Abstract
Effects of antimicrobial peptides (AMP) added to diets on aquatic animal health and body function are influenced by multiple factors such as animal species, initial body weight, the dosage of AMP and feeding duration. However, there is limited knowledge on the relationship between these factors and the body function of aquatic animals. Here, we aimed to perform multiple meta-analyses to investigate the effects of dietary AMP on growth performance (feed conversion ratio [FCR], specific growth rate [SGR]), enzyme activity (superoxide dismutase activity [SOD], lysozyme activity [LSA]), disease resistance (cumulative survival rate [CSR], the expression of immune-related genes [GENE]) and the abundance of gut microbiota (MICRO) from a pool of empirical studies. Additionally, the dose-effect model was applied to determine the optimal AMP dose, initial body weight and feeding duration to maximize body function. To conduct the meta-analyses, we included 34 publications that estimated 705 effect sizes across 21 fish, 2 shrimp and 2 shellfish species. The results confirmed that the inclusion of AMP in the diet can significantly improve SGR, SOD, LSA, CSR and GENE and decrease FCR for aquatic animals. Interestingly, our findings implied a slight positive effect of AMP on MICRO albeit with a limited number of studies available on fish gut microbial communities. Although no significant linear or quadratic relationship was predicted by meta-regression, the dose-effect indicated that the optimal AMP doses for FCR, SGR, SOD and LSA were 707.5, 750.0, 1,050.0 and 937.5 mg/kg, respectively. Taken together, fish with an initial body weight of 30 g could be fed with a dose of 600 to 800 mg/kg for 2 mo when AMP-supplemented diets were applied in aquaculture, which can effectively improve body function and health while lowering aquafeed costs. In addition, more studies should address fish gut microbiota to delimitate the influence of dietary AMP on MICRO in the future.
Keywords: Fish, AMP, Oral administration, Feed additive, Dose-effect, Immune
1. Introduction
Proliferating human populations and the demands for nutritious food have led to the realization that aquaculture is one of the most environmentally sustainable ways to produce food and protein (Naylor et al., 2021). In 2018, world aquaculture supplied around 82.1 million tonnes of fish, encompassing more than 425 farmed species, and bringing it to an all-time high (FAO, 2020). Despite impressive achievements, the aquaculture industry still faces persistent challenges. In particular, drug resistance and residues from indiscriminate use of antibiotics are one of the major concerns for sustainable aquaculture (Cabello, 2006; Dong et al., 2007; Karunasagar et al., 2020). Recently, FAO published a detailed five-year plan (from 2021 to 2025) to restrict the use of antibiotic-like drugs or medical-important antibiotics in animal production (FAO, 2021). Although antibiotic-free aquaculture might be overly optimistic as a goal, fortunately, numerous studies are being done to find alternatives to antibiotics. Antimicrobial peptides (AMP), promising alternatives to antibiotics (FAO, 2021), also known as cationic host defense peptides originated from virtually all domains of life, are naturally occurring innate-immune peptides with immunocompetence and homeostasis against bacteria, viruses, fungi, and parasites (Hancock, 2001; Wang et al., 2016, 2022). Currently, AMP are explored to be used in food preservation (Said et al., 2019), as therapeutic drugs for human beings (Mookherjee et al., 2020), and as feed additives for functional nutrition for animals (Silveira et al., 2021).
Functional nutrition involves not only providing an appropriate amount of nutrients for critical body functions but also considering its influence on host defense and disease resistance (Smits et al., 2021). This knowledge can establish AMP-dietary strategies to reduce the demand for antibiotics while still increasing disease resistance in animal nutrition. In aquaculture, the administration of AMP-supplemented diets to strengthen fish health and improve their resistance to invading pathogens has yielded promising results in recent years. Hitherto, 34 papers have been collected on the investigation of the effects of dietary AMP in aquatic animals, covering 16 AMP that were applied in 25 species, and lactoferrin and cecropin were the most studied AMP as feed additives (Fig. 1A). Among them, 27 reports involved the pathogen-infection experiments, and the results indicated that these AMPs exhibited inhibitory effects on 12 pathogens, dominated by Aeromonas hydrophila (Fig. 1B). An increasing number of studies suggested that the dietary supplementation of AMP boosted growth performance (Ge et al., 2020; Liu et al., 2020; Jan et al., 2021), antioxidant and immuno-physiological responses (Morshedi et al., 2021; Wang et al., 2021), and disease resistance (Esmaeili et al., 2019; Dai et al., 2020; Hu et al., 2021) in fish. Likewise, these positive effects were observed in shrimp and shellfish (Chand et al., 2006; Chen et al., 2020; Gyan et al., 2020; Mi et al., 2022). Recently, taking advantage of 16 S amplicon sequencing technology, the identification of intestinal microbiota and health is not limited to morphological observation and bacterial counts. The new sequencing method can effectively reveal changes in the abundance and diversity of gut microflora that can be evaluated by alpha diversity (within-community richness and diversity), including Chao 1 and Observed-species for richness, Shannon, Simpson and Abundance-based Coverage Estimator (ACE) for diversity as well as beta diversity (between-Habitatat diversity) (Liu et al., 2020). These parameters are closely related to the health of aquatic animals, and an increase in the index may mean an improvement in health conditions. Ting et al. (2019) elucidated that the intestinal immunity of grouper (Epinephelus lanceolatus) was improved when tilapia hepcidin 2-3 was applied in aquafeeds, and similar results were observed in the turbot (Scophthalmus maximus L.) using dietary cecropin with a moderate dose (Dai et al., 2020).
Fig. 1.
A summary of the current application of antimicrobial peptides (AMP) as feed additives in aquatic animals. (A) A total of 34 papers focused on the study of AMP additives, which covered 16 kinds of AMP (5 of those without specific names are classified as other AMP), 25 aquatic animals (21 fish, 2 shrimp and 2 shellfish). The inserted numbers on the pie chart indicate the number of publications. For example, 15 indicates that there are 15 papers studying the performance of aquatic animals using lactoferrin as a feed additive, and these publications cover 11 fish species and 1 shrimp. (B) A total of 27 studies related to disease resistance were validated through pathogen-challenge experiments, which included 12 pathogens, covering 21 aquatic animals (18 fish, 2 shrimp and 1 shellfish). For example, 10 demonstrates that 10 papers are focused on disease resistant against Aeromonas hydrophila employing AMP as feed additives, which cover 7 fish, 1 shrimp and 1 eel. APSH-07, an AMP from large yellow croaker; TP4, Tilapia piscidin 4; TH2-3, Tilapia hepcidin 2-3; IsCT, Isalo scorpion cytotoxic peptide; CM11, a small peptide with 11 residues derived from cecropin and melittin; AP1, an AMP from Bacillus subtilis B06; Other AMP, AMP not mentioned by names or sequences.
In aggregate, numerous studies support AMP as additives to effectively promote the growth and non-specific immune response in aquatic animals. However, different and even contradictory findings emerged due to the heterogeneity of independent studies. For example, compared to basal feeds, lactoferrin-added diets did not alter the growth performance or feed efficiency ratio in Nile tilapia (Oreochromis niloticus), orange-spotted grouper (Epinephelus coioides) and African cichlid (Sciaenochromis fryeri) (Yokoyama et al., 2006; Welker et al., 2007; Moradian et al., 2018), when a variety of AMP doses and feeding durations were applied. Eslamloo et al. (2012) and Khuyen et al. (2017) also suggested that dietary lactoferrin did not change the body-weight gain, lysozyme activity, serum peroxidase or bactericidal activity. In addition, Mi et al. (2022) stated that AMP-added diets did not alter the diversity of gut microbiota (Shannon and Simpson indices), but exhibited significantly negative effects on the richness (Chao 1 and Observed-species indices) in Yesso scallop (Patinopecten yessoensis). However, dietary hepcidin 2-3 increased Chao 1 index in E. lanceolatus compared to regular diets (Ting et al., 2019). In this case, if reliable conclusions can be drawn, in addition to conducting more studies to obtain data, it is particularly important to quantitatively analyze and summarize the existing findings.
Therefore, the overarching goal of this study is to quantitatively integrate empirical data regarding the use of AMP in aquaculture nutrition, and aims to: 1) systematically review the data for AMP application in aquaculture to evaluate the effect of AMP-enriched feed on body function and health, including growth performance (feed conversion ratio [FCR] and specific growth rate [SGR]), enzyme activity (superoxide dismutase activity [SOD], lysozyme activity [LSA]), disease resistance (cumulative survival rate [CSR] after pathogen infection, the expression of immune-related genes [GENE]) and the abundance of gut microbiota (MICRO) in aquatic animals; and 2) propose a realistic feeding plan in terms of the body weight of the individuals, the AMP dose, and the feeding duration when AMP-added feed is to be applied to aquaculture.
2. Methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2010).
2.1. Literature search
The complete search strategy was shown in the PRISMA flowchart (Appendix 1; Fig. S1). Searching and collection of literature were carried out on the internet databases including Web of Science (WOS), PubMed, Academic Search Premier (ASP) and Aquatic Sciences and Fisheries Abstracts (ASFA) using keywords (“food additives” OR “immunostimulants” OR “oral” OR “dietary”) AND (“antimicrobial peptide” OR “antimicrobial peptide gene”) AND (“aquaculture” OR “aquatic animals” OR “fish” OR “shellfish” OR “marine” OR “shrimp”) AND (“disease resistance” OR “growth” OR “immun∗” OR “physiological performance” OR “enzyme activit∗” OR “intestine microbio∗” OR “gut microbio∗” OR “microbio × community”), and the language was restricted to English. Additionally, relevant literature from empirical collections was also recruited and labeled as “additional records”. The last retrieval date was 4/5/2022 for these 4 databases. There were 594 articles initially obtained using the above-mentioned keywords and methods. To ensure the quality of the database, only articles from peer-reviewed journals were further assessed. After strict evaluation of abstract and full texts, 34 articles comprising a total of 705 data entries were used to develop the database as shown in Appendix 2.
2.2. Selection criteria
The inclusion criteria were the following. 1) Types and application scope of AMP. In the retrieved publications, AMP must be applied as feed additives for aquatic animals, and the name/type of AMP should be mentioned. If there is no specific description of AMP in one article, we will classify the type of AMP as “other AMP” but still use the available data from this study. 2) Dose and feeding duration of AMP. The dose and feeding duration of AMP used are critical to moderator meta-analysis, therefore, the collected literature should contain the dose of the treatment groups (AMP-supplemented) and the corresponding control group (AMP-less), and feeding duration as well. 3) Type of pathogens. If an article conducts pathogen-challenge experiments, the specific pathogen type and species name should be disclosed.
The exclusion criteria were the following. (1) AMP is not the only feed additive. For example, articles from Lygren et al. (1999), Ren et al. (2007) and Ulloa et al. (2016) used a compound of lactoferrin and vitamin C or soybean as an additive, and these studies were not within our scope of the investigation. In addition, the mixture of 2 AMP (Natucin C and Natucin P) is also out of evaluation (Chen et al., 2016). (2) AMP is not directly employed as a feed additive. Three studies have evaluated the fish performance by feeding the recombinant epinecidin-1 from Escherichia coli (Pan et al., 2012) and Artemia (Ting et al., 2018), and recombinant piscidin 4 from yeast (Pichia pastoris) (Huang et al., 2020), and these studies should also be excluded. (3) Data is incomplete. Some studies met all the above conditions but did not indicate either standard deviation (SD) or standard error (SE), and these 2 parameters could not be calculated from the existing data. Therefore, such studies could not be included in the meta-analysis. In addition, Kumari et al. (2003), Esteban et al. (2005), Chand et al. (2006) and Khuyen et al. (2017) carried out feeding trials with multiple periods, in this case, we only extracted data with the longest feeding duration.
2.3. Information extraction
The following data were extracted from each selected study: author information (first author, year), article title, AMP type, fish species, the dosage of AMP, sample size, initial and final body weight, experimental/feeding duration, and outcome data, including FCR, SGR, SOD, LSA, CSR, GENE or MICRO and corresponding SD or SE as well. Most of the original articles have given the data directly for other parameters, but for SGR, it is calculated using the formula SGR (% per day) = 100 × [ln (mean final body weight) − ln (mean initial body weight)]/time (days) based on the initial, final body weight of one specific aquatic animal and feeding duration from the reports. In addition, SD was calculated according to SD = SE × (n)1/2 if only SE and sample size (n) are presented in one publication. Besides, 4 studies (He et al., 2014; Khuyen et al., 2017; Ting et al., 2019; Gyan et al., 2020) presented the AMP dose as percentages, such as 0.1% and 1%. They were converted to 1,000 and 10,000 mg/kg, respectively. For those articles that only provided figures, we used online ImageJ (https://imagej.nih.gov/ij/) to extract data (mean and SD or SE).
2.4. Statistical analysis
All statistical analyses were performed via R packages in RStudio version 3.6.3 (2020-02-29). R codes were attached at the end of Appendix 1.
2.4.1. Effect size calculations
We aimed to evaluate the effects of AMP-supplemented feeds on aquatic animals' health based on the parameters of growth performance (FCR and SGR), enzyme activity (SOD and LSA), disease resistance (CSR and GENE) and MICRO. Here, Hedges'g (Appendix 1) served as the calculation of effect size for FCR, SGR, SOD, LSA, GENE, and MICRO, because they are continuous variables. However, in the pathogen infection experiment, the individual outcomes appeared as bivariate (dead or alive), and the log response ratio (LRR, Appendix 1) was used to calculate the effect size. First, we calculated the overall mean effect sizes with 95% confidence intervals (CI) and 95% prediction intervals (PI) to compare the effects of basal and AMP-enriched diets for each parameter using random-effect models. If the 95% CI does not span a zero value, it indicates that they are significantly different between these 2 diets. A positive value of mean effect size shows AMP-supplemented diets can improve aquatic animals' performance. Conversely, a negative value indicates that the performance declined with the presence of AMP supplements in a diet. The heterogeneity of studies was estimated with I2 statistic (Higgins and Thompson, 2002), and I2 > 50% indicated a substantial among-study heterogeneity appeared. Moderator tests in the following analysis were conducted.
2.4.2. Publication bias and sensitivity analyses
Funnel plots were produced to evaluate the publication bias, and classical Egger's test was conducted for funnel plot asymmetry. A P value < 0.05 implies that the funnel plot is asymmetric, and potential publication bias may exist. In this case, Tweedie's nonparametric “trim and fill” procedure (Duval and Tweedie, 2000) was taken to further determine whether random-generated studies needed to be implemented to eliminate publication bias. To check the stability of the estimated measures among studies, the influence and leave-one-out diagnostics (Viechtbauer and Cheung, 2010) as a combined approach were implemented to test sensitivity.
2.4.3. Moderator analysis
A large value I2 > 50% indicates that high heterogeneity exists across studies, and requires regrouping for further moderator analysis. Combining the collected data from original publications, we can see the initial body weight (IBW) of animals, the dose of AMP and the feeding duration have potential effects on primary response variables, FCR, SGR, CSR, SOD and LSA. However, there were more than 10 levels of each predictor variable (IBW, AMP dose and feeding duration), therefore, we simplified and regrouped each predictor variable for moderator analysis as the following: 1) Aquatic animals of various weights were used in recruited studies, with an IBW range of 0.21 to 86.83 g. We sub-grouped them into 3 levels: small, IBW ≤ 15 g; medium, 15 < IBW ≤30 g; and large, IBW > 30 g. 2) Although diverse doses of AMP were adopted in these publications, we set the minimum and maximum dose as low and high level respectively, and the remaining dose-groups were medium level for each study. 3) Similarly, different studies usually use various feeding durations ranging from 28 to 84 d. We re-divided them into 3 subgroups: 1 mo (feeding duration ≤35 d), 2 mo (35 < feeding duration ≤65 d) and more than 2 mo (feeding duration > 65 d). Species-moderator analysis was not performed for these 5 response variables due to the large variation in the number of species (21 fish species, 2 shrimp and 2 shellfish).
Immune-related gene expression was highly specific, especially in tissue and time. In addition, different genes also show different expression patterns (Wang et al., 2022). Therefore, gene-, tissue- and time-moderator analysis were assessed for GENE, respectively. Currently, 37 genes in total have been investigated (Appendix 2). To increase the sample size, homologous genes were grouped into broader categories (e.g., interleukin 1 b [il-1b], il-4, il-6, il-8, il-10 and il-11 belong to il gene; signal transducer and activator of transcription 1 [stat 1], stat 2, stat3a, stat3b1, stat3b2, stat 4, stat5b and stat 6 were grouped into stat and so on.), and all genes with the number of effect size below 20 were classified as “Other”. In this case, 6 subgroups (il, stat, tumor necrosis factor α [tnf-α], myxovirus resistant [mx], caspase 3 and other) were set up for the gene-moderator analysis. The liver, kidney, spleen and intestine are important immune organs and the expression of numerous immune-related genes has been widely reported in these tissues. Thus, these 4 tissues were used as different units for the tissue-moderator test. Similarly, 3 groups with a 24-h interval (0, 24 and 48 h) were selected for the time-variant moderation analysis.
With respect to MICRO, microflora structure- and species-moderator analyses were applied to determine whether the effects of dietary AMP on the gut microbiota have specific patterns in terms of microflora structure and species.
2.4.4. Meta-regression and dose–response meta-analysis
As mentioned above, various IBWs of aquatic animals, AMP doses and feeding duration of administration were used in different studies, and these variables were all continuous. Therefore, a meta-regression was conducted to determine if there was a linear or quadratic relationship between IBW, AMP dose, feeding duration, and the primary response parameters (FCR, SGR, SOD and LSA). Furthermore, the dose-effect was determined by harnessing a dose–response meta-analysis to figure out the optimal dose for these 4 parameters.
3. Results
A total of 90 figures from 25 articles including line charts, histograms and boxplots were extracted and used for the current meta-analysis (Appendix 3). The recruited dataset included 705 effect sizes (k = 77 for FCR, k = 66 for SGR, k = 44 for SOD, k = 62 for LSA, k = 49 for CSR, k = 378 for GENE and k = 29 for MICRO) from 34 studies that covered 25 aquatic animals, including 21 fish (Kumari et al., 2003; Esteban et al., 2005; Yokoyama et al., 2006; Welker et al., 2007; Zhou et al., 2008; Welker et al., 2010; Eslamloo et al., 2012; Rahimnejad et al., 2012; He et al., 2014; Wang et al., 2013; Dong et al., 2015; Lin et al., 2015; Khuyen et al., 2017; Moradian et al., 2018; Pagheh et al., 2018; Dong et al., 2019; Esmaeili et al., 2019; Ge et al., 2020; Su et al., 2019; Ting et al., 2019; Zahran et al., 2019; Dai et al., 2020; Li et al., 2020; Liu et al., 2020; Abdel-Wahab et al., 2021; Hu et al., 2021; Jan et al., 2021; Morshedi et al., 2021; Rashidian et al., 2021; Wang et al., 2021), 2 shrimp (Chand et al., 2006; Gyan et al., 2020) and 2 shellfish (Chen et al., 2020; Mi et al., 2022) (Appendix 2).
3.1. Overall effect size summary
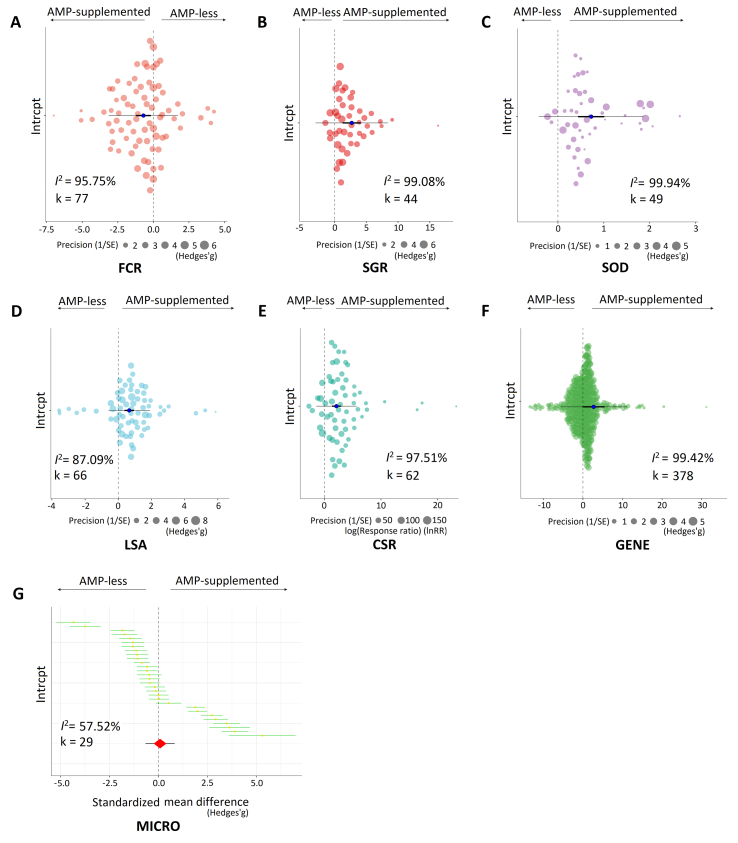
The primary outcomes from the meta-analysis were presented in Table 1. Based on the overall effect size combing all studies for each variable, AMP-supplemented diets showed significant negative effects on FCR (mean effect = −0.70, P = 0.0087, k = 77) (Fig. 2A), and positive effects on SGR (mean effect = 0.66, P < 0.0001, k = 66) (Fig. 2B), SOD (mean effect = 2.70, P = 0.0002, k = 44) (Fig. 2C), LSA (mean effect = 2.11, P < 0.0001, k = 62) (Fig. 2D), CSR (mean effect = 0.73, P < 0.0001, k = 49) (Fig. 2E), GENE (mean effect = 2.70, P = 0.0479, k = 378) (Fig. 2F). However, AMP-enriched feed had a slight positive effect on MICRO (mean effect = 0.09, P = 0.6056, k = 29) (Fig. 2G). Overall, compared with a basal diet, dietary AMP has large positive benefits to diverse parameters of aquatic animals’ health based on the effect size.
Table 1.
Overall effect size calculation outcomes and the results from moderator analysis for growth performance (FCR and SGR), enzyme activity (SOD and LSA), disease resistance (CSR and GENE) and gut microbiota (MICRO).
| Growth performance | Subgroup | k | SMD (Hedges'g) | I2 | 95% CI | P-value | 95% PI |
|---|---|---|---|---|---|---|---|
| FCR | |||||||
| Overall | 77 | −0.70 | 95.75% | −1.22 to −0.18 | 0.0087 | −3.13 to 1.73 | |
| Dose-moderator | Low | 26 | −0.57 | −1.10 to −0.04 | 0.0363 | −3.01 to 1.87 | |
| Medium | 29 | −0.96 | −1.49 to −0.43 | 0.0004 | −3.40 to 1.48 | ||
| High | 22 | −0.55 | −1.08 to −0.01 | 0.0441 | −2.99 to 1.89 | ||
| Duration-moderator | 1 mo | 11 | −0.57 | −2.02 to 0.88 | 0.4402 | −3.46 to 2.32 | |
| 2 mo | 47 | −0.78 | −1.48 to −0.08 | 0.0287 | −3.37 to 1.81 | ||
| >2 mo | 19 | −0.57 | −1.69 to 0.56 | 0.3227 | −3.31 to 2.17 | ||
| IBW-moderator | Small | 41 | −0.61 | −1.34 to 0.12 | 0.0993 | −3.11 to 1.89 | |
| Medium | 22 | −0.39 | −1.37 to 0.60 | 0.4405 | −2.97 to 2.20 | ||
| Large | 14 | −1.41 | −2.61 to −0.21 | 0.0218 | −4.08 to 1.27 | ||
| SGR | |||||||
| Overall | 66 | 0.66 | 87.09% | 0.37 to 0.95 | <0.0001 | −0.59 to 1.91 | |
| Dose-moderator | Low | 24 | 0.55 | 0.25 to 0.85 | 0.0003 | −0.71 to 1.81 | |
| Medium | 23 | 0.97 | 0.67 to 1.27 | <0.0001 | −0.29 to 2.23 | ||
| High | 19 | 0.49 | 0.19 to 0.80 | 0.0014 | −0.77 to 1.76 | ||
| Duration-moderator | 2 mo | 52 | 0.60 | 0.27 to 0.93 | 0.0003 | −0.67 to 1.87 | |
| >2 mo | 14 | 0.89 | 0.26 to 1.52 | 0.0058 | −0.49 to 2.27 | ||
| IBW-moderator | Small | 36 | 0.48 | 0.08 to 0.87 | 0.0191 | −0.81 to 1.76 | |
| Medium | 14 | 0.77 | 0.15 to 1.40 | 0.0156 | −0.60 to 2.15 | ||
| Large | 16 | 0.93 | 0.38 to 1.49 | 0.0010 | −0.41 to 2.28 | ||
| Enzyme activities | |||||||
| SOD | |||||||
| Overall | 44 | 2.70 | 99.08% | 1.27 to 4.13 | 0.0002 | −3.00 to 8.39 | |
| Dose-moderator | Low | 15 | 2.39 | 0.94 to 3.85 | 0.0012 | −3.38 to 8.17 | |
| Medium | 16 | 3.11 | 1.66 to 4.57 | <0.0001 | −2.66 to 8.89 | ||
| High | 13 | 2.74 | 1.28 to 4.19 | 0.0002 | −3.04 to 8.51 | ||
| Duration-moderator | 1 mo | 13 | 5.19 | 3.17 to 7.20 | <0.0001 | 0.30 to 10.07 | |
| 2 mo | 19 | 1.78 | 0.09 to 3.46 | 0.0391 | −2.98 to 6.53 | ||
| >2 mo | 12 | 0.71 | −1.86 to 3.28 | 0.5889 | −4.43 to 5.84 | ||
| IBW-moderator | Small | 19 | 2.43 | 0.12 to 4.75 | 0.0396 | −3.68 to 8.55 | |
| Medium | 10 | 1.31 | −1.96 to 4.58 | 0.4328 | −5.23 to 7.84 | ||
| Large | 15 | 3.67 | 1.34 to 6.00 | 0.0020 | −2.44 to 9.79 | ||
| LSA | |||||||
| Overall | 62 | 2.11 | 97.51% | 1.34 to 2.88 | <0.0001 | −1.48 to 5.70 | |
| Dose-moderator | Low | 22 | 2.37 | 1.63 to 3.11 | <0.0001 | −1.03 to 5.77 | |
| Medium | 20 | 2.91 | 2.17 to 3.66 | <0.0001 | −0.49 to 6.32 | ||
| High | 20 | 1.17 | 0.43 to 1.91 | 0.0019 | −2.23 to 4.58 | ||
| Duration-moderator | 1 mo | 14 | 2.39 | 1.07 to 3.71 | 0.0004 | −1.05 to 5.83 | |
| 2 mo | 39 | 1.48 | 0.56 to 2.40 | 0.0017 | −1.83 to 4.79 | ||
| >2 mo | 9 | 4.08 | 2.22 to 5.93 | <0.0001 | 0.40 to 7.75 | ||
| IBW-moderator | Small | 23 | 2.65 | 1.37 to 3.93 | <0.0001 | −1.16 to 6.46 | |
| Medium | 10 | 1.72 | −0.36 to 3.80 | 0.1054 | −2.43 to 5.87 | ||
| Large | 29 | 1.80 | 0.65 to 2.94 | 0.0021 | −1.97 to 5.56 | ||
| Disease resistance | |||||||
| CSR | |||||||
| Overall | 49 | 0.73 | 99.94% | 0.44 to 1.01 | <0.0001 | −0.41 to 1.86 | |
| Dose-moderator | Low | 17 | 0.56 | 0.28 to 0.85 | <0.0001 | −0.56 to 1.69 | |
| Medium | 18 | 0.93 | 0.64 to 1.21 | <0.0001 | −0.20 to 2.05 | ||
| High | 14 | 0.71 | 0.43 to 0.99 | <0.0001 | −0.41 to 1.83 | ||
| Duration-moderator | 1 mo | 28 | 0.61 | 0.21 to 1.00 | 0.0026 | −0.57 to 1.79 | |
| 2 mo | 21 | 0.86 | 0.44 to 1.28 | <0.0001 | −0.33 to 2.05 | ||
| IBW-moderator | Small | 27 | 0.75 | 0.31 to 1.18 | 0.0008 | −0.49 to 1.98 | |
| Medium | 10 | 0.47 | −0.20 to 1.13 | 0.1700 | −0.87 to 1.80 | ||
| Large | 12 | 0.85 | 0.33 to 1.37 | 0.0013 | −0.41 to 2.12 | ||
| GENE | |||||||
| Overall | 378 | 2.70 | 99.42% | 0.09 to 5.48 | 0.0479 | −5.64 to 11.03 | |
| Gene-moderator | Caspase 3 | 22 | 3.59 | 0.78 to 6.41 | 0.0124 | −4.82 to 12.00 | |
| il | 133 | 2.76 | −0.06 to 5.57 | 0.0547 | −5.65 to 11.17 | ||
| mx | 23 | 2.99 | 0.18 to 5.81 | 0.0371 | −5.42 to 11.40 | ||
| stat | 40 | 2.11 | −0.70 to 4.92 | 0.1412 | −6.30 to 10.52 | ||
| tnf-α | 34 | 2.65 | −0.16 to 5.46 | 0.0650 | −5.76 to 11.06 | ||
| Other | 126 | 2.58 | −0.23 to 5.39 | 0.0721 | −5.83 to 10.99 | ||
| Tissue-moderator | Intestine | 142 | 5.72 | 1.92 to 9.51 | 0.0032 | −1.82 to 13.25 | |
| Kidney | 93 | 1.21 | −1.71 to 4.12 | 0.4173 | −5.92 to 8.33 | ||
| Liver | 46 | 1.10 | −1.82 to 4.01 | 0.4607 | −6.03 to 8.23 | ||
| Spleen | 97 | 0.12 | −2.80 to 3.03 | 0.9347 | −7.01 to 7.25 | ||
| Time-moderator | 0-h | 56 | 1.98 | −1.09 to 5.05 | 0.2060 | −7.20 to 11.16 | |
| 24-h | 239 | 3.35 | 0.28 to 6.42 | 0.0325 | −5.84 to 12.53 | ||
| 48-h | 83 | 2.96 | −0.11 to 6.03 | 0.0587 | −6.22 to 12.14 | ||
| Gut microbiota | |||||||
| MICRO | |||||||
| Overall | 29 | 0.09 | 57.52% | −0.24 to 0.41 | 0.6056 | −0.66 to 0.83 | |
| Structure-moderator | ACE | 6 | 1.01 | −0.60 to 2.63 | 0.2195 | −2.55 to 4.57 | |
| Shannon | 7 | 0.20 | −1.40 to 1.80 | 0.8091 | −3.36 to 3.75 | ||
| Simpson | 7 | 0.90 | −0.70 to 2.51 | 0.2696 | −2.65 to 4.46 | ||
| Chao 1 | 7 | 0.43 | −1.18 to 2.04 | 0.6014 | −3.13 to 3.99 | ||
| Observed-species | 2 | −0.53 | −2.19 to 1.14 | 0.5339 | −4.11 to 3.06 | ||
| Species-moderator | E. lanceolatus | 4 | 0.86 | −1.03 to 2.75 | 0.3749 | −1.79 to 3.50 | |
| P. yessoensis | 4 | −0.80 | −2.68 to 1.07 | 0.3996 | −3.43 to 1.82 | ||
| S. maximus | 16 | −0.74 | −2.59 to 1.11 | 0.4328 | −3.36 to 1.87 | ||
| L. crocea | 5 | 2.51 | 0.65 to 4.37 | 0.0083 | −0.11 to 5.13 | ||
k = the number of effect sizes; SMD = standardized mean difference; I2 = the heterogeneity index across studies; 95% CI = 95% confidence interval; 95% PI = 95% predicted interval; FCR = feed conversion ratio; SGR = specific growth rate; SOD = superoxide dismutase activity; LSA = lysozyme activity; IBW = initial body weight; CSR = cumulative survival rate after pathogen infection; GENE = expression of immune-related genes; MICRO = the abundance of gut microbiota; ACE = Abundance-based Coverage Estimator.
Fig. 2.
Overall effect of AMP-supplemented feeds on individual performance (growth performance, enzyme activity, disease resistance and the abundance of gut microbiota) based on overall effect size. (A) Orchard plot of dietary AMP impact on FCR using the standardized mean difference (SMD, Hedges'g) as the effect size. (B) Orchard plot of dietary AMP impact on SGR using SMD as the effect size. (C) Orchard plot of dietary AMP impact on SOD using SMD as the effect size. (D) Orchard plot of dietary AMP impact on LSA using SMD as the effect size. (E) Orchard plot of dietary AMPs impact on CSR using log10(Response ratio) as the effect size. (F) Orchard plot of dietary AMP impact on GENE using SMD as the effect size. (G) Caterpillar plot shows the overall effect of dietary AMP on MICRO using Hedges'g as the effect size. K indicates the number of effect sizes from the pool of empirical studies. For example, k = 77 shows 77 effect sizes are calculated for FCR from our recruited papers. AMP-less, basal diets without AMP; AMP-supplemented, diets with AMP; I2, the heterogeneity index across studies. SMD is depicted with 95% confidence intervals (CI) and 95% predicted intervals (PI) as scaled effect-size points for each study. Each colorful circle represents a scaled effect size. The dot at the center of each circle is the mean effect size. The diameter of the circles represents the 95% CI of the effect size. AMP = antimicrobial peptide; FCR = feed conversion ratio; SGR = specific growth rate; SOD = superoxide dismutase activity; LSA = lysozyme activity; CSR = cumulative survival rate after pathogen infection; GENE = expression of immune-related genes; MICRO = the abundance of gut microbiota.
In addition, the collected articles exhibited high heterogeneity in all 7 target parameters (I2 = 95.75% for FCR, I2 = 87.09% for SGR, I2 = 99.08% for SOD, I2 = 97.51% for LSA, I2 = 99.94% for CSR, I2 = 99.42% for GENE, I2 = 57.52% for MICRO) (Table 1).
The funnel plots and Egger's test showed that there was potential publication bias for the effect sizes of FCR (P = 0.0307), SOD (P < 0.0001) and LSA (P < 0.0001) (Appendix 1; Fig. S2). In these cases, “trim and fill” was conducted to estimate the number of missing studies in the current meta-analysis for these 3 parameters. Interestingly, the results showed that no extra studies were needed to add and balance the publication bias even though the funnel plots were statistically asymmetrical. Although 1, 1, 3, 1 and 7 outliers of effect sizes were detected in SGR, SOD, LSA, CSR and GENE by influence analysis, respectively (Appendix 1; Fig. S3), leave-one-out analysis confirmed that no particular study carrying a large deviation from the overall level if we removed these unusual cases one by one.
3.2. Moderators
Not surprisingly, in comparison to basal diets, there were statistically significant differences in effect sizes of these parameters for dietary AMP when IBW, AMP dose and feeding duration were regarded as moderators, respectively.
3.2.1. Effects of moderators on growth performance
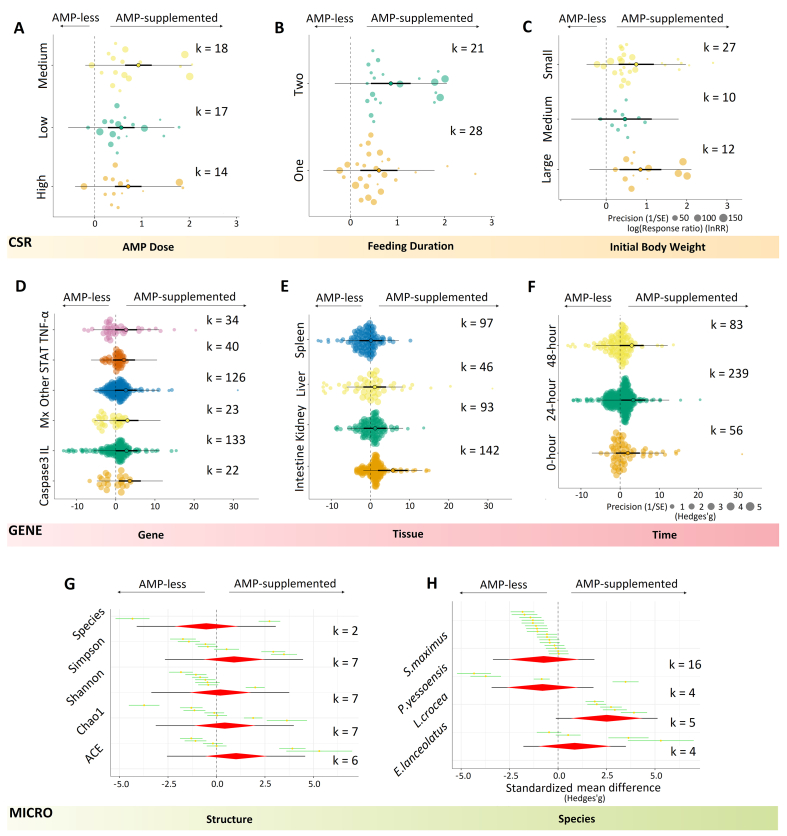
A medium dose of AMP exhibited a higher negative effect on FCR (mean effect = −0.96, P = 0.0004, k = 29), while the low and high doses have the similar effect (mean effect = −0.57, P = 0.0363, k = 26 for the low dose; mean effect = −0.55, P = 0.0441, k = 22 for the high dose) (Fig. 3A). What's more, large individuals usually had the greatest decline in FCR (mean effect = −1.41, P = 0.0218, k = 14), followed by small and medium ones (Fig. 3B). Oral AMP-supplemented feeds had similar effects on FCR for 1 mo (mean effect = −0.57, P = 0.4402, k = 11) and more than 2 mo (mean effect = −0.57, P = 0.3227, k = 47) without statistical difference, however a 2-mo administration period yielded the greatest benefit (mean effect = −0.78, P = 0.0287, k = 19) (Fig. 3C).
Fig. 3.
Moderator-analysis results of the effect of AMP-supplemented feeds on aquatic animal growth performance and enzyme activity. (A) The effect of AMP dose on FCR based on dose-moderator analysis. (B) The effect of initial body weight on FCR based on IBW-moderator analysis. (C) The effect of feeding duration on FCR based on duration-moderator analysis. (D) The effect of AMP dose on SGR based on dose-moderator analysis. (E) The effect of feeding duration on SGR based on duration-moderator analysis. (F) The effect of initial body weight on SGR based on IBW-moderator analysis. (G) The effect of AMP dose on SOD based on dose-moderator analysis. (H) The effect of initial body weight on SOD based on IBW-moderator analysis. (I) The effect of feeding duration on SOD based on duration-moderator analysis. (J) The effect of AMP dose on LSA based on dose-moderator analysis. (K) The effect of feeding duration on LSA based on duration-moderator analysis. (L) The effect of initial body weight on LSA based on IBW-moderator analysis. K indicates the number of effect sizes for each level of these 3 moderators. For example, k = 29 shows 29 effect sizes are calculated for the medium dose of AMP. Here are 3 different levels for each moderator. AMP dose: low, the minimum dose of each study; high, the maximum dose of each study; medium, remaining doses of each study. IBW: small, less than 15 g; medium, from 15 to 30 g; large, above 30 g. Feeding duration: one, less than 35 d; two, from 35 to 65 d; > two, more than 65 d. AMP-less, basal diets without AMP; AMP-supplemented, diets with AMP; AMP = antimicrobial peptide; IBW = initial body weight; FCR = feed conversion ratio; SGR = specific growth rate; SOD = superoxide dismutase activity; LSA = lysozyme activity.
Similarly, the effect size of the medium dose was largest (mean effect = 0.97, P < 0.0001, k = 23) compared to low (mean effect = 0.55, P = 0.0003, k = 24) and high dose (mean effect = 0.49, P = 0.0014, k = 19) for SGR (Fig. 3D). A feeding duration of more than 2 mo can produce a larger effect size (mean effect = 0.89, P = 0.0058, k = 14) than that in 2 mo (mean effect = 0.6, P = 0.0003, k = 52) (Fig. 3E). Moreover, the effect size of a large IBW (mean effect = 0.93, P = 0.001, k = 16) was larger than that in small (mean effect = 0.48, P = 0.0191, k = 36) and medium group (mean effect = 0.77, P = 0.0156, k = 14) for SGR (Fig. 3F).
3.2.2. Effects of moderators on enzyme activity
Doses of AMP, feeding duration and IBW had a diversity of effect sizes for SOD. The results from the dose-moderator analysis were similar to those from SGR, and the medium AMP dose was dominant in effect size (mean effect = 3.11, P < 0.0001, k = 16), followed by the high and low dose (Fig. 3G). Besides, the results indicated that the larger the IBW, the larger the effect size for SOD (mean effect = 3.67, P = 0.002, k = 15 for large individuals; mean effect = 2.43, P = 0.0396, k = 19 for small ones) (Fig. 3H). A 1-mo feeding contributed to the largest effect size (mean effect = 5.19, P < 0.0001, k = 13) for SOD, however, a feeding period of more than 2 mo did not significantly change the results (mean effect = 0.71, P = 0.5889, k = 12) (Fig. 3I).
In addition, effect sizes of LSA were affected significantly by these 3 moderators. For instance, the medium dose increased the effect size (mean effect = 2.91, P < 0.0001, k = 20), which was significantly larger than the overall level (overall effect = 2.11). Nevertheless, the effect size plummeted in the high-dose group (mean effect = 1.17, P = 0.0019, k = 20) compared to lower-dose groups (Fig. 3J). Duration-moderator analysis for LSA indicated that long-term feeding (>2 mo) resulted in a greater effect size (mean effect = 4.08, P < 0.0001, k = 9) than 1 mo (mean effect = 2.39, P = 0.0004, k = 14) (Fig. 3K). Contrary to SOD, small individuals were more likely to produce the large effect size (mean effect = 2.65, P = 0.0004, k = 23) for LSA (Fig. 3L).
3.2.3. Effects of moderators on disease resistance
Although feed supplemented with AMP tended to significantly increase the CSR and GENE at the overall level (overall effect = 0.73, % change = 107.51%, P < 0.0001 for CSR; overall effect = 2.7, P = 0.0479 for GENE), moderators often exhibited different effects. Here, a 107.51% change indicated that AMP-supplemented diets can improve the overall survival rate by 107.51% compared with basal diets. The medium dose contributed to the largest effect size (mean effect = 0.93, % change = 153.45%, P < 0.0001, k = 18) for CSR, followed by high (mean effect = 0.71, % change = 103.40%, P < 0.0001, k = 14) and low dose (mean effect = 0.56, % change = 75.08%, P < 0.0001, k = 17) (Fig. 4A), which were consistent with SOD and LSA. A longer feeding period (2 mo) had a larger effect size (mean effect = 0.86, % change = 136.32%, P < 0.0001, k = 21) than that of a short feeding (1 mo) (mean effect = 0.61, % change = 84.04%, P = 0.0026, k = 28) for CSR (Fig. 4B). Moreover, a large body weight tended to have a large effect (mean effect = 0.85, % change = 133.96%, P = 0.0013, k = 12) on CSR as well (Fig. 4C).
Fig. 4.
Moderator-analysis results of the effect of AMP-supplemented feeds on aquatic animal disease resistance and the abundance of gut microbiota. (A) The effect of AMP dose on CSR based on dose-moderator analysis. (B) The effect of feeding duration on CSR based on duration-moderator analysis. (C) The effect of initial body weight on CSR based on IBW-moderator analysis. (D) The effect of gene type on GENE based on gene-moderator analysis. (E) The effect of tissue on GENE based on tissue-moderator analysis. (F) The effect of time on GENE based on time-moderator analysis. (G) Caterpillar plot shows the effect of different structures on MICRO based on structure-moderator analysis. (H) The effect of fish species on MICRO based on species-moderator analysis. Each green line represents an effect size with a yellow dot (mean effect size). The red rhombus indicates the overall effect size with a mean (centre), 95% CI (left and right borders) and 95% PI (black line through the rhombus). AMP-less, basal diets without AMP; AMP-supplemented, diets with AMP. AMP = antimicrobial peptide; CSR = cumulative survival rate after pathogen infection; GENE = expression of immune-related genes; MICRO = the abundance of gut microbiota.
The increase in caspase 3 gene expression was dominant (mean effect = 3.59, P = 0.0124, k = 22), followed by Mx (mean effect = 2.99, P = 0.0371, k = 23). Although genes IL (mean effect = 2.76, P = 0.0547, k = 133), STAT (mean effect = 2.11, P = 0.1412, k = 40) and TNF-α (mean effect = 2.65, P = 0.065, k = 34) were also up-regulated, these differences were not statistically significant compared to the AMP-less group (Fig. 4D). These expressions were mainly presented in intestine (mean effect = 5.72, P = 0.0032, k = 142), kidney (mean effect = 1.21, P = 0.4173, k = 93), liver (mean effect = 1.1, P = 0.4607, k = 46) and spleen (mean effect = 0.12, P = 0.9347, k = 97) (Fig. 4E) with the highest level at 24 h after pathogen infection (mean effect = 3.35, P = 0.0325, k = 239) (Fig. 4F).
3.2.4. Effects of moderators on gut microflora
Five structure indices of microbial abundance indicated the various effects on MICRO. Structure-moderator analysis indicated that positive effects on ACE (mean effect = 1.01, P = 0.2195, k = 6), Shannon (mean effect = 0.2, P = 0.8091, k = 7), Simpson (mean effect = 0.9, P = 0.2696, k = 7) and Chao 1 (mean effect = 0.43, P = 0.6014, k = 7) when AMP were used as supplements, but a negative effect was detected for Observed-species (mean effect = −0.53, P = 0.5339, k = 2) (Fig. 4G). However, all these effects were not significant in statistics (all P values > 0.05). With respect to species specificity, AMP-added diets showed positive effects for E. lanceolatus (mean effect = 0.86, P = 0.3749, k = 4) and Larimichthys crocea (mean effect = 2.51, P = 0.0083, k = 5) on MICRO, and negative effects for P. yessoensis (mean effect = −0.8, P = 0.3996, k = 4) and S. maximus L (mean effect = −0.74, P = 0.4328, k = 16) (Fig. 4H).
3.3. Summary of meta-regression and dose-effect
Meta-regression indicated there was no significant relationship, linear or quadratic, between moderators and effect size for FCR and SGR (all P > 0.05; Fig. 5A–F). However, only a negative linear relationship between feeding duration and effect size for SOD (estimate = −0.1116, P = 0.0040; Fig. 5G–I) was detected. In addition, the negative linear relationship was observed between AMP dose and effect size for LSA (estimate = −0.0002, P < 0.0001; Fig. 5J), and quadratic relationships between feeding duration and effect size for SOD and LSA (estimate = 0.0008, P = 0.0016 for SOD, Fig. 5H; estimate = 0.0009, P < 0.0001 for LSA, Fig. 5K), IBW and effect size for SOD and LSA (estimate = −0.0004, P < 0.0001 for SOD, Fig. 5I; estimate = 0.0001, P < 0.0001 for LSA, Fig. 5L) were determined. Our meta-regression results indicated that they are difficult to explain biologically due to minuscule estimated values (ranging from −0.0004 to 0.0009) (Appendix 1; Table S1). Given the biological-insignificant results from meta-regression, we performed the dose–response meta-analysis to examine the dose-effect for FCR, SGR, SOD and LSA, and the dose-effect determined that the optimal AMP doses for FCR, SGR, SOD and LSA were 707.5, 750, 1,050 and 937.5 mg/kg, respectively (Fig. 5A, D, 5G, 5J).
Fig. 5.
Dose effect and meta-regression analysis results for growth performance and enzyme activity. (A) The outcome of meta-regression for FCR when AMP dose is involved. The effect size of FCR vs AMP dose, and the dose-effect curve is in red. (B) The outcome of meta-regression for FCR when feeding duration is involved. (C) The outcome of meta-regression for FCR when IBW is involved. (D) The outcome of meta-regression for SGR when AMP dose is involved. The effect size of SGR vs AMP dose, and the dose-effect curve is in red. (E) The outcome of meta-regression for SGR when feeding duration is involved. (F) The outcome of meta-regression for SGR when IBW is involved. (G) The outcome of meta-regression for SOD when AMP dose is involved. The effect size of SOD vs AMP dose, and the dose-effect curve is in red (H) The outcome of meta-regression for SOD when feeding duration is involved. (I) The outcome of meta-regression for SOD when IBW is involved. (J) The outcome meta-regression for LSA when AMP dose is involved. The effect size of LSA vs AMP dose, and the dose-effect curve is in red. (K) The result from meta-regression for LSA when feeding duration is involved. (L) The result from meta-regression for LSA when IBW is involved. AMP = antimicrobial peptide; FCR = feed conversion ratio; SGR = specific growth rate; SOD = superoxide dismutase activity; LSA = lysozyme activity; IBW = initial body weight.
4. Discussion
4.1. AMP-supplemented feed improves health
The positive effects of AMP-supplemented feeds have been demonstrated in individual studies over the past 2 decades. Although there were high heterogeneities (I2 ranged from 57.52% to 99.42%) across the various studies, the data of combining them proved that AMP-enriched feeds can improve health and body function via promoting growth performance, enzyme activity, disease resistance and the abundance of gut microbiota in aquatic animals based on our current meta-analyses. The overall effect size between the AMP-added and basal diets was −0.7 for FCR, 0.66 for SGR, 2.7 for SOD, 2.11 for LSA, 0.73 for CSR, 2.7 for GENE and 0.09 for MICRO (Table 1). This evaluation indicated that dietary AMP had the largest positive effects on enzyme activity, especially SOD and lysozyme activities. Besides, AMP as feed additives tend to boost fish growth performance by improving SGR and decreasing FCR. The comparison with basal diets demonstrated that AMP significantly upregulated GENE, and thus improving the CSR by 107.51% when pathogens invaded. This is the first quantitative synthesis to determine that AMP-supplemented feeds can improve health and bodily function in aquatic animals from a pool of empirical studies.
The intestine is the largest endocrine organ in the fish, which also serves as a place for the digestion and absorption of nutrients (Dong et al., 2019), and the balance of the gut microbiome is linked to the health of aquatic animals. After aquatic animals consume AMP-supplemented feed, endocrine cells of the intestinal epithelium participate in digestion and absorption, influencing the gut microbiota community. Ting et al. (2019) first stated that hepcidin-enriched diets can increase microflora species richness (the Chao 1-and ACE-diversity index) but did not alter species diversity (the Shannon and Simpson index). A little differently, a subsequent study indicated there were no differences in Observed-species but improved species richness and diversity with the inclusion of APSH-07 in regular diets (Ge et al., 2020). However, our present study showed that dietary AMP had a slight effect on MICRO, which was about one seventeenth of the average effect size of other parameters (FCR, SGR, SOD, LSA CSR and GENE) (0.09 vs 1.6). Given our meta-analytic outcomes, the addition of AMP significantly increased the MICRO in L. crocea, but there was no significant improvement in the other 3 aquatic animals based on the effect size. Even though the individual study has shown differences or contradictions in results, our meta-results concluded that AMP had no significant effect on the richness and diversity of gut microbiota when we combined all homogeneous indexes regardless of species. This may be limited to only 4 papers that can be extracted so far, and these studies contributed 29 effect sizes to the current meta-analysis. Another possible explanation is that it is not possible to judge whether it is beneficial or not based on the magnitude of the effect size for the structure of gut microbiota, and the specific types of opportunistic pathogens and beneficial microbes should be identified. For example, a 250 mg/kg dose of cecropin-supplemented diets can enhance gut health by increasing the abundance of Lactobacillus and reducing Bacteroides, even without significant changes in the richness of gut microbiota (Dai et al., 2020). In conclusion, a meta-analysis limited to 4 studies is difficult to obtain accurate and strong evidence that AMP-added diets can improve the composition of intestinal microflora, ideally, more relevant studies should be involved in the future.
4.2. Publication bias and outliers exist but little influence on conclusions
In the present study, the funnel plots of FCR, SOD and LSA exhibited significant asymmetry (P < 0.05), indicating potential publication bias. Nonetheless, publication bias is not the only factor causing funnel asymmetry, but the high heterogeneity of studies also contributes to this (Song et al., 2002; Lin and Chu, 2018). In our meta-analysis, no additional missing studies are needed to impute to adjust publication bias by using the trim and fill analysis (Appendix 1; Fig. S2), which means that the potential bias did not affect our results. Although one or several potential outliers of effect size were detected for SGR, SOD, CSR, LSA and GENE based on the sensitivity analysis, the leave-one-out diagnosis indicated that these unusual cases did not distort our conclusions even if we remove them one by one. We concluded that this outlier-unaffected result benefitted from the large sample size of effect sizes (k = 66 for SGR, k = 44 for SOD, k = 62 for CSR, k = 49 for LSA and k = 378 for GENE). In particular, even if there were 7 outliers of effect size for GENE, the huge sample size (378 vs 7) can completely eliminate the unpredictable influence without changing the conclusions. Furthermore, effect sizes with a large number in our unbiased meta-analysis yielded accurate effect estimates.
4.3. Species, AMP dose, IBW and feeding duration affect AMP's role in health
Species-moderator analysis was not implemented in this study due to differences in the large sample size (21 fish, 2 shrimp and 2 shellfish). Although there are only 4 studies on the application of AMP as an additive in shrimp and shellfish, the researchers also reported their growth-promoting and disease-enhancement effects. Compared to fish, shrimp could still obtain immune boosts even if they are fed with AMP-supplemented feeds with a shorter feeding duration and a lower dose. A previous study indicated that administration of bovine lactoferrin at 100 mg/kg for 1 wk can enhance disease resistance against A. hydrophila in the giant freshwater prawn (Macrobrachium rosenbergii) (Chand et al., 2006). However, a recent publication showed that a longer feeding duration and a higher AMP dose (8 ws and 4,000 mg/kg) were required to enhance the growth and disease resistance of Pacific whiteleg shrimp (Litopenaeus vannamei) (Gyan et al., 2020). We presumed that, in addition to the underlying species- and AMP- specificity, the IBW of the subjects (17.5 vs 0.21 g) contributed significantly to the large variation in these 2 shrimp species. Surprisingly, AMP at doses as low as 7.5 mg/kg was significantly beneficial to abalone (Haliotis discus hannai), but this improvement in growth performance and immunity required a 115-d feeding period (Chen et al., 2020). We suspected that the immune system in shellfish might be too weak to tolerate a high AMP dose compared to fish and shrimp, and in the case of low-dose AMP being used, it is possible to achieve the effect of improving disease resistance or immunity by increasing the effective feeding duration. However, it is inappropriate to draw conclusions based on these limited and controversial results, and more relevant studies on shrimp and shellfish should be conducted to produce robust results.
Accumulate studies revealed that the improvement of health/body function has different requirements on the AMP dose, feeding duration and IBW when AMP is used as a feed additive in aquatic animals. In this vein, it is feasible to determine the optimal combination of these 3 factors and proposal a realistic feeding plan in aquaculture by harnessing meta-analysis and the dose-effect model. For growth performance, medium doses of 600 to 800 mg/kg favored animal growth with a declined FCR when 30-g IBW of individuals were introduced . In addition, the medium dose of AMP was beneficial to enzyme activities with a 2-mo feeding. Although large individuals fed with AMP can effectively improve these parameters, small ones ( <15 g) maximized these efficiencies, and such an effect was manifested after 1 mo. Larger individuals exhibited better immune responses to pathogen invasion when medium dosage and 2-mth feeding were adopted. Taken together, enzyme activity has a faster response than that immune gene activity when AMP is used as a feed additive for aquatic animals. Although more than 2 mo of the feeding period can also significantly improve growth performance and disease resistance, this was not significantly different from the 2-mo feeding. The expression of these immune-associated genes was significantly up-regulated and peaked at 24 h after pathogen infection. Interestingly, compared to basal diets, AMP-enriched feeds promoted the upregulation of immune-related genes even in the absence of pathogens (0-h). Expression of these genes was dominant in the intestine, followed by the kidney, liver and spleen.
4.4. Other potential moderators affect AMP's role in health
Heterogeneity was reduced when the AMP dose, IBW and feeding duration were included as moderators in our overall model. However, even though we incorporated these as moderators into the model, a high degree of heterogeneity still existed. This suggests that other unconsidered variables may also affect the relationship between body function and these factors when AMP is included in the diet. Therefore, there is still a need to analyze potential and reasonable moderators, such as the type of AMP. In addition, waters (marine or freshwater), living habitats (warm or cold water) and feeding diets (carnivorous, omnivorous, herbivorous) potentially affect the role of AMP as a feed additive on aquatic animals’ health.
Firstly, an AMP-moderator test was applied to determine whether different AMP have preferential effects on animal health in aquaculture. For the current study, 8, 7, 10 and 10 AMP were involved in decreasing FCR, increasing SGR, and enhancing SOD and LSA, respectively. We concluded that different AMP contributed to various effects on FCR, SGR, SOD and LSA (Appendix 1; Fig. S4). Although lactoferrin is the most extensively studied feed additive in aquaculture, it is not the most potent AMP in enhancing growth and promoting enzyme activity based on our meta-analysis. By comparison, several recently studied AMP, such as a small peptide with 11 residues derived from cecropin and melittin (CM11), an AMP from large yellow croaker (APSH-07), Tilapia hepcidin 2-3 (TH2-3) and Tilapia piscidin 4 (TP4), dominated the effects on these 4 parameters, respectively. In addition to the type-specificity of AMP, differences in the sample size of these studies may also provide an explanation of the dominant AMP potential. For instance, over 20 effect sizes were applied to the lactoferrin-moderator analysis, but no more than 5 were adopted for other AMP except cecropin. In view of these cases, except for lactoferrin, more studies should be conducted on other types of AMP to balance the sample size, which is conducive to obtaining more reliable meta-analysis results.
In addition to the type of AMP, waters-, habitats- and diets-moderator analyses were respectively adopted to address waters, living habitats and feeding diets potentially affecting the role of AMP as a feed additive on aquatic animal health (Fig. 6). Here, we discovered that adding AMP as a feed additive dramatically enhanced growth performance by lowering FCR and raising SGR in both marine and freshwater species. Besides, this growth-promoting effect was observed in all different-diet aquatic animals, including carnivores, herbivores and omnivores, but this enhanced growth was not significantly different between marine and freshwater species, or among species with varied diets. Nonetheless, compared to cold-water species, warm-water ones showed much better performance in growth. In addition, elevated enzymatic activity was presented in both marine and freshwater animals among different-diet species when AMP were included in diets, but LSA was inhibited in cold-water species. Interestingly, AMP were more effective in improving CSR as feed additives for cold-water or carnivore fish than for warm-water or other feeding species after pathogen infection. With respect to GENE, elevated expression levels of immune-related genes were more significant in warm-water species than in cold-water species; although increased expressions were observed in both herbivores and omnivores animals, these upregulations were not significant. What's more, the effect of AMP on MICRO was significantly affected by the feeding habitats of animals and the water temperatures (warm water vs cold water) they live in. Specifically, AMP had a positive effect on MICRO of warm-water or carnivorous fish, but negative effects were detected in cold-water or herbivorous species.
Fig. 6.
The effect of waters (marine or freshwater), living habitats (warm or cold water) and diets (carnivorous, omnivorous, herbivorous) on aquatic animal health using waters-, habitats- and diets-moderator analysis, respectively. K indicates the number of effect sizes for each level of different moderators. FCR = feed conversion ratio; SGR = specific growth rate; SOD = superoxide dismutase activity; LSA = lysozyme activity; CSR = cumulative survival rate after pathogen infection; GENE = expression of immune-related genes; MICRO = the abundance of gut microbiota; AMP = antimicrobial peptide; AMP-supplemented, diets with AMP. LRR = log response ratio. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Overall, AMP-enriched diets can significantly improve the health of aquatic animals regardless of freshwater or marine species. In contrast to cold-water species, this effect of AMP was more pronounced in warm-water animals. In addition, the effect of AMP-supplemented feed on MICRO is not only affected by the characteristics of AMP itself, but also depends on the feeding habitats of the experimental subjects. Although our preliminary data revealed that various feeding habitats and living water temperatures have different effects on AMP in fish health improvement, multiple species feeding experiments are beneficial to reveal the underlying mechanism.
Additionally, different culture environments, even on the same farm or in the same research unit, physical substrate, biofloc (aggregates of bacteria, phytoplankton, or protozoa in the recirculating systems or in the ponds), and types of microorganisms may be totally different. Future studies should also evaluate the interactions among these factors.
5. Conclusions and perspectives
In conclusion, our multiple meta-analyses demonstrated that AMP-supplemented diets can significantly improve the growth performance, enzyme activity and disease resistance in aquatic animals. These effects are dependent upon the initial body weight of individual, AMP dose and feeding duration to some extent. Although preliminary data indicated that dietary AMP have a statistical-insignificant effect on the abundance of gut microbiota, potential differences in living habitats and feeding habitats may have contributed to this outcome; however, more studies are needed to substantiate this standpoint in the future. Based on our findings combed with previous empirical studies, it is recommended to feed individuals with an initial weight of 30 g at a dose of 600 to 800 mg/kg for 2 consecutive months when AMP are planning to be applied in aquaculture, which is effective in improving body function and health and reducing costs in aquafeeds.
AMP as feed additives are promising substitutes for antibiotics in the disease-resistant enhancement of aquaculture applications. Nevertheless, it is vitally important to understand their potential mechanism to reduce the risk of collateral harm and exert their specificity along with their general broad-spectrum activity if AMP are to be used in aquaculture and fisheries. AMP have a non-specific defensive mechanism that plays an essential part in the innate immune system. Hancock et al. (2016) summarized the multiple mechanisms of AMP against various pathogens, including bacteria, fungi, viruses, and tumor cells. The diversified mechanisms of action include cell membrane disruption, interference and inhibition of nucleic acid synthesis, neutralizing the cell wall and impairing the biosynthesis of peptidoglycan (i.e. lipopolysaccharide or lipoteichoic acid), regulating gene expression, impacting the receptor binding and signaling, induction of the host immune system, which all result in the actual killing of the targets. Such a multi-hit mechanism would favor the application of AMP. However, there are concerns about bacteria resistance to AMP and cross-resistance to endogenous host AMP from applied AMP (Lazzaro et al., 2020); in this regard, the effects of the external applied AMP on aquatic animals should also be evaluated and resolved.
The ultimate goal is to scale up AMP production and application. However, natural organisms from prokaryotes to eukaryotes provide a rich source of AMP according to the antimicrobial peptide database (APD3; https://aps.unmc.edu), but with limited low contents. To solve the problems of the high cost of AMP production, Wang et al. (2022) reviewed the main ways to produce adequate AMP and proposed a possible way to produce AMP which is to generate disease-resistant aquatic animals carrying AMGs via genetic engineering. The only main issue and concern would be the risk of gene-edited animals’ escapement or releasement to the ecosystems and to the receiving populations. The feasible solution is to apply physical and genetic confinement or genetically control the reproductive of the aquatic animals (Su et al., 2014). Other production platform has also emerged, for instance, tobacco (Nicotiana benthamiana) was modeled as a production factory to synthesize channel catfish (Ictalurus punctatus) interleukin-22 (cfIL-22) that upregulated the expression of fibronectin, natural killer lysin-1, and interferon. The sufficient yield (5.4 mg/kg of leaf) and bioactivity of the plant-made recombinant cfIL-22 demonstrated its therapeutic potential for the aquaculture industry (Elkins and Dolan 2021). With the perfection of genome editing and streamlining of synthetic biotechnology, genetically engineered aquatic animals harboring AMP genes and plant-made recombinant AMP would likely be increasingly accepted.
Author contributions
Jinhai Wang: Formal Analysis, Data Curation, Conceptualization, Interpretation of Results, Writing – Original Draft, Funding Acquisition. Alan E. Wilson: Conceptualization, Writing – Review & Editing. Baofeng Su: Writing – Review & Editing, Funding Acquisition. Rex A. Dunham: Supervision; Writing – Review & Editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
Jinhai Wang is supported by a scholarship from the China Scholarship Council. The authors received no financial support from funding agencies in the public, commercial or not-for-profit sectors.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2022.10.001.
Contributor Information
Jinhai Wang, Email: jzw0173@auburn.edu.
Baofeng Su, Email: bzs0014@auburn.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abdel-Wahab M.M., Taha N.M., Lebda M.A., Elfeky M.S., Abdel-Latif H.M.R. Effects of bovine lactoferrin and chitosan nanoparticles on serum biochemical indices, antioxidative enzymes, transcriptomic responses, and resistance of Nile tilapia against Aeromonas hydrophila. Fish Shellfish Immunol. 2021;111:160–169. doi: 10.1016/j.fsi.2021.01.017. [DOI] [PubMed] [Google Scholar]
- Cabello F.C. Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol. 2006;8:1137–1144. doi: 10.1111/j.1462-2920.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- Chand R.K., Sahoo P.K., Kumari J., Pillai B.R., Mishra B.K. Dietary administration of bovine lactoferrin influences the immune ability of the giant freshwater prawn Macrobrachium rosenbergii (de Man) and its resistance against Aeromonas hydrophila infection and nitrite stress. Fish Shellfish Immunol. 2006;21:119–129. doi: 10.1016/j.fsi.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Chen F., Li X., Wu Y., Huang D., Guo Y., Zhang Y., et al. Influences of dietary antimicrobial peptide APSH-07 on the growth performance, immune response and vibriosis resistance of abalone Haliotis discus hannai Ino. Aquacult Nutr. 2020;26:1736–1747. doi: 10.1111/anu.13124. [DOI] [Google Scholar]
- Chen Y.-B., Hu J., Lyu Q.-J., Liu L.-J., Wen L.-F., Yang X.-K., et al. The effects of Natucin C-Natucin P mixture on blood biochemical parameters, antioxidant activity and non-specific immune responses in tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2016;55:367–373. doi: 10.1016/j.fsi.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Dai J., Zheng J., Ou W., Xu W., Ai Q., Zhang W., et al. The effect of dietary cecropin AD on intestinal health, immune response and disease resistance of juvenile turbot (Scophthalmus maximus L.) Fish Shellfish Immunol. 2020;100:117–125. doi: 10.1016/j.fsi.2020.02.052. [DOI] [PubMed] [Google Scholar]
- Dong X.-Q., Qu G.-J., Chen Y.-K., Wang G.-Q., Jiang D. Effects of antimicrobial peptides on growth, morphology of foregut villi and related genes mRNA expression in the common carp (Cyprinus carpio) Aquacult Res. 2019;50:1752–1761. doi: 10.1111/are.14041. [DOI] [Google Scholar]
- Dong X.-Q., Zhang D.-M., Chen Y.-K., Wang Q.-J., Yang Y.-Y. Effects of antimicrobial peptides (AMPs) on blood biochemical parameters, antioxidase activity, and immune function in the common carp (Cyprinus carpio) Fish Shellfish Immunol. 2015;47:429–434. doi: 10.1016/j.fsi.2015.09.030. [DOI] [PubMed] [Google Scholar]
- Dong Y.-H., Wang L.-H., Zhang L.-H. Quorum-quenching microbial infections: mechanisms and implications. Philos Trans R Soc B-Biol Sci. 2007;362:1201–1211. doi: 10.1098/rstb.2007.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Elkins L.L., Dolan M.C. Plant production and functional characterization of catfish interleukin-22 as a natural immune stimulant for aquaculture fish. J Biotechnol. 2021;325:233–240. doi: 10.1016/j.jbiotec.2020.10.017. [DOI] [PubMed] [Google Scholar]
- Eslamloo K., Falahatkar B., Yokoyama S. Effects of dietary bovine lactoferrin on growth, physiological performance, iron metabolism and non-specific immune responses of Siberian sturgeon Acipenser baeri. Fish Shellfish Immunol. 2012;32:976–985. doi: 10.1016/j.fsi.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Esmaeili A., Sotoudeh E., Morshedi V., Bagher D., Dorafshan S. Effects of dietary supplementation of bovine lactoferrin on antioxidant status, immune response and disease resistance of yellowfin sea bream (Acanthopagrus latus) against Vibrio harveyi. Fish Shellfish Immunol. 2019;93:917–923. doi: 10.1016/j.fsi.2019.08.045. [DOI] [PubMed] [Google Scholar]
- Esteban M.A., Rodriguez A., Cuesta A., Meseguer J. Effects of lactoferrin on non-specific immune responses of gilthead seabream (Sparus auratus L.) Fish Shellfish Immunol. 2005;18:109–124. doi: 10.1016/j.fsi.2004.06.003. [DOI] [PubMed] [Google Scholar]
- FAO . 2020. The state of world fisheries and aquaculture 2020. Sustainability in action; pp. 1–244. Rome. [DOI] [Google Scholar]
- FAO . 2021. The FAO action plan on antimicrobial resistance 2021–2025; pp. 1–46. Rome. [DOI] [Google Scholar]
- Ge H., Wang Q., Chen H., Liu G., Pan Y., Chen J., et al. Effects of antimicrobial peptide APSH-07 on the growth performance, anti-oxidation responses, stress resistance and intestine microbiota in large yellow croaker Larimichthys crocea. Aquacult Nutr. 2020;26:715–726. doi: 10.1111/anu.13031. [DOI] [Google Scholar]
- Gyan W.R., Yang Q., Tan B., Jan S.S., Jiang L., Chi S., et al. Effects of antimicrobial peptides on growth, feed utilization, serum biochemical indices and disease resistance of juvenile shrimp, Litopenaeus vannamei. Aquacult Res. 2020;51:1222–1231. doi: 10.1111/are.14473. [DOI] [Google Scholar]
- Hancock R.E.W. Cationic peptides: effectors in innate immunity and novel antimicrobial peptide. Lancet Infect Dis. 2001;1:156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- Hancock R.E.W., Haney E.F., Gill E.E. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol. 2016;16:321–334. doi: 10.1038/nri.2016.29. [DOI] [PubMed] [Google Scholar]
- He Y., Liu B., Xie J., Ge X., Xu P., Lu Y., et al. Effects of antibacterial peptide extracted from Bacillus subtilis fmbJ on the growth, physiological response and disease resistance of Megalobrama amblycephala. Isr J Aquac Bamidgeh. 2014;66:1–10. [Google Scholar]
- Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Huang H.-N., Su B.-C., Tsai T.-Y., Rajanbabu V., Pan C.-Y., Chen J.-Y. Dietary supplementation of recombinant tilapia piscidin 4-expressing yeast enhances growth and immune response in Lates calcarifer. Aquac Rep. 2020;16 doi: 10.1016/j.aqrep.2019.100254. [DOI] [Google Scholar]
- Hu Q.-Y., Wu P., Feng L., Jiang W.-D., Liu Y., Kuang S.-Y., et al. Antimicrobial peptide Isalo scorpion cytotoxic peptide (IsCT) enhanced growth performance and improved intestinal immune function associated with janus kinases (JAKs)/signal transducers and activators of transcription (STATs) signaling pathways in on-growing grass carp (Ctenopharyngodon idella) Aquaculture. 2021;539 doi: 10.1016/j.aquaculture.2021.736585. [DOI] [Google Scholar]
- Jan S.-S., Yang D.-Q., Thammasena R. Effect of antimicrobial peptides on the growth and immunity of swamp eels. J Aquac Fish Health. 2021;10:137–146. doi: 10.20473/jafh.v10i2.22625. [DOI] [Google Scholar]
- Karunasagar I.D., Karunasagar I.N., Bondad-Reantaso M.G. Complexities involved in source attribution of antimicrobial resistance genes found in aquaculture products. Asian Fish Sci. 2020;33:16–21. doi: 10.33997/j.afs.2020.33.S1.003. [DOI] [Google Scholar]
- Khuyen T.D., Mandiki S.N.M., Cornet V., Douxfils J., Betoulle S., Bossier P., et al. Physiological and immune response of juvenile rainbow trout to dietary bovine lactoferrin. Fish Shellfish Immunol. 2017;71:359–371. doi: 10.1016/j.fsi.2017.10.027. [DOI] [PubMed] [Google Scholar]
- Kumari J., Swain T., Sahoo P.K. Dietary bovine lactoferrin induces changes in immunity level and disease resistance in Asian catfish Clarias batrachus. Vet Immunol Immunopathol. 2003;94:1–9. doi: 10.1016/S0165-2427(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Lazzaro B.P., Zasloff M., Rolff J. Antimicrobial peptides: application informed by evolution. Science. 2020;368 doi: 10.1126/science.aau5480. eaau5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Chi S., Cheng X., Wu C., Xu Q., Qu P., et al. Effects of antimicrobial peptides on the growth performance, antioxidant and intestinal function in juvenile largemouth bass, Micropterus salmoides. Aquac Rep. 2020;16 doi: 10.1016/j.aqrep.2019.100252. [DOI] [Google Scholar]
- Lin L., Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74:785–794. doi: 10.1111/biom.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Chen W., Lin S., Luo L. Effects of dietary cecropin on growth, non-specific immunity and disease resistance of tilapia (Oreochromis niloticus × O. aureus) Aquacult Res. 2015;46:2999–3007. doi: 10.1111/are.12457. [DOI] [Google Scholar]
- Liu S., Wang J., Feng Y., Ye Q., Wen L., Xu G., et al. Effects of compound antimicrobial peptides on the growth performance, antioxidant and immune responses and disease resistance of grass carp (Ctenopharyngodon idellus) Fish Shellfish Immunol. 2020;107:163–170. doi: 10.1016/j.fsi.2020.09.042. [DOI] [PubMed] [Google Scholar]
- Liu X., Cao Y., Ouyang S., Wu X. Comparative analysis of gut microbiota diversity in endangered, economical, and common freshwater mussels using 16S rRNA gene sequencing. Ecol Evol. 2020;10:12015–12023. doi: 10.1002/ece3.6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lygren B., Sveier H., Hjeltnes B., Waagbø R. Examination of the immunomodulatory properties and the effect on disease resistance of dietary bovine lactoferrin and vitamin C fed to Atlantic salmon (Salmo salar) for a short-term period. Fish Shellfish Immunol. 1999;9:95–107. doi: 10.1006/fsim.1998.0179. [DOI] [Google Scholar]
- Mi R., Li X., Sun Y., Wang Q., Tian B., Ma S., et al. Effects of microbial community and disease resistance against Vibrio splendidus of Yesso scallop (Patinopecten yessoensis) fed supplementary diets of tussah immunoreactive substances and antimicrobial peptides. Fish Shellfish Immunol. 2022;121:446–455. doi: 10.1016/j.fsi.2021.10.006. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Mookherjee N., Anderson M.A., Haagsman H.P., Davidson D.J. Antimicrobial host defence peptides: functions and clinical potential. Nat Rev Drug Discov. 2020;19:311–332. doi: 10.1038/s41573-019-0058-8. [DOI] [PubMed] [Google Scholar]
- Moradian A.M., Dorafshan S., Heyrati F.P., Ebrahimi E. Effects of dietary bovine lactoferrin on growth, haemato-biochemical parameters, immune functions and tolerance to air exposure stress in the African cichlid Sciaenochromis fryeri. Aquacult Nutr. 2018;24:392–399. doi: 10.1111/anu.12570. [DOI] [Google Scholar]
- Morshedi V., Bojarski B., Hamedi S., Torahi H., Hashemi G., Faggio C. Effects of dietary bovine lactoferrin on growth performance and immuno-physiological responses of Asian sea bass (Lates calcarifer) fingerlings. Probiotics Antimicro Prot. 2021;13:1790–1797. doi: 10.1007/s12602-021-09805-4. [DOI] [PubMed] [Google Scholar]
- Naylor R.L., Hardy R.W., Buschmann A.H., Bush S.R., Cao L., Klinger D.H., et al. A 20-year retrospective review of global aquaculture. Nature. 2021;591:551–563. doi: 10.1038/s41586-021-03308-6. [DOI] [PubMed] [Google Scholar]
- Pagheh E., Marammazi J.G., Agh N., Nouri F., Sepahdari A., Gisbert E., et al. Growth performance, hemato-immunological responses, and digestive enzyme activities in silvery-black porgy (Sparidentex hasta) fed dietary bovine lactoferrin. Probiotics Antimicro Prot. 2018;10:399–407. doi: 10.1007/s12602-017-9340-4. [DOI] [PubMed] [Google Scholar]
- Pan C.-Y., Huang T.-C., Wang Y.-D., Yeh Y.-C., Hui C.-F., Chen J.-Y. Oral administration of recombinant epinecidin-1 protected grouper (Epinephelus coioides) and zebrafish (Danio rerio) from Vibrio vulnificus infection and enhanced immune-related gene expressions. Fish Shellfish Immunol. 2012;32:947–957. doi: 10.1016/j.fsi.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Rahimnejad S., Agh N., Kalbassi M., Khosravi S. Effect of dietary bovine lactoferrin on growth, haematology and non-specific immune response in rainbow trout (Oncorhynchus mykiss) Aquacult Res. 2012;43:1451–1459. doi: 10.1111/j.1365-2109.2011.02947.x. [DOI] [Google Scholar]
- Rashidian G., Moghaddam M.M., Mirnejad R., Azad Z.M. Supplementation of zebrafish (Danio rerio) diet using a short antimicrobial peptide: evaluation of growth performance, immunomodulatory function, antioxidant activity, and disease resistance. Fish Shellfish Immunol. 2021;119:42–50. doi: 10.1016/j.fsi.2021.09.035. [DOI] [PubMed] [Google Scholar]
- Ren T., Koshio S., Ishikawa M., Yokoyama S., Micheal F.R., Uyan O., et al. Influence of dietary vitamin C and bovine lactoferrin on blood chemistry and non-specific immune responses of Japanese eel, Anguilla japonica. Aquaculture. 2007;267:31–37. doi: 10.1016/j.aquaculture.2007.03.033. [DOI] [Google Scholar]
- Said L.B., Fliss I., Offret C., Beaulieu L. In: Encyclopedia of Food Chemistry. Melton L., Shahidi F., Varelis P., editors. 2019. Antimicrobial peptides: the new generation of food additives; pp. 576–582. vol 3, Elsevier; 2019. [DOI] [Google Scholar]
- Silveira R.F., Roque-Borda C.A., Vicente E.F. Antimicrobial peptides as a feed additive alternative to animal production, food safety and public health implications: an overview. Anim Nutr. 2021;7:896–904. doi: 10.1016/j.aninu.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits C.H.M., Li D., Patience J.F., den Hartog L.A. FAO; Rome: 2021. Animal nutrition strategies and options to reduce the use of antimicrobials in animal production; pp. 1–90. FAO animal production and health paper No. 184. [DOI] [Google Scholar]
- Song F.J., Khan K.S., Dinnes J., Sutton A.J. Asymmetric funnel plots and publication bias in meta-analyses of diagnostic accuracy. Int J Epidemiol. 2002;31:88–95. doi: 10.1093/ije/31.1.88. [DOI] [PubMed] [Google Scholar]
- Su B., Peatman E., Shang M., Thresher R., Grewe P., Patil J., et al. Expression and knockdown of primordial germ cell genes, vasa, nanos and dead end in common carp (Cyprinus carpio) embryos for transgenic sterilization and reduced sexual maturity. Aquaculture. 2014;(420–421):S72–S84. doi: 10.1016/j.aquaculture.2013.07.008. [DOI] [Google Scholar]
- Su Y.-L., Chen G., Chen L.-S., Li J.-Z., Wang G., He J.-Y., et al. Effects of antimicrobial peptides on serum biochemical parameters, antioxidant activity and non-specific immune responses in Epinephelus coioides. Fish Shellfish Immunol. 2019;86:1081–1087. doi: 10.1016/j.fsi.2018.12.056. [DOI] [PubMed] [Google Scholar]
- Ting C.-H., Chen Y.-C., Chen J.-Y. Nile tilapia fry fed on antimicrobial peptide Epinecidin-1-expressing Artemia cyst exhibit enhanced immunity against acute bacterial infection. Fish Shellfish Immunol. 2018;81:37–48. doi: 10.1016/j.fsi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Ting C.-H., Pan C.-Y., Chen Y.-C., Lin Y.-C., Chen T.-Y., Rajanbabu V., et al. Impact of Tilapia hepcidin 2-3 dietary supplementation on the gut microbiota profile and immunomodulation in the grouper (Epinephelus lanceolatus) Sci Rep. 2019;9 doi: 10.1038/s41598-019-55509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa P.E., Solís C.J., De la Paz J.F., Alaurent T.G.S., Caruffo M., Hernández A.J., et al. Lactoferrin decreases the intestinal inflammation triggered by a soybean meal-based diet in zebrafish. J Immunol Res. 2016;2016 doi: 10.1155/2016/1639720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer W., Cheung M.W.-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- Wang G., Li X., Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44:D1087–D1093. doi: 10.1093/nar/gkv1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Su B., Dunham R.A. Genome-wide identification of catfish antimicrobial peptides: a new perspective to enhance fish disease resistance. Rev Aquacult. 2022;14:2002–2022. doi: 10.1111/raq.12684. [DOI] [Google Scholar]
- Wang S., Xie S., Zhou A., Zhang C., Wen L., Xu G., et al. Effects of mixed antimicrobial peptide on the growth performance, antioxidant and immune responses and disease resistance of Pengze crucian carp (Carassius auratus var. Pengze) Fish Shellfish Immunol. 2021;114:112–118. doi: 10.1016/j.fsi.2021.04.017. [DOI] [PubMed] [Google Scholar]
- Wang Y.-D., Chang H.-Y., Chen J.-Y., Chen J.-C. Oral administration of bovine lactoferrin inhibits bacterial infection in tilapia and elevates survival after bacterial infection: an examination of its immune-modulating properties. Aquacult Int. 2013;21:75–96. doi: 10.1007/s10499-012-9537-1. [DOI] [Google Scholar]
- Welker T.L., Lim C., Yildirim-Aksoy M., Klesius P.H. Dietary bovine lactoferrin increases resistance of juvenile channel catfish, Ictalurus punctatus, to enteric septicemia. J World Aquacult Soc. 2010;41:28–39. doi: 10.1111/j.1749-7345.2009.00330.x. [DOI] [Google Scholar]
- Welker T.L., Lim C., Yildirim-Aksoy M., Klesius P.H. Growth, immune function, and disease and stress resistance of juvenile Nile tilapia (Oreochromis niloticus) fed graded levels of bovine lactoferrin. Aquaculture. 2007;262:156–162. doi: 10.1016/j.aquaculture.2006.09.036. [DOI] [Google Scholar]
- Yokoyama S., Koshio S., Takakura N., Oshida K., Ishikawa M., Gallardo-Cigarroa F.J., et al. Effect of dietary bovine lactoferrin on growth response, tolerance to air exposure and low salinity stress conditions in orange spotted grouper Epinephelus coioides. Aquaculture. 2006;255:507–513. doi: 10.1016/j.aquaculture.2005.12.001. [DOI] [Google Scholar]
- Zahran E., Risha E., Elbahnaswy S., Mahgoub H.A., Abd El-Moaty A. Tilapia piscidin 4 (TP4) enhances immune response, antioxidant activity, intestinal health and protection against Streptococcus iniae infection in Nile tilapia. Aquaculture. 2019;513 doi: 10.1016/j.aquaculture.2019.734451. [DOI] [Google Scholar]
- Zhou X., Wang Y., Li W. Effect of feeding apidaecin on common carp (Cyprinus carpio) growth performances and immune function. Aquaculture. 2008;279:108–112. doi: 10.1016/j.aquaculture.2008.04.024. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.