Abstract
It was previously reported that female mice resolve a primary Chlamydia trachomatis urogenital infection independent of inducible nitric oxide synthase (iNOS). We now report that although iNOS-deficient (NOS2−/−) mice resolve culture-apparent infection in a fashion similar to that of normal control (NOS2+/+) mice, they sustain significantly increased rates of disease, as assessed by hydrosalpinx formation. PCR amplification of ompA followed by Southern blot detection of amplicands revealed the presence of chlamydial DNA in the lower genital tracts of both NOS2−/− and NOS2+/+ mice at ≥120 days postinfection and in upper genital tract tissues at >120 days postinfection. However, only NOS2−/− mice shed low numbers of viable chlamydiae from the lower genital tract after immunosuppressive treatment at 120 days postinfection. When cultured primary murine lung fibroblasts were activated in the presence of gamma interferon (IFN-γ), inhibition of chlamydial growth occurred in both NOS2+/+ and NOS2−/− cells, but the inhibition was reversible after removal of the cytokine in the NOS2−/− primary cell culture only. The iNOS-independent inhibition was microbistatic but was independent of 2,3-indoleamine dioxygenase activity. We conclude that chlamydial DNA and antigens persist in mice subsequent to culture-apparent resolution. In addition, IFN-γ induces in vivo inhibition of chlamydial growth through microbistatic mechanisms in the absence of iNOS activity, but in the presence of iNOS activity, IFN-γ is microbicidal and effects eradication.
Mice resolve primary chlamydial genital tract infections and become resistant to challenge infections, as assessed by cessation of shedding of viable Chlamydia trachomatis (1, 2). Recovery from chlamydial urogenital infections in mice requires type 1 immune responses that are at least partially dependent on gamma interferon (IFN-γ) (11, 28, 29) and major histocompatibility complex class II antigen processing and presentation (20). A potentially important mechanism of IFN-γ-dependent chlamydial growth inhibition is the production of reactive nitrogen intermediates (RNI) via cytokine-inducible nitric oxide synthase (iNOS). RNI have been shown to restrict chlamydial growth in vitro in murine epithelial cells (16), fibroblasts (19, 27), and macrophages (8). However, RNI could not account for the sterile eradication of C. trachomatis because in the presence of the iNOS inhibitor NG-monomethyl-l-arginine (l-NMMA), chlamydial growth was not completely restored to that of controls. This result indicates that other IFN-γ-inducible mechanisms also might be important for controlling chlamydial infections in mice. It was previously found that in primary murine fibroblast cell cultures derived from iNOS knockout (NOS2−/−) mice, IFN-γ can restrict chlamydial growth via iNOS-independent mechanisms (27). In vivo, NOS2−/− or normal control (NOS2+/+) mice treated with l-NMMA are capable of resolving chlamydial genital tract infections (27). In human systems, the induction of the tryptophan-decyclizing enzyme indoleamine 2,3-dioxygenase (2,3-IDO) has a central role in IFN-γ-mediated growth inhibition (37). However, induction of this enzyme in response to chlamydial infections has yet to be observed in murine systems (8, 27), although the 2,3-IDO gene is present and active in mice in other situations (32, 35, 36).
Persistent chlamydial infections develop in cell cultures in response to low-level IFN-γ treatment of host cells and in response to other stressors (4). Persistent chlamydiae are characterized by their ability to enter a metabolically inactive and noninfectious state in response to stress and by the resumption of growth and the release of infectious elementary bodies upon removal of the stress (4). There is also indirect evidence suggesting that chlamydiae can persist during human ocular (5, 22), genital (13), and joint (31) diseases and in atherosclerotic lesions (18). In subhuman primates, chlamydial DNA and RNA can be detected during a culture-negative state (15). It was previously found that the culture-recoverable mouse pneumonitis (MoPn) biovar of C. trachomatis remains in the normal immunocompetent female mouse genital tract for only up to 5 weeks after culture-apparent clearance of the infection (10). This finding could argue against long-term persistence contributing to potentially chronic genital tract disease in the immunocompetent murine system.
In the studies described here, we used NOS2−/− mice to determine if persistence can be extended in mice that are deficient in the eradicating cytokine-induced activity. We observed exacerbated disease subsequent to infection in the absence of iNOS-derived RNI. Thus, we hypothesize that in the absence of iNOS activity, sterile eradication of chlamydial infection does not occur and that chlamydial antigen and viable chlamydial organisms persist subsequent to culture-apparent resolution of infection. This model may be useful for studying the long-term fate of C. trachomatis in the murine genital tract subsequent to cessation of shedding of viable organisms and for correlating persistent forms of C. trachomatis in the mouse with those previously studied using in vitro systems. Additionally, we seek to define the IFN-γ-inducible mechanism or combination of mechanisms that leads to eradication of murine chlamydial infection in vitro and in vivo.
MATERIALS AND METHODS
Mice.
Mice with a targeted disruption in the iNOS gene (NOS2−/− mice) were obtained under a materials transfer agreement with John Mudgett (Merck & Co., Rahway, N.J.), and a colony was initiated at the Midwestern University Animal Resource Facility. The knockout in these mice was confirmed with randomly selected experimental animals by PCR of genomic DNA using primer pairs for the targeting vector containing the neomycin resistance gene and the NOS2 promoter as described elsewhere (27). B6129F1 control or C57BL/6 wild-type mice (NOS2+/+ mice) were purchased from Taconic Labs (Germantown, N.Y.). All mice were at least 8 weeks of age at the start of each experiment. Knockout mice were housed in microisolator cages under barrier conditions with a 10 h-14 h light-dark cycle and were fed sterile food and water ad libitum. Control mice were housed in standard rodent cages with a similar light-dark cycle.
Chlamydiae.
C. trachomatis MoPn (Weiss) was grown in HeLa 229 cells and maintained by adaptation of previously described methods (7, 11).
Infection and infection assessment.
For primary infection, mice were pretreated with 2.5 mg of DepoProvera (P4; Upjohn, Kalamazoo, Mich.) in 0.1 ml of saline administered subcutaneously 10 and 3 days prior to infection (11). Mice were inoculated intravaginally with 10 μl of MoPn suspension containing 104 inclusion-forming units, equivalent to approximately 200 50% infective doses (10), in SPG buffer (10 mM phosphate, 0.25 M sucrose, 5 mM l-glutamic acid).
To assess shedding of MoPn from the lower urogenital tract, cervicovaginal material was collected using a calcium alginate swab on a flexible aluminum shaft (Fisher Scientific, Pittsburgh, Pa.). Samples were collected at 4, 7, 10, and 14 days postinfection, weekly thereafter until the resolution of infection, and then at several specified time points to include samples just prior to and subsequent to immunosuppression (see Results). All samples were frozen in SPG buffer at −70°C for later batch processing. Just prior to and subsequent to immunosuppression, samples were placed on ice and cultured on the day they were collected in an effort to increase the sensitivity of the cell culture system. MoPn was isolated from thawed swab samples using HeLa 229 cell cultures and enumerated by indirect immunofluorescence as previously described (11).
To assess upper genital tract (UGT) infection by culturing, tissues were excised immediately following sacrifice by removal of the uterine horns and cervix. For some tissues, a small (approximately 10-mg) section consisting of the oviduct and the distal portion of a uterine horn was dissected for total DNA extraction as described below. The remainder was either processed immediately for culturing or frozen in 1 ml of SPG buffer at −80°C. After being thawed, tissues were homogenized, sonicated, and cleared of large debris by low-speed centrifugation (10 min at 500 × g at 4°C). Diluted supernatants of homogenates were plated on HeLa 229 cell monolayers in 24-well plates as described elsewhere (10), and culturing was performed as described above for swab samples.
Assessment of chlamydial DNA.
Swab samples for isolation of viable MoPn and those for detection of DNA were never collected from the same animal on the same day. Instead, either parallel groups of mice were assessed or separate collection dates for the same mice were used to measure these parameters. Vaginal swab samples for chlamydial DNA detection were collected in phosphate-buffered saline (PBS) containing 2.5 μg of amphotericin B (Sigma, St. Louis, Mo.) per ml and were frozen at −20°C until processed. DNA was extracted from either genital swab samples or excised UGT tissues using a commercially prepared kit (QIAMP DNA Mini Kit; Qiagen Inc., Valencia, Calif.).
Chlamydial DNA was amplified by PCR using the oligonucleotides 5′-GGG ACT TCG TTT TTG ATC GT-3′ and 5′-CAT CTT GTT GAG CTG CAA GG-3′ as specific primers for an ∼850-bp region of the ompA gene of MoPn. PCR was performed using 2.5 U of Taq DNA polymerase (Qiagen Taq PCR Master Mix Kit) in 100-μl reaction mixtures containing PCR buffer with 1.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate, 0.2 μM each primer, and 46 μl of the extracted DNA template. The samples were subjected to 35 cycles of 30 s at 96°C, 1 min at 45°C, and 1 min at 72°C in a programmable DNA thermal cycler (LabLine, Inc., Melrose Park, Ill.). PCR products were screened by 1.5% agarose gel electrophoresis for bands of the appropriate size. Southern blot (Sb) hybridization was used to increase the sensitivity of detection of the amplicands to confirm the specificity of the PCR. A 450-bp probe labeled with digoxigenin was made for this purpose (DIG High Prime DNA Labeling and Detection Kit II; Roche Molecular Biochemicals, Indianapolis, Ind.). Hybridization was visualized by chemiluminescence and exposure of blots to BioMax film (Kodak, Rochester, N.Y.). Controls included swab samples and UGT tissues from uninfected mice and were negative. It was determined that Sb hybridization of PCR amplicands could detect between 102 and 103 copies of the ompA gene.
Immunosuppression.
Immunosuppression was used to reveal the presence of viable chlamydiae following culture-apparent recovery from the primary infection (10). To enhance the reactivation of chlamydial shedding subsequent to immunosuppression, mice were treated with P4 at 110 and 117 days postinfection. At 120 days postinfection, mice were treated with 200 mg of cyclophosphamide (Cy)/kg (5 mg/mouse) administered intraperitoneally in approximately 0.2 ml of sterile PBS. On each of the succeeding 9 days, mice were treated with 40 mg of Cy/kg (1 mg/mouse) in 0.2 ml of PBS. Control mice were treated with equivalent volumes of PBS only. To assess the reactivation of chlamydial infection, swab samples were collected at 120, 124, 127, and 130 days postinfection for the isolation of MoPn in HeLa 229 cells as described above. Swab samples for the detection of chlamydial DNA were collected 117, 123, 126, and 129 days postinfection. Also at each time point after immunosuppression, some of the animals were sacrificed by cervical dislocation. UGT tissues (uterine horns and oviduct) were aseptically removed and divided at the bifurcation of the uterine horns. They were then prepared for chlamydial isolation in cultures or for extraction of DNA as described above.
Assessment of pathological outcome.
Hydrosalpinx formation has been used as a means to assess gross UGT pathology in mice subsequent to chlamydial infection, and some researchers have reported its use as a surrogate marker for infertility (24, 34). At the time of necropsy, hydrosalpinx formation was assessed by gross macroscopic or microscopic observation using a dissecting microscope with ×10 magnification. In most cases, hydrosalpinx formation was readily observable without the aid of a microscope. Also noted at necropsy were any organ changes suggestive of disseminated infection or disease, including iliac lymph node adenopathy, splenomegaly, visceral adhesions, and morphological changes in the lungs and uterus.
The development of infertility subsequent to MoPn infection has been used to delineate susceptible and resistant strains of mice and as a further measure of pathological outcome that may be related to hydrosalpinx formation (14, 26). Fifty-six days subsequent to infection, all mice were assessed for fertility with an adaptation of the method of De La Maza et al. (14). Briefly, experimental or control female mice were placed three or four per cage with a proven breeder male. Baseline weights were recorded initially and subsequently 7, 10, 14, and 18 days later. In addition, at the end of each time interval, abdomens were visually checked and palpated for pregnancy. Obviously pregnant mice were sacrificed at 18 days, and embryos in the left and right uterine horns were counted. If a mouse was not obviously pregnant at the end of 21 days, she was monitored for an additional 1 week in the absence of a male and then introduced to a different male who had successfully mated in the first round of breeding. If at the end of the second round the mouse remained not pregnant, she was considered infertile, sacrificed, and necropsied. Fertility rates were compared between groups of infected mice and uninfected age-matched progesterone-treated control mice.
Primary cell cultures.
Lung cells were collected for primary culture experiments for convenience because processed lung tissue yielded consistent and reproducible cultures (27). Single-cell suspensions of lungs from uninfected NOS2−/− and NOS2+/+ mice were derived as described elsewhere and consisted of ≥90% fibroblasts (27). After the cells reached confluence, culture medium was aspirated and the cells were treated with either (i) IFN-γ (50 ng/ml; Pharmingen, San Diego, Calif.) plus lipopolysaccharide (LPS) (100 ng/ml; Sigma), (ii) IFN-γ plus LPS plus 1 mM l-NMMA (Calbiochem, La Jolla, Calif.), (iii) fresh medium plus l-NMMA, or (iv) fresh medium only for 48 h prior to infection. To confirm iNOS activation, nitrite accumulation was assessed by the Greiss reaction (33). Some samples also were tested for the presence of 2,3-IDO as described previously (6). Treatment of cell cultures with IFN-γ plus LPS routinely resulted in marked nitrite accumulation, which could be blocked by the addition of 1 mM l-NMMA (27). Cultures were infected with viable MoPn at a multiplicity of infection of 1.0. Infection was established for 40 h, after which the medium was changed and replenished with medium without IFN-γ and LPS (reactivation). If present during the initial treatment, l-NMMA was also used when the medium was changed. Parallel triplicate cultures were fixed in methanol at the following time points after replenishment of the medium as described above: 0, 24, 48, 60, 72, and 96 h. After fixation in methanol, wells were stained for chlamydial inclusions by indirect fluorescent-antibody staining (11). Control cultures treated and replenished with medium only were used as the basis for percentage-of-control-data calculations.
Statistics.
Rates of hydrosalpinx formation and infertility were compared by Fisher's exact test. Reactivation of infection in vitro was analyzed by a repeated-measures analysis of variance (group and time point postreactivation), and posthoc analysis was completed with a Tukey-Kramer test.
RESULTS
NOS2−/− mice sustain greater pathological outcome subsequent to infection than NOS2+/+ controls.
While it has been reported that iNOS-derived RNI are not essential for the resolution of chlamydial urogenital infection of mice, the role they play in sterile eradication of the pathogen and disease pathogenesis is unknown (27). To address the possible role of RNI in disease pathogenesis, NOS2−/−, C57BL/6 NOS2+/+, and B6129F1 NOS2+/+ mice were infected intravaginally with MoPn. Infection in all mice was confirmed by collection of cervicovaginal swab samples and subsequent culturing. The infection was then monitored with a subset of 10 mice in each group by further collection of swab samples at 4, 7, 10, and 14 days postinfection and every 7 days thereafter until resolution of the infection was confirmed. We found that NOS2−/− mice were capable of resolving infection in a manner not significantly different from that of NOS2+/+ mice (data not shown), confirming previous observations (27).
Table 1 shows the results of gross pathological examination of NOS2−/− and NOS2+/+ mice subsequent to primary infection in two separate experiments. The results are day 56 or later necropsy and fertility results and necropsy results at day 120. These time points represent approximately 3 weeks and 12 weeks, respectively, postinfection resolution in most mice. NOS2−/− and B6129F1 NOS2+/+ mice had similar rates of hydrosalpinx formation at 56 days postinfection that was significantly greater than that of C57BL/6 NOS2+/+ mice. However, when monitored to 120 days postinfection, the rate of hydrosalpinx formation declined in the infected NOS2+/+ mice compared to the NOS2−/− mice. Infertility rates at day 56 or later also correlated well with hydrosalpinx formation. Infertility rates were not assessed in mice at 120 days postinfection due to the possibility that age-related fertility decline would complicate the interpretation of results.
TABLE 1.
Gross pathological outcome subsequent to infection
| Mice | Group | Hydrosalpinx formation at day 56a | Fertility at day 56b | Mean (SD) embryos/mouse | Hydrosalpinx formation at day 120a |
|---|---|---|---|---|---|
| NOS2−/− | Infected | 29/34 (85.3)c | 1/17 (5.9) | 4 (0.0) | 38/46 (82.6)d |
| Uninfected | 0/36 (0.0) | 17/18 (94.4) | 5.7 (2.9) | ND | |
| NOS2+/+ (C57BL/6) | Infected | 16/32 (50.0) | 7/10 (70.0) | 6.3 (2.1) | ND |
| Uninfected | 0/20 (0.0) | 10/10 (100) | 8.8 (3.4) | ND | |
| NOS2+/+ (B6129F1) | Infected | 32/36 (88.9)c | 0/18 (0.0) | 0 (0.0) | 21/54 (38.9) |
| Uninfected | 0/36 (0.0) | 18/18 (100) | 7.1 (3.3) | ND |
Total number of hydrosalpinges formed out of the total number of oviducts assessed (percentage). ND, not done.
Number of fertile mice out of total number tested (percentage).
The P value was 0.033, as determined by Fisher's exact test, when compared to results for C57BL/6 NOS2+/+ mice at day 56; the P value was <0.0001 when compared to results for uninfected controls.
The P value was 0.004, as determined by Fisher's exact test, when compared to results for NOS2+/+ mice at day 120.
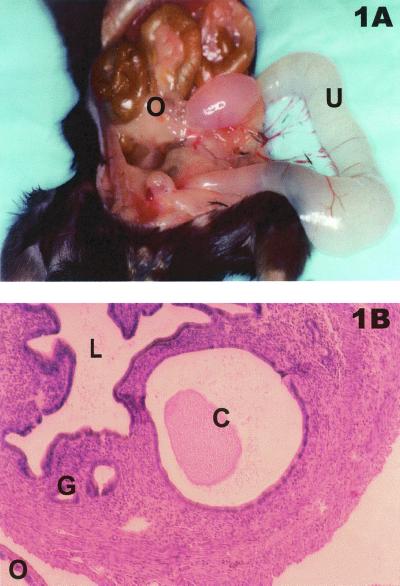
Histologically, the hydrosalpinx comprised a lumen filled with serous fluid but relatively few inflammatory cells at day 56 postinfection. This result is similar to observations made previously (23). However, in several (8 of 23) NOS2−/− mice sacrificed and necropsied at day 120, an exacerbated hydrosalpinx and grossly distended and dilated uteri were observed either unilaterally or bilaterally (Fig. 1A). Hematoxylin-eosin-stained sections revealed that uterine distension and dilation were likely due to the formation of severe endometrial cysts and cystic hyperplasia (Fig. 1B). While uterine cysts and distension occasionally develop postinfection in C57BL/6 NOS2+/+ mice (10), the extent of uterine dilation and distension has not been previously observed by us but is reminiscent of that described by Darville et al. for IFN-γ-deficient mice (12). We did not observe similar results for any B6129F1 NOS2+/+ or C57BL/6 NOS2+/+ mice in these experiments.
FIG. 1.
Gross and histopathological results subsequent to infection in NOS2−/− mice. (A) Necropsy of NOS2−/− mouse 120 days postinfection. U, fluid-filled and grossly distended uterus; O, oviduct showing hydrosalpinx formation. (B) Hematoxylin-eosin-stained histological section of NOS2−/− mouse uterus 120 days postinfection showing a uterine cyst (C) that is characteristic of the histopathological changes observed at necropsy, as seen in panel A. L, lumen of uterus; G, normal mucus gland of uterus; O, oviduct.
Detection of chlamydial nucleic acids subsequent to primary urogenital tract infection.
Considering that exacerbated disease occurred in NOS2−/− mice, we hypothesized that long-term persistent infections occurred due to microbistatic suppression of chlamydial growth. The presence of persistent chlamydiae could potentially provide an impetus for heightened or protracted inflammatory responses and thus greater pathology. To investigate the nature and extent of persistent chlamydial infections, we infected NOS2−/− and NOS2+/+ animals as before. Infection in all mice was confirmed on day 4 postinfection by culturing viable MoPn from cervicovaginal swab samples. Subgroups of NOS2−/−and NOS2+/+ mice were then monitored for 120 days. Additional swab samples were collected, and total DNA was extracted and subjected to PCR amplification targeting a portion of the ompA gene. All PCRs were screened for the presence of bands of the appropriate size on agarose gel electrophoresis and subsequently assessed by Sb hybridization to confirm the specificity of the amplification and to enhance sensitivity when no band was visible on the gels. Table 2 shows the summarized results, and Fig. 2 shows individual results from representative NOS2−/− and NOS2+/+ mice.
TABLE 2.
Detection of viable MoPn and chlamydial DNA during primary and reactivated (day 120) infections
| Mice | Treatmenta | Method | No. of positive mice/total no. tested at the following day(s) postinfection:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3–4 | 7 | 10 | 14 | 21 | 28 | 35 | 42 | 56 | 70 | 84 | 117, 120b | 123, 124 | 126, 127 | 129, 130 | |||
| NOS2−/− | Cy | Culturingc | 15/15 | 5/5 | 5/5 | 5/5 | 4/5 | 2/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/14 | 6/14 | 4/9 | 0/4 |
| PCR-Sb swabd | 5/5 | 4/5 | 4/5 | 1/4 | 4/5 | 4/5 | 5/5 | 5/5 | 2/5 | 5/5 | 5/5 | 13/14 | 1/9 | 1/4 | 0/4 | ||
| PCR-Sb tissuee | 3/3 | 3/4 | 3/3 | ||||||||||||||
| PBS | Culturing | 15/15 | 0/12 | 0/8 | 0/5 | 0/3 | |||||||||||
| PCR-Sb swab | 12/12 | 1/8 | 1/5 | 0/3 | |||||||||||||
| PCR-Sb tissue | 2/3 | 2/2 | 1/3 | ||||||||||||||
| NOS2+/+ | Cy | Culturing | 15/15 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 1/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/14 | 0/14 | 0/9 | 0/5 |
| PCR-Sb swab | 4/5 | 5/5 | 5/5 | 4/5 | 5/5 | 5/5 | 4/5 | 5/5 | 4/5 | 4/5 | 3/5 | 14/14 | 1/14 | 1/9 | 5/5 | ||
| PCR-Sb tissue | 3/3 | 3/3 | 4/4 | ||||||||||||||
| PBS | Culturing | 15/15 | 0/13 | 0/13 | 0/9 | 0/4 | |||||||||||
| PCR-Sb swab | 5/5 | 12/13 | 0/13 | 2/9 | 4/5 | ||||||||||||
| PCR-Sb tissue | 3/3 | 1/3 | 2/4 | ||||||||||||||
Treatments were given at 120 days postinfection.
Separate samples were collected on different days from the same animal. Swab samples were collected for PCR-Sb on the first day shown, and swab samples were collected for culturing on the second day.
Detection of viable MoPn in cervicovaginal swab samples by culturing in HeLa 229 cells.
Detection of chlamydial DNA extracted from cervicovaginal swab samples by PCR followed by Sb hybridization of amplicands.
Detection of chlamydial DNA extracted from UGT tissues by PCR followed by Sb hybridization of amplicands.
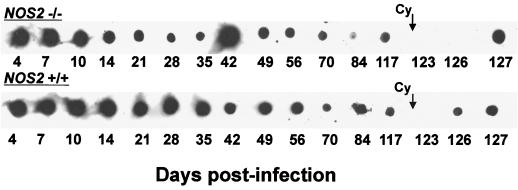
FIG. 2.
Detection of chlamydial DNA subsequent to infection in NOS2−/− and NOS2+/+ mice. PCR followed by Sb hybridization of amplicands was used to detect the presence of MoPn ompA DNA in genital swab extracts (days 4 to 126 postinfection) or in extracts of UGT tissues (day 127). (Top row) Results for sequential samples collected from a single NOS2−/− mouse. (Bottom row) Results for samples collected from a single B6129F1 NOS2+/+ mouse. Shown are typical individual results that are summarized in Table 2. Administration of Cy was initiated at day 120 postinfection.
Interestingly, chlamydial DNA was consistently detected by one or both methods in both NOS2−/− and NOS2+/+ mice for 120 days postinfection. In several cases, Sb hybridization yielded positive reactions when no band was present on agarose gels. Uninfected mice yielded negative results. It thus appears that in this model, chlamydial nucleic acids persist well beyond the ability to culture the organism regardless of whether animals are capable of generating RNI.
Evidence for persistent viable C. trachomatis in NOS2−/− mice subsequent to primary infection.
We also further investigated the nature of chlamydial persistence in this model by seeking to determine if the origin of chlamydial nucleic acids was residual nonviable components of the organisms or viable organisms. It was previously found that infection can be reactivated by the administration of Cy to various strains of NOS2+/+ mice for up to 5 weeks past culture-proven resolution (10). We therefore postulated that the time frame for reactivation would be protracted in NOS2−/− mice compared to that of controls. Animals were again treated at days 110 and 117 with P4 and at day 120 with either Cy or PBS. Genital swab samples were collected for culturing or PCR-Sb hybridization on days 117, 123, 126, and 129 or 130 for all mice. Three to five mice were sacrificed in each group at days 124, 127, and 130, and UGT tissues were excised for culturing, histological assessment, or PCR-Sb hybridization of DNA extracts. Table 2 also shows the summarized results of this experiment. We observed renewed shedding of viable chlamydial elementary bodies in 6 of 14 NOS2−/− mice treated with Cy but in none of the PBS-treated NOS2−/− or similarly treated NOS2+/+ animals. It should be noted that only small numbers of inclusions were observed in these cultures (<102), whereas during the peak of the primary infection, between 105 and 107 inclusion-forming units are normally isolated per swab sample (10).
Interestingly, while chlamydial DNA was detected in extracts of swab samples from nearly all animals at day 117 after primary infection, by day 123 we could no longer detect shedding of chlamydial DNA from the lower genital tract (Table 2). This finding included the Cy-treated NOS2−/− mice from which viable organisms had been isolated. This finding was likely due to the effect of P4 pretreatment, which often results in a great deal of mucus, a known inhibitor of PCR (9). Although the presence of large amounts of mucus was also observed following P4 treatment in the primary infection, the effect on PCR was not observed, likely due to the large numbers of organisms present during that time frame.
Reactivation of MoPn infection in primary cell cultures derived from NOS2−/− and NOS2+/+ mice.
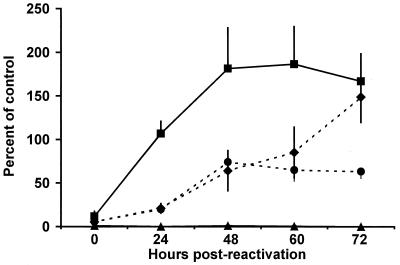
It was previously shown that primary cell cultures derived from NOS2−/− mice restrict chlamydial growth upon stimulation with IFN-γ and LPS (27). The results shown in Fig. 3 support and extend these findings. Primary cell cultures derived from NOS2−/− or NOS2+/+ mice were treated for 48 h with IFN-γ and LPS. NOS2+/+ cells showed iNOS activity, as demonstrated by the Greiss assay (Table 3). NOS2−/− cells showed no evidence of iNOS activity, and activity was substantially reduced in NOS2+/+ cells when incubated in the presence of 1 mM l-NMMA. Forty-eight hours posttreatment, cultures were infected with MoPn. At 40 h postinfection (0 h postreactivation), medium was removed and replaced with medium containing neither IFN-γ nor LPS. Beginning at 24 h postreactivation, chlamydial replication was restored in all NOS2−/− cell cultures and in NOS2+/+ cells in which iNOS was chemically blocked by the presence of 1 mM l-NMMA. These results indicate that IFN-γ induces as-yet-undefined mechanisms that, in conjunction with RNI, are microbicidal for MoPn but that, in the absence of RNI, are microbistatic. Cells also were tested for 2,3-IDO, a known IFN-γ-induced microbistatic enzyme, but neither NOS2−/− nor NOS2+/+ cells were observed to catabolize tryptophan after cytokine activation (Table 3).
FIG. 3.
Reactivation of chlamydial replication in vitro subsequent to removal of IFN-γ and LPS. Primary lung fibroblasts from either NOS2−/− mice (diamonds and circles) or NOS2+/+ mice (triangles and squares) were treated with IFN-γ and LPS for 48 h in the absence (diamonds and triangles) or presence (circles and squares) of 1 mM l-NMMA. Cell monolayers were then infected with MoPn at a multiplicity of infection of 1.0. After an additional 40 h, medium was removed and replenished with medium without IFN-γ or LPS (0 h postreactivation). Triplicate samples were stained for the presence of inclusions at each time point. Percentage-of-control-data calculations were based on controls treated with medium only. Differences observed for IFN-γ- and LPS-treated NOS2+/+ mouse-derived cells were found to be significant when the main effects of group and time point were compared by a two-factor analysis of variance. Error bars indicate standard deviations.
TABLE 3.
iNOS and 2,3-IDO activities in primary murine lung cells from NOS2−/− and NOS2+/+ micea
| Mice | Treatment | Nitrite (μM/ml)b |
|---|---|---|
| NOS2−/− | None | 0.56 |
| l-NMMA | 0.64 | |
| IFN-γ + LPS | 1.15 | |
| IFN-γ + LPS + l-NMMA | 1.05 | |
| NOS2+/+ | None | 0.64 |
| l-NMMA | 0.67 | |
| IFN-γ + LPS | 46.8 | |
| IFN-γ + LPS + l-NMMA | 12.1 |
DISCUSSION
The present findings, taken together with previous observations (27), indicate that while iNOS activity is not essential to resolution of the infection, it may provide a degree of protection against chronic but not acute disease. We also conclude that disease susceptibility varies in the wild-type mice used as controls in most gene knockout mouse studies (C57BL/6 and B6129F1) and should be a planning factor in study designs that assess disease outcome. Hydrosalpinx formation may not necessarily represent a permanent state in mice in that significantly lower rates of disease were noted for wild-type B6129F1 NOS2+/+ mice at day 120 than at day 56 postinfection, thus indicating a tendency toward resolution of this particular outcome in immunocompetent mice.
The reactivation data indicate that although iNOS-derived RNI undoubtedly contribute to the eradication of chlamydial infection, other, unidentified cytokine-mediated mechanisms also exist. In the presence of RNI, the cumulative effects are microbicidal for MoPn, but in the absence of iNOS activity, they are microbistatic. This conclusion was indicated by reactivation of infection subsequent to removal of IFN-γ only in primary cell cultures derived from NOS2−/− mice or in those derived from NOS2+/+ mice but treated with l-NMMA. This conclusion was further confirmed by the ability to reactivate the infection in vivo for prolonged periods of time and in a larger proportion of NOS2−/− mice than of NOS2+/+ mice.
While the exact contributions of additional IFN-γ-inducible mechanisms remain undefined, known mechanisms may include restriction of iron or some other key nutrients (17, 21, 30) or production of defensins, protegrins, or other antimicrobial peptides (38). Others have reported low 2,3-IDO activity in the RAW264.7 murine macrophage line upon IFN-γ treatment, but added tryptophan failed to reverse chlamydial growth inhibition (8). Given previous results (27) and the data presented here, we conclude that 2,3-IDO induction and subsequent tryptophan catabolism are not important factors in this model. In light of the recent findings by Perry et al. (25) that MoPn is less sensitive to the effects of IFN-γ than human strains of C. trachomatis, delineation of the exact IFN-γ-inducible mechanisms that possess microbicidal and microbistatic activities for various chlamydiae is of utmost importance. This is true not only for the interpretation of results in the murine model but also for defining key protective immune mechanisms evoked during human chlamydial infections and other animal models of chlamydial infections.
Our finding of persistent chlamydial DNA subsequent to the resolution of primary infection supports and extends the findings of Beale using the murine model (3) and Holland et al. using the primate model of chlamydial ocular infection (15). It also lends credence to the use of this model for the study of chlamydial pathogenesis because chlamydial DNA or other chlamydial components are often isolated from culture-negative patients (5, 13, 22, 31). In addition, although Cy administration is known to reactivate chlamydial infection for a few weeks after primary infection (3, 10), reactivation out to 120 days postinfection, as documented here, provides evidence that true long-term persistence is possible using NOS2−/− mice. We recognize that treatment with Cy reflects a harsh, global form of suppression, and we understand the need to develop reactivation methods that are less severe and more informative. For example, specific in vivo depletion with monoclonal antibodies may be more useful in defining cell types or cytokines that are responsible for suppressing chlamydial infection subsequent to culture-proven resolution of infection in either NOS2−/− or NOS2+/+ mice.
It is interesting that NOS2−/− mice sustained more severe pathological outcome subsequent to infection than NOS2+/+ controls. Viewed through the paradigm that persistent chlamydial antigen induces immunopathological host responses, it is tempting to link enhanced disease outcome in NOS2−/− mice to the persistence of potentially viable but nonculturable chlamydiae. However, we have not yet conclusively associated these two observations, and they may ultimately represent independent outcomes in this model. Certainly, establishing a clear cause-and-effect relationship between chlamydial persistence and exacerbated disease may prove difficult. Irrespective of whether this is an attainable goal or not, our model of chlamydial persistence in NOS2−/− mice may have greater utility in characterizing the biological and antigenic nature of persistent chlamydial infections in vivo. For example, in vivo host and pathogen gene profiling studies may be possible since genomic information has become available to do these types of studies.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI37807 (to K.H.R.) and AI19782 (to G.I.B.).
REFERENCES
- 1.Barron A L, Rank R G, Moses E B. Immune response in mice infected in the genital tract with mouse pneumonitis agent (Chlamydia trachomatis biovar) Infect Immun. 1984;44:82–85. doi: 10.1128/iai.44.1.82-85.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barron A L, White H J, Rank R G, Soloff B L, Moses E B. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981;143:63–66. doi: 10.1093/infdis/143.1.63. [DOI] [PubMed] [Google Scholar]
- 3.Beale A S. Does Chlamydia trachomatis MoPn enter a microbiologically-inapparent state during experimental infection of the mouse genital tract? Microb Pathog. 1997;22:99–112. doi: 10.1006/mpat.1996.0096. [DOI] [PubMed] [Google Scholar]
- 4.Beatty W L, Morrison R P, Byrne G I. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobo L D, Novak N, Munoz B, Hsieh Y H, Quinn T, West S. Severe disease in children with trachoma is associated with persistent Chlamydia trachomatis infection. J Infect Dis. 1997;176:1524–1530. doi: 10.1086/514151. [DOI] [PubMed] [Google Scholar]
- 6.Byrne G I, Lehmann L K, Landry G J. Induction of tryptophan catabolism is the mechanism for gamma interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986;53:347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen B, Stout R, Campbell W F. Nitric oxide production: a mechanism of Chlamydia trachomatis inhibition in interferon-γ treated RAW264.7 cells. FEMS Immunol Med Microbiol. 1996;14:109–120. doi: 10.1111/j.1574-695X.1996.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 9.Clad A, Naudascher I, Flecken U, Freidank H M, Petersen E E. Evidence of labile inhibitors in the detection of Chlamydia trachomatis in cervical specimens by polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 1996;15:744–747. doi: 10.1007/BF01691963. [DOI] [PubMed] [Google Scholar]
- 10.Cotter T W, Miranpuri G S, Ramsey K H, Poulsen C E, Byrne G I. Reactivation of chlamydial genital tract infection in mice. Infect Immun. 1997;65:2067–2073. doi: 10.1128/iai.65.6.2067-2073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darville T, Andrews C W, Kishen L R, Rank R G, Williams D M. Transforming growth factor-β is associated with increased pathology in γ-interferon gene knockout mice infected with Chlamydiae. In: Stephens R S, Byrne G I, Christiansen G, Clarke I N, Grayston J T, Rank R G, Saikku P, Schachter J, Stamm W E, editors. Chlamydial infections: Proceedings of the Ninth International Symposium on Human Chlamydial Infections. Calif: San Francisco; 1998. pp. 407–410. [Google Scholar]
- 13.Dean D, Suchland R J, Stamm W E. Evidence for long-term cervical persistence of Chlamydia trachomatis by omp1 genotyping. J Infect Dis. 2000;182:909–916. doi: 10.1086/315778. [DOI] [PubMed] [Google Scholar]
- 14.De La Maza L M, Pal S, Khamesipour A, Peterson E M. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–2097. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland S M, Hudson A P, Bobo L, Whittum-Hudson J A, Viscidi R P, Quinn T C, Taylor H R. Demonstration of chlamydial RNA and DNA during a culture-negative state. Infect Immun. 1992;60:2040–2047. doi: 10.1128/iai.60.5.2040-2047.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igietseme J U. The molecular mechanism of T-cell control of Chlamydia in mice: role of nitric oxide. Immunology. 1996;87:1–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Igietseme J U, Ananaba G A, Candal D H, Lyn D, Black C M. Immune control of chlamydial growth in the human epithelial cell line RT4 involves multiple mechanisms that include nitric oxide induction, tryptophan catabolism and iron deprivation. Microbiol Immunol. 1998;42:617–625. doi: 10.1111/j.1348-0421.1998.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 18.Kol A, Sukhova G K, Lichtman A H, Libby P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation. 1998;98:300–307. doi: 10.1161/01.cir.98.4.300. [DOI] [PubMed] [Google Scholar]
- 19.Mayer J, Woods M L, Vavrin Z, Hibbs J B. Gamma interferon-induced nitric oxide production reduces Chlamydia trachomatis infectivity in McCoy cells. Infect Immun. 1993;61:491–498. doi: 10.1128/iai.61.2.491-497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray H W, Granger A M, Teitelbaum R F. Gamma interferon-activated human macrophages and Toxoplasma gondii, Chlamydia psittaci, and Leishmania donovani: antimicrobial role of limiting intracellular iron. Infect Immun. 1991;59:4684–4686. doi: 10.1128/iai.59.12.4684-4686.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ormsby H L, Thompson G A, Cousineau G G, Lloyd L A, Hassard J. Topical therapy in inclusion conjunctivitis. Am J Ophthalmol. 1952;35:1811–1814. doi: 10.1016/0002-9394(52)92022-9. [DOI] [PubMed] [Google Scholar]
- 23.Pal S, Fielder T J, Peterson E M, De La Maza L M. Analysis of the immune response in mice following intrauterine infection with the Chlamydia trachomatis mouse pneumonitis biovar. Infect Immun. 1993;61:772–776. doi: 10.1128/iai.61.2.772-776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal S, Peterson E M, De La Maza L M. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatis genital challenge. Infect Immun. 1996;64:5341–5348. doi: 10.1128/iai.64.12.5341-5348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry L L, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell H D. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J Immunol. 1999;162:3541–3548. [PubMed] [Google Scholar]
- 26.Ramsey K H, DeWolfe J L, Salyer R D. Disease outcome subsequent to primary and secondary urogenital infection with murine or human biovars of Chlamydia trachomatis. Infect Immun. 2000;68:7186–7189. doi: 10.1128/iai.68.12.7186-7189.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsey K H, Miranpuri G S, Poulsen C E, Marthakis N B, Braune L M, Byrne G I. Inducible nitric oxide synthase does not affect resolution of murine chlamydial genital tract infections or eradication of chlamydiae in primary murine cell culture. Infect Immun. 1998;66:835–838. doi: 10.1128/iai.66.2.835-838.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rank R G, Ramsey K H, Pack E A, Williams D M. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rank R G, Soderberg L S F, Barron A L. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect Immun. 1985;48:847–849. doi: 10.1128/iai.48.3.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raulston J E. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect Immun. 1997;65:4539–4547. doi: 10.1128/iai.65.11.4539-4547.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumacher H R., Jr Reactive arthritis. Rheum Dis Clin North Am. 1998;24:261–273. doi: 10.1016/s0889-857x(05)70008-9. [DOI] [PubMed] [Google Scholar]
- 32.Sekkai D, Guittet O, Lemaire G, Tenu J P, Lepoivre M. Inhibition of nitric oxide synthase expression and activity in macrophages by 3-hydroxyanthranilic acid, a tryptophan metabolite. Arch Biochem Biophys. 1997;340:117–123. doi: 10.1006/abbi.1997.9913. [DOI] [PubMed] [Google Scholar]
- 33.Stuehr D J, Marletta M A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci USA. 1985;82:7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su H, Messer R, Whitmire W, Fischer E, Portis J C, Caldwell H D. Vaccination against chlamydial genital tract infection after immunization with dendritic cells pulsed ex vivo with nonviable Chlamydiae. J Exp Med. 1998;188:809–818. doi: 10.1084/jem.188.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takikawa O, Tagawa Y, Iwakura Y, Yoshida R, Truscott R J. Interferon-gamma-dependent/independent expression of indoleamine 2,3-dioxygenase. Studies with interferon-gamma-knockout mice. Adv Exp Med Biol. 1999;467:553–557. doi: 10.1007/978-1-4615-4709-9_68. [DOI] [PubMed] [Google Scholar]
- 36.Tatsumi K, Higuchi T, Fujiwara H, Nakayama T, Egawa H, Itoh K, Fujii S, Fujita J. Induction of tryptophan 2,3-dioxygenase in the mouse endometrium during implantation. Biochem Biophys Res Commun. 2000;274:166–170. doi: 10.1006/bbrc.2000.3115. [DOI] [PubMed] [Google Scholar]
- 37.Thomas S M, Garrity L F, Brandt C R, Schobert S C, Feng G S, Taylor M W, Carlin J M, Byrne G I. γ IFN-mediated antimicrobial response: indoleamine 2,3-dioxygenase-deficient mutant host cells no longer inhibit intracellular Chlamydia spp. or Toxoplasma growth. J Immunol. 1993;150:5529–5534. [PubMed] [Google Scholar]
- 38.Yasin B, Harwig S S L, Lehrer R I, Wagar E A. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infect Immun. 1996;64:709–713. doi: 10.1128/iai.64.3.709-713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]