFR; estimated glomerular filtration rate LDL; Low Density Lipoprotein HDL; High Density Lipoprotein | |
The TTDE parameters are listed in Table 2. All patients had a preserved LVEF ≥ 50%, and the mean LVEF was 61% (53–67%). Mean LVGLS was −18.7% (-20.5% – −16.9%). The CCTA results are listed in Table 2. On CCTA, 53 patients (31.3 %) had no visible CAD. For the remaining patients, non-obstructive CAD (1% to 49% stenosis) was present in 66 patients (39.0%), whereas obstructive CAD, defined as the presence of ≥ 50% diameter stenosis, was detected in 50 patients (29.6%). Among the patients with obstructive CAD, 10 (5.9%) exhibited ≥ 50% stenosis in multivessel CAD. Mean SSS and SIS were 2.0 (0–5) and 4.0 (0–36), respectively, while mean EAT volume was 116.1 mL (22.9–282.5 mL).
Table 2.
Echocardiography and CCTA findings.
| TTDE findings | |
|---|---|
| LVEF, % | 61 (53–67) |
| LVEDD, mm | 45.6 (34.3–56.1) |
| LVESD, mm | 28.6 (15.7–39.0) |
| IVSWT, mm | 8.3 (7.6–9.1) |
| LVPWT, mm | 8.4 (7.5–9.0) |
| LA, mm | 36.1 (32.7–39.7) |
| LVMI, g/mm2 | 61 (53–67) |
| Dct, msec | 209.5 (175.4–242.7) |
| E/A ratio | 0.91 (0.74–1.2) |
| E/e’ ratio | 9.93 (8.25–11.9) |
| TRPG, mmHg | 20.6 (15.7–25.0)) |
| LVGLS, % | −18.7 (-20.5 – −16.9) |
| CCTA and abdominal CT findings | |
| CACS | 23,2 (0–4020) |
| 0, n (%) | 70 (41%) |
| 1–99, n (%) | 46 (27%) |
| 100–399, n (%) | 30 (18%) |
| >400, n (%) | 23 (14%) |
| Non obstructive CAD, n (%) | 85 (50%) |
| Obstructive CAD, n (%) | 44 (26%) |
| LMCA, n (%) | 7 (4.1%) |
| LAD, n (%) | 34 (20%) |
| LCX, n (%) | 17 (10%) |
| RCA, n (%) | 12 (7.1%) |
| Multi-vessel CAD | 10 (5.9) |
| SSS | 2.0 (0–5) |
| SIS | 4.0 (0–36) |
| EAT volume (ml) | 116.1 (22.9–282.5) |
| Visceral adipose tissue area (ml) | 93.7 (9.4–244.5) |
| Subcutaneous adipose tissue area (ml) | 137.8 (3.6–709.0) |
| Values are presented mean ± standard deviation, or median (inter-quartile range), or n (%). LVEF; left ventricular ejection fraction LVEDd; left ventricular end-diastolic diameter LVESD; left ventricular end-systolic diameter IVSWT; Interventricular septum wall thickness LVPWT; left ventricular posterior wall thickness LA; Left Atrial LVMI; Left ventricular mass index Dct; Deceleration time TRPG; tricuspid regurgitant pressure gradient GLS; Global longitudinal strain CAD; coronary artery disease SSS; stenosis severity score SIS; segment involvement score EATV; Epicardial adipose tissue volume | |
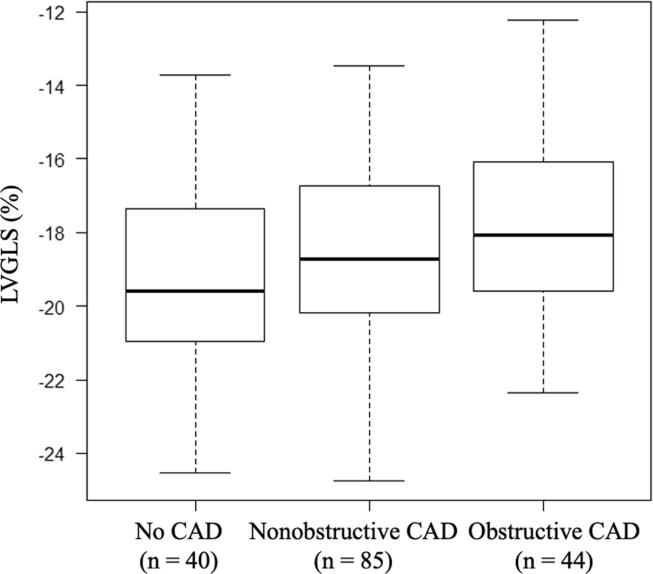
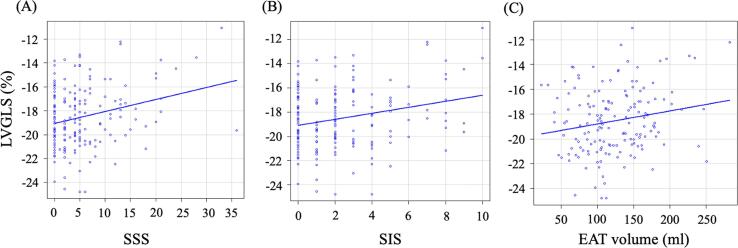
Fig. 2 shows the LVGLS values stratified by CAD categories. LVGLS tended to be higher in obstructive CAD (-17.9 ± 2.7 %) and even in nonobstructive CAD (-18.4 ± 2.6%) as compared to that of no CAD patients (-19.3 ± 2.4%) (p = 0.050, one-way ANOVA). We evaluated the correlations between the LVGLS and SSS, SIS, and EAT volumes (Fig. 3). Pearson’s correlation analysis showed that there was a significant correlation between LVGLS and SSS (r = 0.25, p = 0.001), SIS (r = 0.24, p = 0.002), and EAT volume (r = 0.19, p = 0.017). There were no significant correlations between the location of obstructive CAD and LVGLS.
Fig. 2.
Association of LVGLS with patients with CAD stratified by CAD categories.
Fig. 3.
Correlations between CAD extent or EAT volume and LVGLS. Spearman correlation demonstrated a significant correlation between SSS and LVGLS (A), between SIS and LVGLS (B), and between EAT volume and LVGLS (C).
Univariate linear regression analysis demonstrated that there was no significant association of hypertension (β, 0.28; 95% confidence interval [CI], −0.69–1.25; p = 0.57), diabetes mellitus (β, 0.91; 95%CI, −0.01–1.83; p = 0.054), dyslipidemia (β, 0.79; 95%CI, −0.11–1.70; p = 0.08) or chronic kidney disease (β, −0.09; 95%CI, −1.01–0.81; p = 0.83) with LVGLS. Table 3 shows the univariate and multivariate linear regression analyses for the relationship between LVGLS and clinical parameters, TTDE, and CCTA findings. In the multivariable model, LVEF, LV mass index, and EAT volume were independently associated with LVGLS; however, obstructive CAD was not (Model 1 in Table 3). In contrast, the multivariable models demonstrated that SSS (model 3) and SIS (model 4) were positively correlated with LVGLS, independent of other parameters.
Table 3.
Linear regression models for determinants of LVGLS.
| Univariate model | Multivariable model 1 | Multivariable model 2 | Multivariable model 3 | Multivariable model 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Beta (95%CI) | P | Beta (95%CI) | P | Beta (95%CI) | P | Beta (95%CI) | P | Beta (95%CI) | P |
| LVEF, per 1% increase | −7.04 (-12.34–-1.75) | 0.009 | −17.0 (−20.9 − -13.1) | 0.015 | −6.50 (-11.87–1.13) | 0.017 | −6.04 (-11.21 − -0.87) | 0.022 | −6.08 (11.2–0.87) | 0.022 |
| Age, per 1 year increase | −0.030 (-0.061–-0.0002) |
0.050 | −0.02 (-0.05–0.005) | 0.103 | −0.04 (-0.07 − -0.004) | 0.029 | −0.04 (-0.07 − -0.012) | 0.006 | −0.04 (-0.07–0.01) | 0.007 |
| Male | 1.696 (0.88–2.51) | <0.001 | 1.06 (0.20–1.92) | 0.016 | 0.99 (0.13–1.85) | 0.024 | 0.68 (0.16–1.53) | 0.112 | 0.74 (-0.10–1.60) | 0.085 |
| LVMI, per 1 g/m2 increase | 0.042 (0.0168–0.067) | 0.001 | 0.03 (0.01–0.06) | 0.002 | 0.034 (0.01–0.05) | 0.005 | 0.03 (0.01–0.05) | 0.003 | 0.03 (0.01–0.05) | 0.003 |
| EAT volume, per 1 mL increase | 0.011 (0.002–0.019) | 0.015 | 0.010 (0.002–0.0195) | 0.021 | 0.01(0.001–0.019) | 0.024 | 0.009 (0.0005–0.01) | 0.039 | 0.009 (0.0004–0.01) | 0.039 |
| Obstructive CAD | 0.368 (-0.552–1.288) | 0.430 | 0.54 (-0.33–1.42) | 0.224 | ||||||
| Log (CACS + 1) | 0.230 (-0.139–0.600) |
0.220 | 0.35 (−0.06–0.76) | 0.097 | ||||||
| SSS, per 1 increase | 0.115 (0.054–0.176) | <0.001 | 0.12 (0.05–0.18) | <0.001 | ||||||
| SIS, per 1 increase | 0.297 (0.137–0.457) | <0.001 | 0.27 (0.10–0.44) | 0.001 | ||||||
| CAD; Coronary artery disease SSS; stenosis severity score SIS; segment involvement score EATV; Epicardial adipose tissue volume LVMI; Left ventricular mass index | ||||||||||
4. Discussion
In this study, using CCTA and TTDE, we found a significant association between EAT volume and LVGLS assessed using speckle echocardiography in patients with preserved LVEF without LV regional wall motion abnormalities. Furthermore, semi-quantification of CAD extent or severity was significantly associated with LVGLS, even after adjusting for age, EAT volume, LVEF, and LV mass index. Our observations indicate that assessment of both the extent of CAD and EAT volume is crucial for risk stratification of patients at a higher risk of LV dysfunction in this population.
4.1. CAD as a cause of LV dysfunction
The mechanisms mediating the increased risk of subclinical LV dysfunction are multifactorial. It has been well established that CAD is an underlying cause of not only heart failure with reduced ejection fraction (HFrEF) but also heart failure with preserved ejection fraction (HFpEF) [5], [6], [22]. Hwong et al. demonstrated that patients with HFpEF with angiographically proven CAD exhibited a higher risk of LV function deterioration than those without [22]. However, there is limited knowledge regarding whether CAD is associated with subclinical LV dysfunction.
Several potential mechanisms have been proposed to explain the association between CAD and subclinical LV dysfunctions [7]. A recent study conducted by Rush et al. demonstrated that the majority of patients with HFpEF exhibited epicardial obstructive CAD, CMD, or both [23]. Stankovic et al. demonstrated that critical coronary stenosis can cause LVGLS deterioration [10]. In line with this, our findings expand previous knowledge of the association between CAD, even in nonobstructive CAD, and subclinical LV dysfunction by highlighting the importance of the extent and severity of CAD. Impairment of LVGLS in patients with nonobstructive CAD may be explained by CMD, which is well correlated with LVGLS [24], [25]. Identifying patients with nonobstructive CAD who are at a higher risk of cardiovascular events and heart failure has attracted attention. Nakanishi et al. demonstrated that impairment of the coronary flow reserve, a marker of CMD, provides prognostic value for ACS and heart failure in patients without obstructive CAD in the left ascending coronary artery on CCTA [26]. Given the technical limitations of noninvasive assessment of CMD and the advantages of widespread CCTA imaging in daily clinical practice, CAD extent may offer an explanation for subclinical LV dysfunction in the pre-heart failure population.
4.2. Markers in identifying subclinical LV dysfunction
Numerous studies have shown that EAT plays a key role in the pathophysiology of cardiovascular diseases [12], [19]. Maimaituxun et al. demonstrated that increased EAT volume is associated with LVGLS impairment (≤18%) [27]. Although the underlying mechanisms between EAT accumulation and LV dysfunction remain unclear, increased inflammation, fibrosis, and autonomic dysregulation have been implicated as potential mechanisms linking EAT to LV dysfunction [28]. LVGLS assessed by speckle-tracking echocardiography is a marker of subclinical LV dysfunction, with diagnostic and prognostic implications for pre-heart failure [8], [9].
A key challenge is the identification of patients at a higher risk of developing LV dysfunction leading to heart failure. In our study, comorbidities were not found to be associated with LVGLS deterioration. The biomarker or imaging marker approach is an interesting perspective for understanding the potential mechanisms of epicardial fat accumulation and myocardial fibrosis and the risk stratification of patients with HFpEF [12], [13], [14]. Seo et al. demonstrated that HFpEF patients with CAD had greater deterioration in left ventricular ejection fraction and mitral annulus early diastolic velocity (e’), indicating that myocardial ischemia with a positive stress test may contribute to greater deterioration of diastolic dysfunction [29]. Harada et al. have shown that levels of serum fatty-acid binding protein 4, which is highly expressed in adipocytes and secreted in response to lipolytic signals, correlate with cardiac structural and functional abnormalities in patients with HFpEF, providing prognostic implications [30]. Further studies are required to investigate the clinical significance of LVGLS in association with biomarkers in patients with CAD leading to HFpEF.
4.3. Therapeutic implication for identification of subclinical LV dysfunction
Over the past few decades, large clinical studies have shown that pharmacological therapies, including renin-angiotensin system inhibitors, β-blockers, sodium-glucose co-transporter (SGLT)-2 inhibitors, mineral corticoid receptor antagonists, and angiotensin receptor-neprilysin inhibitors, are effective in the treatment of patients with HFrEF. However, pharmacological therapeutic strategies for HFpEF are limited [31]. Sato et al. demonstrated that SGLT2 inhibitors can reduce EAT volume with a correlation between EAT volume, body weight, and inflammatory biomarkers [32]. Considering the significant associations between EAT, CAD extent and severity, and subclinical LV dysfunction, our observations suggest that EAT could serve as a potential therapeutic target to prevent subclinical LV dysfunction leading to cardiovascular events. Further studies are necessary to investigate whether medical therapy targeting the EAT can prevent the deterioration of LV function and cardiovascular events in this population.
4.4. Study limitations
This study had some limitations. First, there may have been selection bias because this was an observational study consisting of a relatively small number of patients who underwent CCTA and TTDE. Previous studies have demonstrated that HFpEF includes multiple etiologies of heart failure. Phenotyping of the disease according to etiology can provide further insights into the pathophysiology and management of HFpEF [16]. Furthermore, our study consisted of patients with normal weight/overweight rather than obese patients who are well known to have HFpEF [33]. Second, this study did not include patients with atrial fibrillation who were not suitable for LVGLS assessment using speckle-tracking echocardiography. In addition, focal LV wall motion abnormalities contribute to the impairment of LVGLS, and this study did not include patients with focal asynergy of the LV [10]. Therefore, our observations cannot be generalized to patients with atrial fibrillation or regional wall motion abnormalities caused by severe coronary luminal stenosis. Finally, CMD, especially HFpEF, was not assessed as a potent cause of subclinical HF in the present study. CMD can account for up to two-thirds of symptomatic ischemic heart disease patients without epicardial coronary artery stenosis [16]. Löffler et al. demonstrated that HFpEF patients had a higher prevalence of CMD and diffuse fibrosis with decreased exercise tolerance than healthy controls, although LVGLS tended to be higher in the latter [34]. Further prospective studies are warranted to comprehensively assess the causal effects of CAD extent, EAT accumulation, and CMD on heart failure development.
5. Conclusion
The present study demonstrates that EAT volume and CAD extent, even in the less advanced stage of atherosclerosis, can influence subclinical LV function. CCTA may help to investigate the underlying causes of subclinical LV dysfunction in patients with preserved LVEF without LV wall motion abnormalities.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
of grant support: none.
Any potential conflicts of interest, including related consultancies, shareholdings and funding grants: none
References
- 1.Knuuti J., Wijns W., Saraste A., Capodanno D., Barbato E., Funck-Brentano C., Prescott E., Storey R.F., Deaton C., Cuisset T., Agewall S., Dickstein K., Edvardsen T., Escaned J., Gersh B.J., Svitil P., Gilard M., Hasdai D., Hatala R., Mahfoud F., Masip J., Muneretto C., Valgimigli M., Achenbach S., Bax J.J. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndrome. Eur. Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D.M., Larson M.G., Leip E.P., Beiser A., D’Agostino R.B., Kannel W.B., Murabito J.M., Vasan R.S., Benjamin E.J., Levy D. Lifetime risk for developing congestive heart failure: the framingham heart study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 3.Lala A., Desai A.S. The role of coronary artery disease in heart failure. Heart Failure. Clin. 2014;10:353–365. doi: 10.1016/j.hfc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., Djousse L., Elkind M.S.V., Ferguson J.F., Fornage M., Khan S.S., Kissela B.M., Knutson K.L., Kwan T.W., Lackland D.T., Lewis T.T., Lichtman J.H., Longenecker C.T., Loop M.S., Lutsey P.L., Martin S.S., Matsushita K., Moran A.E., Mussolino M.E., Perak A.M., Rosamond W.D., Roth G.A., Sampson U.K.A., Satou G.M., Schroeder E.B., Shah S.H., Shay C.M., Spartano N.L., Stokes A., Tirschwell D.L., VanWagner L.B., Tsao C.W., Wong S.S., Heard D.G. Heart disease and stroke statistics—2020 update: a report from the American Heart Association, 2020. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 5.Maragiannis D., Schutt R.C., Gramze N.L., Chaikriangkrai K., McGregor K., Chin K., Nabi F., Little S.H., Nagueh S.F., Chang S.M. Association of left ventricular diastolic dysfunction with subclinical coronary atherosclerotic disease burden using coronary artery calcium scoring. Thromb. 2015;22:1278–1286. doi: 10.5551/jat.29454. [DOI] [PubMed] [Google Scholar]
- 6.Dunlay S.M., Roger V.L., Redfield M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 7.John J.E., Claggett B., Skali H., Solomon S.D., Cunningham J.W., Matsushita K., Konety S.H., Kitzman D.W., Mosley T.H., Clark D., Chang P.P., Shah A.M. Coronary artery disease and heart failure with preserved ejection fraction: the ARIC study. J. Am. Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.021660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.T. Kuznetsova, N. Cauwenberghs, J. Knez, W.-Y. Yang, L. Herbots, J. D’hooge, F. Haddad, L. Thijs, J.-U. Voigt J.A. Staessen, Additive prognostic value of left ventricular systolic dysfunction in a population-based cohort, Circ. Cardiovasc. Imag. 9 (2016). <https://doi.org/10.1161/CIRCIMAGING.116.004661>. [DOI] [PubMed]
- 9.Park J.J., Park J.B., Park J.H., Cho G.Y. Global longitudinal strain to predict mortality in patients with acute heart failure. J. Am. Coll. Cardiol. 2018;71:1947–1957. doi: 10.1016/j.jacc.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 10.Stankovic I., Putnikovic B., Cvjetan R., Milicevic P., Panic M., Kalezic-Radmili T., Mandaric T., Vidakovic R., Cvorovic V., Neskovic A.N. Visual assessment vs. strain imaging for the detection of critical stenosis of the left anterior descending coronary artery in patients without a history of myocardial infarction. Eur. Heart J. Cardiovasc. Imag. 2015;16:402–409. doi: 10.1093/ehjci/jeu206. [DOI] [PubMed] [Google Scholar]
- 11.Abdelrahman K.M., Chen M.Y., Dey A.K., Virmani R., Finn A.V., Khamis R.Y., Choi A.D., Min J.K., Williams M.C., Buckler A.J., Taylor C.A., Rogers C., Samady H., Antoniades C., Shaw L.J., Budoff M.J., Hoffmann U., Blankstein R., Narula J., Mehta N.N. Coronary computed tomography angiography from clinical uses to emerging technologies: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;76:1226–1243. doi: 10.1016/j.jacc.2020.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakanishi K., Fukuda S., Tanaka A., Otsuka K., Taguchi H., Yoshikawa J., Shimada K. Epicardial adipose tissue accumulation is associated with renal dysfunction and coronary plaque morphology on multidetector computed tomography. Circ. J. 2015;80:196–201. doi: 10.1253/circj.CJ-15-0477. [DOI] [PubMed] [Google Scholar]
- 13.Ansaldo A.M., Montecucco F., Sahebkar A., Dallegri F., Carbone F. epicardial adipose tissue and cardiovascular diseases. Int. J. Cardiol. 2019;278:254–260. doi: 10.1016/j.ijcard.2018.09.089. [DOI] [PubMed] [Google Scholar]
- 14.Nalliah C.J., Bell J.R., Raaijmakers A.J.A., Waddell H.M., Wells S.P., Bernasochi G.B., Montgomery M.K., Binny S., Watts T., Joshi S.B., Lui E., Sim C.B., Larobina M., O’Keefe M., Goldblatt J., Royse A., Lee G., Porrello E.R., Watt M.J., Kistler P.M., Sanders P., Delbridge L.M.D., Kalman J.M. epicardial adipose tissue accumulation confers atrial conduction abnormality. J. Am. Coll. Cardiol. 2020;76:1197–1211. doi: 10.1016/j.jacc.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Cavalcante J.L., Tamarappoo B.K., Hachamovitch R., Kwon D.H., Alraies M.C., Halliburton S., Schoenhagen P., Dey D., Berman D.S., Marwick T.H. Association of epicardial fat, hypertension, subclinical coronary artery disease, and metabolic syndrome with left ventricular diastolic dysfunction. J. Cardiol. 2012;110:1793–1798. doi: 10.1016/j.amjcard.2012.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zawadzka M.M., Grabowski M., Kapłon-Cieślicka A. Phenotyping in heart failure with preserved ejection fraction: a key to find effective treatment. Adv. Clin. Exp. Med. 2022;31:1163–1172. doi: 10.17219/acem/149728. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi K., Fukuda S., Tanaka A., Otsuka K., Taguchi H., Shimada K. Relationships between periventricular epicardial adipose tissue accumulation, coronary microcirculation, and left ventricular diastolic dysfunction. Can. J. Cardiol. 2017;33:1489–1497. doi: 10.1016/j.cjca.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Otsuka K., Nakanishi K., Shimada K., Nakamura H., Inanami H., Nishioka H., Fujimoto K., Kasayuki N., Yoshiyama M. Ankle-brachial index, arterial stiffness, and biomarkers in the prediction of mortality and outcomes in patients with end-stage kidney disease. Cardiol. 2019;42:656–662. doi: 10.1002/clc.23188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaura H., Otsuka K., Ishikawa H., Shirasawa K., Fukuda D., Kasayuki N. Determinants of non-calcified low-attenuation coronary plaque burden in patients without known coronary artery disease: a coronary CT angiography study. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.824470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otsuka K., Ishikawa H., Kono Y., Oku S., Yamaura H., Shirasawa K., Hirata K., Shimada K., Kasayuki N., Fukuda D. Aortic arch plaque morphology in patients with coronary artery disease undergoing coronary computed tomography angiography with wide-volume scan. Coronary Artery Dis. 2022;33:531–539. doi: 10.1097/MCA.0000000000001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hecht H.S., Blaha M.J., Kazerooni E.A., Cury R.C., Budoff M., Leipsic J., Shaw L. CAC-DRS: coronary artery calcium data and reporting system. expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT) J. Cardiovasc. Comput. Tomogr. 2018;12:185–191. doi: 10.1016/j.jcct.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Hwang S.-J., Melenovsky V., Borlaug B.A. Implications of coronary artery disease in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2014;63:2817–2827. doi: 10.1016/j.jacc.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 23.C.J. Rush, C. Berry, K.G. Oldroyd, J.P. Rocchiccioli, M.M. Lindsay, R.M. Touyz, C.L. Murphy, T.J. Ford, N. Sidik, M.B. McEntegart, N.N. Lang, P.S. Jhund, R.T. Campbell, J.J. V McMurray, M.C. Petrie, Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction., 6 (2021) 1130–1143. <https://doi.org/10.1001/jamacardio.2021.1825>. [DOI] [PMC free article] [PubMed]
- 24.Clemmensen T.S., Løgstrup B.B., Eiskjaer H., Poulsen S.H. Coronary flow reserve predicts longitudinal myocardial deformation capacity in heart transplant patients. Echocardiography. 2016;33:562–571. doi: 10.1111/echo.13123. [DOI] [PubMed] [Google Scholar]
- 25.Sucato V., Galassi A.R., Novo S., Saladino A., Evola S., Novo G. Correlation between longitudinal strain analysis and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Microcirculation. 2020;27:e12605. doi: 10.1111/micc.12605. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi K., Fukuda S., Shimada K., Miyazaki C., Otsuka K., Maeda K., Miyahana R., Kawarabayashi T., Watanabe H., Yoshikawa J., Yoshiyama M. Impaired coronary flow reserve as a marker of microvascular dysfunction to predict long-term cardiovascular outcomes, acute coronary syndrome, and development of heart failure. Circ J. 2012;76:1958–1964. doi: 10.1253/circj.cj-12-0245. [DOI] [PubMed] [Google Scholar]
- 27.G. Maimaituxun, K. Kusunose, H. Yamada, D. Fukuda, S. Yagi, Y. Torii, N. Yamada, T. Soeki, H. Masuzaki, M. Sata, M. Shimabukuro, Deleterious effects of epicardial adipose tissue volume on global longitudinal strain in patients with preserved left ventricular ejection fraction, Front. Cardiovasc. Med. 7 (2020) 607825. <https://doi.org/10.3389/fcvm.2020.607825>. [DOI] [PMC free article] [PubMed]
- 28.Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022:1–14. doi: 10.1038/s41569-022-00679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo J.H., Hong D., Youn T., Lee S.H., Choi K.H., Kim D., Park T.K., Lee J.M., Bin Song Y., Choi J.O., Hahn J.Y., Choi S.H., Gwon H.C., Jeon E.S., Yang J.H. Prognostic implications of coronary artery disease and stress tests in patients with elevated left ventricular filling pressure and preserved ejection fraction. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.955731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada T., Sunaga H., Sorimachi H., Yoshida K., Kato T., Kurosawa K., Nagasaka T., Koitabashi N., Iso T., Kurabayashi M., Obokata M. Pathophysiological role of fatty acid-binding protein 4 in Asian patients with heart failure and preserved ejection fraction. ESC Heart Fail. 2020;7:4256–4266. doi: 10.1002/ehf2.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anker S.D., Butler J., Filippatos G., Ferreira J.P., Bocchi E., Böhm M., Brunner-La Rocca H.-P., Choi D.-J., Chopra V., Chuquiure-Valenzuela E., Giannetti N., Gomez-Mesa J.E., Janssens S., Januzzi J.L., Gonzalez-Juanatey J.R., Merkely B., Nicholls S.J., Perrone S.V., Piña I.L., Ponikowski P., Senni M., Sim D., Spinar J., Squire I., Taddei S., Tsutsui H., Verma S., Vinereanu D., Zhang J., Carson P., Lam C.S.P., Marx N., Zeller C., Sattar N., Jamal W., Schnaidt S., Schnee J.M., Brueckmann M., Pocock S.J., Zannad F., Packer M. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 32.Sato T., Aizawa Y., Yuasa S., Kishi S., Fuse K., Fujita S., Ikeda Y., Kitazawa H., Takahashi M., Sato M., Okabe M. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc. Diabetol. 2018;17:6. doi: 10.1186/s12933-017-0658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He J., Yang W., Wu W., Sun X., Li S., Yin G., Zhuang B., Xu J., Zhou D., Zhang Y., Wang Y., Zhu L., Sharma P., Sirajuddin A., Teng Z., Kureshi F., Zhao S., Lu M. Clinical features, myocardial strain and tissue characteristics of heart failure with preserved ejection fraction in patients with obesity: a prospective cohort study. EClinical Med. 2023;55 doi: 10.1016/j.eclinm.2022.101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Löffler A.I., Pan J.A., Balfour P.C., Shaw P.W., Yang Y., Nasir M., Auger D.A., Epstein F.H., Kramer C.M., Gan L.M., Salerno M. Frequency of coronary microvascular dysfunction and diffuse myocardial fibrosis (Measured by Cardiovascular Magnetic Resonance) in patients with heart failure and preserved left ventricular ejection fraction. Am. J. Cardiol. 2019;124:1584–1589. doi: 10.1016/j.amjcard.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]