Abstract
Purpose
The aim of this study is to evaluate whether concentrated growth factor (CGF) and photobiomodulation (PBMT) can show synergistic effect on bone healing process.
Methods
In vivo osteogenesis studies were performed in a rabbit critical-sized calvarial defect model. Four 8 mm critical-sized bone defects were created on each rabbit calvarium, and these 4 defects were randomly divided into 4 groups: 1-control (defect filled with autologous blood clot); 2-CGF (defect filled with CGF); 3-LLLT (defect filled with autologous blood clot and received Nd:YAG low-level laser irradiation); 4-CGF + LLLT (defect filled with CGF and received LLLT). 15 Japanese big-ear white rabbits were operated on using the same procedure in this study. Then, 5 rabbits were selected randomly and sacrificed at 4th, 6th and 8th week postoperatively and respectively. The calvariums were harvested and scanned by micro-CT. The volumes of new bone formation of these defects were calculated by analyzing the micro-CT image. Data were analyzed as mean values of each group, comparisons were made for statistical analysis with the group and among the 4 groups using analysis of variance (ANOVA, P < 0.05).
Results
At the 4th, 6th and 8th weeks, compared with the control group, the volume of new bone formed in each experimental group was significantly increased. Both CGF and LLLT can accelerate bone healing, but the effect of LLLT is better than that of CGF, and the difference between the two is statistically significant (P < 0.01). There was no statistically significant difference in the osteogenic effect between the combined application of CGF + LLLT and the application of CGF alone. And the osteogenic effect of the former two groups was weaker than that obtained by laser irradiation alone.
Conclusions
Both CGF and LLLT can promote osteogenesis effectively, but the combination of the two did not show a synergistic effect. The pro-osteogenic effect of Nd:YAG low-level laser irradiation is superior to that of CGF, and also superior to the combined effect of the two.
Keywords: Low-level laser therapy, Photobiomodulation, Concentrated growth factor, Nd:YAG laser, Calvarial defect
Abbreviations: CGF, concentrated growth factor; PBMT, photobiomodulation; LLLT, low-level laser therapy
1. Introduction
Usually the bone tissue has a strong regeneration ability and can be completely repaired after fractures. However, when the bone defect is so large that it exceeds the bone tissue's self-repairing ability, it cannot be completely repaired by itself. This phenomenon is commonly seen in large bone defects caused by trauma, tumor resection, osteonecrosis or segmental bone defects [1]. These non-healing bone defects may cause deformity and dysfunction of the body. It is necessary to explore some new therapeutic methods to promote bone healing in the field of bone tissue engineering.
The regeneration and repair of bone tissue relies on osteogenesis, osteoinduction and osteoconduction. Adequate vascularity is quite required in these processes, and avascularity plays an important role in the pathogenesis of critical defects. During bone repair, mesenchymal stem cells present in blood vessels, bone marrow, and periosteum differentiate into osteoprogenitor cells, which can differentiate into osteocytes to promote bone healing under the action of osteoinductive factors. Osteoinductive factors [2] including bone morphogenetic protein, transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF), are transported to the bone defect through the blood. In bone defect healing, the osteoconductive scaffold is important and necessary, which is first provided by the hematoma and then by the cartilage callus. So autogenous bone is regarded as the gold standard [3] for graft materials for bone defect healing as it provides the three elements necessary to generate and maintain bone: osteoprogenitor cells for osteogenesis, osteoinductive factors for osteoinduction, and scaffolds for osteoconduction [4]. However, autologous bone graft requires incision in other parts of the body, some adverse reactions such as pain, scar, nerve injury may occur in the donor parts. In addition, bone graft surgery requires the operator to have higher surgical skills and may prolong the operation time. At present, synthetic bone graft materials can be used clinically to promote bone healing [5].
In recent years, platelet concentrate products have been used in the field of tissue repair, especially in the field of wound healing. In stomatology, platelet concentrate products have been successfully used in alveolar ridge preservation after tooth extraction, bone cavity filling after jaw cyst excision, wisdom tooth extraction, and periodontal surgery [6,7]. After activation, platelets can release many powerful growth factors/cytokines, including platelet-derived growth factor, TGF-β, VEGF, etc., which can promote cell proliferation, matrix synthesis, and collagen deposition; platelet concentrate products also contain adhesion factors, such as fiber Protein, fibronectin and vitronectin, which can build the three-dimensional structure required for tissue repair and provide a scaffold for the migration of repair cells. Concentrate growth factor (CGF) is the third-generation platelet concentrate product [8], which contains a larger, denser and more growth factor-rich fibrin matrix, has greater tensile strength and viscosity, and has stronger regenerative and osteogenic induction properties. Meanwhile, it can provide an excellent guiding scaffold for bone tissue regeneration.
On the other hand, many scholars are trying to explore non-invasive methods with osteoinductive ability. They found that low-level laser therapy (LLLT), also known as photobiomodulation (PBM), has an effect on cell metabolism without irreversible damage to biological tissues [9]. When low-level laser light is absorbed by tissue, it can enhance mitochondrial and alkaline phosphatase (ALP) activity in stem cells, promote DNA or RNA synthesis and induce their differentiation [10,11]. There are many types of lasers currently used in LLLT, such as He–Ne laser, GaAlAs laser, Nd:YAG laser, etc. Previous experiments revealing the effect of lasers on osteogenesis are controversial, and the difference in results may be attributed to the different absorption properties of biological tissues for lasers with different wavelength, or due to the different parameters set in the laser therapy [9,12].
In our study, a critical bone defect model of the rabbit skull was established, and the differences among CGF filling, 1064 nm Nd:YAG low-level laser irradiation, and the combination of the two were compared in the treatment of bone defects. The rabbit calvariums were harvest at the 4th, 6th, and 8th week. The bone defects were scanned by Micro-CT and the volumes of new bone formation were analyzed.
2. Material and methods
2.1. Critical-sized calvarial defect model of the rabbit
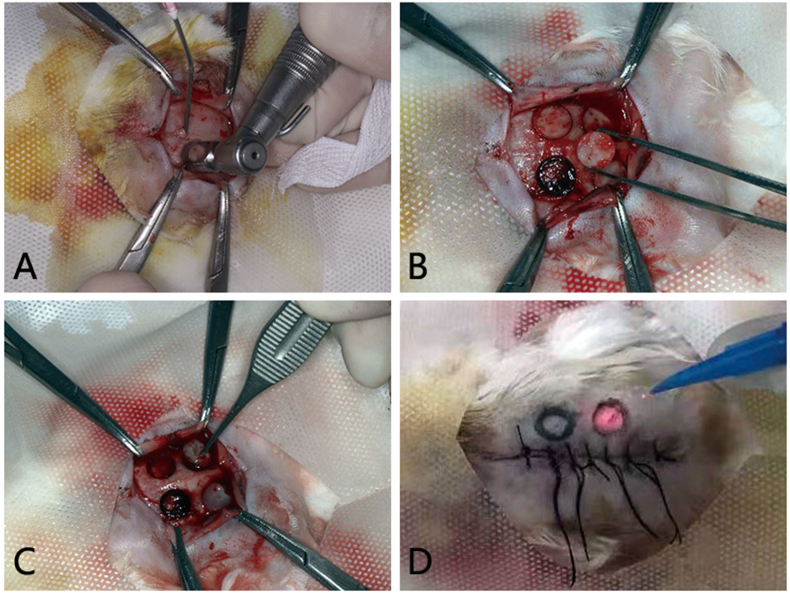
All animals were kept in a pathogen-free environment and fed ad lib, and all procedures were conducted in accordance with the “Guiding Principles in the Care and Use of Animals” (China). Our animal experiment was under the supervision and assessment by the Laboratory Animal Ethics Committee, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, and passed the ethical review (No. SBQDL-2022-068). SPF Japanese white rabbits aged 5 months (total n = 15) were used for this model. All animals were weighed, fixed in a rabbit fixing frame, and subjected to general anesthesia through the ear vein (3% sodium pentobarbital solution, 1 ml/kg). The calvarial skin was prepared and then disinfected with 5% betadine. An incision was made along the sagittal suture, the periosteum was elevated, and four 8 mm diameter calvarial bone defects without dural perforation were created using a trephine (φ6/8 mm, 1000prm). The four 8 mm critical-sized bone defects were created on each rabbit calvarium (Fig. 1A and B), with a 3 mm or further separation between them, and were randomly divided into 4 groups: 1-control (defect filled with autologous blood clot); 2-CGF (defect filled with CGF); 3-LLLT (defect filled with autologous blood clot and received Nd:YAG low-level laser irradiation); 4-CGF + LLLT (defect filled with CGF and LLLT). The wound was closed, and all rabbits were given penicillin (400,000 IU, im, qd) 3 days postoperatively to prevent infection.
Fig. 1.
Prepare of four 8 mm critical-sized bone defects on rabbit calvarium. (A) 8 mm diameter trephine used in our operation. (B) Calvarial bone defects without dural perforation were created. (C) Bone defects filled with CGF. (D) The wound was closed, and Nd:YAG laser irradiation was performed on the marked bone defect area according to the experimental design.
2.2. CGF preparation
After the rabbit was anesthetized and fixed, 18 ml of blood was rapidly collected from the middle ear artery of the rabbit using a 20-ml single use syringe. Then, the blood was equally divided, put into two standard disposable 10-ml non-anticoagulant tubes, equilibrated into a centrifuge, centrifuged by the matching centrifuge device (Medifuge200, Silfradentsrl, Sofia, Italy), and the acceleration and deceleration processes were adjusted automatically due to the device's feature [13]. The final product is gel-like, the middle layer in the form of membrane containing the concentrated growth factors was separated from the red blood cell layer (at the bottom) by cutting with a pair of scissors, and then fill it into the target bone defect area (Fig. 1C). In this experiment, the middle layer was trimmed as the CGF graft material, which could not only fill the calvarial defects to meet the requirements of the scaffold, but also obtain a high concentration of growth factors.
2.3. Experimental design of laser irradiation
The calvarial defects in LLLT group and CGF + LLLT group were received Nd:YAG (1064 nm, Fidelis AT, Fotona, Ljubljana, Slovenia) low-level laser irradiation once a day with 7 days after operation. After the incision was closed, a round was drawn on the rabbit calvarial skin with a marker pen, the center of which corresponds to the center of the calvarial bone defect, and the distance from the outer circle to the center of the round is 4 mm, and the laser spot irradiation covered the entire marking round. The entire bone defect area was vertically irradiated by a pulsed Nd:YAG laser through a 320 μm diameter optical fiber in a non-focused way at a distance of 1–2 cm from it (Fig. 1D). In the rabbit calvarial model output power was set as 3 W. Other parameters, including the light spot diameter (8 mm), pulse repetition rate (15 pps), a total fluence rate (344J/cm2), and irradiation time (60s) were remained constant at output power of 3 W [14].
2.4. Micro-computed tomography (Micro-CT) scanning
5 rabbits were selected randomly and sacrificed at 4th week, 6th week and 8th week postoperatively and respectively (total n = 15). The calvarias were harvested and scanned by Micro-CT (viva CT 80, SCANCO MEDICAL, Switzerland). The CT scan parameters were sat at 70 kVp, 114 μA, 8 W. All samples were scanned (thin cut 10.4 μm, exposure time 400 ms, 2048*2048pxl), and the original data were stored in DICOM format.
2.5. vol calculation of new bone formation in the critical-sized calvarial defect
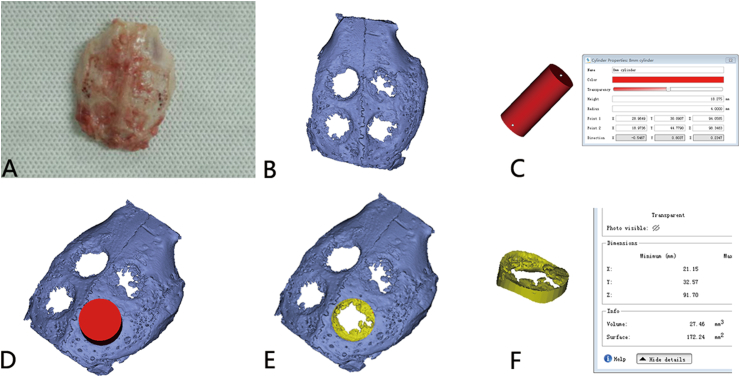
The DICOM data were then input and reconstructed by Mimics Research software (Version21.0; Materialise, Leuven, Belgium). A cylinder with a diameter of 8 mm was created, and then the edge of the cylinder was completely overlapped with the edge of the calvarial defect area, and the volume of new bone was obtained through Boolean operation and calculated (Fig. 2A–F). In Mimics software, the predefined threshold of micro-CT HU value was set at a range from 1000HU to 6000HU. On this condition, the bone in the non-defective area of calvarium can be displayed normally. The 3D-constructed bone within this threshold range in each defect region can be defined as new bone formed.
Fig. 2.
Demonstration volume of the new bone formed. (A) Calvaria harvested from the rabbit. (B) Calvaria scanned by micro-CT and then reconstructed. (C) A cylinder with a diameter of 8 mm was created. (D) The volume of new bone was obtained through Boolean operation. (E) New bone formed in the defect area is isolated. (F) New bone formed in the defect area is calculated.
2.6. Statistical analysis
SPSS 21.0 package software was used for statistical analysis. The mean and standard deviation (SD) of the formation bone volumes were analyzed. Comparisons were made for statistical analysis with the group and among the 4 groups using analysis of variance (ANOVA, P < 0.05).
3. Results
3.1. Osteogenic effect from the morphology of three-dimensional CT reconstruction
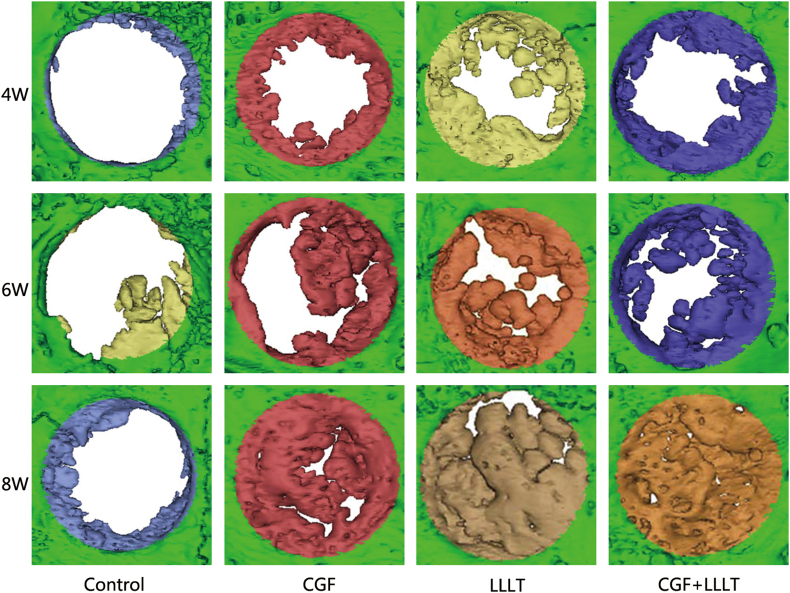
The osteogenic effect from the morphology of three-dimensional CT reconstruction are showed in Fig. 3. From the three-dimensional reconstruction image of Micro-CT, the bone regeneration effect of the four groups is visually compared, and it can be seen that:
Fig. 3.
General view of new bone volume of different group and different time. Three representative rabbits were selected from each of the 5 rabbits sacrificed at 4 W, 6 W and 8 W for display.
In the control group: At the 4th week, a small amount of new bone was formed at the edge of the original bone defect, and at the 6th and 8th week, there was still only a small amount of new bone. In the other 3 groups: At the 4th week, new bone was formed at the edge of the original bone defect, which was significantly increased compared with the control group. At 6th week, the range of new bone continued to increase. And at 8th week, most areas of original bone defect were basically occupied by new bone, and only small bone defect areas were left.
From the three-dimensional reconstruction images of each group, it can be also found that in the process of bone healing, bone repair is formed from the edge of the defect to the center. At the forefront of bone tissue regeneration, there are many scattered new bone islands secondary to the ossification center.
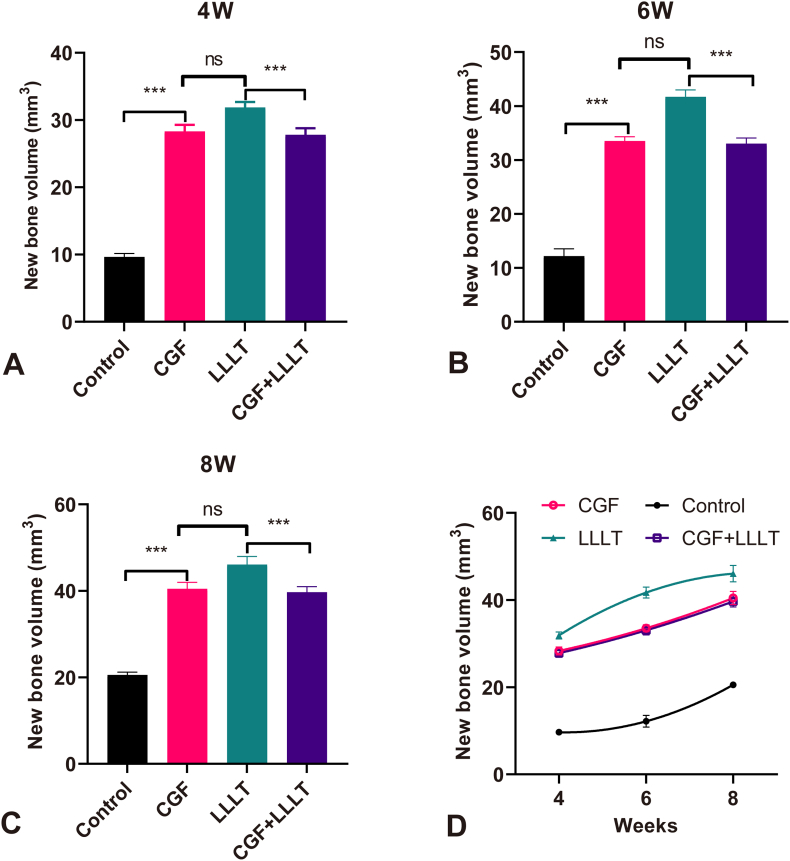
3.2. Volume of new bone formation in different groups
The volume of new bone formed in each group at different weeks is shown in Table 1. Compared with the control group, the volume of new bone formed in each experimental group was significantly increased at 4 W (Fig. 4A), 6 W (Fig. 4B) and 8 W (Fig. 4C), respectively, indicating that CGF, LLLT, and CGF + LLLT can promote osteogenesis effectively. The new bone volume in each group at different weeks is shown in Fig. 4D. Both CGF and LLLT can accelerate bone healing, but the effect of LLLT is better than that of CGF, and the difference between the two is statistically significant (P < 0.01). The combination of CGF and LLLT also accelerated bone healing, but did not show a synergistic effect. There was no statistically significant difference in the osteogenic effect between the combined application of CGF + LLLT and the application of CGF alone. And the osteogenic effect of the former two groups was weaker than that obtained by laser irradiation alone. From the comparison of the bone volume in the 4th week, the one in the control group was the least, and the one in the other three groups was much more than that in the control group (almost 3 times). It can be speculated that CGF and LLLT can play a better role in the first 4 weeks of new bone formation. However, in the following 4–8 weeks, the curves of new bone formation in the three experimental groups gradually slowed down, and the upward trend was less than that in the control group.
Table 1.
The volume of new bone formed in each group calculated from Micro-CT(X ± S).
| Week | New bone volume (mm3) |
|||
|---|---|---|---|---|
| Control | CGF | LLLT | CGF + LLLT | |
| 4 W | 9.66 ± 0.49 | 28.33 ± 0.96 AC | 31.88 ± 0.81 ABD | 27.82 ± 0.96 AC |
| 6 W | 12.16 ± 1.36 | 33.52 ± 0.79 AC | 41.70 ± 1.28 ABD | 33.04 ± 1.04 AC |
| 8 W | 20.55 ± 0.66 | 40.46 ± 1.51 AC | 46.05 ± 1.89 ABD | 39.68 ± 1.27 AC |
Note. In the same week, values with no letter mean no significant difference (P > 0.05), while values with capital letter superscripts mean significant difference (P < 0.01). A compared with control group, B compared with CGF group, C compared with LLLT group, D compared with CGF + LLLT group.
Fig. 4.
Compared with every group, except CGF and CGF + LLLT, the other groups had statistical difference. (A) New bone volumes at 4 W. (B) New bone volumes at 6 W. (C) New bone volumes at 8 W. (D) Comparison of overall new bone volumes.
4. Discussion
This study is mainly based on the following considerations to select the Japanese big-eared white rabbit calvaria to establish a critical bone defect model. Firstly, compared to long bones, calvarias allows the creation of larger bone defects without the risk of fracture and generally does not interfere with the daily activities of laboratory animals. Secondly, the operation on the calvaria has a clear field of view, high reproducibility, and easy quality control. Thirdly, the CGF in this experiment was derived from autologous blood concentration, requiring 18–20 ml of blood to be collected, and the amount of blood collected from rodents hardly meet the experimental conditions.
Critical bone defect refers to the minimum size of bone defect that cannot be repaired completely by itself during the entire life of animals or during the experimental observation period [15]. In recent years, several scholars have chosen to establish a rabbit calvarial defect model in the research on bone regeneration. However, the size of the bone defects is not uniform, and the lack of consistency leads to differences in the final experimental results. Kim et al. prepared 8 mm diameter rabbit calvarial full-thickness bone defects to study the ability of Nd:YAG laser on the regeneration of bone defects, and found that the high-intensity laser could accelerate bone regeneration at 8 weeks, but could not completely heal [14]. Drawing on the experience of previously published papers, this study selected 8 mm as the critical bone defect [12,14,16]. Observation period of this study was 8 weeks, and the 8 mm diameter bone defect was in line with the critical bone defect.
This study requires blood collection of 18–20 ml. For rabbits, maximum blood collection volume may be calculated as 15% of total blood volume (8% of body weight) every 2 weeks, and 18–20 ml blood collection is safe and feasible for a 2 kg rabbit(24 ml). There is a thicker and brighter red artery in the middle of the rabbit ear, which is the middle ear artery. It should be fully expanded and congested, and blood collection should be completed at one time before spastic contraction. Cardiac blood collection can provide 20–25 ml one time, which also meet the experimental requirements, but the heart and lungs are easily injured during the operation, and even life-threatening. In our experiment, 13 rabbits were successfully collected through the middle ear artery. Another 2 rabbits failed to collect blood through the middle ear artery, and the experimenter switched to cardiac blood collection. None of the animals died during the entire experimental period.
Platelet rich fibrin (PRF) and CGF are both platelet concentrate products, but the centrifugation and production process are different. PRF is the second-generation platelet concentrate product, and CGF is the third. PRF or CGF used alone can accelerate the healing of bone defects [17,18], which is the same as our experimental results. Reviews have concluded that the association of PBM and bone substitutes presented positive effects for bone healing in animal studies [5,19,20]. Bone substitutes traditionally include biosilicate, mineral tri-oxide aggregate (MTA), ceramic materials, deproteinized bovine bone graft. PRF or CGF combined with other biomaterials are considered to give better results for bone healing [21,22]. The application of this combination is widely used in dental surgery [23,24]. The results of previous studies showed that the combined with PRF and LLLT did not show a synergistic effect in the process of bone healing [25,26,27]. Only when bone graft material was added to the bone defect area, PRF combined with LLLT in the treatment of bone defect showed synergistic effect to a certain extent [28]. The literatures of CGF combined with LLLT for the treatment of bone defects are hardly to see. Our experiments concluded that CGF combined with LLLT did not show synergistic effects in the bone healing, either.
In this study, the LLLT group showed a significant positive effect on bone regeneration. Based on the conclusions reported in the previous literatures, we are almost certain that the setting of laser parameters will have a significant impact on the final result in low-energy laser therapy. The inconsistency of these conclusions may be due to the parameter setting of LLLT, such as differences in irradiation regimen, wavelength, power, energy density, treatment duration, treatment intervention time, application methods. Nd:YAG laser was selected as the laser for low-level laser treatment, because Nd:YAG laser has its unique characteristics in bone regeneration. First, the long wavelength (1064 nm) of Nd:YAG laser allows laser penetration skin/muscle to reach the bone tissue, thereby removing obstacles for light to penetrate biological tissue [14,29]. Second, Nd:YAG laser irradiation can up-regulate the expression of recombinant human insulin-like growth factor 1 (IGF-1) and BMP-2 cytokines, with a clear photobiomodulatory effect [30]. Third, the use of high-power lasers can shorten the laser exposure time and achieve the required laser intensity in a short time, which provides convenience for clinical treatment.
According to the clinical use of Fotona laser equipment, the power setting of the 3 mm diameter spot in LLLT therapy in vivo is generally 1.5 W. In this experiment, as the diameter of the laser spot increases (8 mm), the energy density will decrease. If we continue to use 1.5 W, we need to extend the irradiation time. In addition, Kimi et al. used Nd:YAG pulsed laser to study the effect of high-power pulsed laser on bone regeneration [14]. The same energy density (344 J/cm2) was used to irradiate the 8 mm skull bone defect of rabbits with 0.75 W and 3 W output power respectively. It was found that both 0.75 W and 3 W could promote bone formation, and there was no difference between the two power levels. Considering the above factors, a 3 W Nd:YAG laser was selected in this experiment. However, in vitro cell experiments, the irradiation power can be reduced to several hundred milliwatts because there is no soft tissue barrier [29]. Or in the process of photodynamic experiments, it can also be reduced to hundreds of milliwatts due to the strong absorption of laser with specific wavelength by photosensitizer.
The results showed that there were still large bone defects in the control group at the end of 8th week, indicating that the critical bone defect model was successfully prepared. The use of micro-CT scanning and three-dimensional reconstruction is helpful to observe the effect of various treatment factors on the clinical osteogenesis more intuitively. It should be pointed out that all the bone defects on the harvested calvarial specimens seem to have healed (Fig. 2A), while the three-dimensional reconstructed calvarial specimens still seem to have large defects (Fig. 2B). The reason for this difference is that some new bones cannot be visualized on CT due to short formation time and insufficient calcification. These bones usually do not have the strength and hardness compared with normal calcified bone, and are not included in the statistics of this experiment. The evaluation of bone regeneration can be discussed as a percentage of the original defect by the volume of new bone. The thickness of the calvaria in every rabbit is different, and the thickness of different parts in the same rabbit calvaria is also not uniform. Limited by the experimental conditions, this experiment cannot obtain the accurate volume of the original bone defect. The 60 bone defect models prepared in this experiment were all 8 mm in diameter and full-thickness calvarial defects, and were then randomly grouped to reduce errors that may be caused by non-uniformity of thickness. Therefore, the influence of each factor on the outcome was evaluated by measuring the change of the new bone volume rather than the percentage. The influence of various factors on the outcome was evaluated by measuring the change of new bone volume.
All specimens in the control group had only a small amount of bone formation at 4, 6, and 8 weeks after surgery. This study confirmed that the regeneration capacity of bone tissue itself is limited. It is necessary to use some adjuvant treatments for increasing the regeneration capacity of bone tissue. Both CGF and LLLT can promote osteogenesis, especially in the early stage of bone healing. CGF promotes bone healing by providing scaffolds and high concentrations of growth factors, which are exogenous effects. LLLT mainly enhances cell metabolism and promotes bone healing through photobiomodulation, which seems an endogenous effect. As we know, external factors are the conditions for change, internal factors are the basis for changes, and external factors act through internal factors. However, this is not enough to explain that the ability of LLLT to promote bone regeneration is better than that of CGF, and there may be other undetermined mechanisms that need to be further studied in follow-up experiments. The results also showed that the osteogenic effect of CGF + LLLT group is no different from that of CGF group, and the osteogenic effect of LLLT alone is the best. It can be concluded that the combined application of CGF and LLLT did not show a synergistic effect, but CGF may inhibit the osteopromoting effect of LLLT. It is speculated that the reason may be due to CGF reducing the absorption of laser light in the irradiated area. The main absorption sources of Nd:YAG laser are hemoglobin containing pigments, microorganisms, organic compounds. When Nd:YAG laser is absorbed by cells containing these pigments, it may increase the activity of mitochondria and alkaline phosphatase in osteoblasts, and promote DNA or RNA synthesis. CGF is a platelet concentrate, and the bottom red blood cell layer has been discarded after centrifugation. When the bone defect area is filled with CGF, due to the lack of such pigments, the laser absorption is weakened. Therefore, the photobiomodulation effect of LLLT in the CGF + LLLT group significantly weakened or disappeared.
Several limitations existed in the current study. First, the minimum distance between the four defects is 3 mm, and there is not enough evidence to prove whether this distance can rule out the influence of CGF on bone healing in adjacent defect regions. Before this study started, we had reviewed several literatures on the use of platelet concentrate products for the repair of calvarial defects in rabbits, and the distance between adjacent defects was not uniform and hardly to see in all those literatures [31,32,33,34]. According to the figures in their published papers, the distances between these four defects with a diameter of 8 mm are all relatively near. On the other hand, in clinical treatment, after the extraction of two adjacent teeth, the distance between the edges of adjacent tooth sockets is usually less than 3 mm. Using CGF to cover the tooth socket or perform alveolar ridge preservation, there is no obvious contamination observed in adjacent tooth sockets. Additionally, CGF gel is a translucent jelly with a tough and elastic texture, and a smooth surface, which slowly releases growth factors in the body. A relatively closed healing space can be obtained after placing CGF into the skull defect and closing the wound with periosteum and scalp soft tissue based on clinical experience. Second, the histomorphological analysis of this study is missed, our conclusion is only based on the volume of new bone from Micro-CT, and the explanation is quite limited.
5. Conclusion
Both CGF and LLLT can promote osteogenesis effectively, but the combination of the two did not show a synergistic effect. The pro-osteogenic effect of Nd:YAG low-level laser irradiation is superior to that of CGF, and also superior to the combined effect of the two.
Funding statement
Professor Kuanshou Zhang was supported by the National Natural Science Foundation of China (62175135).Pengfei Xin was supported by Program of State Key Laboratory of Quantum Optics and Quantum Optics Devices, Shanxi University [KF202105], and Fund for Shanxi Bethune Hospital "Beacon Project" Talent Training [2022FH18].
References
- 1.Mauffrey C., Barlow B.T., Smith W. Management of segmental bone defects. J. Am. Acad. Orthop. Surg. 2015;23(3):143–153. doi: 10.5435/JAAOS-D-14-00018. [DOI] [PubMed] [Google Scholar]
- 2.Chen G., Deng C., Li Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012;8(2):272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Gareta E., Coathup M.J., Blunn G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone. 2015;81:112–121. doi: 10.1016/j.bone.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Hing K.A. Bone repair in the twenty-first century: biology, chemistry or engineering? Philos Trans A Math Phys Eng Sci. 2004;362(1825):2821–2850. doi: 10.1098/rsta.2004.1466. [DOI] [PubMed] [Google Scholar]
- 5.Inchingolo F., et al. Innovative concepts and recent breakthrough for engineered graft and constructs for bone regeneration: a literature systematic review. Materials. 2022;15(3) doi: 10.3390/ma15031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes G.V.O., et al. Autologous Platelet Concentrate of 2(nd) and 3(rd) generations efficacy in the surgical treatment of gingival recession: an overview of systematic reviews. J. Indian Soc. Periodontol. 2021;25(6):463–479. doi: 10.4103/jisp.jisp_515_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torul D., Omezli M.M., Kahveci K. Evaluation of the effects of concentrated growth factors or advanced platelet rich-fibrin on postoperative pain, edema, and trismus following lower third molar removal: a randomized controlled clinical trial. J Stomatol Oral Maxillofac Surg. 2020;121(6):646–651. doi: 10.1016/j.jormas.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Bernardi S., et al. Histological characterization of Sacco's concentrated growth factors membrane. Int. J. Morphol. 2017;35:114–119. [Google Scholar]
- 9.Zein R., Selting W., Benedicenti S. Effect of low-level laser therapy on bone regeneration during osseointegration and bone graft. Photomed Laser Surg. 2017;35(12):649–658. doi: 10.1089/pho.2017.4275. [DOI] [PubMed] [Google Scholar]
- 10.de Freitas L.F., Hamblin M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quant. Electron. 2016;22(3) doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolukbasi Ates G., et al. Photobiomodulation effects on osteogenic differentiation of adipose-derived stem cells. Cytotechnology. 2020;72(2):247–258. doi: 10.1007/s10616-020-00374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yilmaz B.T., et al. In vivo efficacy of low-level laser therapy on bone regeneration. Lasers Med. Sci. 2022;37(4):2209–2216. doi: 10.1007/s10103-021-03487-8. [DOI] [PubMed] [Google Scholar]
- 13.Durmuşlar M.C., et al. Histological evaluation of the effect of concentrated growth factor on bone healing. J. Craniofac. Surg. 2016;27(6):1494–1497. doi: 10.1097/SCS.0000000000002873. [DOI] [PubMed] [Google Scholar]
- 14.Kim K., et al. High-intensity Nd:YAG laser accelerates bone regeneration in calvarial defect models. J Tissue Eng Regen Med. 2015;9(8):943–951. doi: 10.1002/term.1845. [DOI] [PubMed] [Google Scholar]
- 15.Nauth A., et al. Critical-size bone defects: is there a consensus for diagnosis and treatment? J. Orthop. Trauma. 2018;32(Suppl 1):S7–S11. doi: 10.1097/BOT.0000000000001115. [DOI] [PubMed] [Google Scholar]
- 16.Biguetti C.C., et al. Effect of low-level laser therapy on intramembranous and endochondral autogenous bone grafts healing. Microsc. Res. Tech. 2012;75(9):1237–1244. doi: 10.1002/jemt.22056. [DOI] [PubMed] [Google Scholar]
- 17.Sharma R., et al. Platelet-rich fibrin as an aid to soft- and hard-tissue healing. J Maxillofac Oral Surg. 2021;20(3):496–501. doi: 10.1007/s12663-019-01317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arıcan G., et al. Micro-ct findings of concentrated growth factors (cgf) on bone healing in masquelet's technique-an experimental study in rabbits. Arch. Orthop. Trauma. Surg. 2022;142(1):83–90. doi: 10.1007/s00402-020-03596-z. [DOI] [PubMed] [Google Scholar]
- 19.Magri A.M.P., et al. Bone substitutes and photobiomodulation in bone regeneration: a systematic review in animal experimental studies. J. Biomed Mater. Res. A. 2021;109(9):1765–1775. doi: 10.1002/jbm.a.37170. [DOI] [PubMed] [Google Scholar]
- 20.Hanna R., et al. Effects of photobiomodulation on bone defects grafted with bone substitutes: a systematic review of in vivo animal studies. J. Biophot. 2021;14(1):e202000267. doi: 10.1002/jbio.202000267. [DOI] [PubMed] [Google Scholar]
- 21.Tiwari U.O., et al. Comparative analysis of platelet-rich fibrin, platelet-rich fibrin with hydroxyapatite and platelet-rich fibrin with alendronate in bone regeneration: a cone-beam computed tomography analysis. J. Conserv. Dent. 2020;23(4):348–353. doi: 10.4103/JCD.JCD_228_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mijiritsky E., et al. Use of PRP, PRF and CGF in periodontal regeneration and facial rejuvenation-A narrative review. Biology. 2021;10(4) doi: 10.3390/biology10040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaid T., et al. Clinical and radiographic evaluation of demineralized freeze-dried bone allograft with concentrated growth factor versus concentrated growth factor alone in the treatment of intrabony defects. Med Pharm Rep. 2021;94(2):220–228. doi: 10.15386/mpr-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keranmu D., et al. Clinical application of concentrate growth factors combined with bone substitute in Alveolar ridge preservation of anterior teeth. BMC Oral Health. 2022;22(1):54. doi: 10.1186/s12903-022-02091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arakeeb M.A.A., et al. Effect of combined application of growth factors and diode laser bio-stimulation on the osseo integration of dental implants. Open Access Maced J Med Sci. 2019;7(15):2520–2527. doi: 10.3889/oamjms.2019.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sleem S., et al. Evaluation of the bio-stimulatory effect of platelet rich fibrin augmented by diode LASER compared to platelet rich fibrin alone on dental implant replacing posterior mandibular teeth. Randomised clinical trial: split mouth study. Open Access Maced J Med Sci. 2019;7(5):869–875. doi: 10.3889/oamjms.2019.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thalaimalai D.B.R., et al. Effect of low-level laser therapy and platelet-rich fibrin on the treatment of intra-bony defects. J. Laser Med. Sci. 2020;11(4):456–463. doi: 10.34172/jlms.2020.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemaid S., et al. Enhancement of healing of periodontal intrabony defects using 810 nm diode laser and different advanced treatment modalities: a blind experimental study. Open Access Maced J Med Sci. 2019;7(11):1847–1853. doi: 10.3889/oamjms.2019.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuka Y., et al. Effects of Nd:YAG low-level laser irradiation on cultured human osteoblasts migration and ATP production: in vitro study. Laser Med. Sci. 2019;34(1):55–60. doi: 10.1007/s10103-018-2586-6. [DOI] [PubMed] [Google Scholar]
- 30.Kim I.S., et al. High power-pulsed Nd:YAG laser as a new stimulus to induce BMP-2 expression in MC3T3-E1 osteoblasts. Laser Surg. Med. 2010;42(6):510–518. doi: 10.1002/lsm.20870. [DOI] [PubMed] [Google Scholar]
- 31.Knapen M., et al. Effect of leukocyte- and platelet-rich fibrin (L-PRF) on bone regeneration: a study in rabbits. Clin. Implant Dent. Relat. Res. 2015;17(Suppl 1):e143–e152. doi: 10.1111/cid.12146. [DOI] [PubMed] [Google Scholar]
- 32.Acar A.H., et al. Micro-computed tomography and histomorphometric analysis of the effects of platelet-rich fibrin on bone regeneration in the rabbit calvarium. Arch. Oral Biol. 2015;60(4):606–614. doi: 10.1016/j.archoralbio.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Kim B.J., Kim S.K., Lee J.H. Bone regeneration of demineralized dentin matrix with platelet-rich fibrin and recombinant human bone morphogenetic protein-2 on the bone defects in rabbit calvaria. Maxillofac Plast Reconstr Surg. 2021;43(1):34. doi: 10.1186/s40902-021-00320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y.K., et al. Micro-CT and histomorphometric study of bone regeneration effect with autogenous tooth biomaterial enriched with platelet-rich fibrin in an animal model. Scanning. 2021;2021 doi: 10.1155/2021/6656791. [DOI] [PMC free article] [PubMed] [Google Scholar]