Highlights:
-
•
13% experienced death without progression (DWP) after CRT for NSCLC.
-
•
Baseline comorbidity and heart dose were associated with DWP.
-
•
Dose to ventricles, but not atria, was associated with DWP.
-
•
Associations with DWP were distinct from those observed with survival.
Keywords: Radiotherapy, NSCLC, Toxicity, Cardiac toxicity, Cardiac dosimetry
Abstract
Background and purpose
Prior studies have examined associations of cardiovascular substructure dose with overall survival (OS) or cardiac events after chemoradiotherapy (CRT) for non-small cell lung cancer (NSCLC). Herein, we investigate an alternative endpoint, death without cancer progression (DWP), which is potentially more specific than OS and more sensitive than cardiac events for understanding CRT toxicity.
Materials and methods
We retrospectively reviewed records of 187 patients with locally advanced or oligometastatic NSCLC treated with definitive CRT from 2008 to 2016 at a single institution. Dosimetric parameters to the heart, lung, and ten cardiovascular substructures were extracted. Charlson Comorbidity Index (CCI), excluding NSCLC diagnosis, was used to stratify patients into CCI low (0–2; n = 66), CCI intermediate (3–4; n = 78), and CCI high (≥5; n = 43) groups. Primary endpoint was DWP, modeled with competing risk regression. Secondary endpoints included OS. An external cohort consisted of 140 patients from another institution.
Results
Median follow-up was 7.3 years for survivors. Death occurred in 143 patients (76.5 %), including death after progression in 118 (63.1 %) and DWP in 25 (13.4 %). On multivariable analysis, increasing CCI stratum and mean heart dose were associated with DWP. For mean heart dose ≥ 10 Gy vs < 10 Gy, DWP was higher (5-year rate, 16.9 % vs 6.7 %, p = 0.04) and OS worse (median, 22.9 vs 34.1 months, p < 0.001). Ventricle (left, right, and bilateral) and pericardial but not atrial substructure dose were associated with DWP, whereas all three were inversely associated with OS. Cutpoint analysis identified right ventricle mean dose ≥ 5.5 Gy as a predictor of DWP. In the external cohort, we confirmed an association of ventricle, but not atrial, dose with DWP.
Conclusion
Cardiovascular substructure dose showed distinct associations with DWP. Future cardiotoxicity studies in NSCLC could consider DWP as an endpoint.
Introduction:
Patients with locally advanced non-small cell lung cancer (LA-NSCLC) treated with chemoradiotherapy (CRT) receive considerable radiotherapy (RT) dose to cardiopulmonary structures frequently in the setting of multiple baseline co-morbid conditions. RTOG 0617 demonstrated inferior overall survival (OS) with higher dose RT, believed to be at least partially attributable to a higher heart dose [1], [2]. Subsequent work has described independent associations of heart dose with OS, cardiac events, and immunosuppression [3], [4], [5], [6], [7], [8].
There is considerable interest in understanding which cardiovascular substructures are most important to protect during RT planning. Prior studies have examined associations of cardiovascular substructure dose with OS or cardiac events [9], [10], [11], [12], [13]. However, challenges arise with both endpoints. First, because most patients die from NSCLC progression, OS is dominated by disease progression events that may mask RT effects on cardiovascular substructures despite best efforts to control for confounders. Second, cardiac events after RT often may not be fatal and thus cannot fully explain the association between heart dose and worse OS. Cardiac events are also difficult to record retrospectively. Selection of a suitable endpoint is critical to accurately determine which regions of the heart to prioritize for dose sparing during RT planning.
Death without progression (DWP), defined as death in the absence of NSCLC progression, is another endpoint to consider [14]. DWP is potentially more specific than OS (ie, selects out death from NSCLC progression), and potentially more sensitive than cardiac-specific death (ie, includes a variety of non-cancer causes of death) for understanding CRT toxicity. DWP may be a better endpoint to show the impact of CRT on survival isolated from anti-cancer effects. With controlled cancer, the relative impact of baseline comorbidity or CRT toxicity on longevity is expected to increase.
Accordingly, we hypothesized that both baseline comorbidity and dose to cardiovascular substructures would correlate with DWP. Additionally, we hypothesized that cardiovascular substructure dose would show distinct associations with DWP from those seen with OS.
Materials and methods
Patients
We retrospectively reviewed an institutional database of patients with locally advanced or oligometastatic (1 metastatic lesion) NSCLC treated with definitive concurrent or sequential CRT between December 2008 and November 2016 at the University of Pennsylvania. All patients in this cohort underwent baseline positron emission tomography and magnetic resonance imaging of the brain, and 4-dimensional computed tomography (CT) simulation. No patient received consolidation immunotherapy as the period predated the results of the PACIFIC trial [15]. We excluded those who received prior thoracic RT or thoracic RT doses < 50 Gy, and those who died during RT. The final cohort included 187 patients.
Treatment
RT was delivered with 3-dimensional conformal radiotherapy (3D-CRT), intensity-modulated radiotherapy (IMRT), or proton therapy (either pencil beam scanning [PBS] or passive scattering) to a prescription dose of 60–74 Gy in 1.8–2 Gy per fraction. Daily image guidance consisted of either kilovoltage imaging (proton therapy) or cone beam CT (photon therapy). RT dose constraints were as follows: spinal cord maximum dose ≤ 50 Gy, mean lung dose < 20 Gy, lung V20 < 37 %, and mean heart dose ≤ 26 Gy.
Follow-up
Follow-up CT chest and clinical visits were performed every 2–3 months for the first year after RT, every 4–6 months for the following two years, and every 6–12 months thereafter.
Study endpoints
The primary endpoint was DWP, defined as death in the absence of NSCLC progression on last CT chest and clinical visit. All clinical records (eg, hospitalization, clinic and telephone notes, imaging, death certificates) were reviewed to determine cause of DWP. For patients who died with a recent (defined according to the follow-up section above) stable CT chest but no notes describing a cause of death, cause of DWP was “unknown”. Secondary endpoints were OS and death after cancer progression. All endpoints were measured from the start of RT to the event of interest.
Baseline comorbidity
Baseline cardiovascular comorbidity (any cardiac condition, cerebrovascular accident [CVA] or peripheral arterial disease [PAD]), pulmonary comorbidity (chronic obstructive pulmonary disease [COPD], asthma, interstitial lung disease, obstructive sleep apnea, or pulmonary embolism), and Charlson Comorbidity Index (CCI), excluding NSCLC diagnosis, were manually extracted from medical records [16]. CCI assigns points for the following: age, myocardial infarction, congestive heart failure, PAD, CVA or transient ischemic attack, dementia, COPD, connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, hemiplegia, chronic kidney disease, leukemia, lymphoma, solid tumor (NSCLC excluded for this analysis), and acquired immunodeficiency syndrome. CCI was grouped into CCI low (0–2), CCI intermediate (3–4), and CCI high (≥5) based on approximate terciles and previously used cutpoints [17], [18]. Prior work suggests these cutpoints predict the risk of mortality in patients without cancer [17], [18]; because we were interested in the impact of baseline comorbidity on non-cancer deaths, these cutpoints were deemed appropriate for use in our study.
Dosimetric parameters
Heart and lung minus gross tumor volume contours were reviewed and manually re-contoured, as necessary. Mean heart dose (MHD), heart volume receiving ≥ 5 Gy (V5), heart V30, heart V50, and mean lung dose (MLD) were then extracted from the Eclipse treatment planning software (Varian Medical Systems, Palo Alto, CA).
RT plans were exported to MIM (version 7.1.4, MIM Software, Cleveland, OH). Ten cardiovascular substructures – atria (bilateral), right atrium, left atrium, ventricles (bilateral), right ventricle, left ventricle, pericardium, aorta, superior vena cava, and pulmonary artery – were auto-segmented using a previously validated deep learning model [19], [20]. Cardiovascular substructures were then manually reviewed and edited based on a validated cardiac contouring atlas [21]. Dosimetric parameters (mean dose, V5, V30, V50) to each substructure were extracted, chosen based on prior work assessing the significance of cardiovascular substructure dose for NSCLC [7], [10].
Statistical analysis
The cumulative incidence method was used to model DWP and death after progression. For the former, disease progression was considered a competing event, and for the latter, DWP was considered a competing event. For DWP, patients with incomplete follow-up prior to death were censored on the date of the last CT chest or clinical encounter.
Gray’s test was used to compare the cumulative incidence of DWP among CCI strata and mean heart dose cutpoint of 10 Gy. The latter cutpoint was chosen based on Atkins et al [8]. Fine-Gray regression was used to assess associations of patient-, tumor- and treatment-related factors with DWP. Significant factors on univariable analysis (p < 0.05) were considered for inclusion in multivariable models. Because of a limited number of DWP events (25), multivariable models included 2 variables. CCI strata was preferentially included in multivariable models as CCI is a combined measure of age and a variety of comorbidities. Given collinearity between different normal tissue dosimetric parameters, only one such parameter (treated as a continuous covariate) was included in each model.
Exploratory associations between cardiovascular substructure dose and DWP were assessed separately from the above process. For each cardiovascular substructure (heart and 10 substructures), associations between dosimetric parameters (mean, V5, V30, V50) and DWP were assessed, and the candidate parameter with the lowest significant p value was promoted to multivariable analysis. MLD was also included. Benjamini-Hochberg procedure was used to correct for multiple (45) comparisons, accepting a false discovery rate of 5 %. Multivariable models with 2 variables (CCI and different dosimetric parameters) were generated and ranked by Akaike information criterion (AIC) to determine the “best fit” models (lowest AIC). In a sensitivity analysis, we included 3 variables per model (age, internal target volume [ITV], and different dosimetric parameters) to see if the ordering of models changed. Cutpoint analysis was done with Contal and O’Quigley method [22].
For OS, the Kaplan-Meier method and Cox regression were used. Given the greater number of OS (versus DWP) events, multivariable models for OS preferentially included individual measures of comorbidity rather than CCI, along with one normal tissue dosimetric parameter. The exploratory associations described above were repeated in a similar fashion for cardiovascular substructure dose and OS.
All hypothesis tests were two-sided and p < 0.05 was considered statistically significant. Analyses were performed using SAS OnDemand for Academics.
External cohort
In an independent cohort of 140 patients from Rutgers Cancer Institute of New Jersey, associations between cardiac substructure dosimetric parameters and both DWP and OS were assessed in a similar fashion to the analysis described above. Details of this cohort have been previously published [7]. Mean, V5, and V30 to the heart, atria, right atrium, left atrium, ventricles, right ventricle, and left ventricle were included. Pericardium, aorta, superior vena cava, and pulmonary artery substructures, as well as CCI, were not available.
Results
Baseline characteristics
Baseline characteristics are shown in Table 1. Median age was 67 years. Cardiovascular, pulmonary, and either cardiovascular or pulmonary comorbidities were present in 94 (50.3 %), 78 (41.7 %), and 127 patients (67.9 %), respectively. CCI was low (0–2), intermediate (3–4), and high (5–9) in 66 (35.3 %), 78 (41.7 %), and 43 patients (23 %), respectively. Median RT prescription dose was 66.6 Gy. Proton therapy was used in 98 (52.4 %; n = 9 PBS) and photon therapy in 89 patients (47.6 %, n = 68 IMRT). Median mean heart dose was 8.1 Gy.
Table 1.
Baseline characteristics (N = 187).
| Characteristic | N (%) |
|---|---|
| Age (median, IQR) | 67 (59–73) |
| Sex | |
| Female | 99 (52.9) |
| Male | 88 (47.1) |
| ECOG PS | |
| 0 | 70 (37.4) |
| 1 | 102 (54.5) |
| 2 | 15 (8.0) |
| Smoking, pack-years (median, IQR) | 35 (15–50) |
| Cardiovascular comorbidity | 94 (50.3) |
| Coronary artery disease | 34 (18.2) |
| Pulmonary comorbidity | 78 (41.7) |
| Cardiovascular or pulmonary comorbidities | 127 (67.9) |
| CCI (median, IQR) | 3 (2–4) |
| 0–2 (low) | 66 (35.3) |
| 3–4 (intermediate) | 78 (41.7) |
| 5–9 (high) | 43 (23.0) |
| Histology | |
| Adenocarcinoma | 147 (78.6) |
| Squamous cell carcinoma | 29 (15.5) |
| Other | 11 (5.9) |
| AJCC Stage (7th edition) | |
| IIA-B | 4 (2.1) |
| IIIA | 119 (63.6) |
| IIIB | 62 (33.2) |
| IV (oligometastatic) | 2 (1.1) |
| T stage | |
| x, 1–2 | 108 (57.8) |
| 3–4 | 79 (42.2) |
| N stage | |
| 0–2 | 144 (77.0) |
| 3 | 43 (23.0) |
| Left-sided primary | 74 (39.6) |
| RT dose, Gy (median, IQR) | 66.6 (66.6–66.6) |
| RT technique | |
| Proton therapy | 98 (52.4) |
| PBS-PT | 9 (4.8) |
| PS-PT | 89 (47.6) |
| Photon therapy | 89 (47.6) |
| IMRT | 68 (36.4) |
| 3D-CRT | 21 (11.2) |
| Internal target volume (cc; median, IQR) | 242.9 (165.3–427.2) |
| Mean heart dose (Gy; median, IQR) | 8.1 (4.8–17.8) |
| Mean lung dose (Gy; median, IQR) | 16.8 (13.5–19.3) |
| Chemotherapy regimen | |
| Carboplatin/paclitaxel | 106 (56.7) |
| Cisplatin/etoposide | 57 (30.5) |
| Other | 24 (12.8) |
| Chemotherapy sequence | |
| Concurrent | 184 (98.4) |
| Sequential | 3 (1.6) |
IQR, interquartile range; PS, performance status; CCI, Charlson Comorbidity Index; RT, radiotherapy; PT, proton therapy; PBS, pencil beam scanning; PS, passive scattering; IMRT, intensity-modulated radiotherapy; 3D-CRT, 3-dimensional conformal radiotherapy.
Death without progression
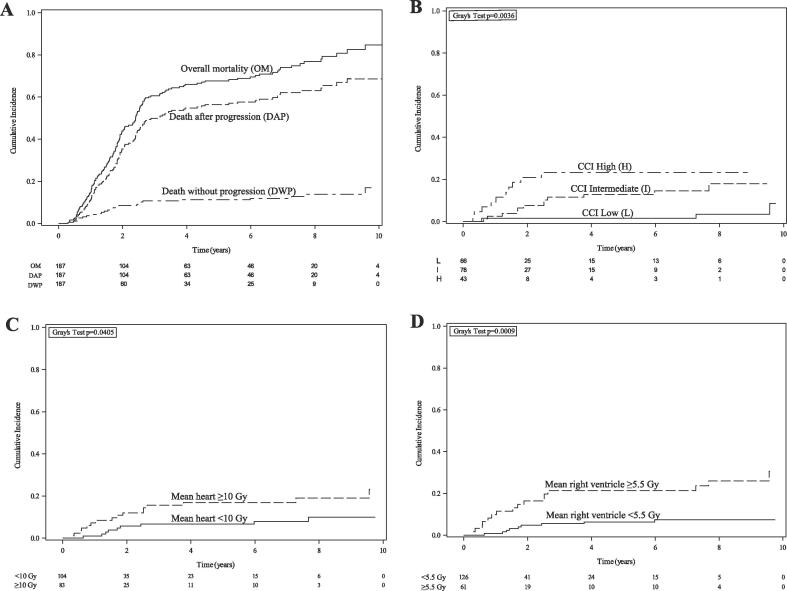
Median follow-up was 29.9 months (interquartile range [IQR], 16.4–71.8) for all patients and 7.3 years (IQR, 5.7–8.7) for survivors. Death occurred in 143 (76.5 %), including death after progression in 118 (63.1 %), and DWP in 25 patients (13.4 %) (Fig. 1A). 1-, 2-, and 5-year cumulative incidence of DWP was 3.7 %, 8.6 %, and 11.2 %, respectively. DWP was attributed to underlying comorbidity (n = 6, 24 %), infection (n = 6, 24 %), out-of-hospital cardiopulmonary arrest (n = 4, 16 %), chemoradiation toxicity (n = 2, 8 %), and unknown (n = 7, 28 %) (Table 2). Of the 25 patients with DWP, baseline cardiovascular, pulmonary, and either cardiovascular or pulmonary comorbidities were present in 15 (60 %), 14 (56 %), and 20 patients (80 %), respectively (Supplementary Table 1).
Fig. 1.
(A) Cumulative incidence of overall mortality, death after progression, and death without progression. (B-D) Death without progression stratified by (B) Charlson Comorbidity Index (CCI) (low = 0–2; intermediate = 3–4; high = 5–9), (C) mean heart dose ≥ 10 Gy, and (D) mean right ventricle dose ≥ 5.5 Gy.
Table 2.
Presumed causes of death without progression (N = 25).
| Cause of death | N |
|---|---|
| Pre-existing comorbidity | |
| COPD (pre-existing) | 1 |
| COPD (pre-existing) + pulmonary embolism (new) | 1 |
| Heart failure (pre-existing) | 1 |
| COPD (pre-existing) + heart failure (new) | 1 |
| Heart failure (pre-existing) + renal failure (pre-existing CKD) +/- pneumonitis | 1 |
| IPF (pre-existing) + pneumonia | 1 |
| Infection | |
| Pneumonia | 4 |
| Undifferentiated sepsis | 2 |
| Out-of-hospital cardiopulmonary arrest | 4 |
| Chemoradiation toxicity | |
| Pneumonitis +/- pneumonia | 1 |
| Esophagopleural fistula | 1 |
| Unknown | 7 |
COPD, chronic obstructive pulmonary disease; IPF, interstitial pulmonary fibrosis.
DWP (Fig. 1B-C), but not death after progression (Supplementary Fig. 1A-B), increased with higher CCI strata and MHD ≥ 10 Gy (5-year DWP rate, 16.9 % vs 6.7 %, p = 0.04). On univariable analysis, age, ECOG performance status, CCI strata, and MHD were associated with an increased risk of DWP (Table 3). On multivariable analysis, MHD (sHR 1.06/Gy, 95 % CI 1.02–1.10, p = 0.002) retained significance when paired with CCI strata (sHR 2.60/stratum, 95 % CI 1.50–4.49, p < 0.001). There was no significant interaction between CCI strata and MHD (interaction p = 0.73). There was a significant interaction between proton therapy and MHD (interaction p = 0.024): MHD was associated with DWP among those receiving photon therapy (sHR 1.06/Gy, 95 % CI 1.03–1.10, p < 0.001) but not proton therapy (sHR 0.87/Gy, 95 % CI 0.73–1.03, p = 0.1).
Table 3.
Fine-Gray regression for death without progression and Cox regression for overall survival.
| Variable | Univariable |
Multivariable |
||
|---|---|---|---|---|
| Death without progression | sHR (95 % CI) | P | sHR (95 % CI) | P |
| Age (y) | 1.07 (1.03–1.11) | <0.001 | ||
| Female sex | 0.97 (0.45–2.11) | 0.94 | ||
| ECOG PS (stratum) | 2.16 (1.21–3.85) | 0.009 | ||
| Cardiovascular comorbidity | 1.90 (0.85–4.25) | 0.12 | ||
| Pulmonary comorbidity | 1.93 (0.88–4.22) | 0.099 | ||
| CCI (stratum) | 2.23 (1.35–3.69) | 0.002 | 2.60 (1.50–4.49) | <0.001 |
| Adenocarcinoma (vs all else) | 1.06 (0.40–2.82) | 0.90 | ||
| N3 (vs all else) | 1.68 (0.72–3.92) | 0.23 | ||
| Left-sided primary | 1.00 (0.45–2.21) | 1 | ||
| RT dose (Gy) | 1.14 (0.97–1.33) | 0.11 | ||
| Proton therapy (vs all else) | 0.50 (0.22–1.12) | 0.092 | ||
| Proton therapy (vs IMRT) | 0.50 (0.21–1.17) | 0.11 | ||
| Internal target volume (per 100 cc) | 0.97 (0.84–1.12) | 0.67 | ||
| Mean heart dose (Gy) | 1.05 (1.01–1.08) | 0.007 | 1.06 (1.02–1.10) | 0.002 |
| Mean lung dose (Gy) | 0.97 (0.89–1.06) | 0.45 | ||
| Carbo/taxol (vs all else) | 2.03 (0.85–4.82) | 0.11 | ||
| Overall survival | HR (95 % CI) | P | HR (95 % CI) | P |
| Age (y) | 1.03 (1.02–1.05) | <0.001 | 1.04 (1.02–1.06)2 | <0.001 |
| Female sex | 0.82 (0.59–1.14) | 0.24 | ||
| ECOG PS (stratum) | 1.67 (1.25–2.30) | <0.001 | 1.59 (1.18–2.16)2 | 0.003 |
| Cardiovascular comorbidity | 1.28 (0.92–1.78) | 0.15 | ||
| Pulmonary comorbidity | 1.42 (1.02–1.98) | 0.040 | 1.48 (1.05–2.09)2 | 0.027 |
| CCI (stratum) | 1.67 (1.33–2.09) | <0.001 | ||
| Adenocarcinoma (vs all else) | 0.89 (0.60–1.33) | 0.89 | ||
| N3 (vs all else) | 1.21 (0.82–1.78) | 0.34 | ||
| Left-sided primary | 1.07 (0.77–1.50) | 0.68 | ||
| RT dose (Gy) | 0.97 (0.91–1.03) | 0.36 | ||
| Proton therapy (vs all else) | 1.09 (0.78–1.53) | 0.61 | ||
| Proton therapy (vs IMRT) | 1.12 (0.78–1.61) | 0.54 | ||
| Internal target volume (per 100 cc) | 1.08 (1.03–1.14) | 0.002 | 1.10 (1.03–1.16)2 | 0.002 |
| Mean heart dose (Gy) | 1.02 (1.01–1.04) | 0.002 | 1.03 (1.01–1.05)1 | 0.010 |
| Mean lung dose (Gy) | 1.04 (1.00–1.09) | 0.041 | 1.05 (1.01–1.10)2 | 0.027 |
| Carbo/taxol (vs all else) | 1.11 (0.80–1.55) | 0.52 | ||
sHR, subdistribution hazard ratio; y, years; PS, performance status; CCI, Charlson Comorbidity Index; RT, radiotherapy; IMRT, intensity-modulated radiotherapy; carbo/taxol, carboplatin/paclitaxel; HR, hazard ratio.
Model with mean heart dose, age, ECOG PS, pulmonary comorbidity, internal target volume.
Model with mean lung dose, age, ECOG PS, pulmonary comorbidity, internal target volume.
Overall survival
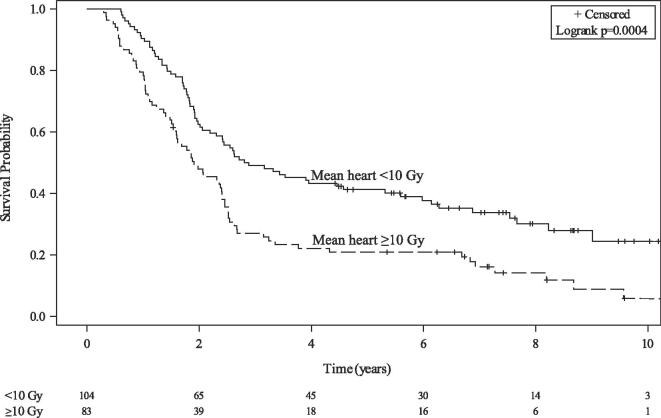
Median OS was 28.9 months (95 % CI, 23.5–31.6) and lower for those with MHD ≥ 10 Gy (median, 22.9 vs 34.1 months, p < 0.001) (Fig. 2). On multivariable analysis, MHD (HR 1.03/Gy, 95 % CI 1.01–1.05, p = 0.010) was associated with inferior OS after accounting for age, ECOG performance status, pulmonary comorbidity, and ITV. MLD was also associated with inferior OS in a separate multivariable model (Table 3).
Fig. 2.
Overall survival stratified by mean heart dose ≥ 10 Gy.
Associations between cardiovascular substructure dose and either DWP or OS
On univariable analysis, dose to the right ventricle, left ventricle, ventricles, pericardium, and heart were associated with DWP (Supplementary Table 2). Mean right ventricle dose (AIC 229; lowest), ventricles V5 (AIC 235; second lowest), heart V5, mean pericardium dose, and left ventricle V5 were associated with an increased risk of DWP in separate multivariable models that included CCI (Table 4). Model rankings were similar using an alternative set of multivariable models that incorporated age and ITV instead of CCI (Supplementary Table 3). Mean right ventricle cutpoint of 5.5 Gy was identified; this threshold predicted for DWP (Fig. 1D) but not death after progression (Supplementary Fig. 1C).
Table 4.
Exploratory associations between cardiovascular substructure dose and either death without progression or overall survival1.
| Dosimetric parameter | Multivariable analysis2 |
||
|---|---|---|---|
| Death without progression | sHR (95 % CI) | P | AIC |
| Mean right ventricle dose (Gy) | 1.08 (1.04–1.11) | <0.001 | 229 |
| Ventricles V5 (%) | 1.02 (1.01–1.03) | <0.001 | 235 |
| Heart V5 (%) | 1.02 (1.01–1.04) | 0.002 | 236 |
| Mean pericardium dose (Gy) | 1.07 (1.03–1.11) | <0.001 | 236 |
| Left ventricle V5 (%) | 1.02 (1.01–1.03) | 0.002 | 239 |
| Overall survival | HR (95 % CI) | P | AIC |
| Left atrium V5 (%) | 1.01 (1.00–1.02) | 0.003 | 1277 |
| Atria V5 (%) | 1.01 (1.00–1.02) | 0.004 | 1278 |
| Pericardium V5 (%) | 1.01 (1.00–1.02) | 0.008 | 1279 |
| Right atrium V5 (%) | 1.01 (1.00–1.01) | 0.010 | 1280 |
| Mean heart dose (Gy) | 1.03 (1.01–1.05) | 0.010 | 1280 |
| Right ventricle V5 (%) | 1.01 (1.00–1.01) | 0.016 | 1281 |
| Left ventricle V50 (%) | 1.02 (1.00–1.03) | 0.014 | 1281 |
| Ventricles V5 (%) | 1.01 (1.00–1.01) | 0.053 | 1283 |
sHR, subdistribution hazard ratio; AIC, Akaike information criterion; Vx, volume receiving ≥ x Gy; HR, hazard ratio.
Shown are only those dosimetric parameters that were significant on univariable analysis. In addition to 44 total cardiovascular dosimetric parameters (11 cardiovascular structures, 4 parameters per structure), mean lung dose was tested.
For death without progression, each dosimetric parameter was tested in a separate multivariable model with Charlson Comorbidity Index. For overall survival, each dosimetric parameter was tested in a separate multivariable model with age, ECOG performance status, pulmonary comorbidity, and internal target volume.
On univariable analysis, dose to the left atrium, right atrium, atria, pericardium, heart, right ventricle, left ventricle, and ventricles were associated with OS (Supplementary Table 2). Left atrium V5 (AIC 1277; lowest), atria V5 (AIC 1278; second lowest), pericardium V5, right atrium V5, mean heart dose, right ventricle V5, left ventricle V50, and ventricles V5 were associated with inferior OS in separate multivariable models (Table 4).
External cohort
In the external cohort, median follow-up was 18.7 months (IQR, 8.2–36.6) for all patients and 36.6 months (IQR, 26.6–51.9) for survivors. DWP occurred in 19 of 140 patients (13.6 %). Right ventricle V30, ventricles V30, heart V30, and left ventricle V30 were associated with DWP after adjusting for age (Supplementary Table 4), whereas doses to atria, right atrium, and left atrium were not. Mean right ventricle dose ≥ 5.5 Gy was associated with numerically but not significantly higher cumulative incidence of DWP (2-year rate, 13.4 % vs 6.3 %, p = 0.35). No cardiac substructure dosimetric parameters were associated with OS.
Discussion
Among patients with NSCLC treated with CRT, we describe three main findings: 1) DWP occurred in 13 % of patients; 2) After controlling for baseline comorbidity burden, MHD and dose to specific cardiovascular substructures (right ventricle, ventricles, left ventricle, pericardium) were associated with DWP; and 3) Cardiovascular substructure dose showed associations with DWP distinct from those seen with OS. For DWP, the critical structures identified in the primary cohort were confirmed in the external cohort, but additional work is needed to determine the optimal dose-volume constraints for these structures. Our results support DWP as an endpoint to consider in studies assessing the significance of cardiovascular substructure dose for NSCLC.
Patients with NSCLC frequently present with baseline cardiopulmonary comorbidities (67.9 % in this cohort), and comorbidity burden is a known negative prognostic factor [23], [24]. In our cohort, both CCI and MHD ≥ 10 Gy were associated with an increased risk of DWP, but not death after progression, supporting the utility of DWP in assessing the effects of comorbidity and CRT toxicity on longevity. Although pre-existing comorbidity was listed as a cause of DWP in only 6/25 patients, it likely contributed, at least partially, to DWP among many of the remaining cases (based on Supplementary Table 1). No significant interaction was observed between CCI strata and MHD (ie, the association between MHD and DWP did not differ based on CCI stratum); however, power was limited.
Prior studies attempting to identify the most dose-sensitive cardiovascular substructures in LA-NSCLC have focused on OS and cardiac events [9], [11], [12], [13] (Supplementary Table 5). In our cohort, we found that right ventricle, ventricles, heart, pericardium, and left ventricle dose were associated with both DWP and OS, whereas left atrium, atria, and right atrium dose were associated with OS but not DWP. Thus, DWP may be a more specific endpoint and can provide additional dose-toxicity information beyond OS. Though mean right ventricle dose had the lowest AIC for prediction of DWP, this finding is hypothesis-generating given the limited number of events and lack of more robust model-selection methods.
RT likely has a multi-faceted effect on the cardiovascular system that could explain an association with DWP. RT potentially leads to early microvascular changes and perfusion defects [25], [26], impaired ventricle ejection fraction [27], and may affect circulating immune cells in the blood to increase the risk of immunosuppression [6], [28]. Additionally, the association with right ventricle dose raises the question of underdiagnosed pulmonary hypertension in this patient population [29]. Conceivably, any of these factors could weaken a patient’s cardiopulmonary and immune system and increase their susceptibility to succumb to infection, comorbidity exacerbation, or another event unrelated to cancer progression.
Efforts to reduce RT dose to the heart appear warranted. Based on our findings and those from Atkins et al. [8], we advocate for a mean heart dose < 10 Gy, below the currently recommended threshold of 20 Gy [30]. Efforts toward plan optimization and standardization of cardiac dose constraints may reduce cardiac dose without comprising tumor coverage or other normal tissue constraints [31], [32]. Specific recommendations for cardiovascular substructure dose thresholds appear less obvious. For select tumors abutting the heart, proton therapy may significantly reduce heart dose [33]. Notably, in our cohort proton therapy was associated with a marginally lower risk of DWP, lower MHD (median, 6.7 Gy vs 15 Gy, Wilcoxon rank-sum p < 0.001), and lower dose to multiple cardiovascular substructures (eg, right ventricle mean dose 0.03 Gy vs 7.7 Gy, p < 0.001, Supplementary Table 6). Given the possibility of selection bias and non-significant difference in DWP, these observations should be interpreted with caution. A secondary analysis of the ongoing RTOG 1308 trial could assess differences in DWP between the proton and photon groups.
Patients in this cohort were treated prior to approval of consolidation with durvalumab. With improved disease control and OS seen with durvalumab [15], the risk of NSCLC progression and death from NSCLC is expected to decrease and the relative importance of baseline comorbidity and heart dose on longevity may increase.
There are limitations to this work. First, the study was retrospective with a limited number of DWP events, restricting the ability to control for potential confounders beyond CCI (eg, chemotherapy regimen) and introducing the possibility of unmeasured confounders. Given the limited number of events, we restricted our analysis to four dosimetric parameters per cardiovascular structure (mean, V5, V30, V50), realizing that other parameters (e.g., maximum dose, minimum dose to the hottest x% volume [Dx%]) may be more predictive. Second, DWP does not account for toxicity or death from intercurrent disease that may occur after disease progression (limiting sensitivity), and includes death from causes such as second cancers or accidents unrelated to comorbidity or CRT (limiting specificity). However, we did not observe any causes of DWP that were clearly unrelated to comorbidity or CRT. Furthermore, DWP removes the often-subjective nature of attributing causes of death (i.e., any patient without disease progression at the time of death experiences DWP). Third, this work assumes that heart dose should not increase the risk of disease progression, but emerging evidence suggests that effective dose to immune cells, which factors in heart dose, may be associated with worse disease control [34], [35]. Fourth, left anterior descending coronary artery (LAD) dose statistics were not available as LAD was not included in our autocontouring model. LAD V15 has been associated with major adverse cardiac events and worse OS after CRT [11], [36], and should be included in future cardiotoxicity studies. Fifth, the external cohort should not be interpreted as a validation cohort since certain variables were unavailable (e.g., CCI) and different dose metrics emerged as predictive (e.g., RV V30 instead of mean). Nevertheless, it suggests a degree of external validity to our findings.
Conclusion
In conclusion, baseline comorbidity, MHD, and dose to several cardiovascular substructures were associated with DWP after CRT for NSCLC. Future studies should consider using DWP as an endpoint when assessing the significance of cardiovascular substructure dose.
Funding
There was no research support for this study
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2023.100581.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bradley J.D., Paulus R., Komaki R., Masters G., Blumenschein G., Schild S., et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial p. Lancet Oncol. 2015;16(2):187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley J.D., Hu C., Komaki R.R., Masters G.A., Blumenschein G.R., Schild S.E., et al. Long-Term Results of NRG Oncology RTOG 0617: Standard- Versus High-Dose Chemoradiotherapy With or Without Cetuximab for Unresectable Stage III Non–Small-Cell Lung Cancer. J Clin Oncol. 2020;38(7):706–714. doi: 10.1200/JCO.19.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dess R.T., Sun Y., Matuszak M.M., Sun G., Soni P.D., Bazzi L., et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non–Small-Cell Lung Cancer. J Clin Oncol. 2017;35(13):1395–1402. doi: 10.1200/JCO.2016.71.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speirs C.K., Dewees T.A., Rehman S., Molotievschi A., Velez M.A., Mullen D., et al. Heart Dose Is an Independent Dosimetric Predictor of Overall Survival in Locally Advanced Non-Small Cell Lung Cancer. J Thorac Oncol. 2017;12(2):293–301. doi: 10.1016/j.jtho.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 5.Wang K., Eblan M.J., Deal A.M., Lipner M., Zagar T.M., Wang Y., et al. Cardiac Toxicity After Radiotherapy for Stage III Non–Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387–1394. doi: 10.1200/JCO.2016.70.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contreras J.A., Lin A.J., Weiner A., Speirs C., Samson P., Mullen D., et al. Cardiac dose is associated with immunosuppression and poor survival in locally advanced non-small cell lung cancer. Radiother Oncol. 2018;128(3):498–504. doi: 10.1016/j.radonc.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Yegya-Raman N., Wang K., Kim S., Reyhan M., Deek M.P., Sayan M., et al. Dosimetric Predictors of Symptomatic Cardiac Events After Conventional-Dose Chemoradiation Therapy for Inoperable NSCLC. J Thorac Oncol. 2018;13(10):1508–1518. doi: 10.1016/j.jtho.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkins K.M., Rawal B., Chaunzwa T.L., Lamba N., Bitterman D.S., Williams C.L., et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients With Lung Cancer. J Am Coll Cardiol. 2019;73(23):2976–2987. doi: 10.1016/j.jacc.2019.03.500. [DOI] [PubMed] [Google Scholar]
- 9.McWilliam A., Kennedy J., Hodgson C., Vasquez Osorio E., Faivre-Finn C., van Herk M. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur J Cancer. 2017;85:106–113. doi: 10.1016/j.ejca.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 10.Wang K., Pearlstein K.A., Patchett N.D., Deal A.M., Mavroidis P., Jensen B.C., et al. Heart dosimetric analysis of three types of cardiac toxicity in patients treated on dose-escalation trials for Stage III non-small-cell lung cancer. Radiother Oncol. 2017;125(2):293–300. doi: 10.1016/j.radonc.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkins K.M., Chaunzwa T.L., Lamba N., Bitterman D.S., Rawal B., Bredfeldt J., et al. Association of Left Anterior Descending Coronary Artery Radiation Dose With Major Adverse Cardiac Events and Mortality in Patients With Non-Small Cell Lung Cancer. JAMA Oncol. 2021;7(2):206. doi: 10.1001/jamaoncol.2020.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thor M., Deasy J.O., Hu C., Gore E., Bar-Ad V., Robinson C., et al. Modeling the Impact of Cardiopulmonary Irradiation on Overall Survival in NRG Oncology Trial RTOG 0617. Clin Cancer Res. 2020;26(17):4643–4650. doi: 10.1158/1078-0432.CCR-19-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McWilliam A., Khalifa J., Vasquez Osorio E., Banfill K., Abravan A., Faivre-Finn C., et al. Novel Methodology to Investigate the Effect of Radiation Dose to Heart Substructures on Overall Survival. Int J Radiation Oncol Biol Phys. 2020;108(4):1073–1081. doi: 10.1016/j.ijrobp.2020.06.031. [DOI] [PubMed] [Google Scholar]
- 14.Machtay M., Lee J.H., Shrager J.B., Kaiser L.R., Glatstein E. Risk of Death From Intercurrent Disease Is Not Excessively Increased by Modern Postoperative Radiotherapy for High-Risk Resected Non–Small-Cell Lung Carcinoma. J Clin Oncol. 2001;19(19):3912–3917. doi: 10.1200/JCO.2001.19.19.3912. [DOI] [PubMed] [Google Scholar]
- 15.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 16.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y.-Q., Gou R., Diao Y.-S., Yin Q.-H., Fan W.-X., Liang Y.-P., et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58–66. doi: 10.1631/jzus.B1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jędrzejczyk M., Foryś W., Czapla M., Uchmanowicz I. Relationship between Multimorbidity and Disability in Elderly Patients with Coexisting Frailty Syndrome. Int J Environ Res Public Health. 2022;19(6):3461. doi: 10.3390/ijerph19063461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apte A.P., Iyer A., Thor M., Pandya R., Haq R., Jiang J., et al. Library of deep-learning image segmentation and outcomes model-implementations. Phys Med. 2020;73:190–196. doi: 10.1016/j.ejmp.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haq R., Hotca A., Apte A., Rimner A., Deasy J.O., Thor M. Cardio-pulmonary substructure segmentation of radiotherapy computed tomography images using convolutional neural networks for clinical outcomes analysis. Physics Imaging Radiation Oncol. 2020;14:61–66. doi: 10.1016/j.phro.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng M., Moran J.M., Koelling T., Chughtai A., Chan J.L., Freedman L., et al. Development and Validation of a Heart Atlas to Study Cardiac Exposure to Radiation Following Treatment for Breast Cancer. Int J Radiation Oncol Biol Phys. 2011;79(1):10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Contal C., O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30(3):253–270. [Google Scholar]
- 23.Islam K.M.M., Jiang X., Anggondowati T., Lin G., Ganti A.K. Comorbidity and Survival in Lung Cancer Patients. Cancer Epidemiol Biomark Prev. 2015;24(7):1079–1085. doi: 10.1158/1055-9965.EPI-15-0036. [DOI] [PubMed] [Google Scholar]
- 24.Tammemagi C.M., Neslund-Dudas C., Simoff M., Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer. 2003;103(6):792–802. doi: 10.1002/ijc.10882. [DOI] [PubMed] [Google Scholar]
- 25.Marks L.B., Yu X., Prosnitz R.G., Zhou S.M., Hardenbergh P.H., Blazing M., et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63(1):214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P., Hu X., Yue J., Meng X., Han D., Sun X., et al. Early detection of radiation-induced heart disease using 99mTc-MIBI SPECT gated myocardial perfusion imaging in patients with oesophageal cancer during radiotherapy. Radiother Oncol. 2015;115(2):171–178. doi: 10.1016/j.radonc.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Hatakenaka M., Yonezawa M., Nonoshita T., Nakamura K., Yabuuchi H., Shioyama Y., et al. Acute cardiac impairment associated with concurrent chemoradiotherapy for esophageal cancer: magnetic resonance evaluation. Int J Radiat Oncol Biol Phys. 2012;83(1):e67–e73. doi: 10.1016/j.ijrobp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Ladbury C.J., Rusthoven C.G., Camidge D.R., Kavanagh B.D., Nath S.K. Impact of Radiation Dose to the Host Immune System on Tumor Control and Survival for Stage III Non-Small Cell Lung Cancer Treated with Definitive Radiation Therapy. Int J Radiation Oncol Biol Phys. 2019;105(2):346–355. doi: 10.1016/j.ijrobp.2019.05.064. [DOI] [PubMed] [Google Scholar]
- 29.Yang X., Wang L., Lin L., Liu X. Elevated Pulmonary Artery Systolic Pressure is Associated with Poor Survival of Patients with Non-Small Cell Lung Cancer. Cancer Manag Res. 2020;12:6363–6371. doi: 10.2147/CMAR.S260857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NCCN Clinical Practice Guidelines in Oncology - Non-Small Cell Lung Cancer - Version 03.2022. 2022.
- 31.Herr D.J., Hochstedler K.A., Yin H., Dess R.T., Matuszak M., Grubb M., et al. Effect of Education and Standardization of Cardiac Dose Constraints on Heart Dose in Patients With Lung Cancer Receiving Definitive Radiation Therapy Across a Statewide Consortium. Pract Radiat Oncol. 2022;12(5):e376–e381. doi: 10.1016/j.prro.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Turtle L., Bhalla N., Willett A., Biggar R., Leadbetter J., Georgiou G., et al. Cardiac-sparing radiotherapy for locally advanced non-small cell lung cancer. Radiat Oncol. 2021;16(1) doi: 10.1186/s13014-021-01824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yegya-Raman N., Zou W., Nie K., Malhotra J., Jabbour S.K. Advanced radiation techniques for locally advanced non-small cell lung cancer: intensity-modulated radiation therapy and proton therapy. J Thorac Dis. 2018;10(S21):S2474–S2491. doi: 10.21037/jtd.2018.07.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCall N.S., McGinnis H.S., Janopaul-Naylor J.R., Kesarwala A.H., Tian S., Stokes W.A., et al. Impact of radiation dose to the immune cells in unresectable or stage III non-small cell lung cancer in the durvalumab era. Radiother Oncol. 2022;174:133–140. doi: 10.1016/j.radonc.2022.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Jin J.-Y., Hu C., Xiao Y., Zhang H., Paulus R., Ellsworth S.G., et al. Higher Radiation Dose to the Immune Cells Correlates with Worse Tumor Control and Overall Survival in Patients with Stage III NSCLC: A Secondary Analysis of RTOG0617. Cancers. 2021;13(24):6193. doi: 10.3390/cancers13246193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKenzie E., Zhang S., Zakariaee R., Guthier C.V., Hakimian B., Mirhadi A., et al. Left Anterior Descending Coronary Artery Radiation Dose Association With All-Cause Mortality in NRG Oncology Trial RTOG 0617. Int J Radiation Oncol Biol Phys. 2022 doi: 10.1016/j.ijrobp.2022.11.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.