Abstract
Progressive pulmonary infection is the dominant clinical feature of cystic fibrosis (CF), but the molecular basis for this susceptibility remains incompletely understood. To study this problem, we developed a model of chronic pneumonia by repeated instillation of a clinical isolate of Burkholderia cepacia (genomovar III, ET12 strain), an opportunistic gram-negative bacterium, from a case of CF into the lungs of Cftr m1unc−/− (Cftr−/−) and congenic Cftr+/+ controls. Nine days after the last instillation, the CF transmembrane regulator knockout mice showed persistence of viable bacteria with chronic severe bronchopneumonia while wild-type mice remained healthy. The histopathological changes in the lungs of the susceptible Cftr−/− mice were characterized by infiltration of a mixed inflammatory-cell population into the peribronchiolar and perivascular spaces, Clara cell hyperplasia, mucus hypersecretion in airways, and exudation into alveolar airspaces by a mixed population of macrophages and neutrophils. An increased proportion of neutrophils was observed in bronchoalveolar lavage fluid from the Cftr−/− mice, which, despite an increased bacterial load, demonstrated minimal evidence of activation. Alveolar macrophages from Cftr−/− mice also demonstrated suboptimal activation. These observations suggest that the pulmonary host defenses are compromised in lungs from animals with CF, as manifested by increased susceptibility to bacterial infection and lung injury. This murine model of chronic pneumonia thus reflects, in part, the situation in human patients and may help elucidate the mechanisms leading to defective host defense in CF.
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the gene encoding the CF transmembrane regulator (CFTR), a transmembrane glycoprotein responsible for chloride conductance in epithelial cells. Progressive pulmonary disease is the dominant clinical feature of CF and accounts for 95% of the morbidity and mortality (11, 51). Despite intensive study, the mechanisms responsible for this enhanced susceptibility to infection remain incompletely understood and a source of controversy. Absence or dysfunction of CFTR leads to alterations in the microenvironment of the lung that are manifest early in life as inflammation of the distal airways (25), but whether this is cause or consequence of infection remains controversial (4). Reported abnormalities in the pulmonary environment in the lungs of animals with CF include altered fluid and ionic fluxes across the respiratory epithelium, excessive luminal mucus, diminished mucociliary clearance, altered patterns of epithelial surface glycosylation, and diminished activity of bactericidal factors such as lysozyme, lactoferrin, defensins, and cathelicidins (6, 11, 18, 34, 41, 54, 61). The relative contributions of these factors to airway infection in CF remain unknown.
Whatever the proximate cause of the susceptibility to infection, the milieu of the lungs of animals with CF provides a favorable niche for bacterial infection with certain opportunistic pathogens such as Staphylococcus aureus and eventually resistant gram-negative organisms such as Pseudomonas aeruginosa. A subpopulation consisting mainly of adolescent and adult patients also develop chronic infection with resistant gram-negative bacterium Burkholderia cepacia. The clinical outcome after acquisition of B. cepacia is variable, ranging from an asymptomatic culture-positive state to a devastating syndrome of fatal necrotizing pneumonia and septicemia (cepacia syndrome) (12, 16, 60). Why CF predisposes patients to acquisition of B. cepacia is incompletely understood. Host lung factors such as underlying lung damage (16, 23), repeated exposure (15, 28, 37, 53), and specific bacterial factors, such as presence of cable pili (46–48), production of extracellular enzymes (30, 39), and the ability of some strains of B. cepacia to replicate intracellularly (3, 33, 43), all appear to contribute to the propensity to persistent infection.
One feature that has thwarted the identification of virulence properties is that B. cepacia is not a single clonal strain. Rather, it consists of a complex of strains that belong to one of five or more genetic groups or genomovars (2, 62). The most common groups cultured from sputa from CF patients are genomovars II, III, and IV (30). (Genomovars II and IV have been renamed Burkholderia multivorans and Burkholderia stabilis, respectively [2, 62].) Within genomovar III, the highly transmissible strain (the ET12 strain [59]) expresses cable pili and its associated 22-kDa adhesin (46, 48). This is the strain most commonly cultured from CF patients in Canada and the United Kingdom and is the one most commonly associated with the cepacia syndrome (17, 32). Since this strain was shown to bind most strongly to mucins, to epithelial cells, and to tissue sections from lungs of CF patients (45, 47, 49), we have used this strain to develop an animal model of lung infection.
Given the difficulties in studying the pathogenesis of bacterial infection in CF patients, murine models have been used as an experimental model system. CFTR-deficient (knockout) mice have been used to study experimental infection (7, 8, 10, 14, 19, 35). While these studies have provided important insights into the pathogenesis of CF lung disease, many have focused primarily on the acute responses to infection and some have relied on the use of agar-entrapped bacteria (14, 19) to assure the retention of bacteria in the lungs by mechanical means. In general, compared to wild-type mice, Cftr−/− mice demonstrate decreased bacterial clearance, an excessive inflammatory response, and significant mortality when challenged with pathogenic organisms including S. aureus, P. aeruginosa, and B. cepacia, indicating that the absence of CFTR may increase the susceptibility of mice to infection with these opportunistic pathogens. In contrast, several recent studies have found that Cftr−/− mice were able to clear P. aeruginosa from their lungs as well as wild-type controls (7, 8, 35). These discrepancies may be explained by the complex genetic backgrounds of the mouse strains used, including the presence of alternative chloride channels, the different methods of bacterial delivery to the lungs, dietary factors, and the nutritional state of the mice. Thus, the relationship between CFTR expression, the pulmonary inflammatory response, and bacterial clearance remains uncertain.
Our goal was to develop a model of chronic pulmonary infection with B. cepacia in mice with CF without the need for bacterial entrapment or use of immunosuppressive agents. We hypothesized that the ineffective inflammatory responses observed in CF patients would be manifest in this model and that the propensity for enhanced pulmonary inflammation and injury would also depend on bacterial virulence. To investigate the latter, we used two strains of B. cepacia, one clinical isolate from the highly transmissible genomovar III (ET12) strain and an environmental type strain isolated from onion rot (ATCC 25416; genomovar I), to compare their virulence properties in mice. We compared the pulmonary inflammatory response to repeated administration of bacteria in both Cftr−/− and Cftr+/+ mice to judge the role of the Cftr−/− phenotype in enhancing susceptibility to lung infection.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A clinical isolate of B. cepacia BC7 was obtained from sputum of a CF patient at the Hospital for Sick Children in Toronto, Ontario, Canada. This patient died with the cepacia syndrome within 1 month of acquisition of the organism (45). Isolate BC7 is an ET12 strain, belongs to genomovar III, has been classified as randomly amplified polymorphic DNA type 2, carries the epidemic DNA marker designated BCESM, and expresses surface cable pili (32, 46, 47). ATCC 25416 is an environmental type strain isolated from onion rot, belongs to genomovar I, and was purchased from the American Type Culture Collection (Manassas, Va.) (32). Both isolates were stored at −80°C. In preparing the inoculum for infecting animals, B. cepacia isolates were subcultured on brain heart infusion (BHI) agar (Becton Dickinson Co., Cockeysville, Md.) and single colonies were inoculated into 10 ml of tryptic soy broth (Difco Labs, Detroit, Mich.) and grown overnight on an orbital shaker at 150 rpm at 37°C. Bacteria were harvested by centrifugation at 6,000 × g for 10 min, and the bacterial pellet was suspended in sterile phosphate-buffered saline (PBS) to a concentration of 109 CFU/ml. Viable bacterial counts were measured by plating serial dilutions of bacteria on B. cepacia selective agar (20) or BHI agar plates.
Experimental animals.
Long-surviving liquid-fed Cftrm1UNC knockout (i.e., Cftr−/−) mice (24) and their littermate wild-type Cftr+/+ controls, age 6 to 8 weeks, were utilized in the study. Wild-type mice were also liquid fed during the experimental protocol to minimize differences that could occur due to diet or nutritional status. Genotyping was done as previously described (56), and only homozygotes (Cftr+/+ and Cftr−/−) were used in this study. Mice were divided into three groups. In group 1, comprising 12 Cftr+/+ and 11 Cftr−/− mice, animals received only PBS. In group 2, comprising 12 Cftr+/+ and 19 Cftr−/− mice, animals were infected with isolate BC7. In group 3, comprising four Cftr+/+ and six Cftr−/− mice, animals were infected with isolate ATCC 25416. Experiments were carried out according to protocols approved by the animal care committee at the Hospital for Sick Children. The animals were housed in a clean conventional area free of pathogens in sterile microisolator cages until they reached the required age. Littermate wild-type controls were maintained under identical conditions. Mice were transferred 24 h before the start of an experiment to a containment unit and housed in the same area throughout the experiment.
Infection of mice.
Mice were anesthetized lightly using the inhalant anesthetic enflurane. To achieve intrapulmonary delivery, PBS (50 μl) or PBS containing B. cepacia (107 CFU) was instilled dropwise intranasally and allowed to be aspirated into the lungs. To ensure maximum delivery of bacteria into the lungs, mice were held with their mouths closed during the instillation. This technique was based on the results of preliminary experiments using 99mTc-labeled bovine serum albumin, which demonstrated maximum pulmonary delivery with minimal delivery to the gastrointestinal tract (data not shown). Additional studies using nasal instillation of a suspension of iron-dextran followed by Pearl's Prussian blue staining of the fixed and sectioned lungs demonstrated that the suspension was distributed equally to all lobes of the lungs and reached the alveoli (data not shown). Bacteria were administered on days 0, 3, 6, and 9, and the mice were sacrificed on day 18 by intraperitoneal injection of 0.3 ml of a 6.5-mg/ml solution of sodium pentobarbital and exsanguination.
BAL.
Bronchoalveolar lavage (BAL) was conducted by instilling three 1-ml aliquots of sterile PBS via a cannula placed into the trachea and secured with ligatures. The average volume of BAL fluid recovered was 2.6 ml. The concentration of cells in BAL fluid was determined using a hemocytometer. Fifty microliters of BAL fluid was sedimented in a cytocentrifuge (Shandon Inc., Pittsburgh, Pa.), fixed with methanol, and stained using a modified Wright-Giemsa stain (Diff-Quick; Dade Diagnostics, Aquanda, Puerto Rico). The percentage of each cell type was determined by counting a total of 300 cells/slide. The cells in the remaining BAL fluid were sedimented by centrifugation and saved for analyses as outlined below. The supernatant was immediately mixed with a protease inhibitor cocktail (Boehringer Mannheim, Toronto, Ontario, Canada) and stored on ice until used. Lungs, blood, and spleens were also collected for analyses.
Flow-cytometric analysis.
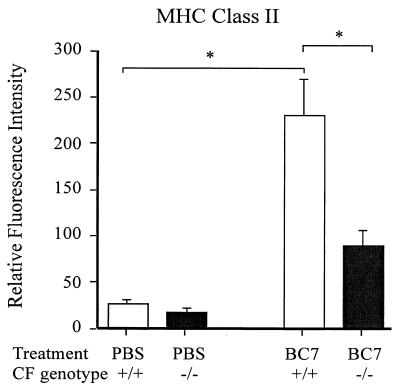
Cells obtained from the BAL fluid were incubated with 20% fetal bovine serum (FBS) for 30 min and washed with 10% FBS in PBS prior to addition of antibodies. As indicated in the legend to Fig. 6, cells were labeled with fluorescein isothiocyanate (FITC)-conjugated anti-CD11c and phycoerythrin-conjugated anti-major histocompatibility complex class II (MHC-II) (BD-Pharmingen Canada, Mississauga, Ontario, Canada) in 10% FBS in PBS, washed, and fixed with 1.6% paraformaldehyde. Two-color flow cytometry was performed using a FACScan flow cytometer equipped with CELLQuest software (Becton Dickinson, San Jose, Calif.). Macrophages were identified using a combination of light-scattering properties and surface expression of CD11c. The level of MHC-II surface expression was quantified on this population of cells. For assessment of oxidant production, cells were incubated with 10−5 M dihydrorhodamine (Molecular Probes, Eugene, Oreg.) for 5 min at 37°C followed by fixation with 1.6% paraformaldehyde. The fluorescence of the reduction product, rhodamine 1-2-3, was evaluated by one-color flow cytometry as a measure of oxidant production as previously described (63). Surface expression of F4/80, CD4, and CD8 on BAL cells was determined using the appropriate primary conjugated antibodies (BD-Pharmingen Canada). For all analyses, a minimum of 10,000 cells were measured per condition, and all values are expressed as relative fluorescence indices based on the geometric mean of the gated populations as described above.
FIG. 6.
Surface expression of MHC-II molecules on alveolar macrophages recovered by BAL. Cells from 100 μl of BAL fluid were incubated with 20% FBS for 30 min, washed with 10% FBS, and incubated with fluorescein isothiocyanate-conjugated anti-CD11c (not shown) and phycoerythrin-conjugated anti-MHC-II. Cells were washed to remove excess antibodies and fixed with 1.6% paraformaldehyde, and fluorescence was quantified by flow cytometry. Macrophages were gated by a combination of light scattering and CD11c staining. The level of MHC-II expression was assessed on the gated cells. Asterisk, statistically significant difference between the groups, as determined by ANOVA with correction for multiple comparisons (Sheffe).
Determination of bacterial persistence.
BAL fluid, blood, lungs, and spleens were individually collected from each animal under aseptic conditions. Tissues were homogenized in 2 ml of sterile PBS using a tissue homogenizer (Polytron PT10/35; Brinkman Instruments, Mississauga, Ontario, Canada). Serial 10-fold dilutions of tissue homogenates, BAL fluid, and blood were plated on blood agar and/or B. cepacia selective agar plates. The number of CFU was determined after 72 h of incubation at 37°C. Identity of the bacteria recovered from animals was routinely confirmed by the Clinical Microbiology Department at the Hospital for Sick Children. When mice were infected with isolate BC7, a PCR with gene-specific primers for cblA (48) was used to confirm the identity of the recovered bacteria.
Histopathological evaluation.
Lungs were inflated with air, flushed via the pulmonary artery with PBS followed by 5% paraformaldehyde, and then fixed by immersion in 10% neutral buffered formalin overnight. Tissues were processed and embedded in paraffin, and 5-μm-thick sections were stained with hematoxylin and eosin, periodic acid-Schiff (PAS), Giemsa, or trichrome stain. Slides were blinded for genotype of the mice and the treatment given and scored by a veterinary pathologist using a semiquantitative scale in the range of 0 to 5 (10). Zero on this scale indicated no inflammatory change, while 5 represented severe inflammation with tissue destruction.
Immunofluorescence detection of B. cepacia in lungs and BAL cells.
Lung sections from each mouse were deparaffinized and rehydrated in graded alcohol and water. Sections were heated in 10 mM sodium citrate buffer, pH 6.0, for 90 s at 121°C under pressure for antigen retrieval (52). Sections were washed with water, equilibrated in Tris-buffered saline (10 mM Tris buffer [pH 7.5] containing 0.15 M NaCl) and blocked with 5% normal donkey serum for 2 h at room temperature. Sections were incubated overnight at 4°C with polyclonal antibody R418 (1:1,000 dilution), which recognizes B. cepacia of all genomovars (44), and washed to remove excess antibody, and the bound antibody was detected by anti-rabbit immunoglobulin G conjugated with Cy3 fluorophore (1:250 dilution) (Jackson ImmunoResearch Lab, West Grove, Pa.). Sections were counterstained with Mayer's hematoxylin. When BAL fluid was used, cells were harvested by cytocentrifuge onto a slide, fixed in cold methanol, blocked with 5% normal donkey serum, and treated with anti-B. cepacia antibody R418 as described above.
Measurement of cytokines by ELISA.
The levels of murine tumor necrosis factor alpha (TNF-α), KC/N51, gamma interferon (IFN-γ), and macrophage inflammatory protein 2 (MIP-2) in BAL fluid were measured by a sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems, Minneapolis, Minn.). All samples were analyzed in triplicate in a blinded fashion and compared with known standards.
EMSA.
Nuclear levels of transcription factors NF-κB and CREB were measured by electrophoretic mobility shift assays (EMSA) essentially as described previously (38). In brief, 5 μg of protein from nuclear extracts of whole lungs was preincubated with nonspecific DNA competitor poly(dI-dC) (5 mg; Pharmacia, Piscataway, N.J.) for 10 min at room temperature. The 32P-radiolabeled probe containing the NF-κB3 site of the murine TNF-α gene promoter (mTNFα κB3) was incubated for an additional 20 min at room temperature. DNA-protein complexes were resolved on a 5% nondenaturing polyacrylamide (60:1 cross-link)–Tris-glycine gel, and autoradiographs were prepared by exposure at −70°C using a Kodak X-OMAT film. To demonstrate specificity of the protein-DNA complex, a 125 M excess of unlabeled probe was added to the nuclear extract before adding the radiolabeled probe. The sequence of the plus strand of the oligonucleotide was 5′-CAAACAGGGGGCTTTCCCTCCTC-3′.
Statistical analysis.
Data that are normally distributed are expressed as mean values ± standard errors of the means (SEM). For these data, an unpaired Student t test with Bonferroni correction for multiple comparisons or analysis of variance (ANOVA) with correction for multiple comparisons (Sheffe) was used for statistical comparison of sample means as indicated in the figure legends. Nonparametric analysis using the Wilcoxon rank test was conducted on data that were not normally distributed. For these data, the medians and ranges of the values are illustrated. A P value of <0.05 was considered to be significant.
RESULTS
Initial deposition of B. cepacia in the lungs of Cftr−/− and Cftr+/+ mice.
To establish that intranasal instillation efficiently delivered bacteria to the lungs of experimental mice, 50 μl of PBS containing 107 CFU of B. cepacia isolate BC7 was instilled once intranasally into three Cftr−/− and three Cftr+/+ mice. Mice were sacrificed 2 h later, and the lungs were harvested, homogenized, serially diluted, and plated on B. cepacia isolation agar to determine the number of viable bacteria. There was no observed difference between the three Cftr−/− and the three Cftr+/+ mice in the initial deposition rate, which was consistently over 10% (i.e., >106 CFU/lung). Bacteria were detected by the anti-B. cepacia antibody in all regions of the lungs (data not shown). Control mice that were given only intranasal PBS demonstrated no immunoreactivity with the anti-B. cepacia antibody.
Establishment of chronic pulmonary infection with B. cepacia.
The next objective was to establish a more chronic pulmonary infection with B. cepacia in mice with a method that permitted physiologically relevant adhesive interactions between the bacteria and respiratory epithelium without the need for bacterial entrapment in agar beads. To accomplish this, sterile PBS or B. cepacia (107 bacteria per mouse) was administered on days 0, 3, 6, and 9 and the mice were sacrificed on day 18. Two strains of B. cepacia, a clinical isolate from the highly transmissible genomovar III, ET12 strain and an environmental type strain isolated from onion rot (ATCC 25416; genomovar I), were used to compare their virulence properties in mice. Due to technical difficulties with the administration of the anesthetic, 1 to 3 mice died in each group during the intranasal instillation procedure, and these mice were removed from the study leaving 9 Cftr+/+ and 10 Cftr−/− mice in group 1 (PBS), 10 Cftr+/+ and 16 Cftr−/− mice in group 2 (B. cepacia isolate BC7), and 3 Cftr+/+ and 4 Cftr−/− mice in group 3 (B. cepacia isolate ATCC 25416). Two of the 16 Cftr−/− mice in group 2, which were infected with isolate BC7, died within a few hours after the fourth instillation on day 9, and at necropsy the lungs from both of these mice were characterized grossly by complete consolidation. The bacterial loads in these two mice were 2.3 × 106 and 9.7 × 106 CFU/g of lungs at the time of death. All of the remaining mice were sacrificed on day 18 and were used to generate data presented in this report.
The weights of the Cftr−/− mice (mean = 15.3 ± 0.9 g) were slightly less than those of Cftr+/+ controls (mean = 17.6 ± 0.8 g) at the beginning of the experiment. Both Cftr−/− and Cftr+/+ mice treated with PBS gained weight (mean weight gains for Cftr−/− and Cftr+/+ mice, 2.8 ± 0.2 and 3.1 ± 0.3 g, respectively). In contrast, Cftr−/− mice infected with isolate BC7 gained less weight during the experiment (1.6 ± 0.2 g) than Cftr+/+ controls (2.9 ± 0.4 g). Additionally, the Cftr−/− mice treated with the BC7 strain appeared lethargic compared to wild-type controls. Both the Cftr−/− and Cftr+/+ mice infected with isolate ATCC 25416 gained weight (mean weight gains for Cftr−/− and Cftr+/+ mice, 2.7 ± 0.3 and 3.0 ± 0.4 g, respectively) and appeared well.
Persistence of viable B. cepacia in the lungs.
To determine the extent of bacterial persistence, lungs from each animal were dissected under aseptic conditions, homogenized, and plated on Burkholderia cepacia selective agar (BCSA) or blood agar plates. The lungs of all Cftr−/− animals that had received isolate BC7 harbored from 1 × 104 to 5 × 105 (median of 5.3 × 104) CFU of viable bacteria/g of lungs. In contrast, only three out of the eight Cftr+/+ mice infected with B. cepacia isolate BC7 had viable bacteria in their lungs and the counts were very low (<104 CFU/g). The other five Cftr+/+ mice had no detectable B. cepacia. By the Wilcoxon rank test, the difference between mouse groups was statistically significant (P < 0.001). None of the mice infected with isolate ATCC 25416 yielded positive lung cultures. Thus, even 9 days after the last exposure of mice to bacteria, ET12 strain BC7 was more persistent than environmental type strain ATCC 25416, and Cftr−/− mice were more susceptible to infection than Cftr+/+ mice. No viable B. cepacia organisms were found in the spleen, blood, or BAL fluid of either Cftr−/− or Cftr+/+ mice infected with either BC7 or ATCC 25416.
Instillation of B. cepacia results in bronchopneumonia.
On visual inspection, the lungs of both Cftr+/+ and Cftr−/− mice treated with PBS alone or B. cepacia ATCC 25416 were grossly normal. Lungs of Cftr+/+ mice infected with BC7 appeared healthy, with only occasional areas of atelectasis. In contrast, the lungs from Cftr−/− mice infected with BC7 were found to be friable and showed diffuse areas of consolidation and atelectasis. The lungs of both Cftr−/− and Cftr+/+ mice infected with ATCC 25416 demonstrated minimal evidence of pathology.
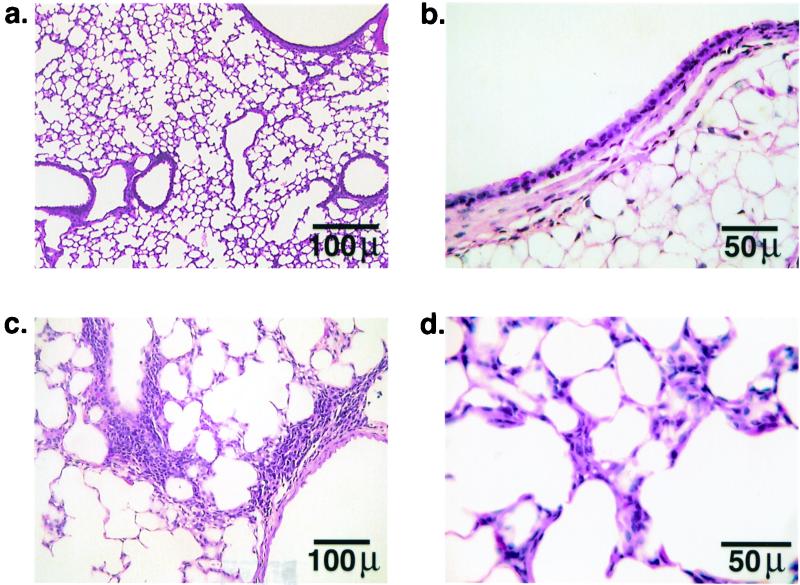
By histological examination, Cftr+/+ mice given PBS alone showed no evidence of inflammation of airway or lung parenchyma (Fig. 1a). Bronchi and bronchioles were lined by a constitutively normal population of ciliated and nonciliated (Clara) columnar epithelial cells with randomly distributed PAS-positive mucus-secreting cells (Fig. 1b). Mice of genotype Cftr−/− treated with PBS (not presented) also exhibited mostly normal lungs, although a few small, scattered areas of peribronchiolar and perivascular mononuclear cell cuffing were noted. Occasionally there were also small random multifocal patches of interstitial thickening characterized by fibroblast hypertrophy and mono- and polymorphonuclear inflammatory cell infiltration (data not shown). Thus lungs from Cftr−/− mice contained minor inflammatory changes in the absence of infection, presumably due to the effects of repeated instillation of PBS. Cftr+/+ mice infected with isolate BC7 or ATCC 25416 were similar to each other in showing mostly normal and functional lungs, as seen in control, PBS-treated mice. However, in a few scattered areas there was peribronchiolar and perivascular inflammation (Fig. 1c). The epithelia of bronchioles were normal, but there were occasional small areas of hypertrophy of resident interstitial cells with associated infiltration by mononuclear inflammatory cells, consistent with pneumonitis (Fig. 1d). Therefore both BC7 and ATCC 25416 appeared to have caused a mild inflammatory response in Cftr+/+ mice.
FIG. 1.
Lung histology of Cftr+/+ mice after repeated exposure to PBS or B. cepacia isolate BC7. (a, c, and d) Hematoxylin- and eosin-stained sections; (b) PAS/Alcian blue-stained section. Representative sections of Cftr+/+ mice treated with PBS showing normal lung histology (a) with few PAS-positive goblet cells in the bronchiolar epithelia (b). Cftr+/+ mice exposed to BC7 demonstrated occasional perivascular and peribronchiolar inflammation (c) and small areas with mild hypertrophy of resident interstitial cells (d).
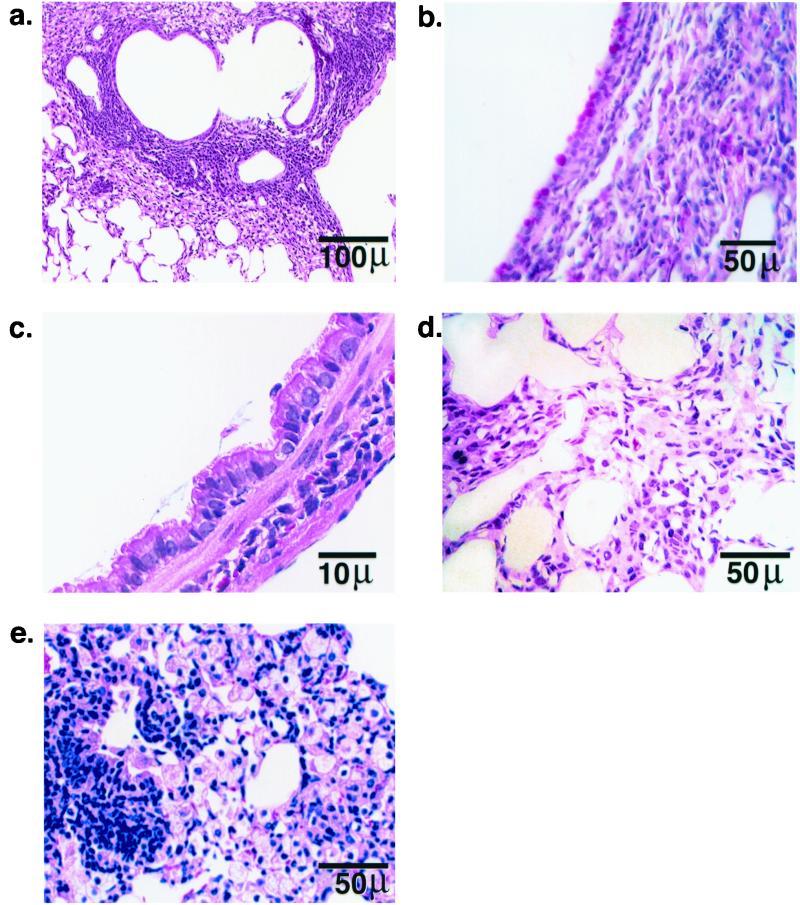
The histological appearance of the lungs of Cftr−/− mice treated with isolate BC7 was considerably different. There were almost no normal areas of lung remaining, and in many regions there was complete pneumonic consolidation (Fig. 2a). Moderate-to-severe infiltration by lymphoplasmacytic cells was observed in most of the peribronchiolar and perivascular spaces. Epithelia of the affected airways exhibited striking Clara cell hyperplasia, prominent PAS-reactive domed apical hypersecretion, and mucus-like material over the luminal surface (Fig. 2b and c). The majority of the parenchyma was characterized by moderate-to-severe infiltration of alveolar septa by a mixed population of inflammatory cells composed predominantly of macrophages with some neutrophils (Fig. 2d). The airspace epithelium showed marked hypertrophy of type II pneumocytes and exudation by foamy vacuolated macrophages (Fig. 2e). None of the sections showed evidence of fibrosis. In contrast, Cftr−/− mice infected with isolate ATCC 25416 did not show any marked pathological changes and resembled Cftr+/+ mice infected with the same isolate (not illustrated). Therefore clinical isolate BC7, but not isolate ATCC 25416, elicited a severe inflammatory response only in Cftr−/− mice. These observations may reflect differences between strains or the importance of bacterial virulence factors.
FIG. 2.
Representative lung sections of Cftr−/− mice after repeated exposure to B. cepacia isolate BC7. (a, c, d) Hematoxylin- and eosin-stained sections; (b) PAS/Alcian blue-stained section; (e) section stained with Giemsa. The histological sections demonstrate hypertrophy of PAS-positive cells in bronchiolar epithelia, hypertrophy of Clara cells, mucus retention in airways, inflamed parenchyma characterized by marked hypertrophy of resident interstitial cells with infiltration by macrophages and neutrophils, and consolidation of alveolar airspaces by an exudation of inflammatory cells and debris.
To quantify these changes, the lung sections were examined by a veterinary pathologist who was blinded to the genotype of the mice and the treatment given. Severity scores for each mouse are given in Table 1. ATCC 25416-infected Cftr+/+ and Cftr−/− mice and BC7-infected Cftr+/+ mice showed very-mild-to-moderate inflammation in the lungs, with the score ranging from 1 to 3. Cftr−/− mice infected with isolate BC7 showed moderate-to-severe bronchiolitis and pneumonia, with the score consistently averaging between 4 and 5. Thus the ET12 strain (isolate BC7), in addition to its greater pulmonary persistence 9 days after the last nasal instillation, also caused much more severe inflammation than did the environmental type strain (ATCC 25416).
TABLE 1.
Histopathological scores of mice infected with B. cepacia
| Genotype and treatment | Scorea for:
|
||||
|---|---|---|---|---|---|
| Bronchiolitis | Respiratory epithelial cell hyperplasia | Mucus retention | Alveolitis and consolidation | Edema and exudation | |
| Cftr+/+ | |||||
| PBS | 0 | 1 | 1 | 0 | 0 |
| 1 | 1 | 0 | 0 | 1 | |
| BC7 | 3 | 2 | 2 | 3 | 3 |
| 2 | 2 | 2 | 2 | 2 | |
| ATCC 25416 | 2 | 2 | 1 | 2 | 1 |
| 3 | 2 | 1 | 2 | 1 | |
| Cftr−/− | |||||
| PBS | 2 | 1 | 2 | 1 | 1 |
| 1 | 1 | 1 | 1 | 1 | |
| BC7 | 5 | 4 | 4 | 5 | 4 |
| 4 | 4 | 3 | 4 | 4 | |
| 5 | 4 | 4 | 5 | 5 | |
| ATCC 25416 | 2 | 2 | 3 | 3 | 3 |
| 3 | 2 | 1 | 1 | 0 | |
Each row represents the scores from a single mouse.
Localization of B. cepacia in the lungs.
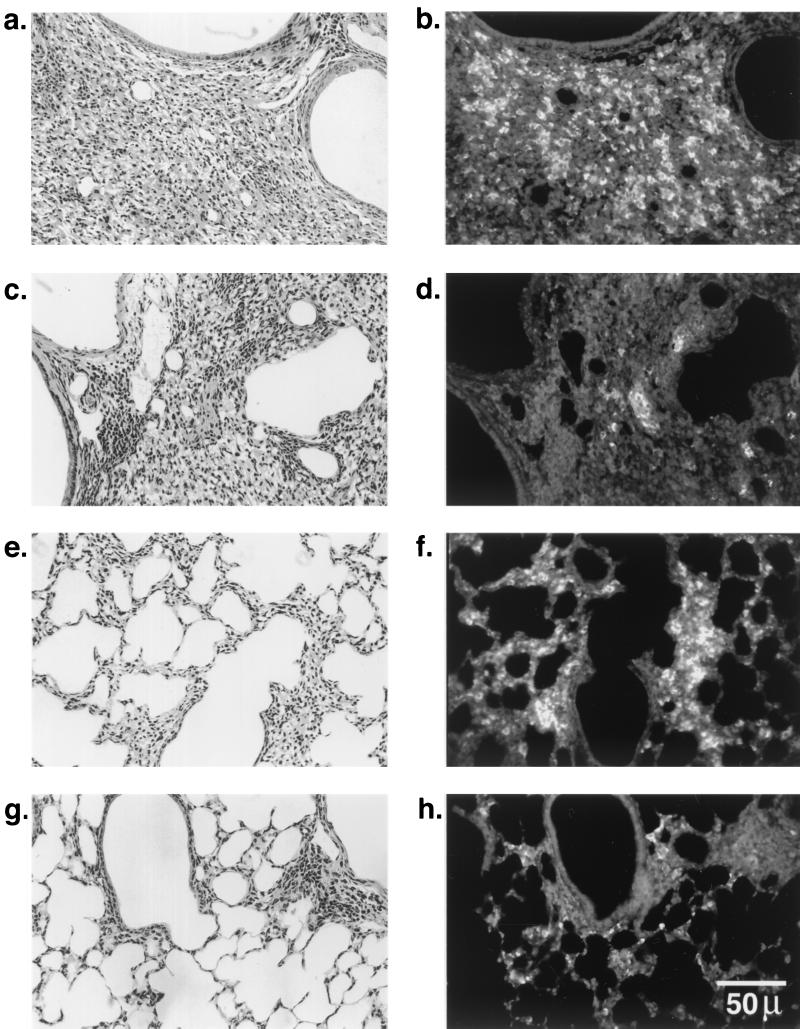
To determine the anatomical site of the persistent bacteria, the lungs were examined by immunofluorescence microscopy using an antibody specific for B. cepacia. For both genotypes, mice that were infected with isolate ATCC 25416 harbored no bacteria in their lungs, despite evidence of mild inflammation. In contrast, BC7-infected Cftr−/− mice demonstrated bacteria in the consolidated and inflamed peribronchiolar and perivascular areas (Fig. 3a and b) and in the thickened alveolar septa and in areas of consolidation (Fig. 3e and f). Cftr+/+ mice also showed bacteria in inflamed peribronchiolar areas (Fig. 3c and d) and thickened alveolar septa (Fig. 3g and h), but the density was much lower that for that for Cftr−/− mice.
FIG. 3.
Immunolocalization of B. cepacia in the lungs of Cftr+/+ and Cftr−/− mice infected with isolate BC7. Paraffin sections (5 μm thick) were deparaffinized, rehydrated, heated in 10 mM sodium citrate buffer, pH 6.0, blocked with 5% normal donkey serum, and incubated overnight at 4°C with anti-B. cepacia antibody (diluted 1:1,000), and the bound antibody was detected by CY-3-conjugated anti-rabbit immunoglobulin G. (b and d) Bacteria in inflamed bronchoalveolar areas of Cftr−/− and Cftr+/+ mouse lungs, respectively; (f and h) bacteria in infiltrated and inflamed parenchyma of Cftr−/− and Cftr+/+ mouse lungs, respectively; (a, c, e, and g) hematoxylin- and eosin-stained sections corresponding to panels b, d, f, and h, respectively.
Characteristics of BAL fluid cell populations.
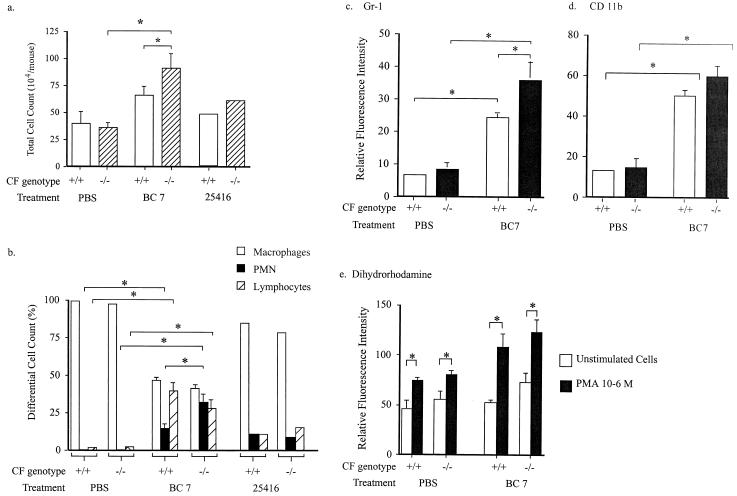
The cellular characteristics of BAL fluid were examined 9 days after the final instillation of bacteria. The total number of cells present in BAL fluid was significantly greater for Cftr−/− than for Cftr+/+ mice treated with isolate BC7, and numbers for both mouse types were greater than those for the corresponding mice treated with PBS or isolate ATCC 25416 (Fig. 4a). In mice treated with PBS, macrophages comprised the majority of alveolar cells in both Cftr−/− and Cftr+/+ mice (Fig. 4b). However, there was a significantly higher proportion of neutrophils (31.8%) in Cftr−/− mice than in Cftr+/+ mice (14.1%; P < 0.02) infected with isolate BC7. The neutrophilia, as assessed by Wright-Giemsa staining of cytospins, corresponded to an increase in surface expression of myeloid differentiation antigen Ly-6G (GR-1) (13) in BAL cells as determined by flow cytometry (Fig. 4c).
FIG. 4.
(a and b) Total and differential cell counts in BAL fluid from Cftr+/+ and Cftr−/− mice treated with PBS or B. cepacia (either BC7 or ATCC 25416). BAL fluid was harvested from each mouse by washing with three 1-ml aliquots of PBS via a cannulated trachea. Cells from 100 μl of BAL fluid were cytocentrifuged onto a slide, fixed with methanol, and stained with a modified Wright-Giemsa stain. Total numbers of cells and different cell types were determined by counting a minimum of 300 cells/slide. (a) Total numbers of cells; (b) differential cell counts. Bars, cell averages from two (ATCC 25416) and seven (PBS and BC7) animals in each group. SEM were calculated only for groups that had more than four animals. Asterisk, statistically significant difference between the groups as determined by ANOVA with correction for multiple comparisons (Sheffe). (c) Surface expression of myeloid marker Ly-6G (Gr-1) on cells recovered in BAL fluid from Cftr+/+ and Cftr−/− mice treated with PBS or B. cepacia isolate BC7. Analysis was done by flow cytometry as outlined in Materials and Methods. Asterisk, statistically significant differences between the groups as determined by ANOVA with correction for multiple comparisons (Sheffe). (d and e) Assessment of neutrophil activation in cells recovered by BAL. Shown is oxidant production, as indicated by surface expression of β2 integrin CD11b (d) and oxidation of dihydrorhodamine (e) and by neutrophils recovered by BAL from Cftr+/+ and Cftr−/− mice treated with PBS or B. cepacia isolate BC7. Analysis was done by flow cytometry as outlined in Materials and Methods. Asterisk, statistically significant differences between the groups as determined by ANOVA with correction for multiple comparisons (Sheffe).
Despite the increased percentage of neutrophils in BAL fluid of Cftr−/− mice, there was no significant difference in the degree of neutrophil activation between Cftr−/− and Cftr+/+ mice infected with B. cepacia, as assessed by determining surface expression of β2 integrin CD11b/CD18 (Fig. 4d) or oxidant production (Fig. 4e). Importantly, these cells were capable of activation when removed from the milieu of the Cftr−/− lung, because exposure of the neutrophils recovered by lavage to phorbol-12-myristate-13-acetate, a potent neutrophil-activating agent, resulted in increased oxidant production (Fig. 4e). Thus, despite the presence of increased numbers of viable bacteria in the lungs and increased numbers of neutrophils in the BAL fluid of Cftr−/− mice, there was no evidence of enhanced neutrophil activation in the lung, which might be anticipated under these circumstances.
Analysis of the cells recovered in BAL fluid also revealed an increased proportion of lymphocytes from both Cftr+/+ and Cftr−/− mice treated with BC7 (Fig. 4b). Further analysis of lymphocyte subtypes demonstrated that the majority (>80%) of these cells were neither T nor B cells.
Association of B. cepacia with the cells of BAL fluid.
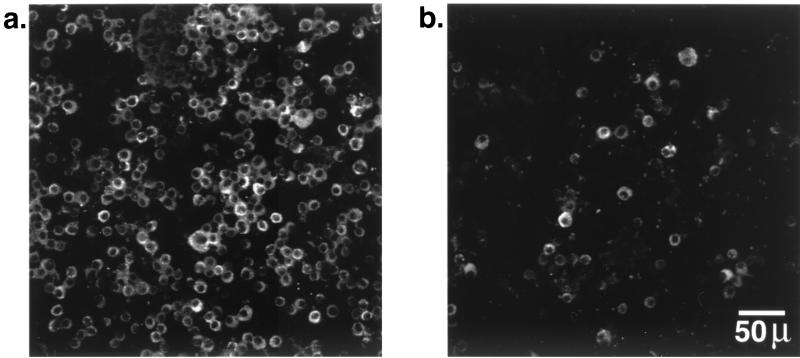
In the cells recovered by BAL lavage from Cftr+/+ mice infected with isolate BC7, a significant number of macrophages were associated with immunoreactive B. cepacia (Fig. 5a). In contrast, in comparable Cftr−/− mice, there were relatively fewer macrophages associated with bacteria (Fig. 5b), suggesting that alveolar macrophages from Cftr+/+ mice may be more efficient in phagocytosing bacteria than those from Cftr−/− mice.
FIG. 5.
Association of B. cepacia with alveolar macrophages in Cftr+/+ and Cftr−/− mice infected with BC7. Cells from 100 μl of BAL fluid were cytocentrifuged onto a slide, fixed in cold methanol, blocked with 5% normal donkey serum, and treated with anti-B. cepacia antibody as described for Fig. 4. Shown is the association of bacteria with alveolar macrophages harvested from the lungs of Cftr+/+ (a) and Cftr−/− (b) mice.
Assessment of macrophage activation.
To assess the extent of macrophage activation, the levels of surface expression of F4/80 and MHC-II molecules (markers of macrophage activation) were determined by flow cytometry. CD11c expression was also measured as a marker for the total macrophage population. PBS control and BC7-infected Cftr−/− and Cftr+/+ mice did not differ in their levels of CD11c surface expression (data not shown). A minor percentage (<15%) of macrophages from all groups expressed F4/80, and no significant differences between Cftr−/− and Cftr+/+ mice infected with isolate BC7 were noted (data not shown). In contrast, macrophages from BC7-infected Cftr+/+ mice expressed 3.5 times more MHC-II than macrophages from similarly treated Cftr−/− mice (Fig. 6). These results indicate that, despite an enhanced pulmonary bacterial load, the alveolar macrophages recovered from Cftr−/− mice demonstrated suboptimal activation compared with those from Cftr+/+ mice.
Transcription factor activation.
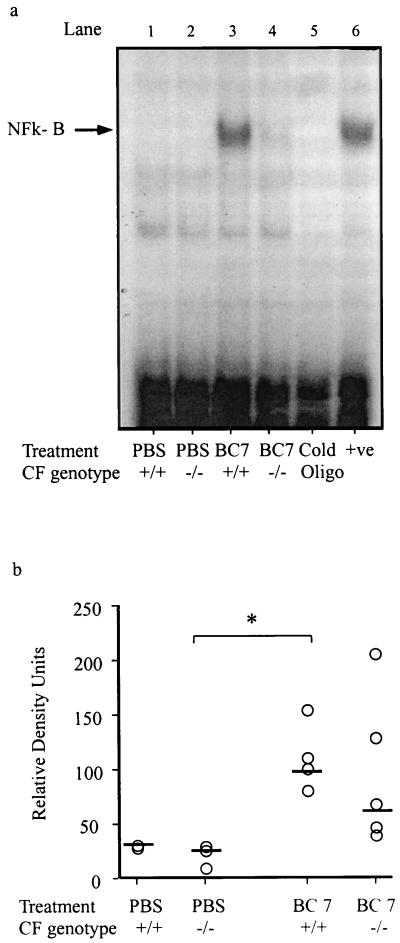
The transcription of many genes involved in acute inflammation (e.g., interleukin-1 [IL-1] and IL-6, TNF-α, and intercellular adhesion molecule 1 genes) is regulated by transcription factors such as CREB and NF-κB (5, 27). To determine if the observed differences in the inflammatory response could be accounted for by variations in this aspect of inflammatory gene regulation, the nuclear translocation of these factors was determined in whole-lung extracts using EMSA. Low levels of nuclear translocation of CREB were present in all groups and did not differ between Cftr+/+ and Cftr−/− mice (data not shown). The pattern of NF-κB translocation was more complex (Fig. 7). In all Cftr+/+ mice treated with isolate BC7, increased levels of nuclear NF-κB were present compared with those in PBS controls. In five of seven Cftr−/− mice, the levels of nuclear NF-κB were very low, while in two Cftr−/− mice high levels of NF-κB, similar to those in wild-type mice, were present.
FIG. 7.
Nuclear translocation of NF-κB. Nuclear levels of transcription factor NF-κB were measured by EMSA as described in Materials and Methods. (a) Representative autoradiogram of EMSA illustrating specific NF-κB binding activity. Lanes 1 and 2, Cftr+/+ and Cftr−/− mice, respectively, treated with PBS; lanes 3 and 4, Cftr+/+ and Cftr−/− mice, respectively, treated with BC7; lane 5, control with an excess of cold oligonucleotides to demonstrate specificity of the probe; lane 6, positive control. (b) Histogram summarizing the results of seven or eight experiments with nuclear extracts from lungs from Cftr+/+ and Cftr−/− mice treated with PBS or B. cepacia isolate BC7.
Lung cytokine levels.
The levels of selected cytokines (TNF-α, IFN-γ, MIP-2, and KC) in BAL fluid were measured by ELISA (Table 2). The levels of TNF-α and IFN-γ were variable and did not differ significantly between the groups. The levels of KC and MIP-2 were elevated in both Cftr+/+ and Cftr−/− mice infected with isolate BC7 compared with those in PBS-treated controls. Notably, the levels of the CXC chemokine KC were elevated greater than twofold in Cftr−/− mice compared to those in Cftr+/+ mice infected with BC7 (P = 0.02 by t test). The elevation in the level of KC in Cftr−/− mice is consistent with the neutrophil predominance in BAL fluid (Fig. 4b).
TABLE 2.
Cytokine levels in BAL fluid
| Cytokine | Mean cytokine concna (pg/ml of BAL fluid) for mice of indicated genotypeb ± SEM after treatment with:
|
|||
|---|---|---|---|---|
| PBS
|
BC7
|
|||
| +/+ | −/− | +/+ | −/− | |
| TNF-α | 4.3 ± 2.3 | 4.2 ± 1.4 | 13.1 ± 5.2 | 10.9 ± 1.3 |
| IFN-γ | 30.5 ± 9.6 | 20.7 ± 30.1 | 50.4 ± 22.5 | 47 ± 5.5 |
| KC | 203 ± 100 | 190 ± 118 | 250 ± 76 | 666 ± 194∗ |
| MIP-2 | 80 ± 40 | 90 ± 24 | 342 ± 66∗∗∗ | 326 ± 60∗∗ |
Statistical analysis was by unpaired t test. ∗, P = 0.02 for Cftr−/− mice versus Cftr+/+ mice treated with BC7 and for Cftr−/− mice treated with BC7 versus Cftr−/− mice treated with PBS; ∗∗, P = 0.02 for Cftr−/− mice versus Cftr−/− mice treated with PBS; ∗∗∗, P = 0.005 for Cftr+/+ mice versus Cftr+/+ mice treated with BC7.
+/+, Cftr+/+; −/−, Cftr−/−. n = four to six mice for each group.
DISCUSSION
In the present study, we have demonstrated that a clinical isolate of B. cepacia, BC7, belonging to the highly transmissible genomovar III, ET12 strain (28, 59), from a case of CF persisted preferentially in the lungs of mice with CF 9 days after the last instillation without the use of bacterium-immobilizing agents. The Cftr−/− mice demonstrated an enhanced pulmonary inflammatory response but one that was apparently less effective than that for Cftr+/+ mice. On the other hand, an environmental type strain of B. cepacia (isolate ATCC 25416), which is predominantly a plant pathogen, did not persist or cause excessive inflammation in either Cftr−/− or Cftr+/+ mice. Taken together, these observations suggest that the absence of functional CFTR in the lung creates a milieu that interferes with macrophage and neutrophil function and thereby impedes the clearance of a clinically relevant strain of B. cepacia from the lung. Additionally, the predilection for enhanced pulmonary inflammation and injury was dependent in part on bacterial virulence. It is noteworthy that only two Cftr−/− mice died of lung infection, indicating that, similar to the human CF patient population, few mice with CF succumb to the initial stages of the infection. Rather, most mice with CF develop a more persistent infection that promotes an excessive and prolonged inflammatory response leading to progressive lung injury, as indicated by the pathological changes observed in infected Cftr−/− mice.
There was a significantly increased proportion of neutrophils present within the airspace of Cftr−/− mice infected with B. cepacia isolate BC7. The mechanism of this enhanced neutrophil infiltration and accumulation could involve enhanced recruitment, diminished clearance, or a combination thereof. Enhanced neutrophil recruitment could be due to the increased numbers of bacteria remaining in the lungs of mice with CF, which would promote the release of chemoattractant molecules such as the CXC chemokine KC from pulmonary macrophages, lymphocytes, and epithelial cells. However, it is also possible that intrinsic differences in the lungs of mice with CF predispose them to the increased recruitment and/or the decreased clearance of neutrophils. Several hypotheses have been proposed to account for the enhanced predilection of lungs of animals with CF for infection and excessive inflammation including (i) increased airway surface liquid absorption leading to depletion of the periciliary liquid layer and diminished mucociliary clearance (34), (ii) failure of airway epithelial cells in animals with CF to ingest bacteria and be sloughed, resulting in enhanced bacterial retention (40), (iii) abnormal surface properties of airway epithelial cells in animals with CF, leading to enhanced mucus secretion, adherence, and retention of bacteria in the lung (42), and (iv) leukocytes that fail to ingest and/or kill bacteria in the milieu of the epithelial lining fluid of animals with CF.
Although the present study did not address these mechanisms directly, our observations suggest that a contributing factor is defective activation and microbicidal function of pulmonary neutrophils and macrophages. Moreover, our model reflects the clinical situation where increased neutrophils are commonly seen in CF patients in association with chronic infection and advanced lung injury (25, 26). Despite their abundance in the lungs of animals with CF, the intrapulmonary neutrophils are apparently less effective at reducing the bacterial burden in CF. Moreover, despite the presence of viable bacteria in the lungs of animals with CF, minimal evidence of enhanced activation, as assessed by oxidant production and surface expression of CD11b, was observed in the Cftr−/− mice. In this regard, it is noteworthy that in vitro exposure of neutrophils to lipopolysaccharide (LPS) from an ET12 strain of B. cepacia (isolate J2315, genomovar III) results in cell activation including NADPH oxidase activity and enhanced surface expression of CD11b (21). One possible explanation for these apparently discrepant observations is that live bacteria (as opposed to purified LPS) release toxins that prevent optimal neutrophil activation. Additionally, it is possible that factors present in the milieu of the lungs of animals with CF prevent complete activation of neutrophils. Because we studied these facets of neutrophil function at only a single time point (9 days) after the establishment of infection, a more comprehensive examination of neutrophil activation is now required.
Macrophages are also important effectors of lung defense as they are both bactericidal and have critical immune system-activating functions, including antigen presentation and orchestration of the pulmonary immune response by virtue of cytokine and chemokine production (65). We observed that, despite a much larger bacterial burden in the lungs of Cftr−/− mice, the levels of MHC-II surface expression, an indicator of macrophage activation, were much lower in these mice than in their wild-type counterparts. There are at least two possible explanations for these observations. One is that specific (but as yet uncharacterized) virulence factors of B. cepacia directly limit MHC expression and other aspects of macrophage activation as a means of evading the innate immune system. Similar evasion of host defenses has been demonstrated for other respiratory pathogens (36). A second possibility is that the unique milieu of the lungs of animals with CF may impede macrophage activation and microbicidal activity.
The localization of B. cepacia in the lungs of Cftr−/− mice to the alveolar septum in our studies is noteworthy and is in contrast to the usual situation in human CF patients, where chronic airway infection predominates (11). However, the localization of B. cepacia to the alveolar septum in human CF patients has recently been reported (44) and may reflect evasion by the virulent bacteria of host defense systems, allowing movement of the bacteria across the epithelial barrier and into the interstitial space. This translocation may presage the development of pneumonia and eventually systemic dissemination with the development of cepacia syndrome, a devastating condition associated with a high mortality (12, 60).
Animal models for CF have proven to be useful in studying the physiological significance of mutations in the Cftr locus that predispose animals to chronic lung infections and subsequent inflammatory lung injury. To date, various models have shown a predilection of the lungs of mice with CF for infection with several opportunistic pathogens including P. aeruginosa, Haemophilus influenzae, S. aureus, and B. cepacia (10, 14, 19). However, to establish bacterial infection in rodent lungs, some investigators have used immobilizing agents such as agar beads (14, 19) which bypass colonization mechanisms such as interactions between bacterial adhesins and respiratory epithelial cells (42, 46, 47, 49, 50). Most studies to date have focused on the host response to infection with P. aeruginosa and S. aureus, while information regarding the host response to B. cepacia is more limited, in part because of the difficulties of establishing a pulmonary infection with this organism. One study, however, compared the acute responses to challenge with aerosolized B. cepacia or S. aureus, without the use of agar beads, in Cftr+/+ and Cftr−/− mice (10). These Cftr−/− mice, generated by targeted insertional mutagenesis of exon 10 (Cftrm1HGU), were repeatedly exposed to aerosolized S. aureus and B. cepacia in separate experiments. It is notable that the B. cepacia used in this study (J2315; genomovar III) was also an ET12 strain. Bacterial clearance was significantly impaired, and the histopathological abnormalities were more pronounced for both organisms in CFTR-deficient mice than in wild-type controls. Mice infected with S. aureus demonstrated bronchitis and bronchiolitis, whereas those infected with B. cepacia demonstrated severe bronchopneumonia. The pathological changes seen with B. cepacia are similar but more severe than those seen in our model. To achieve these results, the experimental protocol involved aerosolization of bacteria daily for an entire month. This represents a much greater bacterial load than that used in our study.
In contrast, when another strain of CFTR-deficient mice (Cftrm1UNC; S489X null mutant) was challenged with S. aureus, no differences in bacterial clearance between CFTR-deficient mice and the controls were demonstrated (55). In this study, no mechanical immobilizing agents were used to increase retention of the bacteria in the lungs. However, when these same Cftrm1UNC mice were given P. aeruginosa enmeshed in agar beads (19), an increased mortality and elevated levels of inflammatory cytokines (TNF-α, MIP-2, and KC) were noted early in the course of infection in mice with CF compared to controls. No differences in either bacterial burden or in the composition of inflammatory cells in BAL were noted, however, between mice with CF and wild-type control mice. In our study, pathological changes were much more severe in the Cftr−/− mice than in the Cftr+/+ mice treated with B. cepacia, a difference that may reflect the repetitive-exposure regimen using intranasal instillation.
In our model, no viable B. cepacia organisms were found in the spleens or blood of either Cftr−/− or Cftr+/+ mice infected with either B. cepacia clinical isolate BC7 (ET12 strain) or the environmental type strain (isolate ATCC 25416). This lack of systemic dissemination is in contrast to the report by Speert and colleagues, who infected IFN-γ knockout mice with B. multivorans (genomovar II) isolated from a patient with chronic granulomatous disease and recovered viable bacteria from the spleen (58). This discrepancy may reflect variations in the route of administration of the bacteria (intranasal versus intraperitoneal), differences in the pathogenicities of the two strains of B. cepacia (ET12 strain of genomovar III versus B. multivorans) used, or the generalized compromise of the immune system in the IFN-γ knockout mice compared to the apparently localized compromise of pulmonary host defenses in the CF knockout mice.
It is not clear why CF predisposes patients to acquisition of B. cepacia. Host lung factors undoubtedly play an important role, and B. cepacia infections usually occur in patients with lung damage due to chronic infection by P. aeruginosa (16, 30). Indeed, human CF patients are frequently infected by several different bacteria, and this polymicrobial infection may alter the host response to B. cepacia (21). In our murine studies, we did not attempt to model this more-complex situation. Another factor to be considered in humans is one of repeated exposure to B. cepacia. Epidemic-like spread of B. cepacia usually occurs in a pattern reflecting close person-to-person social contacts, suggesting that recurrent exposure to the pathogen is important in increasing susceptibility to infection (15, 28, 37, 53). Our murine system with repetitive exposure to B. cepacia was designed to model this environment. It should be noted that even apparently immunocompetent patients can develop B. cepacia pneumonia if host defenses are overwhelmed (1, 29, 64).
Specific bacterial factors are also assumed to play an important role in determining the clinical severity of B. cepacia infections in CF patients. Bacterial virulence factors that have been proposed include surface cable pili, which are expressed by some B. cepacia isolates of the ET12 strain and which mediate adherence to mucins and epithelial cells (46, 47, 49, 50), extracellular proteases (57), lipases (31), siderophores (9), hemolysins (22), LPS (21, 66), and melanin-like pigment (67). The ability of some strains of B. cepacia to enter epithelial cells and macrophages and to replicate intracellularly (3, 33, 43) and their resistance to phagocytic killing (67) are also likely to contribute to virulence.
Infection with B. cepacia remains an important clinical problem for CF patients in many centers, yet this pathogen and the host response to this infection remain poorly understood. In this study, we have reported the development and characterization of a murine model of chronic pneumonia due to B. cepacia that leads to bacterial persistence and chronic pulmonary inflammation consistent with clinical CF disease. Importantly, we provide evidence that there is suboptimal activation of pulmonary neutrophils and macrophages in the milieu of the lungs of animals with CF that may contribute to bacterial persistence. Further studies using this model will allow a better understanding of the host-microbe interaction for B. cepacia and the ineffective, yet exaggerated, inflammatory response that characterizes this disease. Given the lack of adequate therapy for treating B. cepacia infection, this model may also allow for the development and testing of novel therapies to limit bacterial and immune-system-mediated lung damage.
ACKNOWLEDGMENTS
This work was supported by operating grants from the National Institutes of Health (P50 DK49096–06 SCORE) to G. P. Downey and the Canadian Cystic Fibrosis Foundation to J. Forstner. G. P. Downey is the R. Fraser Elliott Chair in Transplantation Research for the Toronto General Hospital of the University Health Network and is the recipient of a Canada Research Chair in respiration from the Canadian Institutes of Health Research.
REFERENCES
- 1.Belchis D A, Simpson E, Colby T. Histopathologic features of Burkholderia cepacia pneumonia in patients without cystic fibrosis. Mod Pathol. 2000;13:369–372. doi: 10.1038/modpathol.3880060. [DOI] [PubMed] [Google Scholar]
- 2.Brisse S, Verduin C M, Milatovic D, Fluit A, Verhoef J, Laevens S, Vandamme P, Tummler B, Verbrugh H A, van Belkum A. Distinguishing species of the Burkholderia cepacia complex and Burkholderia gladioli by automated ribotyping. J Clin Microbiol. 2000;38:1876–1884. doi: 10.1128/jcm.38.5.1876-1884.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns J L, Jonas M, Chil E Y, Clark D K, Berger A, Griffith A. Invasion of respiratory epithelial cells by Burkholderia (Pseudomonas) cepacia. Infect Immun. 1996;64:4054–4059. doi: 10.1128/iai.64.10.4054-4059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantin A. Cystic fibrosis lung inflammation: early, sustained, and severe. Am J Respir Crit Care Med. 1995;151:939–941. doi: 10.1164/ajrccm.151.4.7697269. [DOI] [PubMed] [Google Scholar]
- 5.Chandra G, Cogswell J P, Miller L R, Godlevski M M, Stinnett S W, Noel S L, Kadwell S H, Kost T A, Gray J G. Cyclic AMP signaling pathways are important in IL-1 beta transcriptional regulation. J Immunol. 1995;155:4535–4543. [PubMed] [Google Scholar]
- 6.Cheng P W, Boat T F, Cranfill K, Yankaskas J R, Boucher R C. Increased sulfation of glycoconjugates by cultured nasal epithelial cells from patients with cystic fibrosis. J Clin Investig. 1989;84:68–72. doi: 10.1172/JCI114171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chroneos Z C, Wert S E, Livingston J E, Hassett D J, Whitsett J A. Role of cystic fibrosis transmembrane conductance regulator in pulmonary clearance of Pseudomonas aeruginosa in vivo. J Immunol. 2000;165:3941–3950. doi: 10.4049/jimmunol.165.7.3941. [DOI] [PubMed] [Google Scholar]
- 8.Cressman V L, Hicks E M, Funkhouser W K, Backlund D C, Koller B H. The relationship of chronic mucin secretion to airway disease in normal and CFTR-deficient mice. Am J Respir Cell Mol Biol. 1998;19:853–866. doi: 10.1165/ajrcmb.19.6.3194. [DOI] [PubMed] [Google Scholar]
- 9.Darling P, Chan M, Cox A D, Sokol P A. Siderophore production by cystic fibrosis isolates of Burkholderia cepacia. Infect Immun. 1998;66:874–877. doi: 10.1128/iai.66.2.874-877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson D J, Dorin J R, McLachlan G, Ranaldi V, Lamb D, Doherty C, Govan J, Porteous D J. Lung disease in the cystic fibrosis mouse exposed to bacterial pathogens. Nat Genet. 1995;9:351–357. doi: 10.1038/ng0495-351. [DOI] [PubMed] [Google Scholar]
- 11.Davis P B, editor. Pathophysiology of the lung disease in cystic fibrosis. New York, N.Y: Marcel Dekker; 1993. [Google Scholar]
- 12.Dobbin C J, Soni R, Jelihovsky T, Bye P T P. Cepacia syndrome occurring following prolonged colonization with Burkholderia cepacia. Aust N Z J Med. 2000;30:288–289. doi: 10.1111/j.1445-5994.2000.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 13.Fleming T J, Fleming M L, Malek T R. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 14.Gossellin D, Stevenson M M, Cowley E A, Griesenbach U, Eidelman D H, Boule M, Tam M, Kent G, Skamene E, Tsui L, Radzioch D. Impaired ability of Cftr knockout mice to control lung infection with Pseudomonas aeruginosa. Am J Respir Crit Care Med. 1998;157:1253–1262. doi: 10.1164/ajrccm.157.4.9702081. [DOI] [PubMed] [Google Scholar]
- 15.Govan J R, Brown P H, Maddison J, Doherty C J, Nelson J W, Dodd M, Greening A P, Webb A K. Evidence for transmission of Pseudomonas cepacia by social contact in cystic fibrosis. Lancet. 1993;342:15–19. doi: 10.1016/0140-6736(93)91881-l. [DOI] [PubMed] [Google Scholar]
- 16.Govan J R, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govan J R, Hughes J E, Vandamme P. Burkholderia cepacia: medical, taxonomic and ecological issues. J Med Microbiol. 1996;45:395–407. doi: 10.1099/00222615-45-6-395. [DOI] [PubMed] [Google Scholar]
- 18.Guggino W B. Cystic fibrosis and the salt controversy. Cell. 1999;96:607–610. doi: 10.1016/s0092-8674(00)80570-x. [DOI] [PubMed] [Google Scholar]
- 19.Heeckeren A N, Walenga R, Konstan M W, Bonfield T, Davis P B, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Investig. 1997;100:2810–2815. doi: 10.1172/JCI119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry D A, Campbell M E, LiPuma J J, Speert D P. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J Clin Microbiol. 1997;35:614–619. doi: 10.1128/jcm.35.3.614-619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes J E, Stewart J, Barclay G R, Govan J R. Priming of neutrophil respiratory burst activity by lipopolysaccharide from Burkholderia cepacia. Infect Immun. 1997;65:4281–4287. doi: 10.1128/iai.65.10.4281-4287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchison M L, Poxton I R, Govan J R. Burkholderia cepacia produces a hemolysin that is capable of inducing apoptosis and degranulation of mammalian phagocytes. Infect Immun. 1998;66:2033–2039. doi: 10.1128/iai.66.5.2033-2039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isles A, Maclusky I, Corey M, Gold R, Prober C, Fleming P, Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 24.Kent G, Oliver M, Foskett J K, Frndova H, Durie P, Forstner J, Forstner G G, Riordan J R, Percy D, Buchwald M. Phenotypic abnormalities in long-term surviving cystic fibrosis mice. Pediatr Res. 1996;40:233–241. doi: 10.1203/00006450-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Khan T Z, Wagnener J S, Bost T, Martinez J, Accurso J, Riches D W H. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 26.Konstan M W. Treatment of airway inflammation in cystic fibrosis. Curr Opin Pulm Med. 1996;2:452–456. [PubMed] [Google Scholar]
- 27.Ledebur H C, Parks T P. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J Biol Chem. 1995;270:933–943. doi: 10.1074/jbc.270.2.933. [DOI] [PubMed] [Google Scholar]
- 28.Ledson M J, Gallagher M J, Corkill J E, Hart C A, Walshaw M J. Cross infection between cystic fibrosis patients colonised with Burkholderia cepacia. Thorax. 1998;53:432–436. doi: 10.1136/thx.53.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledson M J, Gallagher M J, Walshaw M J. Chronic Burkholderia cepacia bronchiectasis in a noncystic fibrosis individual. Thorax. 1998;53:430–432. doi: 10.1136/thx.53.5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LiPuma J J. Burkholderia cepacia. Management issues and new insights. Clin Chest Med. 1998;19:473–486. doi: 10.1016/s0272-5231(05)70094-0. [DOI] [PubMed] [Google Scholar]
- 31.Lonon M K, Woods D E, Straus D C. Production of lipase by clinical isolates of Pseudomonas cepacia. J Clin Microbiol. 1988;26:979–984. doi: 10.1128/jcm.26.5.979-984.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahenthiralingam E, Coenye T, Chung J W, Speert D P, Govan J R, Taylor P, Vandamme P. Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J Clin Microbiol. 2000;38:910–913. doi: 10.1128/jcm.38.2.910-913.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin D W, Mohr C D. Invasion and intracellular survival of Burkholderia cepacia. Infect Immun. 2000;68:24–29. doi: 10.1128/iai.68.1.24-29.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsui H, Grubb B R, Tarran R, Randell S H, Gatzy J T, Davis C W, Boucher R C. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 35.McCray P B, Jr, Zabner J, Jia H P, Welsh M J, Thorne P S. Efficient killing of inhaled bacteria in DeltaF508 mice: role of airway surface liquid composition. Am J Physiol. 1999;277:L183–L190. doi: 10.1152/ajplung.1999.277.1.L183. [DOI] [PubMed] [Google Scholar]
- 36.McGuirk P, Mills K H. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur J Immunol. 2000;30:415–422. doi: 10.1002/1521-4141(200002)30:2<415::AID-IMMU415>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 37.Muhdi K, Edenborough F P, Gumery L, O'Hickey S, Smith E G, Smith D L, Stableforth D E. Outcome for patients colonised with Burkholderia cepacia in a Birmingham adult cystic fibrosis clinic and the end of an epidemic. Thorax. 1996;51:374–377. doi: 10.1136/thx.51.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nathens A B, Bitar R, Davreux C, Bujard M, Marshall J C, Dackiw A P, Watson R W, Rotstein O D. Pyrrolidine dithiocarbamate attenuates endotoxin-induced acute lung injury. Am J Respir Cell Mol Biol. 1997;17:608–616. doi: 10.1165/ajrcmb.17.5.2661. [DOI] [PubMed] [Google Scholar]
- 39.Nelson J W, Butler S L, Krief D, Govan R W. Virulence factors of Burkholderia cepacia. FEMS Immunol Med Microbiol. 1944;8:89–98. doi: 10.1111/j.1574-695X.1994.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 40.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaskas J R, Goldberg J B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilewski J M, Frizzell R A. Role of CFTR in airway disease. Physiol Rev. 1999;79(Suppl.):S215–S255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- 42.Saiman L, Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Investig. 1993;92:1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saini L S, Galsworthy S B, John M A, Valvano M A. Intracellular survival of Burkholderia cepacia complex isolates in the presence of macrophage cell activation. Microbiology. 1999;145:3465–3475. doi: 10.1099/00221287-145-12-3465. [DOI] [PubMed] [Google Scholar]
- 44.Sajjan U, Corey M, Humar A, Tullis E, Cutz E, Ackerley C, Forstner J. Immunolocalization of Burkholderia cepacia in the lungs of cystic fibrosis patients. J Med Microbiol. 2001;50:535–546. doi: 10.1099/0022-1317-50-6-535. [DOI] [PubMed] [Google Scholar]
- 45.Sajjan U S, Corey M, Karmali M, Forstner J F. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Investig. 1991;89:648–656. doi: 10.1172/JCI115631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sajjan U S, Forstner J F. Identification of the mucin-binding adhesin of isolated Pseudomonas cepacia from patients with cystic fibrosis. Infect Immun. 1992;60:1434–1440. doi: 10.1128/iai.60.4.1434-1440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sajjan U S, Forstner J F. Role of a 22-kilodalton pilin protein in binding of Pseudomonas cepacia to buccal epithelial cells. Infect Immun. 1993;61:3157–3163. doi: 10.1128/iai.61.8.3157-3163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sajjan U S, Sun L, Goldstein R, Forstner J F. Cable (Cbl) type II pili of cystic fibrosis-associated Burkholderia (Pseudomonas) cepacia: nucleotide sequence of the cblA major subunit pilin gene and novel morphology of the assembled appendage fibers. J Bacteriol. 1995;177:1030–1038. doi: 10.1128/jb.177.4.1030-1038.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sajjan U S, Sylvester F A, Forstner J. Cable-piliated Burkholderia cepacia binds to cytokeratin 13 of epithelial cells. Infect Immun. 2000;68:1787–1795. doi: 10.1128/iai.68.4.1787-1795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sajjan U S, Wu Y, Kent G, Forstner J. Preferential adherence of cable-piliated B. cepacia to respiratory epithelia of CF knockout mice and human CF lung explants. J Med Microbiol. 2000;49:875–885. doi: 10.1099/0022-1317-49-10-875. [DOI] [PubMed] [Google Scholar]
- 51.Schwiebert E M, Erik M, Dale J, Fuller C. Cystic fibrosis: a multiple exocrinopathy caused by dysfunctions in a multifunctional transport protein. Am J Med. 1998;104:576–590. doi: 10.1016/s0002-9343(98)00119-3. [DOI] [PubMed] [Google Scholar]
- 52.Shi S-R, Key M, Kalra K L. Antigen retrieval in formalin-fixed paraffin-embedded tissues: an enhanced method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 53.Smith D L, Gumery L B, Smith E G, Stableforth D E, Kaufmann M E, Pitt T L. Epidemic of Pseudomonas cepacia in an adult cystic fibrosis unit: evidence of person-to-person transmission. J Clin Microbiol. 1993;31:3017–3022. doi: 10.1128/jcm.31.11.3017-3022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 55.Snouwaert J N, Brigman K K, Latour A M, Iraj E, Schwab U, Gilmour M I, Koller B H. A murine model of cystic fibrosis. Am J Respir Crit Care Med. 1995;151:S59–S64. doi: 10.1164/ajrccm/151.3_Pt_2.S59. [DOI] [PubMed] [Google Scholar]
- 56.Snouwaert J N, Brigman K K, Latour A M, Malouf N N, Boucher R C, Smithies O, Koller B H. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 57.Sokol P A, Kooi C, Hodges R S, Cachia P, Woods D E. Immunization with a Pseudomonas aeruginosa elastase peptide reduces severity of experimental lung infections due to P. aeruginosa or Burkholderia cepacia. J Infect Dis. 2000;181:1682–1692. doi: 10.1086/315470. [DOI] [PubMed] [Google Scholar]
- 58.Speert D P, Steen B, Halsey K, Kwan E. A murine model for infection with Burkholderia cepacia with sustained persistence in the spleen. Infect Immun. 1999;67:4027–4032. doi: 10.1128/iai.67.8.4027-4032.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun L, Jiang R Z, Steinbach S, Holmes A, Campanelli C, Forstner J, Sajjan U, Tan Y, Riley M, Goldstein R. The emergence of a highly transmissible lineage of cbl+ Pseudomonas (Burkholderia) cepacia causing CF centre epidemics in North America and Britain. Nat Med. 1995;1:661–666. doi: 10.1038/nm0795-661. [DOI] [PubMed] [Google Scholar]
- 60.Tablan O C, Chobra T L, Schidlow D V, White J W, Hardy K A, Gilligan P H, Morgan W M, Chow L A, Martone W J, Jarvis W R. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr. 1985;107:382–387. doi: 10.1016/s0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]
- 61.Travis S M, Conway B A, Zabner J, Smith J J, Anderson N N, Singh P K, Greenberg E P, Welsh M J. Activity of abundant antimicrobials of the human airway. Am J Respir Cell Mol Biol. 1999;20:872–879. doi: 10.1165/ajrcmb.20.5.3572. [DOI] [PubMed] [Google Scholar]
- 62.Vandamme P, Holmes B, Vancanneyt M, Coenye T, Hoste B, Coopman R, Revets H, Lauwers S, Gillis M, Kersters K, Govan J R. Occurrence of multiple genomovars of Burkholderia cepacia in cystic fibrosis patients and proposal of Burkholderia multivorans sp. Int J Syst Bacteriol. 1997;47:1188–1200. doi: 10.1099/00207713-47-4-1188. [DOI] [PubMed] [Google Scholar]
- 63.Waddell T K, Fialkow L, Chan C K, Kishimoto T K, Downey G P. Potentiation of the oxidative burst of human neutrophils. A signaling role for L-selectin. J Biol Chem. 1994;269:18485–18491. [PubMed] [Google Scholar]
- 64.Waterer G W, Jones C B, Wunderink R G. Bacteremic community-acquired pneumonia in an immunocompetent adult due to Burkholderia cepacia. Chest. 1999;116:1842–1843. doi: 10.1378/chest.116.6.1842. [DOI] [PubMed] [Google Scholar]
- 65.Zhang P, Summer W R, Bagby G J, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]
- 66.Zughaier S M, Ryley H C, Jackson S K. Lipopolysaccharide (LPS) from Burkholderia cepacia is more active than LPS from Pseudomonas aeruginosa and Stenotrophomonas maltophilia in stimulating tumor necrosis factor alpha from human monocytes. Infect Immun. 1999;67:1505–1507. doi: 10.1128/iai.67.3.1505-1507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zughaier S M, Ryley H C, Jackson S K. A melanin pigment purified from an epidemic strain of Burkholderia cepacia attenuates monocyte respiratory burst activity by scavenging superoxide anion. Infect Immun. 1999;67:908–913. doi: 10.1128/iai.67.2.908-913.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]