Abstract
The endothelium is a specific target for Bartonella henselae, and endothelial cell infection represents an important step in the pathogenesis of cat scratch disease and bacillary angiomatosis. Mechanisms of Bartonella-endothelial cell interaction as well as signaling pathways involved in target cell activation were analyzed. B. henselae strain Berlin-1, isolated from bacillary angiomatosis lesions of a human immunodeficiency virus-infected patient, potently stimulated human umbilical cord vein endothelial cells (HUVEC), as determined by NF-κB activation and enhanced adhesion molecule expression. These effects were accompanied by increased PMN rolling on and adhesion to infected endothelial cell monolayers, as measured in a parallel-plate flow chamber assay. Monoclonal antibodies against E-selectin significantly reduced PMN rolling and adhesion. In our hands, B. henselae Berlin-1 was substantially more active than the typing strain B. henselae ATCC 49882. E-selectin and ICAM-1 upregulation occurred for up to 9 days, as verified by Northern blotting and cell surface enzyme-linked immunosorbent assay. Induction of adhesion molecules was mediated via NF-κB activation and could be blocked by a specific NF-κB inhibitor. Additional studies indicated that B. henselae-induced effects did not require living bacteria or Bartonella lipopolysaccharides. Exposure of HUVEC to purified B. henselae outer membrane proteins (OMPs), however, reproduced all aspects of endothelial cell activation. In conclusion, B. henselae, the causative agent of cat scratch disease and bacillary angiomatosis, infects and activates endothelial cells. B. henselae OMPs are sufficient to induce NF-κB activation and adhesion molecule expression followed by enhanced rolling and adhesion of leukocytes. These observations identify important new properties of B. henselae, demonstrating its capacity to initiate a cascade of events culminating in a proinflammatory phenotype of infected endothelial cells.
Bartonella henselae, a gram-negative, fastidious, rod-shaped bacterium, can cause several human diseases, the most prominent being cat scratch disease, a persistent, necrotizing lymphadenitis (2, 4, 38). The domestic cat is the main reservoir of B. henselae, and the cat flea, Ctenocephalides felis, has been established as the vector in cat-to-cat transmission (15). An increasing number of other clinical manifestations such as endocarditis, osteolytic lesions, pulmonary nodules, neuroretinitis, and fever of unknown origin have been documented (1, 7, 11, 24, 43). In immunocompromised patients B. henselae infection can result in bacillary peliosis hepatitis (BPH). Another typical Bartonella-related disease is bacillary angiomatosis (BA), which is characterized by lesions resembling those produced by Kaposi's sarcoma (27–29).
Judged from the histology of BPH and BA lesions bacteria are in direct contact with the endothelium, probably promoting endothelial cell proliferation and angiogenesis. Therefore, endothelial cells appear to be a specific and unique target of the genus Bartonella (17). A detailed analysis of bacteria-endothelial cell interaction is vital for understanding the pathophysiology of these Bartonella-induced emerging diseases.
Exposure of endothelial cells to the bacterium has been shown to result in aggregation, engulfment, and subsequent internalization of the rods, forming a singular and well-defined host cellular structure, the invasome (18; for a review see reference 19), but uptake by phagocytosis has also been described (8, 18). B. henselae-related virulence factors and the target cell reaction upon infection are poorly defined (5, 9, 39).
Rolling and adhesion of circulating polymorphonuclear leukocytes (PMN) to endothelial cells, an early step in an acute inflammatory reaction, are governed by several adhesion molecules such as selectins and intercellular adhesion molecule 1 (ICAM-1) (12, 32). Multiple receptor-ligand pairs act sequentially and in an overlapping manner to effect initial attachment, rolling, firm adhesion, and transmigration of leukocytes. Several receptor-ligand interactions are involved in PMN adhesion to activated endothelial cells: interactions between E-selectin and sialyl Lewis carbohydrate-containing molecules (34, 47), P-selectin and P-selectin glycoprotein ligand 1, and ICAM-1 and β2-integrins (23).
A prerequisite for expression of E-selectin and other proinflammatory mediators is activation and subsequent translocation of NF-κB, which is involved in the transcription of genes associated with the immune response (33, 45, 50).
The main objective of this study was to assess the ability of B. henselae to infect and to activate cultured human endothelial cells. For this purpose we used the first European B. henselae isolate obtained from BA lesions of a human immunodeficiency virus (HIV)-infected patient (Berlin-1) (3). We compared this isolate with highly passaged phenotypically and genotypically indiscernible typing strain ATCC 49882 (Houston-1). We focused on endothelial signal transduction resulting in enhanced adhesion molecule expression and PMN-endothelial cell interaction. To further characterize effects induced by B. henselae (Berlin-1), we also made use of enriched outer membrane protein (OMP) preparations.
The data presented indicate that B. henselae bacteria as well as B. henselae-derived OMPs induce an NF-κB-dependent upregulation of E-selectin and ICAM-1 in endothelial cells, which in turn results in enhanced PMN rolling and adhesion.
(This work was done in partial fulfillment of the requirements for a Ph.D. by O. Fuhrmann.)
MATERIALS AND METHODS
Materials.
Tissue culture plastic ware was obtained from Becton Dickinson (Heidelberg, Germany) and Nunc (Wiesbaden, Germany). MCDB 131 medium, Hanks balanced salt solution, phosphate-buffered salt solution (PBS), trypsin-EDTA solution, HEPES, and fetal calf serum (FCS) were from GIBCO (Karlsruhe, Germany). Collagenase (CLS type II) was purchased from Worthington Biochemical Corp. (Freehold, N.J.). Specific inhibitor of NF-κB translocation caffeic acid phenethyl ester (CAPE) (40) was obtained from Biomol (Hamburg, Germany). Tumor necrosis factor alpha (TNF-α) was from R&D Biosystems (Wiesbaden, Germany). Lipopolysaccharide (LPS) from Salmonella enterica serovar Abortus equi was a kind gift from C. Galanos, Freiburg, Germany. All other reagents were purchased from Sigma Chemical Co. (Munich, Germany).
Abs.
Purified freeze-dried monoclonal antibodies (MAbs) directed against E-selectin (1.2B6), vascular cell adhesion molecule 1 (VCAM-1) (1G11), and ICAM-1 (15.2) were from Dianova (Hamburg, Germany), and horseradish peroxidase-conjugated polyclonal sheep anti-mouse immunoglobulin G (IgG) antibodies (Abs) were from Amersham Pharmacia (Freiburg, Germany). All Abs used were azide free.
Rabbits were inoculated with 3.5 × 107 CFU of heat-inactivated B. henselae Houston-1 (ATCC 49882) in 1 ml of saline intravenously. Blood was collected 4 weeks postinoculation (p.i.), and serum samples were prepared according to standard protocols. The titer of Bartonella-specific IgG Abs was measured by indirect immunofluorescence test of the preinoculation and p.i. serum samples and found to be <100 (detection limit) and 10,000, respectively. The hyperimmune serum did not show any cross-reaction at a dilution of 1:100 with other bacterial species tested.
Preparation of HUVEC.
Cells were isolated from umbilical cord veins and identified according to the method of Jaffe et al. (25). Briefly, cells obtained from collagenase digestion were washed, resuspended, and cultivated in MCDB 131–10% FCS and seeded into flasks. Confluent monolayers were split once, and first-passage human umbilical cord vein endothelial cells (HUVEC) were used.
Isolation of human PMN.
Heparinized human donor blood was centrifuged in a discontinuous Percoll gradient to yield a PMN fraction of >97% purity as previously described (31).
Bacterial strains and growth media.
B. henselae strain Berlin-1, isolated from bacillary angiomatosis lesions of an HIV-infected patient in Germany, has been characterized previously (3). Low-passage bacteria were grown to logarithmic phase in brucella broth supplemented with 7% Fildes (Unipath Ltd., Basingstoke, United Kingdom) and 250 μg of hemin (EGA-Chemie, Steinhausen, Germany)/ml.
B. henselae strains ATCC 49882 and ATCC 49793 were obtained from the American Type Culture Collection (ATCC; Manassas, Va.). They had originally been isolated from blood of HIV-infected patients. All ATCC Bartonella strains were grown on Columbia agar (Difco Laboratories, Detroit, Mich.) supplemented with 7% defibrinated sheep blood (Oxoid, Wesel, Germany) in a humidified atmosphere at 37°C and 5% CO2.
B. henselae OMP preparation.
OMPs were enriched by sarcosyl treatment of total-membrane preparations (20, 49). Bacteria were grown in supplemented brucella broth and harvested during the logarithmic phase by 30 min of centrifugation at 3,000 × g at 4°C. After being washed twice with ice-cold PBS, bacteria were resuspended in 30 ml of PBS. DNase and RNase (1 mg each) were added to the solution, followed by sonication (15 cycles at 2 min each; Branson Sonifier; duty cycle, 50%; output, 5) on ice. To remove residual bacteria, the suspension was centrifuged for 15 min at 3,000 × g, followed by additional centrifugation of the clarified supernatant at 20,000 × g for 30 min at 4°C (Beckman; J2–21, JA 20 rotor). The pellet was then resuspended in 1 ml of PBS. After that, the suspension was mixed on ice with 1 ml of sarcosyl (2% in PBS)/ml of pellet. Incubation for 1 h on ice was followed by centrifugation at 20,000 × g for 30 min (Beckman; J2–21, JA 20 rotor). The final membrane pellet, containing enriched OMPs, was then resuspended in 1 ml of PBS and stored in aliquots at −70°C. Protein concentration was determined using the Bradford protein assay (6). Ten micrograms of protein in an equal volume of sample buffer was heated at 95°C for 3 min and analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Bartonella infection assay.
Prior to infection with B. henselae, HUVEC (passage 1) were washed three times with MCDB 131 medium without supplements or antibiotics. Bacterial concentrations were adjusted to 108 bacteria/ml, and appropriate dilutions were prepared. For infection, bacteria were added in a bacteria-to-eukaryotic-cell ratio of 50:1 and centrifuged on a HUVEC monolayer at 1,500 × g for 5 min. After 2 h plates were washed extensively with plain medium and subsequently incubated in MCDB 131–2% FCS. Medium was replaced every 24 h. At times indicated in the figure legends cells were processed for NF-κB electrophoretic mobility shift assay (EMSA), Northern blotting for E-selectin and ICAM-1, cell surface enzyme-linked immunosorbent assay (ELISA), and neutrophil rolling and adhesion assays under defined flow conditions. Where indicated in the figure legends, NF-κB-specific inhibitor CAPE was added 1 h before bacterial infection. In some experiments, inactivation of B. henselae was achieved by heating (65°C for 30 min). LPS was inactivated by addition of 50 μg of polymyxin B/ml immediately before exposure to HUVEC.
Immunofluorescence analysis of B. henselae infection.
After stimulation of HUVEC grown on glass chamber slides (Falcon CultureSlide; Becton Dickinson, Rutherford, N.J.) with 50 bacteria/endothelial cell for the appropriate time, cells were fixed with 4% paraformaldehyde for 15 min. Permeabilization of the cell membrane was achieved by addition of 0.1% Triton X-100 in PBS for 5 min. The primary polyclonal rabbit anti-B. henselae Ab (1:200) was added for 30 min. Thereafter cells were washed three times with PBS and exposed to an ALEXA488-conjugated goat anti-rabbit Ig Ab and/or ALEXA568-phalloidin-conjugated anti-human F-actin Ig Ab (Molecular Probes, Eugene, Oreg.) for 15 min. After the cells were washed three times with PBS, coverslips were sealed and examined in an IMT-2 fluorescence microscope (Olympus Optical; equipped with an Olympus OM-4 camera) with an Olympus 60× objective.
Confocal laser scanning microscopy.
Confocal laser scanning microscopy was performed as described by Dehio et al. (18). The samples stained for immunofluorescence were viewed with a TCS NT confocal laser scanning microscope (Leica Lasertechnik, Heidelberg, Germany) equipped with an argon-krypton mixed-gas laser. In triple stainings, the three channels were recorded simultaneously. The corresponding images were digitally processed with Photoshop, version 5.0 (Adobe Systems, Mountain View, Calif.).
Preparation of nuclear extracts and EMSA.
Nuclear proteins were extracted as described by Jonat et al. (26). For the NF-κB EMSA, nuclear proteins (1 μg) were incubated with a 32P-labeled double-stranded consensus oligonucleotide probe 5′-AGT TGA GGG GAC TTT CCC AGGC-3′ (sense strand). Briefly, 0.07 pmol of NF-κB consensus oligonucleotides in 10 mM HEPES (pH 7.9)–50 mM KCl–0.2 mM EDTA–2.5 mM dithiothreitol–50 pmol of salmon sperm DNA–10% glycerol–0.05% NP-40 was incubated with 1 μg of soluble HUVEC nuclear proteins. The binding reaction was performed for 30 min at room temperature. Protein-DNA complexes were resolved on 7% native acrylamide gels run in 5 mM Tris (pH 8.3)–38 mM glycine before vacuum drying and autoradiography. Specificity was shown by addition of a 20-fold excess of unlabeled competitor consensus oligonucleotides.
Northern blot analysis.
RNA was extracted using the guanidinium isothiocyanate method described by Chomczynski and Sacchi (14). Total RNA was quantified by measuring absorbance at 260 nm with a Uvikon 860 spectrophotometer (Kontron, Neufahrn, Germany). RNA samples (10 μg/lane) were electrophoresed on denaturing 1% formaldehyde-agarose gels and transferred onto a Magna nylon membrane (MSI, Westborough, Mass.) and fixed by exposure to UV radiation using a Hoefer UVC 500 cross-linker.
cDNA probes were labeled with [α-32P]dCTP (>3,000 Ci/mmol) by random priming (Rediprime DNA labeling system; Amersham Pharmacia) and added to the prehybridization chambers at 106 cpm/ml and incubated for 12 to 16 h at 42°C. The E-selectin cDNA probe was a kind gift from D. Simmons (Institute of Molecular Medicine, Oxford, United Kingdom). The ICAM-1 cDNA probe was prepared by reverse transcription-PCR with custom-designed primer pair 5′-AAAGGATGGCACTTTCCCAC-3′ and 5′-TTCCCCTCTCATCAGGCTAGAC-3′ (Amersham Pharmacia). The 598-bp cDNA fragment of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained as previously described (22). Following hybridization, the filters were washed to a stringency of 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS for 30 min at 55°C. Membranes were autoradiographed overnight at −70°C by exposure to Hyperfilm MP (Amersham Pharmacia). After exposure, blots were stripped in 50% formamide–10 mM NaH2PO4 for 1 h at 70°C before subsequent rehybridization. To account for a difference in loading or transfer of the RNA, hybridization was performed with a 32P-labeled GAPDH cDNA probe.
Cell surface ELISA for E-selectin and ICAM-1 expression.
E-selectin and ICAM-1 expression on endothelial cells preincubated with B. henselae was determined by cell surface ELISA (46). Confluent pretreated HUVEC monolayers in 96-well flat-bottom microtiter plates were washed and finally fixed with 4% paraformaldehyde for 15 min. Human Ig was used to reduce nonspecific binding, and primary Abs were added for 30 min. Thereafter, cells were washed three times and exposed to a horseradish peroxidase-conjugated rabbit anti-mouse Ig Ab for 30 min. After the cells were washed, o-phenylenediamine was added for 5 min. Data are indicated as optical densities at 492 nm.
Neutrophil rolling and adhesion assay under flow conditions.
Leukocyte rolling and adhesion were determined using a parallel-plate flow chamber as described by Lawrence and Springer (32) and were evaluated as described previously (30). Confluent endothelial monolayers grown on coverslips (Thermanox; 22 by 60 mm; Nunc) were preincubated with live B. henselae as described above. A suspension of 3 × 106 leukocytes/ml was perfused through the chamber at a constant wall shear stress of 1.0 dyne/cm2 (syringe pump sp100i; WPI, Sarasota, Fla.). The field of observation was chosen randomly, and interactions were visualized using a phase-contrast video microscope (with a KP-C551 charge-coupled device color camera; Hitachi, Rodgau, Germany) and videotaped (HR-S7000EG; JVC, Friedberg, Germany) during the entire time period of leukocyte perfusion. Images were recorded in real time and played back at six- or ninefold-slower speed. The tape was paused to mark the locations of cells, and the displacements of the centers of individual cells were measured 2 to 4 s later. Rolling was expressed as the number of rolling cells/high-power field during a 3-min observation period (47). Leukocytes were considered to be adherent after 30 s of stable contact with the monolayer. Adhesion was determined after 5 min of perfusion by analysis of 10 to 12 high-power (40×) fields from videotape (32).
Statistical methods.
Depending on the number of groups and number of different time points studied, data were analyzed by a two-way analysis of variance. Main effects were then compared by an F probability test. A P value of <0.05 was considered significant.
RESULTS
Infection of HUVEC by B. henselae.
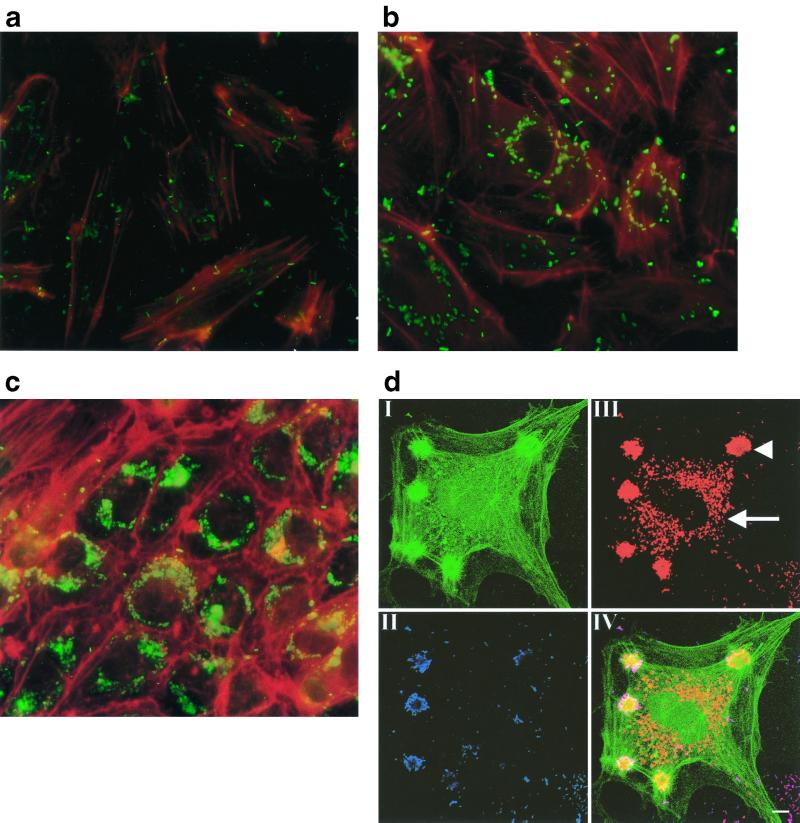
During BA, bacteria are in contact with the endothelium, apparently promoting endothelial cell proliferation and angiogenesis. We exposed HUVEC to recently described B. henselae strain Berlin-1 (3). This isolate is phenotypically and genotypically indiscernible from typing strain ATCC 49882 but differs with respect to its low passage number and growth conditions. Exposure of HUVEC to B. henselae Berlin-1 resulted in bacterial adhesion and internalization. Singular bacteria appeared to be internalized by phagocytosis within 2 h (Fig. 1a). After 24 h of exposure, perinuclear formation of internalized bacteria was observed (Fig. 1b). By 7 days p.i., the internalized bacteria form a dense area around the nucleus (Fig. 1c), clearly demonstrating the ability of B. henselae to replicate within endothelial cells, whereas lysis of cells was not observed. Confocal laser scanning microscopy (Fig. 1d) clearly demonstrated the intracellular localization of the bacteria and, furthermore, gave evidence for the internalization of B. henselae Berlin-1 by an invasome-like mechanism, as described for B. henselae ATCC 49882 and ATCC 49793 (18).
FIG. 1.
Infection of HUVEC by B. henselae Berlin-1 is shown in double-immunofluorescence experiments. HUVEC were grown on glass chamber slides and exposed to B. henselae in a bacteria/cell ratio of 50:1. After the appropriate time, cells were fixed with paraformaldehyde and permeabilized with 0.1% Triton X-100 in PBS. Anti-human F-actin MAbs and anti-B. henselae Abs were added; this was followed by incubation with fluorescein isothiocyanate (FITC)- or rhodamine-conjugated secondary Abs. At 2 h p.i., singular bacteria were phagocytosed (a). At 24 h p.i. perinuclear localization of internalized bacteria was evident (b). At 7 days p.i. dense areas of bacteria were visible (c). Representative pictures from five independent experiments are shown. Magnification, ×540. Specimens were immunocytochemically stained for extracellular bacteria, intracellular bacteria, and the actin cytoskeleton and analyzed by confocal laser scanning microscopy (d). Prior to the permeabilization of the host cell membranes, extracellular bacteria were labeled with anti-B. henselae antiserum and Texas red-conjugated secondary Abs (II; blue). Following permeabilization, all bacteria were labeled with anti-B. henselae antiserum and Cy5-conjugated secondary Abs (III; red). The actin cytoskeleton was stained with FITC-labeled phalloidin to indicate the location of cellular structures (I; green). IV, overlay of all three channels. Intracellular bacteria appear red due to the absence of a signal in the blue channel. In contrast, extracellular bacteria appear purple as a result of the superimposition of signals in both the red and blue channels. Arrow, perinuclear localized bacteria; arrowhead, bacterial aggregate within the invasome. Bar, 10 μm.
B. henselae induced rolling and adhesion of PMN on HUVEC.
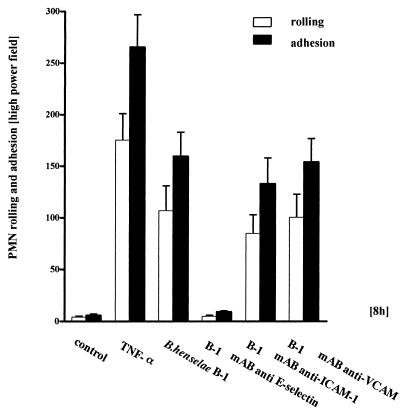
Exposure of HUVEC to B. henselae resulted in a profound cell activation with subsequently enhanced PMN rolling and adhesion at 8 h p.i. as determined under flow at a shear rate of 1 dyne/cm2. B. henselae turned out to be almost as potent as TNF-α (20 ng/ml), which was used as a positive control (Fig. 2). In the presence of an anti-E-selectin MAb, PMN rolling and adhesion on B. henselae-stimulated cells were reduced by 90%, suggesting that this endothelial adhesion molecule is central for PMN-endothelium interaction in the early phase of B. henselae infection. Experiments with anti-ICAM-1 and anti-VCAM-1 antibodies indicated no decrease of rolling and adhesion of PMN upon blockade of ICAM-1 or VCAM, underscoring the important role of E-selectin at 8 h p.i.
FIG. 2.
PMN rolling and adhesion on Bartonella-exposed HUVEC under flow at a shear rate of 1 dyne/cm2. Bacteria were centrifuged on endothelial cells in four-well plates containing rectangular coverslips. After 8 h coverslips were processed for a laminar-flow adhesion assay. PMN (3 × 10 6 per ml) were injected into the flow system and perfused over endothelial cell monolayers for 5 min using a high-precision syringe pump. When indicated, cells were preincubated with Abs against E-selectin, ICAM-1, or VCAM-1 120 min before measurement. TNF-α (20 ng/ml) was used as a positive control. Rolling PMN (for a definition see Materials and Methods) were counted over a 3-min observation period. Adherent PMN were determined by counting 10 to 12 random high-power fields (see Materials and Methods). B. henselae Berlin-1 was almost as effective as TNF-α. Note that PMN rolling and adhesion were almost completely blocked by an anti-E-selectin MAb. Data are means ± standard errors of the means of three separate experiments.
Bartonella strains ATCC 49882 and ATCC 49793 which have been passaged frequently induced only a marginal endothelial cell activation. Similarly, B. henselae Berlin-1 in passage 20 was only 30 to 40% as active as its low-passage-number parents (data not shown), suggesting that a high passage number is accompanied by loss of rolling- and adhesion-inducing properties. Therefore, low-passage-number B. henselae Berlin-1 was used in the following experiments.
B. henselae increased E-selectin and ICAM-1 expression in HUVEC.
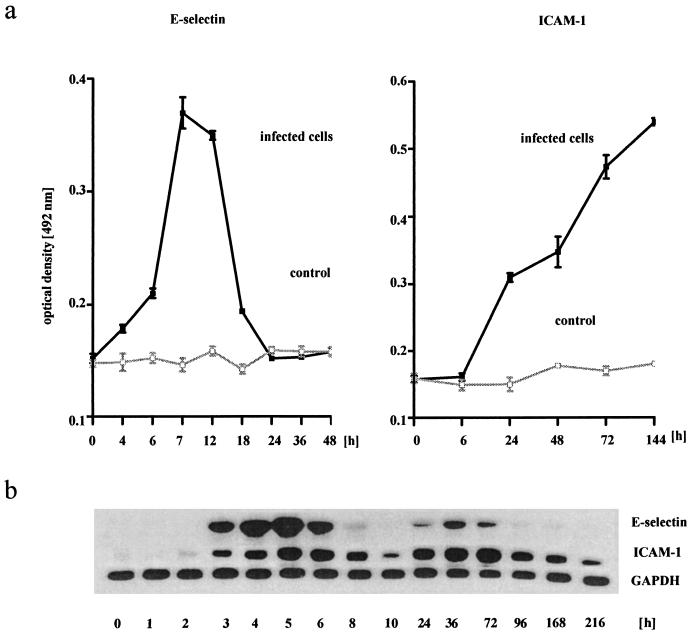
Enhanced rolling and adhesion of PMN on B. henselae-infected HUVEC were accompanied by an increased expression of adhesion molecules on the cell surface (Fig. 3a). E-selectin expression on B. henselae-exposed HUVEC increased at 6 h p.i., peaked at 7 to 12 h, and declined to baseline after 20 to 24 h. Significant ICAM-1 expression occurred at 20 to 26 h p.i. and persisted for up to at least 6 days after initial contact with the bacteria. In contrast, control cells showed no increase in adhesion molecule expression.
FIG. 3.
(a) Enhanced expression of E-selectin and ICAM-1 in B. henselae-infected HUVEC. Bacteria were added to HUVEC monolayers in 96-well plates by centrifugation at 1,500 × g in a bacteria-to-cell ratio of 50:1. After 2 h plates were washed three times with fresh medium. After incubation for another 2 to 144 h, cells were processed for E-selectin or ICAM-1 cell surface ELISA. Note that E-selectin expression peaked at 7 to 12 h, while ICAM-1 increased up to 144 h. Data are means ± standard errors of the means of four separate experiments. (b) Northern blot showing increase of E-selectin and ICAM-1 mRNA in B. henselae-infected HUVEC. Total endothelial RNA was isolated, and levels of E-selectin-, ICAM-1, and GAPDH mRNA were quantitated. Note different time patterns of mRNA expression after B. henselae infection for E-selectin and ICAM-1 and the remarkably long upregulation of ICAM-1. A representative gel (of four independent experiments) is shown.
Northern blot analysis was performed to study mRNA upregulation for E-selectin and ICAM-1 (Fig. 3b). E-selectin mRNA peaked at 4 to 5 h after B. henselae stimulation, declined to almost baseline after 10 h, and was detectable again after 36 h. ICAM-1 mRNA increased by 4 h p.i. and remained elevated for as long as 216 h (9 days).
B. henselae induced NF-κB-dependent adhesion molecule expression in HUVEC.
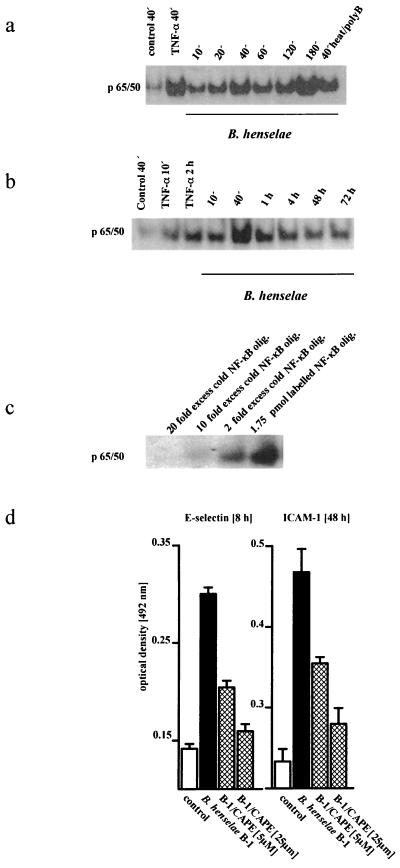
NF-κB is important for regulation of the transcriptional activities of E-selectin and ICAM-1, and multiple NF-κB binding sites have been located in the promoters of the genes for both (21, 36). NF-κB activation and translocation were demonstrated by bandshift assays, which indicated the enhanced capacity of NF-κB to bind to the corresponding consensus oligonucleotides (Fig. 4a). TNF-α was used as a positive control. Exposure of HUVEC to B. henselae resulted in NF-κB activation and translocation within 10 min. This signal transiently peaked at 40 min p.i. and remained elevated for at least 72 h (Fig. 4b). Interestingly, HUVEC stimulated with heat-inactivated bacteria in the presence of polymyxin B displayed undiminished NF-κB activation, suggesting that live bacteria and Bartonella LPS are not central for NF-κB activation in HUVEC (Fig. 4a). Control experiments with a 2- to 20-fold molar excess of cold NF-κB consensus oligonucleotide demonstrated the specificity of the shift (Fig. 4c).
FIG. 4.
(a) B. henselae-induced translocation of transcription factor NF-κB in HUVEC. An EMSA using a 32P-labeled double-stranded NF-κB consensus oligonucleotide was performed. Nuclear proteins were extracted 10 to 180 min p.i. TNF-α was used as a positive control. Note biphasic NF-κB translocation in B. henselae-exposed HUVEC. Moreover, note the undiminished NF-κB signal in HUVEC treated with heat-inactivated polymyxin B (50 μg/ml)-exposed bacteria (right lane), suggesting that living bacteria and LPS are not central to NF-κB-translocation. A representative gel (of five independent experiments) is shown. (b) NF-κB upregulation was detected even at 72 h p.i. (c) Control experiments with a 2- to 20-fold molar excess of an NF-κB consensus oligonucleotide clearly demonstrate the specificity of the shift. (d) B. henselae-induced E-selectin and ICAM-1 expression was dose dependently reduced by NF-κB-specific inhibitor CAPE, as demonstrated by cell surface ELISA. Data are means ± standard errors of the means of three separate experiments.
Preincubation of HUVEC with NF-κB-specific inhibitor CAPE dose dependently reduced E-selectin mRNA and protein expression in B. henselae-exposed cells (Fig. 4d). The E-selectin protein level in cells preincubated with CAPE (5 and 25 μM) for 1 h was reduced dose dependently. Similar results were obtained for ICAM-1, demonstrating that NF-κB is also a pivotal transcription factor in adhesion molecule expression in B. henselae-exposed endothelial cells.
Preparation of B. henselae OMPs.
The immediate endothelial cell activation by B. henselae, even by dead bacteria, as well as the data obtained with polymyxin B pointed to OMPs of Bartonella as key initiators of target cell stimulation.
OMPs of B. henselae Berlin-1 were purified according to a method described by Welch et al. (49). The sarcosyl fraction and a total-membrane preparation were analyzed by SDS-PAGE. Prominent bands of 92, 43, 32, 30, 28, and 23 kDa were identified (Fig. 5). These OMPs compared very well with those purified by Burgess and Anderson (9). These authors also demonstrated specific binding of B. henselae OMPs of 43, 32, and 28 kDa to endothelial cells, suggesting that these OMPs represent B. henselae adhesins.
FIG. 5.
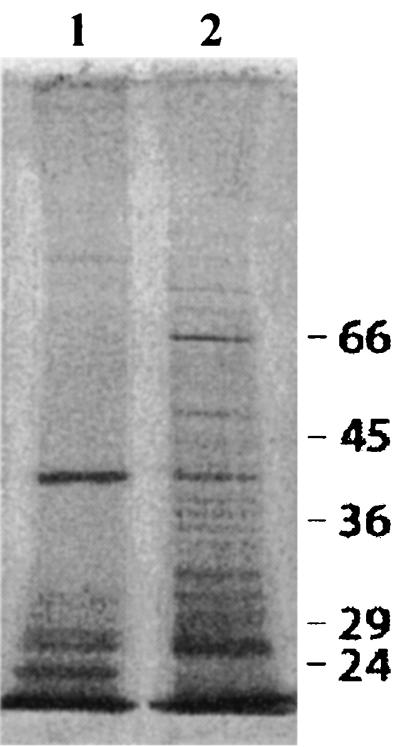
OMP fractions of B. henselae were characterized by SDS–10% PAGE and silver staining. Lane 1, sarcosyl-insoluble outer membrane fraction of B. henselae; lane 2, total protein fraction of B. henselae.
B. henselae OMPs activate endothelial cells.
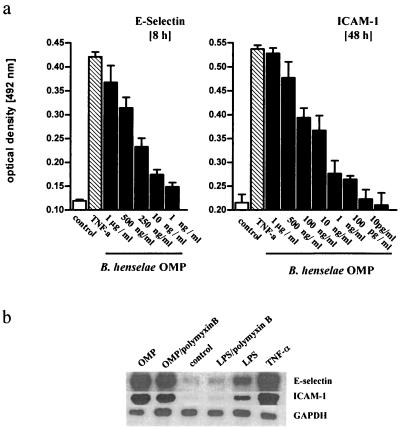
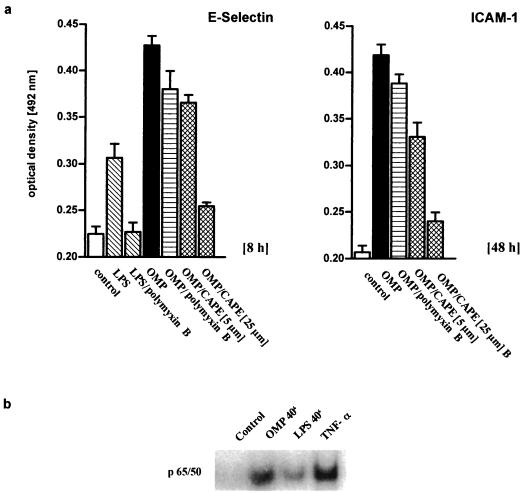
B. henselae Berlin-1 OMPs in the range of 10 pg/ml to 1 μg/ml activated HUVEC dose dependently, as shown by increased E-selectin and ICAM-1 protein expression (Fig. 6a). Data on OMP-induced PMN rolling and adhesion resembled the data obtained for live bacteria (data not shown). OMPs in a concentration of 500 ng/ml were as potent as living B. henselae (compare Fig. 6 and 3) and almost as effective as TNF-α, clearly indicating that OMPs are a potent stimulus and that intracellular infection of HUVEC by B. henselae is not required for induction of adhesion molecule expression. Effects of B. henselae OMPs were not due to LPS, as indicated by Northern blot experiments and cell surface ELISAs, because polymyxin B was very efficient in blocking LPS- but not OMP-induced adhesion molecule expression (Fig. 7a). Furthermore, OMP-related endothelial cell activation was dose dependently reduced by CAPE, suggesting that OMPs also acted via NF-κB (Fig. 7a). Bandshift assays clearly indicate that OMP (500 ng/ml) induced NF-κB activation and translocation, while LPS (S. enterica serovar Abortus equi LPS; 50 μg/ml) had only a minor effect. In these experiments TNF-α (20 ng/ml) was used as a positive control (Fig. 7b).
FIG. 6.
(a) B. henselae Berlin-1 OMPs in the range of 10 pg/ml to 1 μg/ml dose dependently enhanced E-selectin and ICAM-1 expression in HUVEC. OMPs were added to cell monolayers in 96-well plates by centrifugation at 1,500 × g. After 8 (E-selectin) or 48 h (ICAM-1) cells were processed for cell surface ELISA. Data are means ± standard errors of the means of three separate experiments. (b) Northern blot showing increase of E-selectin and ICAM-1 mRNA in HUVEC exposed to OMP, TNF-α (10 ng/ml), and LPS (100 ng/ml) after 5 h of stimulation. OMPs were added in a concentration of 500 ng/ml. When indicated, polymyxin B (50 μg/ml) was added 5 min prior to exposure to OMPs or LPS. A representative blot (from one of three independent experiments) is shown.
FIG. 7.
(a) OMP-induced endothelial adhesion molecule expression was NF-κB dependent and unrelated to LPS. Effects of OMPs (500 ng/ml) on E-selectin and ICAM-1 expression were compared to those for untreated cells. When indicated, cells were pretreated with 5 or 25 μM CAPE 1 h before exposure to OMPs; this resulted in a dose-dependent reduction of adhesion molecule expression. When indicated, polymyxin B was added to OMPs or LPS in a concentration of 50 μg/ml. Data are means ± standard errors of the means of three separate experiments. (b) OMP induced translocation of transcription factor NF-κB in HUVEC. An EMSA was performed as described in Materials and Methods. Nuclear proteins were extracted 40 min p.i. TNF-α was used as a positive control. Note the weak NF-κB signal in LPS-exposed HUVEC compared to that for OMP-treated cells. A representative gel (from three independent experiments) is shown.
DISCUSSION
This study demonstrates that B. henselae infects and activates human endothelial cells. B. henselae strain Berlin-1, isolated from a BA lesion of an HIV-infected patient, was used and was shown to upregulate, via NF-κB translocation, endothelial adhesion molecule expression, followed by increased rolling and adhesion of human PMN. Endothelial cell activation was reproduced by exposure to enriched B. henselae OMPs. These observations identify important new properties of this pathogen, demonstrating its capacity to initiate a cascade of events culminating in the proinflammatory phenotype of infected endothelial cells.
B. henselae strain Berlin-1 has previously been characterized extensively (3). Infection of HUVEC resulted in internalization by phagocytosis and subsequent perinuclear localization of B. henselae. Bacteria seem to slowly replicate within their host cells, forming a dense area around the nucleus. Lysis of the endothelial cells could never be observed, even at 7 days p.i. In addition to phagocytosis of bacteria, we observed invasome-mediated mechanisms of B. henselae Berlin-1 uptake, as demonstrated by confocal laser scanning microscopy (18). With respect to endothelial cell infection, Berlin-1 grown in supplemented brucella broth turned out to be substantially more effective in our hands than plated subcultures or typing strains ATCC 49882 and 49793 on agar plates (data not shown).
Avoidance of high passage number and of growth on solid media, which may be responsible for potential loss of pathogenicity, contributed to the high efficiency of B. henselae Berlin-1. In addition, centrifugation of bacteria on HUVEC monolayers turned out to be necessary, since exposure without prior centrifugation stimulated adhesion molecule expression by only 30%. Moreover, strict use of first-passage human endothelial cells contributed to a reproducible infection and activation of HUVEC.
PMN are known to be important for the control of the early phase of a bacterial infection. Hence, it is conceivable that PMN also contribute to the nonspecific resistance to Bartonella, although no studies of Bartonella-infected PMN-depleted animals have been published.
We analyzed the PMN-endothelial cell interaction at a defined shear rate of 1 dyne/cm2 using a parallel-plate flow chamber. This approach allowed the study of PMN rolling and adhesion under physiological conditions (23, 47). At least two separate receptor-ligand pairs appear to be involved in the PMN interaction with infected endothelial cells (23, 34). E-selectin and ICAM-1 on the endothelium bind to E-selectin ligand 1 and leukocyte β2-integrins, respectively. These adhesion molecules act sequentially and in an overlapping manner to effect leukocyte attachment and adhesion (12). At 8 h after B. henselae infection the PMN-HUVEC interaction was dominated by E-selectin, as shown by E-selectin upregulation and by anti-E-selectin MAb studies (Fig. 2). No significant decrease of rolling and adhesion of PMN was observed upon blockade of ICAM-1 or VCAM-1. In the later phase of infection, an indirect PMN stimulation by Bartonella-exposed endothelial cells via E-selectin binding and subsequent upregulation of β2-integrin, which in turn binds to ICAM-1, is conceivable. This aspect requires further study as does the question of whether B. henselae directly stimulates PMN. Moreover, B. henselae-exposed endothelial cells likely support adhesion of other circulating leukocytes (lymphocytes and monocytes) which contribute to the specific host defense. Additional studies to address the role of VCAM-1 and different interleukins in the leukocyte-endothelial cell interaction are required.
B. henselae virulence factors leading to activation and reorganization of the endothelial cells are of major interest, but unfortunately knowledge about the pathogenic role of Bartonella-specific endotoxin, exotoxins, and pili is very fragmentary (5, 39). Conley et al. (17) localized a stimulus for endothelial cell proliferation in the particulate fraction of B. henselae lysates. In contrast, Maeno et al. (36) suggested a soluble B. henselae-derived growth factor. Garcia et al. (21) reported on a heat-labile (56°C, 30 min) mitogenic factor of Bartonella bacilliformis extract.
Burgess et al. (10) demonstrated a 43-kDa OMP to be the major B. henselae-derived adhesin that specifically interacts with HUVEC.
Our observation that heat-inactivated B. henselae in the presence or absence of polymyxin B was still able to induce NF-κB activation and adhesion molecule expression in HUVEC suggested the sufficiency of dead B. henselae and the requirement for a component other than LPS. This study demonstrates for the first time that a purified and enriched outer membrane fraction of B. henselae can mimic the effects of live bacteria, indicating a prominent role for OMPs as a pathogenic factor in B. henselae-induced endothelial cell activation.
At least nine proteins (28, 30, 35, 43, 58, 61, 79, 92, and 171 kDa) were reported to occur in the sarcosyl-insoluble fraction of B. henselae lysates (9). Five of them (28, 32, 43, 52, and 58 kDa) have been shown to attach to HUVEC, with the 43-kDa OMP being the predominant adhesin (18). Very recent sequence analysis of the 43-kDa OMP by Burgess et al. (10) revealed a 38% identity to Brucella spp. OMP2b porin.
Sarcosyl fractionation of B. henselae Berlin-1 resulted in the resolution of proteins with similar molecular masses (23, 28, 30, 43, and 92 kDa). The differences observed are possibly due to different purification protocols and/or to Bartonella-specific aspects with regard to piliation, strain, passage number, and growth and culture conditions. Further studies to characterize the biological effects of the B. henselae Berlin-1 43-kDa OMP and the role of pili in activation of endothelial cells are in progress.
Colonization of host tissues usually is mediated by microbial adhesins, which facilitate recognition of and binding to specific receptors of host cells (35, 42, 44). Use of purified OMPs will help identify endothelial binding sites. ICAM-1 accumulation at the tips of the endothelial protrusions formed during bacterial engulfment was shown by Dehio et al. via confocal microscopy (18). In this context we propose ICAM-1 as a receptor candidate for the 43-kDa OMP. For rhinovirus, NF-κB-mediated induction of its own receptor ICAM-1 has been demonstrated (41).
Data presented indicate that B. henselae triggered immediate signal transduction cascades in HUVEC. Bacterial attachment was sufficient to initiate this endothelial response; uptake appeared not to be required. NF-κB is an important regulatory element in endothelial cell adhesion molecule expression (37, 45). Induction of E-selectin requires NF-κB binding to at least three of the four positive regulatory domains in the E-selectin promoter region, a situation similar to that for the ICAM-1 promoter (16, 48). Our study, aimed at identifying the possible intracellular signaling steps involved, indicated that NF-κB activation and translocation occurred within 10 to 40 min after B. henselae exposure to HUVEC. In addition, a specific NF-κB inhibitor significantly blocked adhesion molecule expression on the transcriptional level, proving the importance of NF-κB in the early phase of Bartonella-induced endothelial cell activation. Moreover, enriched OMPs (500 ng/ml) induced NF-κB activation and translocation, while LPS (S. enterica serovar Abortus equi LPS; 50 μg/ml) had only a minor effect. Further experiments must include B. henselae LPS, which unfortunately has not been purified yet.
In vivo, endothelial cells appear to be a specific target of B. henselae. In diseases such as BA and BPH bacteria are in contact with the endothelium, thereby promoting endothelial cell proliferation and angiogenesis (13). In this context our observation of prolonged (up to 9 days) upregulation of endothelial adhesion molecule expression might be relevant. Permanently adhering leukocytes and release of angiogenic factors possibly contribute to the vascular lesions described.
The interpretation of our study is limited to cultured human large-vessel endothelial cells. For a detailed analysis of B. henselae-related alterations of endothelial function in clinical disorders it would be desirable to study human microvascular endothelial cells of different organs, especially of lymph nodes and dermal capillaries. The isolation and culture of these cells in sufficient quantities, however, are difficult, and therefore the applicability of the data presented to clinical disorders such as cat scratch disease and BA must be verified in further studies.
In conclusion, infection of HUVEC with B. henselae resulted in internalization and reproduction of bacteria without subsequent lysis of the host cell. Bacterial contact immediately led to NF-κB-dependent upregulation of adhesion molecule expression and subsequently increased PMN rolling and adhesion. The proinflammatory endothelial cell phenotype was reproduced by exposure of cells to purified Bartonella OMP. The data presented suggest that B. henselae-induced endothelial cell activation is an important event in the pathogenesis of Bartonella-related diseases.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft.
We gratefully acknowledge the excellent technical assistance of Heike Geisel. We thank the staff of the Delivery Services of the hospitals Lich and Ehringshausen, as well as the Departments of Gynecology of Humboldt-Hospital, Berlin-Reinickendorf, and Waldkrankenhaus, Berlin-Spandau, for their help in collecting umbilical cords.
REFERENCES
- 1.Abbasi S, Chesney P J. Pulmonary manifestations of cat-scratch disease; a case report and review of the literature. Pediatr Infect Dis J. 1995;14:547–548. doi: 10.1097/00006454-199506000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Anderson B E, Neuman M A. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203–219. doi: 10.1128/cmr.10.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvand M, Wendt C, Regnath T, Ullrich R, Hahn H. Characterization of Bartonella henselae isolated from bacillary angiomatosis lesions in a human immunodeficiency virus-infected patient in Germany. Clin Infect Dis. 1998;26:1296–1299. doi: 10.1086/516348. [DOI] [PubMed] [Google Scholar]
- 4.Bass J, Vincent J M, Person D A. The expanding spectrum of Bartonella infections. II. Cat-scratch disease. Pediatr Infect Dis J. 1997;16:163–179. doi: 10.1097/00006454-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Batterman H J, Peek J A, Loutit J S, Falkow S, Tompkins L S. Bartonella henselae and Bartonella quintana adherence to and entry into cultured human epithelial cells. Infect Immun. 1995;63:4553–4556. doi: 10.1128/iai.63.11.4553-4556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Breathnach A S, Hoare J M, Eykyn S J. Culture-negative endocarditis: contribution of Bartonella infections. Heart. 1997;77:474–476. doi: 10.1136/hrt.77.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouqui P, Raoult D. Bartonella quintana invades and multiplies within endothelial cells in vitro and in vivo and forms intracellular blebs. Res Microbiol. 1996;147:719–731. doi: 10.1016/s0923-2508(97)85119-4. [DOI] [PubMed] [Google Scholar]
- 9.Burgess A W, Anderson B E. Outer membrane proteins of Bartonella henselae and their interaction with human endothelial cells. Microb Pathog. 1998;25:157–164. doi: 10.1006/mpat.1998.0223. [DOI] [PubMed] [Google Scholar]
- 10.Burgess A W, Paquet J Y, Letesson J J, Anderson B E. Isolation, sequencing and expression of Bartonella henselae omp 43 and predicted membrane topology of the deduced protein. Microb Pathog. 2000;29:73–80. doi: 10.1006/mpat.2000.0366. [DOI] [PubMed] [Google Scholar]
- 11.Caniza M A, Granger D L, Wilson K H, Washington M K, Kordick D L, Frush D P, Blitchington R B. Bartonella henselae: etiology of pulmonary nodules in a patient with depressed cell-mediated immunity. Clin Infect Dis. 1995;20:1505–1511. doi: 10.1093/clinids/20.6.1505. [DOI] [PubMed] [Google Scholar]
- 12.Carlos T M, Harlan J M. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 13.Chan J K, Lewin K J, Lombard C M, Teitelbaum S, Dorfman R F. Histopathology of bacillary angiomatosis of lymph node. Am J Surg Pathol. 1991;15:430–437. doi: 10.1097/00000478-199105000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Chomczynski P, Sacchi N. Single step method for RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 15.Chomel B B, Kasten R W, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Nikos-Gurfield A, Abbott R C, Pedersen N C, Koehler J E. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol. 1996;34:1952–1956. doi: 10.1128/jcm.34.8.1952-1956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collins T, Williams A, Johnston G I, Kim J, Eddy R, Shows T, Gimbrone M A, Jr, Bevilacqua M P. Structure and chromosomal location of the gene for endothelial-leukocyte adhesion molecule 1. J Biol Chem. 1991;266:2466–2473. [PubMed] [Google Scholar]
- 17.Conley T, Slater L, Hamilton K. Rochalimaea species stimulate human endothelial cell proliferation and migration in vitro. J Lab Clin Med. 1994;124:521–528. [PubMed] [Google Scholar]
- 18.Dehio C, Meyer M, Berger J, Schwarz H, Lanz C. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J Cell Sci. 1997;110:2141–2154. doi: 10.1242/jcs.110.18.2141. [DOI] [PubMed] [Google Scholar]
- 19.Dehio C. Interaction of Bartonella henselae with vascular endothelial cells. Curr Opin Microbiol. 1999;2:78–82. doi: 10.1016/s1369-5274(99)80013-7. [DOI] [PubMed] [Google Scholar]
- 20.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia F U, Wojta J, Broadley K N, Davidson J M, Hoover R L. Bartonella bacilliformis stimulates endothelial cells in vitro and is angiogenic in vivo. Am J Pathol. 1990;136:1125–1135. [PMC free article] [PubMed] [Google Scholar]
- 22.Giembycz M A, Corrigan C J, Seybold J, Newton R, Barnes P J. Identification of cyclic AMP phosphodiesterases 3, 4 and 7 in human CD4+ and CD8+ T-lymphocytes: role in regulating proliferation and the synthesis of interleukin-2. Br J Pharmacol. 1996;118:1945–1958. doi: 10.1111/j.1476-5381.1996.tb15629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Issekutz A C, Rowter D, Springer T A. Role of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in neutrophil transendothelial migration. J Leukoc Biol. 1999;65:117–126. doi: 10.1002/jlb.65.1.117. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs R F, Schutze G E. Bartonella henselae as a cause of prolonged fever and fever of unknown origin in children. Clin Infect Dis. 1998;26:80–84. doi: 10.1086/516256. [DOI] [PubMed] [Google Scholar]
- 25.Jaffe E A, Nachman R L, Becker C G, Mimnick C R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Investig. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonat C, Rahmsdorf H J, Park K K, Cato A C, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 27.Koehler J E, Glaser C A, Tappero J W. Rochalimaea henselae infection. A new zoonosis with the domestic cat as reservoir. JAMA. 1994;271:531–535. doi: 10.1001/jama.271.7.531. [DOI] [PubMed] [Google Scholar]
- 28.Koehler J E, Quinn F D, Berger T G, LeBoit P E, Tappero J W. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 29.Koehler J E, Sanchez M A, Garrido C S, Whitfeld M J, Chen F M, Berger T G, Rodriguez-Barradas M C, LeBoit P E, Tappero J W. Molecular epidemiology of Bartonella infections in patients with bacillary angiomatosis-peliosis. N Engl J Med. 1997;337:1876–1883. doi: 10.1056/NEJM199712253372603. [DOI] [PubMed] [Google Scholar]
- 30.Krüll M, Klucken A C, Wuppermann F N, Fuhrmann O, Magerl C, Seybold J, Hippenstiel S, Hegemann J H, Jantos C A, Suttorp N. Signal transduction pathways activated in endothelial cells following infection with Chlamydia pneumoniae. J Immunol. 1999;162:4834–4841. [PubMed] [Google Scholar]
- 31.Krüll M, Dold C, Hippenstiel S, Rosseau S, Lohmeyer J, Suttorp N. Escherichia coli hemolysin and Staphylococcus aureus a-toxin potently induce neutrophil adhesion to cultured human endothelial cells. J Immunol. 1996;157:4133–4140. [PubMed] [Google Scholar]
- 32.Lawrence M B, Springer T. Leucocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 33.Lewis H, Kaszubska W, DeLamarter J F, Whelan J. Cooperativity between two NF-κB complexes, mediated by high-mobility-group protein I (Y), is essential for cytokine-induced expression of the E-selectin promoter. Mol Cell Biol. 1994;14:5701–5709. doi: 10.1128/mcb.14.9.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ley K, Allietta M, Bullard D C, Morgan S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ Res. 1998;83:287–294. doi: 10.1161/01.res.83.3.287. [DOI] [PubMed] [Google Scholar]
- 35.Lingwood C A. Glycolipid receptors for verotoxin and Helicobacter pylori: role in pathology. Biochim Biophys Acta. 1999;1455:375–386. doi: 10.1016/s0925-4439(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 36.Maeno N, Oda H, Yoshiie K, Wahid M R, Fujimura T, Matayoshi S. Live Bartonella henselae enhances endothelial cell proliferation without direct contact. Microb Pathog. 1999;27:419–427. doi: 10.1006/mpat.1999.0315. [DOI] [PubMed] [Google Scholar]
- 37.Manning A M, Chen C C. Transcriptional regulation of endothelial cell adhesion molecules: a dominant role for NF-κB. Agents Actions Suppl. 1995;47:135–141. doi: 10.1007/978-3-0348-7343-7_12. [DOI] [PubMed] [Google Scholar]
- 38.Maurin M, Birtles R, Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis. 1997;16:487–506. doi: 10.1007/BF01708232. [DOI] [PubMed] [Google Scholar]
- 39.Minnick M F, Mitchell S J, McAllister S. Cell entry and the pathogenesis of Bartonella infections. Trends Microbiol. 1996;4:343–347. doi: 10.1016/0966-842x(96)10055-x. [DOI] [PubMed] [Google Scholar]
- 40.Natarajan K, Singh S, Burke T R, Jr, Grunberger D, Aggarwal B B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papi A, Johnston S L. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappa B-mediated transcription. J Biol Chem. 1999;274:9707–9720. doi: 10.1074/jbc.274.14.9707. [DOI] [PubMed] [Google Scholar]
- 42.Prasadarao N V, Wass C A, Hacker J, Jann K, Kim K S. Adhesion of S-fimbriated Escherichia coli to brain glycolipids mediated by sfaA gene-encoded protein of S-fimbriae. J Biol Chem. 1993;268:10356–10363. [PubMed] [Google Scholar]
- 43.Reed J B, Scales D K, Wong T M, Lattuada C P, Jr, Dolan M J, Schwab I R. Bartonella henselae neuroretinitis in cat scratch disease. Diagnosis, management, and sequelae. Ophthalmology. 1998;105:459–466. doi: 10.1016/S0161-6420(98)93028-7. [DOI] [PubMed] [Google Scholar]
- 44.Scheuerpflug I, Rudel T, Ryll R, Pandit J, Meyer T F. Roles of PilC and PilE proteins in pilus-mediated adherence of Neisseria gonorrhoeae and Neisseria meningitidis to human erythrocytes and endothelial and epithelial cells. Infect Immun. 1999;67:834–843. doi: 10.1128/iai.67.2.834-843.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schindler U, Baichwal V R. Three NF-κB binding sites in the human E-selectin gene required for maximal tumor necrosis factor alpha-induced expression. Mol Cell Biol. 1994;14:5820–5831. doi: 10.1128/mcb.14.9.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarzer N, Nöst R, Seybold J, Parida S K, Fuhrmann O, Krüll M, Schmidt R, Newton R, Hippenstiel S, Domann E, Chakraborty T, Suttorp N. Two distinct phospholipases C of Listeria monocytogenes induce ceramide generation, nuclear factor-κB activation, and E-selectin expression in human endothelial cells. J Immunol. 1998;161:3010–3018. [PubMed] [Google Scholar]
- 47.Sriramarao P, Norton C R, Borgstrom P, DiScipio R G, Wolitzky B A, Broide D H. E-selectin preferentially supports neutrophil but not eosinophil rolling under conditions of flow in vitro and in vivo. J Immunol. 1996;157:4672–4680. [PubMed] [Google Scholar]
- 48.Vorarberger G, Schäfer R, Stratowa C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5′-regulatory region: induction by cytokines and phorbol ester. J Immunol. 1991;147:2777–2786. [PubMed] [Google Scholar]
- 49.Welch D F, Hensel D M, Pickett D A, San Joaquin V H, Robinson A, Slater L N. Bacteremia due to Rochalimaea henselae in a child: practical identification of isolates in the clinical laboratory. J Clin Microbiol. 1993;31:2381–2386. doi: 10.1128/jcm.31.9.2381-2386.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitley M Z, Thanos D, Read M A, Maniatis T, Collins T. A striking similarity in the organization of the E-selectin and beta interferon gene promoters. Mol Cell Biol. 1994;14:6464–6475. doi: 10.1128/mcb.14.10.6464. [DOI] [PMC free article] [PubMed] [Google Scholar]