Introduction
Radiation therapy is commonly used in the management of primary and metastatic tumors of the central nervous system. Historically, fractionated external beam radiation has been the primary treatment modality. Stereotactic radiosurgery (SRS), including Gamma Knife radiosurgery, delivers high doses of radiation and has been increasingly used for the treatment of intracranial metastatic and benign conditions. While both modalities are well established as safe and effective treatment options, they have been associated with rare side effects including radiation edema, cyst formation, neuropathies, hydrocephalus, and tissue necrosis.1, 2, 3, 4
The use of radiation therapy in patients with multiple sclerosis (MS) is controversial. There are several case series and reports of patients with MS experiencing severe or more frequent adverse effects after radiation treatment intended for tumor control.5, 6, 7, 8, 9, 10, 11, 12 These studies describe the occurrence of demyelinating and nondemyelinating injuries near the radiation field. While SRS has been described in patients with MS as safe in the treatment of benign conditions including vestibular schwannoma and trigeminal neuralgia, these treatments may be associated with less parenchymal exposure and thus less representative of tumor treatment.13,14 To better understand the potential for and duration of radiation toxicity in patients with MS receiving tumoricidal doses, we report a case of a woman with a history of MS and breast cancer who developed concerning radiographic and clinical changes after Gamma Knife treatment to a progressive cerebellar lesion.
Case Summary
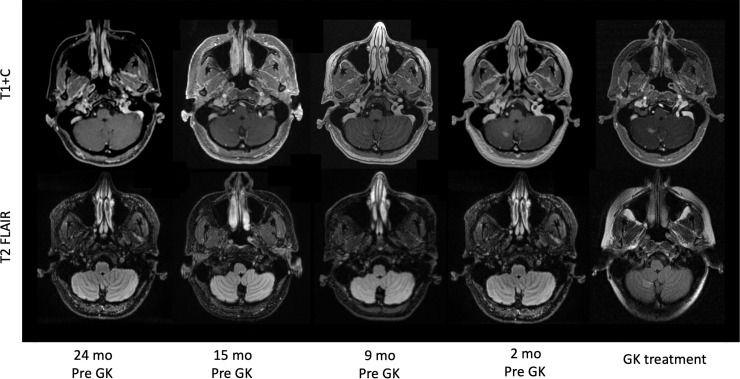
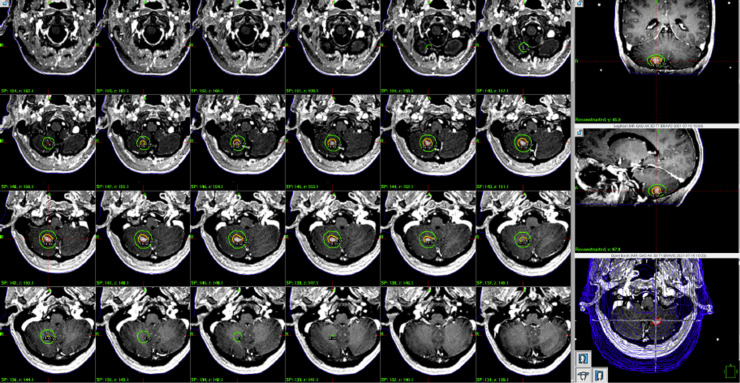
A 44-year-old woman with a history of MS with stability of known lesions and intraductal hormone receptor-positive breast carcinoma treated with bilateral mastectomy and neoadjuvant/adjuvant systemic therapy presented with a slowly progressive right cerebellar mass on surveillance imaging. Three years after initial cancer diagnosis, she was found to have 2 new intracranial lesions: a right parietal lesion that was stable and appeared demyelinating in origin, and a right cerebellar lesion that progressed in size and enhancement over 15 months (Fig. 1). She remained neurologically asymptomatic. Although a biopsy of the right cerebellar lesion was deferred, given the progressive nature of the lesion and an appearance atypical of MS lesions, the cerebellar mass was considered most likely metastatic in nature. Her existing MS lesions remained stable after the patient discontinued disease modifying treatments at the time of cancer diagnosis. She underwent Gamma Knife radiosurgery to the single right cerebellar lesion to 19 Gy in 1 fraction prescribed to the 74% isodose line, with a maximum dose of 25.7 Gy (Fig. 2). The prescription volume was 1.18 cc while the 12-Gy volume was 3.5 cc, well below established safe thresholds for single-fraction SRS.15 The Radiation Therapy Oncology Group conformity index was 2.05.16 There were no adverse effects noted in the weeks immediately following treatment.
Figure 1.
Progression of right cerebellar lesion demonstrating enhancement without associated T2/FLAIR changes. Abbreviations: GK = Gamma Knife.
Figure 2.
Gamma knife radiosurgery treatment plan.
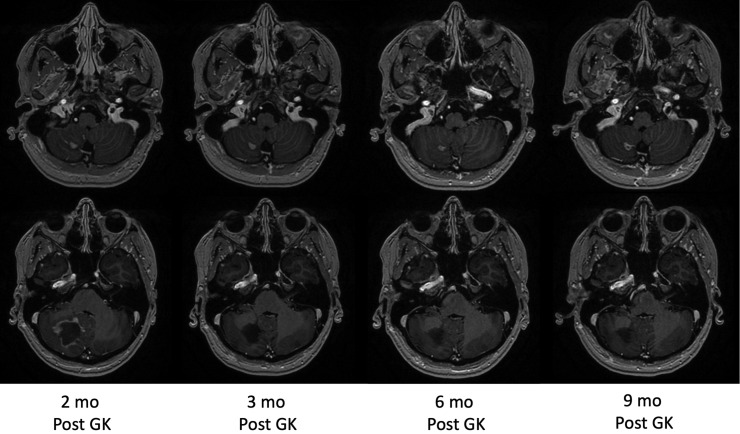
Two months postradiosurgery, the patient reported new-onset headaches. Repeat magnetic resonance imaging of the brain with and without contrast showed interval decrease in the size of the right cerebellar lesion, though with new right leptomeningeal enhancement with an irregularly shaped liquefied cavity near but separate from the treated lesion (Fig. 3). Interdisciplinary imaging review favored radiation toxicity rather than a new demyelinating plaque. She was started on high-dose dexamethasone with significant improvement in the extensive inflammatory changes after a 1-month course of steroids. Subsequent imaging performed every 3 months continued to demonstrate resolution of inflammatory changes. Twelve months after her Gamma Knife radiosurgery, she remained off steroids and disease modifying treatments without evidence of tumor recurrence or MS flare.
Figure 3.
Interval decrease of treated right cerebellar lesion (top row) with new enhancing liquefactive cavity (bottom row) after Gamma Knife (GK) treatment. Patient was treated with high-dose steroids between 2 and 3 months post Gamma Knife radiosurgery.
Discussion
In this case presentation, Gamma Knife radiosurgery was used to treat a presumed malignant lesion in a patient with a history of metastatic breast cancer and long-standing stable MS without active demyelinating disease. She developed severe radiographic and clinical changes suggestive of radiation toxicity but not new or progressive demyelinating changes. The interval development of a liquefied cavity and leptomeningeal enhancement were consistent with cyst formation and radiation necrosis, which can be an uncommon side effect of SRS.1,4,17
The presenting symptom of headache without focal neurologic deficits was also most consistent with radiation sequelae and not acute MS exacerbation. Her symptoms ultimately resolved with a 1-month course of steroids. Based on this case, we recommend close monitoring for clinical changes posttreatment with SRS among patients with a history of MS. However, while radiation toxicity may occur, this case suggests that subacute radiation toxicity is reversible and can be adequately managed with steroids. As such, MS should not be a contraindication to Gamma Knife radiosurgery for patients with limited intracranial metastases.
Our experience builds on previous data showing associations of increased radiation toxicity among patients with MS treated for intracranial lesions. Several clinical syndromes have been associated with increased radiation toxicity including collagen vascular disease and germline defects of DNA damage repair.18, 19, 20 In Table 1, we summarize prior outcomes among patients with MS treated with tumoricidal doses of radiation therapy for malignant and benign central nervous system tumors.5, 6, 7, 8, 9, 10, 11, 12,21,22 Several case series report the development of symptomatic focal demyelination injury in the radiation field seen on posttreatment imaging or autopsy.6,9,10,21 Others have reported the development of widespread white matter lesions without associated symptoms consistent with radiographically isolated MS after radiation treatment.22 Some lesions were treated as presumed tumors though later identified as demyelinating plaques, with increased neurologic toxicity following treatment, which highlights the difficulties in radiographic interpretation of intracranial lesions in patients with known MS.7,9 Outcomes in patients who received radiation to demyelinating lesions ranged from minimal toxicity to profound neurologic defects and death. Although interpretation of prior studies on the interaction between radiation and MS is limited by heterogenous clinical situations, radiation therapy approaches, and small cohort sizes, they raise concern for increased radiation adverse effects or MS exacerbation with the use of radiation therapy among patients with MS.
Table 1.
Toxicity outcomes among patients with multiple sclerosis treated with tumoricidal radiation doses
| Study | Number of study patients | Disease | Radiation therapy | Outcomes |
|---|---|---|---|---|
| Lampert (1959)5 | 1 | Basal cell carcinoma of external auditory canal | 57.5 Gy in 20 fractions | Obtundation, death 3 mo after treatment |
| McMeekin (1969)6 | 1 | Glomus jugulare tumor | 72 Gy in 45 fractions | Rapid neurologic deterioration within 2 mo of radiation, autopsy consistent with demyelinating injury in radiation field |
| Peterson (1993)7 | 5 | Demyelinating lesions (identified retrospectively as not tumors) | 40-60 Gy | 4/5 patients experienced unexpectedly poor neurologic outcome |
| Murphy (2003)8 | 1 | Metastatic adenoid cystic carcinoma | 50 Gy in 25 fractions | Clinical and radiographic findings consistent with acute multiple sclerosis exacerbation within 10 w of treatment |
| Miller (2006)9 | 15 | 9/15 primary brain tumors, 4/15 metastatic tumors, 2/15 retroactively found to be demyelinating lesions | 30-55.8 Gy (median 50 Gy) | Grade 4+ neurotoxicity in 6/15 patients at 1 y |
| Daniels (2009)10 | 1 | Pituitary adenoma | Gamma knife marginal dose 25 Gy | Optic neuritis within 3 mo of treatment |
| Lowell (2011)11 | 6 | 3 metastatic tumors, 3 primary tumors | Gamma knife marginal dose 12-21 Gy | 3/6 patients with grade 2 + toxicities: 6th nerve palsy (grade 2), 7th nerve palsy (grade 3), AMS/hydrocephalus/diffuse cerebral atrophy with leukoaraiosis (grade 3) |
| Milic (2016)21 | 2 | Oligodendroglioma | 56 Gy in 30 fractions | New onset white matter lesions consistent with acute demyelination 2 and 4 mo after treatment |
| Borges (2021)22 | 1 | Suprasellar germinoma | 24 Gy in 15 fractions (whole ventricular radiation) | Multiple white matter lesions in posterior fossa and supratentorium consistent with inflammatory lesions |
| Wallerius (2021)12 | 1 | Vestibular schwannoma | 25 Gy in 5 fractions | Grade 2 toxicity and radiation edema consistent with multiple sclerosis exacerbation |
The mechanism for frequency of complication occurrence among patients with MS is unknown though may be related to pre-existing demyelination, inflammation, or vascular changes, even without presence of active demyelination at the time of treatment.12 In part, the observed toxicities among patients with MS may be associated with a lower threshold for developing radiation-induced demyelination. Radiation has been shown to damage both progenitor and mature oligodendrocytes, reducing the current and future pool of myelin-producing cells.23 In line with this theory, guinea pigs who underwent radiation therapy to the spinal cord were found to have 2 nadirs of myelin basic protein in the treatment field, which corresponded with depletion of precursor and mature oligodendrocytes, respectively.24 Given the destruction of oligodendrocytes in MS, these patients may be more susceptible to additional insult from radiation.25 Another possible mechanism for toxicity is via increased sensitivity to radiation. Reduced expression of ataxia-telangiectasia serine/threonine kinase seen in some patients with MS affects the DNA damage repair pathway and thus increases radiation sensitivity.26 Furthermore, patients with MS have been reported to develop hydrocephalus after radiation,11 possibly related to chronic inflammation and increased concentration of proteins in the cerebrospinal fluid from necrotic tumors that decrease cerebrospinal fluid resorption.8,27
Interestingly, despite associations between MS and increased toxicities with radiation therapy, SRS has been studied as a treatment in medically refractory MS and trigeminal neuralgia. In a retrospective series of 15 patients with medically refractory MS-related tremors, 13 patients experienced tremor improvement after Gamma Knife thalamotomy treatment to a median maximum dose of 140 Gy.13 Two patients experienced temporary paresis associated with imaging changes of thalamic target and a third patient developed thalamic cyst requiring eventual placement of Ommaya reservoir. Radiosurgery has also been successfully used in the management of trigeminal neuralgia in MS patients with no clear MS exacerbations, though less white matter is exposed to radiation because of the inferior location of the treatment target.14 Despite the potential for adverse effects, radiosurgery continues to have a role for treatment of nontumor pathologies in patients with MS.
Given the potential for more significant side effects in patients with MS receiving radiation therapy, patients should be closely followed posttreatment with a low threshold for early intracranial imaging. However, adverse radiation effect may be effectively managed with steroids and MS should not be a contraindication to consideration of Gamma Knife radiosurgery in appropriately selected patients. Despite some differences in radiographic appearance of demyelinating plaques compared with injuries of differing pathogeneses, it remains difficult to identify the etiology of neurotoxicity in these patients. Early initiation of treatment may reverse clinical and radiographic symptoms and elucidate the mechanism of injury.
Conclusion
Intracranial radiation in patients with MS has been associated with adverse effects with several reports of demyelinating injury within the radiation field. We describe a case of nondemyelinating lesion injury adjacent to a cerebellar lesion treated with Gamma Knife reflective of adverse radiation effect that resolved with steroid management. Radiosurgery continues to have a role in the management of multiple pathologies in patients with MS. Prompt evaluation and treatment may reverse clinical and radiographic findings associated with toxicity in patients with MS.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Xu N, Shu H, Marcus R, et al. Radiation necrosis after stereotactic radiosurgery for metastatic brain disease. Int J Radiat Oncol Biol Phys. 2007;69:S163. [Google Scholar]
- 2.Varlotto JM, Flickinger JC, Niranjan A, et al. Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2003;57:452–464. doi: 10.1016/s0360-3016(03)00568-6. [DOI] [PubMed] [Google Scholar]
- 3.Carr CM, Benson JC, DeLone DR, et al. Intracranial long-term complications of radiation therapy: An image-based review. Neuroradiol. 2021;63:471–482. doi: 10.1007/s00234-020-02621-7. [DOI] [PubMed] [Google Scholar]
- 4.Ilyas A, Chen CJ, Ding D, et al. Cyst formation after stereotactic radiosurgery for brain arteriovenous malformations: A systematic review. J Neurosurg. 2018;128:1354–1363. doi: 10.3171/2016.12.JNS162478. [DOI] [PubMed] [Google Scholar]
- 5.Lampert P, Tom M, Rider W. Disseminated demyelination of the brain following Co60 (gamma) radiation. Arch Pathol. 1959;68:322–330. [PubMed] [Google Scholar]
- 6.McMeekin R, Hardman J, Kempe L. Multiple sclerosis after x-radiation: Activation by treatment of metastatic glomus tumor. Arch Otolaryngol. 1969;90:617–621. doi: 10.1001/archotol.1969.00770030619017. [DOI] [PubMed] [Google Scholar]
- 7.Peterson K, Rosenblum MK, Powers JM, et al. Effect of brain irradiation on demyelinating lesions. Neurology. 1993;43 doi: 10.1212/wnl.43.10.2105. 2105-2105. [DOI] [PubMed] [Google Scholar]
- 8.Murphy CB, Hashimoto SA, Graeb D, et al. Clinical exacerbation of multiple sclerosis following radiotherapy. Arch Neurol. 2003;60:273–275. doi: 10.1001/archneur.60.2.273. [DOI] [PubMed] [Google Scholar]
- 9.Miller RC, Lachance DH, Lucchinetti CF, et al. Multiple sclerosis, brain radiotherapy, and risk of neurotoxicity: The Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 2006;66:1178–1186. doi: 10.1016/j.ijrobp.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Daniels TB, Pollock BE, Miller RC, et al. Radiation-induced optic neuritis after pituitary adenoma radiosurgery in a patient with multiple sclerosis: Case report. J Neuro-Oncology. 2008;93:263–267. doi: 10.1007/s11060-009-9860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowell D, Tatter SB, Bourland JD, et al. Toxicity of Gamma Knife radiosurgery in the treatment of intracranial tumors in patients with collagen vascular diseases or multiple sclerosis. Int J Radiat Oncol Biol Phys. 2011;81:e519–e524. doi: 10.1016/j.ijrobp.2011.02.056. [DOI] [PubMed] [Google Scholar]
- 12.Wallerius K, Collins S, Forsthoefel M, et al. Increased radiosurgery toxicity associated with treatment of vestibular schwannoma in multiple sclerosis. Otol Neurotol. 2021;42:e489–e494. doi: 10.1097/MAO.0000000000002977. [DOI] [PubMed] [Google Scholar]
- 13.Raju SS, Niranjan A, Monaco EA, et al. Stereotactic radiosurgery for medically refractory multiple sclerosis–related tremor. J Neurosurg. 2018;128:1214–1221. doi: 10.3171/2017.1.JNS162512. [DOI] [PubMed] [Google Scholar]
- 14.Spina A, Nocera G, Boari N, et al. Efficacy of Gamma Knife radiosurgery in the management of multiple sclerosis-related trigeminal neuralgia: A systematic review and meta-analysis. Neurosurg Rev. 2021;44:3069–3077. doi: 10.1007/s10143-021-01507-3. [DOI] [PubMed] [Google Scholar]
- 15.Milano MT, Grimm J, Niemierko A, et al. Single- and multifraction stereotactic radiosurgery dose/volume tolerances of the brain. Int J Radiat Oncol Biol Phys. 2021;110:68–86. doi: 10.1016/j.ijrobp.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw E, Kline R, Gillin M, et al. Radiation therapy oncology group: Radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993;27:1231–1239. doi: 10.1016/0360-3016(93)90548-a. [DOI] [PubMed] [Google Scholar]
- 17.Vellayappan B, Tan CL, Yong C, et al. Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol. 2018;8:395. doi: 10.3389/fonc.2018.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hölscher T, Bentzen SM, Baumann M. Influence of connective tissue diseases on the expression of radiation side effects: A systematic review. Radiother Oncol. 2006;78:123–130. doi: 10.1016/j.radonc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Morris MM, Powell SN. Irradiation in the setting of collagen vascular disease: Acute and late complications. J Clin Oncol. 1997;15:2728–2735. doi: 10.1200/JCO.1997.15.7.2728. [DOI] [PubMed] [Google Scholar]
- 20.Pollard JM, Gatti RA. Clinical radiation sensitivity with DNA repair disorders: An overview. Int J Radiat Oncol Biol Phys. 2009;74:1323–1331. doi: 10.1016/j.ijrobp.2009.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milic M, Rees JH. Acute demyelination following radiotherapy for glioma: A cautionary tale. Pract Neurol. 2017;17:35–38. doi: 10.1136/practneurol-2016-001432. [DOI] [PubMed] [Google Scholar]
- 22.Borges A, Garcez D, Pedro C, et al. Chemoradiation induced multiple sclerosis-like demyelination. eNeurologicalSci. 2021;22 doi: 10.1016/j.ensci.2021.100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belka C, Budach W, Kortmann RD, et al. Radiation induced CNS toxicity – Molecular and cellular mechanisms. Br J Cancer. 2001;85:1233–1239. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang CS, Mason KA, Withers HR, et al. Alteration in myelin-associated proteins following spinal cord irradiation in guinea pigs. Int J Radiat Oncol. 1992;24:929–937. doi: 10.1016/0360-3016(92)90477-y. [DOI] [PubMed] [Google Scholar]
- 25.Cudrici C, Niculescu T, Niculescu F, et al. Oligodendrocyte cell death in pathogenesis of multiple sclerosis: Protection of oligodendrocytes from apoptosis by complement. 2006;43:123–132. doi: 10.1682/jrrd.2004.08.0111. [DOI] [PubMed] [Google Scholar]
- 26.Deng X, Ljunggren-Rose A, Maas K, et al. Defective ATM-p53-mediated apoptotic pathway in multiple sclerosis. Ann Neurol. 2005;58:577–584. doi: 10.1002/ana.20600. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien T, Paine M, Matotek K, et al. Apparent hydrocephalus and chronic multiple sclerosis: A report of two cases. Clin Exp Neurol. 1994;30:137–143. [PubMed] [Google Scholar]