Abstract
Introduction
People affected by diabetes are at higher risk for complications from certain vaccine-preventable diseases. Suboptimal vaccination coverages are reported in this population sub-group. The purpose of this study is to estimate the proportion of diabetic patients who express hesitation to the COVID-19 vaccine worldwide.
Methods
Seven studies were included in the meta-analysis and systematic review, selected from scientific articles available in the MEDLINE/PubMed, Google Scholar and Scopus databases from 2020 to 2022. The following terms were used for the search strategy: (adherence OR hesitancy OR compliance OR attitude) AND (covid* OR SARS*) AND (vaccin* OR immun*) AND (diabet*).
Results
The vaccine hesitation rate among persons with diabetes was 27.8 % (95 %CI = 15.6–41.9 %). In the comparison of vaccine hesitancy between sexes and educational status, the RRs were 0.90 (95 %CI = 0.71–1.15) and 0.88 (95 %CI = 0.76–1.02), respectively. The main reasons of unwillingness were lack of information, opinion that the vaccine was unsafe or not efficient, and fear of adverse events.
Conclusions
In order to achieve a high vaccination coverage, multifactorial approach is needed, which requires major social, scientific and health efforts. The success of the vaccination campaign in this population depends on the capillarity and consistency of the interventions implemented.
Keywords: Diabetes, High-risk patients, Vaccine compliance, COVID-19, SARS-CoV-2, Mandatory vaccination
1. Introduction
People affected by diabetes (both type 1 and type 2) are at increased risk for complications and death from some vaccine-preventable diseases. They are vulnerable to infections because of hyperglycemia, impaired immune function, vascular complications and comorbidities such as hypertension, dyslipidemia, and cardiovascular disease [1]. In 2019, an estimated 463 million people with diabetes live worldwide, representing 9.3 % of the global adult population (20–79 years); this number is expected to increase to 578 million (10.2 %) in 2030 and 700 million (10.9 %) in 2045 [2]. As reported by the Center for Disease Control and Prevention (CDC), strongly recommended vaccinations in diabetic adults are diphtheria-tetanus-pertussis (Tdpa), influenza, hepatitis B, pneumococcal and Herpes zoster vaccines [3].
People with diabetes have a 3- to 5-fold higher risk of becoming infected with SARS-CoV-2 and dying from COVID-19 than healthy people [4]. The reason for this epidemiological scenario is related to the angiotensin-converting enzyme 2 (ACE2), which is a receptor for SARS-CoV-2 in the human body. Hence, the frequent use of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blockers (ARB) in the therapy of diabetic patients and high level of angiotensin II in the same population could explain this higher risk of SARS-COV-2-related infection and disease [1]. A 2020 meta-analysis showed that diabetes was associated with a poor outcome comprising mortality, severe COVID-19, acute respiratory distress syndrome, and disease progression [5]. So, the CDC recommends with high priority COVID-19 vaccination for people with diabetes [6].
Despite the fact that most COVID-19 vaccine trials have not enrolled people with diabetes, post-marketing studies have demonstrated a high effectiveness and safety profile in this population [7], [8], [9]. Nevertheless, low vaccine coverage (VC) is reported in this population sub-group [10]; a cross-sectional study from 2022 [10] showed, in a sample of 11,573 Arab patients affected by diabetes a basal routine VC of 55.5 %. The authors focused on vaccine hesitancy as a major determinant of the success (or otherwise) of the COVID-19 vaccination campaign in this high-risk group.
Vaccine hesitancy is a phenomenon already known in diabetics; in fact, many scientific studies have reported low flu VCs and high levels of vaccine hesitancy in this population [11], [12], [13]; the main reasons for low vaccination adherence were concerns about vaccine safety, belief that the vaccine is not necessary or effective, lack of recommendations by healthcare professionals, low knowledge of vaccines, access issues, and conflicting advice [11], [12], [13].
To estimate the proportion of people with diabetes expressing COVID-19 vaccine hesitancy worldwide, we conducted a systematic narrative review of relevant literature and a meta-analysis. Determinants of vaccine compliance and options suggested by these studies to deal with vaccine hesitation were also analyzed.
2. Methods
2.1. Search strategy and selection criteria
The Scopus, MEDLINE/PubMed and Google Scholar (first 100 records) databases were systematically searched. Research articles, brief reports, commentaries, and letters published between January 1, 2020 and March 21, 2022 were included in our search. The following terms were used for the search strategy: (adherence OR hesitancy OR compliance OR attitude) AND (covid* OR SARS*) AND (vaccin* OR immun*) AND (diabet*). Studies in English with full text were included. Abstracts without full text, systematic reviews, meta- analyses and all studies focusing on issues unrelated to the purpose of this review (vaccine knowledge, adverse vaccine reactions, etc.) were excluded. When necessary, study authors were contacted for additional information. References of all articles were reviewed for further study. The list of papers was independently screened by title and/or abstract by two reviewers (FPB and PS) who applied the predefined inclusion/exclusion criteria. Discrepancies were recorded and resolved by consensus.
Extracted data included year of study publication, sample size, number of hesitant subjects, country, and management options for hesitant subjects.
2.2. Quality assessment
The quality of the selected studies was assessed according to the STROBE checklist, which includes 22 methodological questions [14]. Eligible short reports, commentaries, and letters describing cross-sectional studies and their quality were also assessed using the STROBE checklist. Quality assessment was not performed for studies without full text. Studies assessed according to the STROBE checklist had a minimum and maximum possible score of 0 and 44, respectively, and were classified as low quality (<15.5), moderate quality (15.5–29.5), or high quality (30–44).
The risk of bias for each study was assessed independently by two researchers (FPB and PS). Discrepancies were recorded and resolved by consensus.
2.3. Pooled analysis
A meta-analysis was performed to estimate vaccine hesitation in persons with diabetes; a separate analysis was carried out using only high-quality papers. Moreover, vaccine hesitancy was compared according to sex (male vs female) and educational status (college or more vs lower education) as determinants, calculating the risk ratio (RR) and 95 % confidence interval (95 %CI). The choice of these determinants was not random, but they represented the only two factors investigated in at least three of the selected studies; sub-analysis for high-quality papers was not performed because of the small number of studies.
The pooled proportion in the meta-analysis was calculated using the Freeman-Tukey double arcsine transformation to stabilize variances, and the DerSimonian-Laird weights for random effects models, with the estimate of heterogeneity obtained from the inverse-variance fixed-effects model. The pooled prevalence and the associated 95 % Wald confidence interval were plotted, and a forest plot was drawn. The I2 statistic was calculated as a measure of the proportion of the overall variance attributable to heterogeneity between studies rather than to chance. Heterogeneity between studies in different groups was also assessed. A p-value < 0.05 was considered to indicate statistical significance of heterogeneity.
A sensitivity analysis was conducted to evaluate stability; among the studies included in this systematic review, one study at a time was excluded, and the subsequent conclusion based on the others was then re-evaluated to avoid severe distortions.
Statistical analysis was conducted using STATA MP17.
Strategies to increase vaccination compliance among diabetics and suggested strategies to address vaccine hesitancy were collected from all available studies and their respective findings were compared, with particular attention to the evidence presented in several of the included papers.
3. Results
3.1. Identification of relevant studies
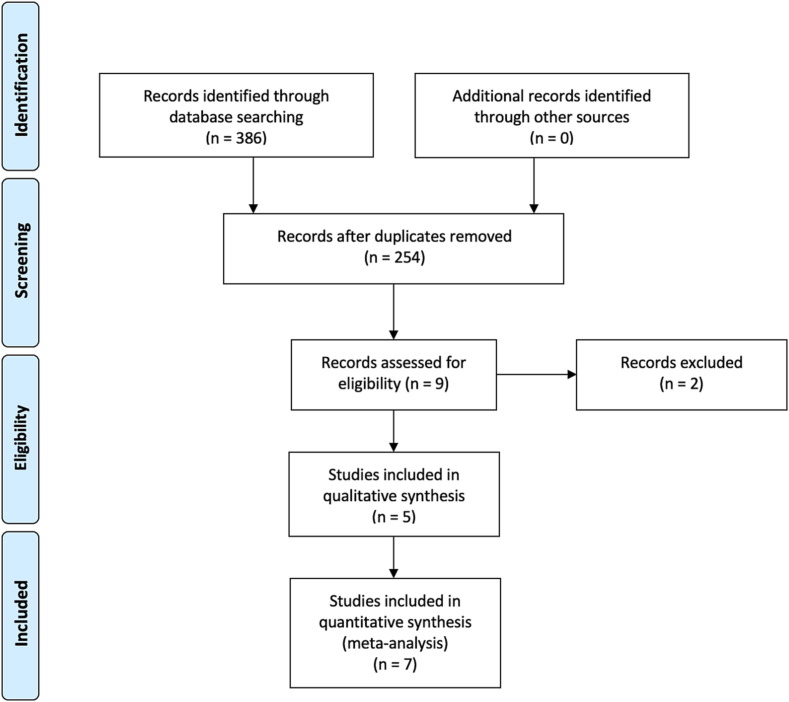
The flow-chart, constructed following the PRISMA guide [15] (Fig. 1 ), shows the process of article selection. According to the aforementioned inclusion criteria, 7 articles were identified in MEDLINE/PubMed, 6 in Scopus and one in Google Scholar. After exclusion of duplicate articles in the two databases, there were 9 eligible studies [16], [17], [18], [19], [20], [21], [22], [23], [24]. Of these, two [23], [24] were excluded because additional information was requested from the authors, but they did not respond. Thus, overall, 7 studies were eligible [16], [17], [18], [19], [20], [21], [22], of which five were included in the quantitative analysis [17], [18], [20], [21], [22] (Table 1 ). The remaining 254 studies did not match the inclusion criteria.
Fig. 1.
Flow-chart of the bibliographic research.
Table 1.
Characteristics of the selected studies included in meta-analysis and systematic review.
| First author | Year | Quality | Hesitant patients (n) | Total sample | Study period | Country |
|---|---|---|---|---|---|---|
| Tsai R [16]§ | 2022 | h | 266 | 1400 | January–February 2021 | more countries |
| Wang Y [17] | 2022 | h | 273 | 483 | April–August 2021 | China |
| Aldossari KK [18] | 2021 | h | 200 | 709 | March–May 2021 | Saudi Arabia |
| Asadi-Pooya AA [19]*,§ | 2021 | m | 2 | 127 | late 2020 | Iran |
| Guaraldi F [20] | 2021 | h | 185 | 1176 | January 2021 | Italy |
| Nachimuthu S [21]** | 2021 | m | 133 | 179 | March–April 2021 | India |
| Scoccimaro D [22]* | 2021 | m | 92 | 502 | January–April 2021 | Italy |
Short report.
Letter to editor.
Not included in systematic review.
3.2. Quality assessment
The STROBE checklist was applied appropriately to the included studies; 57.1 % of eligible papers were determined to be of high quality (Table 1). The impact of study quality was assessed in a sub-analysis.
3.3. Pooled analysis
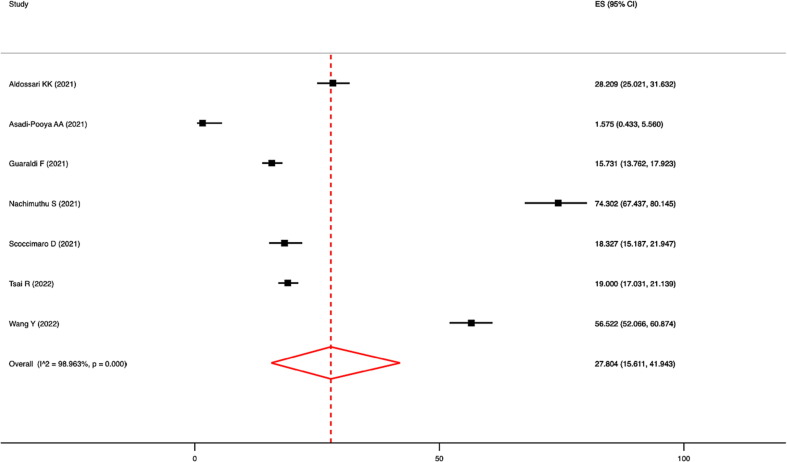
Meta-analysis showed that the prevalence of vaccine hesitancy was 27.8 % (95 %CI: 15.6–41.9 %; I2 = 99.0 %; p-value for heterogeneity < 0.0001; Fig. 2 ). Based on high-quality articles only, the pooled prevalence was 28.8 % (95 %CI = 15.3–44.5 %; I2 = 99.0 %; p < 0.0001).
Fig. 2.
Forest plot of the pooled prevalence of vaccine hesitancy.
In the comparison of vaccine hesitation between sexes (males vs females), the RR was 0.90 (95 %CI = 0.71–1.15; I2 = 58.0 %; p = 0.090). In the comparison based on educational status (college or more vs lower education) the RR was 0.88 (95 %CI = 0.76–1.02; I2 = 0.0 %; p = 0.770).
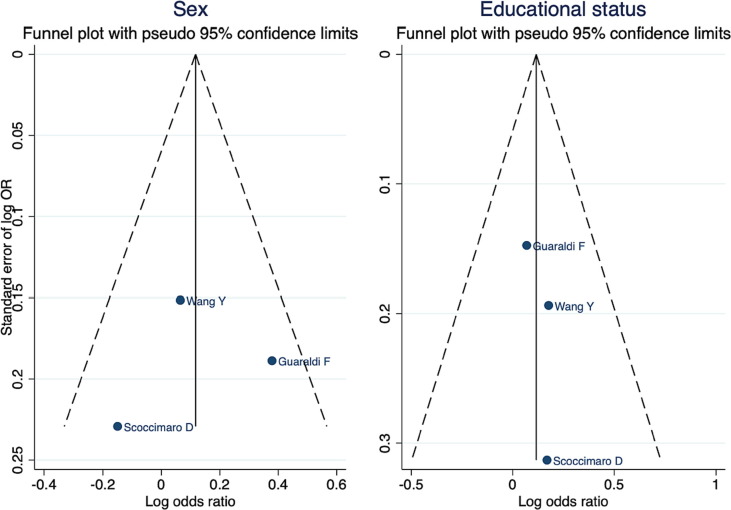
Sensitivity analysis did not show severe study-specific distortion. In the publication bias analysis, there was no obvious asymmetry in the funnel plots and no strong evidence of publication bias (Fig. 3 ). The p-value in Egger’s test was 0.854 for the sub-analysis based on sex and 0.498 for the sub-analysis based on educational status.
Fig. 3.
Funnel plots with pseudo 95 % confidence limits.
3.4. Determinants of vaccination compliance and suggested strategies to address vaccination hesitancy
All studies concluded that vaccine hesitancy is a crucial issue in the management of the COVID-19 pandemic. Many determinants of hesitancy were investigated; most studies reported that the main reasons were lack of information about vaccination, opinion that the vaccine was unsafe or not efficient, and fear of adverse events (including potentially long-term ones) [17], [18], [20], [21], [22]. Moreover, the role of pharmaceutical companies in influencing vaccine policy decisions, the uncertainty associated with the rapid COVID-19 vaccine development process, and the lack of clinical trials focused on patients with diabetes [18] were also determinants of poor attitudes.
Health status seemed to play a major role in the willingness of diabetics. Aldossari KK et al. [18] reported that patients with a history of diabetes for more than 5 years (and so with a higher probability of diabetes complications) were more inclined towards vaccine uptake than those with a history of <5 years; on the other hand, Nachimuthu S et al. [21] reported that a chronic condition was indicated by diabetic patients as a reason for declining vaccination. Scoccimaro D et al. [22] evidenced that hesitation was associated with poor glucose and lipid control and obesity, suggesting that the negative attitude might be more prevalent in subjects with lower adherence to medical prescriptions and/or less concern for their health; as a consequence, those with the highest risk of severe COVID-19 might be the least prone to vaccination [22].
Family members and friends of patients play a primary role in vaccination compliance; in fact, the opinions and behaviors of people in their environment influenced patients’ willingness to be vaccinated, including the vaccination status of relatives [17]. Concern about the risk associated with COVID-19 disease and its impact on their health is a determinant of better attitude [22], as false beliefs regarding the risk of SARS-CoV-2 infection among diabetics may lead to vaccine hesitation [17]. Many studies have reported that higher education and information from scientific sources and institutional websites were associated with better acceptance [17], [18], [20]. Deepening the sources of information about vaccines and COVID-19, the role of social media is discussed; Aldossari KK et al. [18] reported that subjects who used mass media or the Internet as their main source of information showed higher levels of hesitancy, while Wang Y et al. [22] reported that receiving vaccination messages on social media from trustworthy sources such as scientists or politicians could promote vaccine acceptance.
The role of having received a previous vaccination is questioned; Scoccimaro D et al. [22] reported that hesitancy for other vaccines was not associated with COVID-19 vaccine hesitancy, Aldossari KK et al. [18] evidenced that no history of influenza vaccine was associated with COVID-19 vaccine uptake, while Guaraldi F et al. [20] reported that previous vaccination increased readiness for COVID-19 vaccine, although having experienced adverse effects following previous vaccination was a negative predictor.
Regarding age, higher levels of compliance have been reported in older patients [20]; the role of sex is more debated, with one study [20] reporting better compliance in males while another study [18] in females.
In this light, few experiences are cited by the authors to achieve better immunization coverage. Two authors [17], [21] focused on the role of healthcare providers; indeed, the degree of confidence in healthcare professionals to provide reliable and trustworthy information regarding COVID-19 vaccine safety seems to be a strong predictor of vaccine acceptance. Given this, it is important that health providers are constantly informed about the latest scientific updates regarding COVID-19 vaccination, as well as current data regarding the risks of infection for diabetics [17].
Much attention is paid to reporting evidence-based data of vaccine safety and efficacy to minimize vaccine hesitancy, as well as education and specific guidelines are needed to overcome this resistance [17], [18], [22]; thus, public health strategies should prioritize providers and public education regarding the adverse effects of COVID-19 and evolving vaccine safety data in this group. As reported by Wang Y et al. [17] providing SARS-CoV-2 vaccine safety and side effect reports, from patients with diabetes who have been vaccinated, could allay these concerns. Additionally, the medium- and long-term effects are still unclear, and there is a need for the healthcare sector to further investigate the long-term immune response of diabetic patients to COVID-19 vaccination in the future, thereby improving the relevant cognition of diabetic patients as well as making more scientific decisions about vaccination behavior.
Furthermore, better use of mass media, social media, and the internet to disseminate evidence-based facts about the vaccine and COVID-19 is advocated [17]; encouraging patients with diabetes to accept the COVID-19 vaccine through (social) media campaigns may increase vaccination rates in this population.
Finally, the exclusion of diabetic patients from vaccine trials, leaving clinicians in the position of recommending vaccination to diabetics without evidence of efficacy or safety is a critical issue [18]. Identifying the best way to include these subjects in future vaccine studies rather than excluding them by default seems to be a priority for the scientific community and policy makers.
4. Discussion
Our meta-analysis estimated vaccine hesitancy among persons with diabetes worldwide to be 28 % (95 %CI = 16–42 %); the determinants evaluated (sex and educational status) did not appear to influence the willingness of our considered population.
The systematic review brought out the main determinants of vaccination hesitancy; lack of information about vaccination, opinion that the vaccine is not safe and/or effective, and fear of adverse events are known determinants of vaccination refusal in the scientific literature [11], [12], [13]; indeed, these data confirmed evidence already acquired in the literature for other high-risk groups [25], [26], [27]. On the other hand, the role of pharmaceutical companies in influencing vaccine policy decisions and the uncertainty associated with the rapid COVID-19 vaccine development process as determinants of hesitation are pathognomonic of COVID-19 vaccination compared with other vaccines.
Having a history of the disease or experience of it among family members and friends, fear of complications from COVID-19, and patient safety and protection seemed to increase willingness to vaccinate. Higher education and scientific sources played a fundamental role in the attitudes of diabetics; trust in the scientific community has already been identified has a major determinant of vaccination compliance in the general population [28] and therefore also plays a key role for this this sub-group. The role of social media and the internet is debated; it is well known that fake news in mass media and social media facilitates distrust of vaccines [29], and our systematic review confirmed this mechanism in patients with diabetes [18], [22]. In any case, extensive use of mass media, social media, and the internet to disseminate evidence-based facts about the vaccine and COVID-19 is desirable [22] in order to promote vaccination through classic or modern sources of information, to combat fake news, and to ensure institutional communication based on scientific evidence.
The role of healthcare professionals in promoting immunization has been reported to be crucial in achieving high immunization rates; the degree of confidence in healthcare professionals in providing reliable and trustworthy information regarding the safety of the COVID-19 vaccine appears to be strong predictors of vaccine acceptance. This evidence is also confirmed when considering the literature for other high-risk infectious populations [25], [26], [27].
The main limitation of this meta-analysis was the high heterogeneity across studies, as indicated by the I2 values; however, the use of a random-effects analysis minimized this bias. In addition, the low number of experiences reported in the literature and the suboptimal level of quality make the results of our study difficult to generalize. However, most of the studies considered were conducted in Asia, two in Italy; therefore, evidence from most countries of the world has not been reported in the literature. This critical issue needs to be addressed with a greater focus of international research on vaccine hesitation in the diabetic population, in order to repeat the meta-analysis and systematic review in the future and obtain more robust results; indeed, other determinants of vaccine hesitancy may be investigated, as low knowledge of vaccines, access issues, and conflicting advices. Another limitation was the fact that the definition of “vaccine hesitation” is rather heterogeneous among the studies, yet they all investigated the same topic; therefore, this does not seem to be a critical issue. Moreover, the small number of considerable studies on the topic did not allow us to define adequate determinants to perform sub-analyses. However, a strength of our review and meta-analysis was the sample size resulting from the collation of selected papers, which improved the statistical analysis and provided a better view of anti-COVID-19 vaccine hesitancy among diabetics. In addition, all studies were published as of 2021, so this view is up-to-date and reliable. Finally, to our knowledge, RRs calculated considering gender and education status have never been reported before.
Our study highlighted that approximately 30 % of people with diabetes expressed hesitation to vaccination and the main determinants of vaccination compliance were exposed. Many strategies have been proposed to deal with this criticism, and other approaches could be implemented considering those already suggested for other recommended vaccines in this population [11], [12], [13], as well as for other frail patients [30]. Indeed, the evidence available in the literature has shown that combating vaccine resistance is harsh, and it is too slow as a process considering the rapidity and unpredictability of a pandemic. Health education regarding the anti-COVID19 vaccine should be provided to improve community willingness [31].
Widespread actions on multiple fronts are therefore needed to achieve good outcomes in these patients.
Government and Public Health institutions probably have the main role; in fact, clear and unambiguous communication is needed to express the risk/benefit ratio of the vaccine. Politicians should not be seduced by the vote pool of the no vax and anti-science community, but should rely on scientific evidence and educate the population on data-driven decisions.
Furthermore, HCWs are among the most reliable sources of vaccine information and have direct influence on the immunization decisions of their patients and social contacts [32]. Hence, healthcare professionals need to be empowered to play their role in the immunization campaign and pandemic management; it is also necessary to work on the hesitation of health care professionals, considering that a hesitant provider is less likely to recommend the vaccine to his or her patients [33]. In this perspective, the free offer of vaccine prophylaxis in the hospital, with a close link between the branch specialist (such as diabetologist) and the vaccinologist, would allow to concentrate in a single “hospital vaccine clinic” different diagnostic and therapeutic pathways that provide for the vaccination of subjects at risk for diseases or conditions. In doing so, it is necessary to manage vaccine hesitation in high-risk patients and to take into account the social determinants that influence vaccine adherence.
The role of information sources, particularly social media, must also be questioned. Many studies have reported the risk of vaccine campaign failure due to uncontrolled dissemination of misinformation by the media [34], [35]. Although media content cannot be controlled, it must be taken into account that especially social platforms are the battleground of no-vax groups that, even if small in number, are very organized and able to circulate fake news in a very short time [34]. It is precisely for this reason that public health institutions should ensure proper institutional and scientific communication, especially on social networks.
Finally, high-risk patients or people with chronic diseases have historically been excluded from most vaccine clinical trials due to ethical concerns and the potential for liability. Thus, there is a lack of evidence from phase III studies supporting the safety and efficacy of vaccines in this specific population. Additional protections that could be provided through rulemaking and regulatory development would help overcome these barriers to research on therapies in patients with chronic conditions, especially for those preventive or therapeutic interventions that are accelerated to address a public health emergency.
In conclusion, vaccination hesitancy toward the anti-COVID-19 vaccine among people affected by diabetes is an existing phenomenon. Achieving high vaccination coverage requires a multifactorial approach that demands major social, scientific, and health efforts. The success of vaccination campaigns in this population depends on the capillarity and consistency of the interventions implemented.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The study was founded by Apulia’s Regional Observatory for Epidemiology. The manuscript has not been presented at a meeting.
Data availability
No data was used for the research described in the article.
References
- 1.Jeong I.K., Yoon K.H., Lee M.K. Diabetes and COVID-19: Global and regional perspectives. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed]
- 3.CDC. Diabetes type 1 and type 2 and adult vaccination. Available on: https://www.cdc.gov/vaccines/adults/rec-vac/health-conditions/diabetes.html. Last accessed on 22 March, 2022.
- 4.American Diabetes Association. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl. 1):S37–47. [DOI] [PubMed]
- 5.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. People with certain medical conditions. Available on: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Last accessed on 23 March, 2022.
- 7.Ali H., Alterki A., Sindhu S., Alahmad B., Hammad M., Al-Sabah S., et al. Robust antibody levels in both diabetic and non-diabetic individuals after BNT162b2 mRNA COVID-19 vaccination. Front Immunol. 2021;24(12) doi: 10.3389/fimmu.2021.752233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papadokostaki E., Tentolouris A., Anastasiou I.A., Psichogiou M., Iliaki E., Eleftheriadou I., et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in people with diabetes: a prospective observational study. Vaccines (Basel) 2022;10(3):382. doi: 10.3390/vaccines10030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tourkmani A.M., Bin Rsheed A.M., AlEissa M.S., Alqahtani S.M., AlOtaibi A.F., Almujil M.S., et al. Prevalence of COVID-19 infection among patients with diabetes and their vaccination coverage status in Saudi Arabia: a cross-sectional analysis from a Hospital-based Diabetes Registry. Vaccines (Basel) 2022;10(2):310. doi: 10.3390/vaccines10020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verger P., Bocquier A., Vergélys C., Ward J., Peretti-Watel P. Flu vaccination among patients with diabetes: motives, perceptions, trust, and risk culture - a qualitative survey. BMC Public Health. 2018;18(1):569. doi: 10.1186/s12889-018-5441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bocquier A., Cortaredona S., Fressard L., Loulergue P., Raude J., Sultan A., et al. Trajectories of seasonal influenza vaccine uptake among French people with diabetes: a nationwide retrospective cohort study, 2006–2015. BMC Public Health. 2019;19(1):918. doi: 10.1186/s12889-019-7209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamorano-Leon JJ, Jimenez-Garcia R, Lopez-de-Andres A, de-Miguel-Diez J, Carabantes-Alarcon D, Albaladejo-Vicente R, et al. Low levels of influenza vaccine uptake among the diabetic population in Spain: a time trend study from 2011 to 2020. J Clin Med. 2021;11(1):68. [DOI] [PMC free article] [PubMed]
- 14.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372) doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai R., Hervey J., Hoffman K., Wood J., Johnson J., Deighton D., et al. COVID-19 vaccine hesitancy and acceptance among individuals with cancer, autoimmune diseases, or other serious comorbid conditions: cross-sectional, internet-based survey. JMIR Public Health Surveill. 2022;8(1):e29872. doi: 10.2196/29872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Duan L., Li M., Wang J., Yang J., Song C., et al. COVID-19 vaccine hesitancy and associated factors among diabetes patients: a cross-sectional survey in Changzhi, Shanxi, China. Vaccines (Basel) 2022;10(1):129. doi: 10.3390/vaccines10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aldossari KK, Alharbi MB, Alkahtani SM, Alrowaily TZ, Alshaikhi AM, Twair AA. COVID-19 vaccine hesitancy among patients with diabetes in Saudi Arabia. Diabetes Metab Syndr. 2021;15(5):102271. [DOI] [PMC free article] [PubMed]
- 19.Asadi-Pooya A.A., Barzegar Z., Sadeghian S., Nezafat A., Shahisavandi M., Nabavizadeh S.A. COVID-19 vaccine hesitancy among patients with epilepsy or other chronic conditions. Disaster Med Public Health Prep. 2021;11:1–3. doi: 10.1017/dmp.2021.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guaraldi F., Montalti M., Di Valerio Z., et al. Rate and predictors of hesitancy toward SARS-CoV-2 vaccine among type 2 diabetic patients: results from an Italian survey. Vaccines (Basel) 2021;9(5):460. doi: 10.3390/vaccines9050460. Published 2021 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nachimuthu S, Viswanathan V. Trend in COVID-19 vaccination among people with diabetes: a short study from India. Diabetes Metab Syndr. 2021;15(4):102190. [DOI] [PMC free article] [PubMed]
- 22.Scoccimarro D., Panichi L., Ragghianti B., Silverii A., Mannucci E., Monami M. Sars-CoV2 vaccine hesitancy in Italy: a survey on subjects with diabetes. Nutr Metab Cardiovasc Dis. 2021;31(11):3243–3246. doi: 10.1016/j.numecd.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kassa Mekonnen C., Gizaw Demissie N., Wako Beko Z., Mulu Ferede Y., Kindie A.H. Intent to get vaccinated against COVID-19 pandemic and its associated factors among adults with a chronic medical condition. Int J Afr Nurs Sci. 2022;16 doi: 10.1016/j.ijans.2022.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bongomin F, Olum R, Andia-Biraro I, Nakwagala FN, Hassan KH, Nassozi DR, et al. COVID-19 vaccine acceptance among high-risk populations in Uganda. Ther Adv Infect Dis. 2021;8:20499361211024376.s. [DOI] [PMC free article] [PubMed]
- 25.Bianchi F.P., Vimercati L., Mansi F., De Nitto S., Stefanizzi P., Rizzo L.A., et al. Compliance with immunization and a biological risk assessment of health care workers as part of an occupational health surveillance program: the experience of a university hospital in southern Italy. Am J Infect Control. 2020 Apr;48(4):368–374. doi: 10.1016/j.ajic.2019.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Januszek SM, Faryniak-Zuzak A, Barnaś E, et al. The approach of pregnant women to vaccination based on a COVID-19 systematic review. Medicina (Kaunas). 2021;57(9):977. Published 2021 Sep 17. [DOI] [PMC free article] [PubMed]
- 27.Nabavi SM , Mehrabani M , Ghalichi L , Nahayati MA , Ghaffari M , Ashtari F , et al. COVID-19 Vaccination Willingness and Acceptability in Multiple Sclerosis Patients: A Cross Sectional Study in Iran . Vaccines 10 (1): 135. [DOI] [PMC free article] [PubMed]
- 28.Sturgis P., Brunton-Smith I., Jackson J. Trust in science, social consensus and vaccine confidence. Nat Hum Behav. 2021;5(11):1528–1534. doi: 10.1038/s41562-021-01115-7. [DOI] [PubMed] [Google Scholar]
- 29.Muric G., Wu Y., Ferrara E. COVID-19 vaccine hesitancy on social media: building a public twitter data set of antivaccine content, vaccine misinformation, and conspiracies. JMIR Public Health Surveill. 2021;7(11):e30642. doi: 10.2196/30642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianchi F.P., Stefanizzi P., Spinelli G., Mascipinto S., Tafuri S. Immunization coverage among asplenic patients and strategies to increase vaccination compliance: a systematic review and meta-analysis. Expert Rev Vaccines. 2021;20(3):297–308. doi: 10.1080/14760584.2021.1886085. [DOI] [PubMed] [Google Scholar]
- 31.Wake A.D. The willingness to receive COVID-19 vaccine and its associated factors: “Vaccination Refusal Could Prolong the War of This Pandemic” - a systematic review. Risk Manag Healthc Policy. 2021;21(14):2609–2623. doi: 10.2147/RMHP.S311074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giambi C., Fabiani M., D'Ancona F., Ferrara L., Fiacchini D., Gallo T., et al. Parental vaccine hesitancy in Italy - results from a national survey. Vaccine. 2018;36(6):779–787. doi: 10.1016/j.vaccine.2017.12.074. [DOI] [PubMed] [Google Scholar]
- 33.Biswas N., Mustapha T., Khubchandani J., Price J.H. The nature and extent of COVID-19 vaccination hesitancy in healthcare workers. J Commun Health. 2021;46(6):1244–1251. doi: 10.1007/s10900-021-00984-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianchi F.P., Tafuri S. A public health perspective on the responsibility of mass media for the outcome of the anti-COVID-19 vaccination campaign: the AstraZeneca case. Ann Ig. 2022;34(6):650–655. doi: 10.7416/ai.2022.2499. [DOI] [PubMed] [Google Scholar]
- 35.Signorelli C., Odone A., Conversano M., Bonanni P. Deaths after Fluad flu vaccine and the epidemic of panic in Italy. BMJ. 2015;14(350) doi: 10.1136/bmj.h116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.