Abstract
Wild-type Legionella pneumophila grows in human macrophages within a replicative phagosome, avoiding lysosomal fusion, while nonreplicative mutants are killed in lysosomes. Wortmannin, a phosphatidylinositol 3-kinase (PI3K) inhibitor, blocks phagocytosis of an avirulent mutant, but not of wild-type L. pneumophila, without affecting membrane ruffling and actin polymerization. These results show that wild-type and mutant Legionella strains use different entry pathways. They suggest that PI3Ks are involved in phagocytosis of an avirulent L. pneumophila mutant and regulate the ability of microorganisms to generate a replicative phagosome.
Legionella pneumophila targets specifically alveolar macrophages in vivo and kills human monocytic cell lines such as U937 (23) and HL-60 in vitro (18). It is internalized by monocytes through coiling phagocytosis (14). Bacteria multiply in replicative phagosomes (10) that fail to acidify completely (15) and to acquire markers of the late endosomal-lysosomal pathway (7, 12). Instead, phagosomes containing L. pneumophila associate sequentially with small vesicles, mitochondria, and ribosomes (13), appearing to interact with the endoplasmic reticulum (29). Coiling phagocytosis is not essential for L. pneumophila virulence, since it occurs with heat-killed bacteria and with an avirulent mutant, 25D (16).
The ability to form a replicative phagosome appears to be essential for intracellular multiplication and macrophage killing (17). Characterization of avirulent mutants that have lost these properties led to the description of two sets of genes, the icm locus (intracellular multiplication) (6, 19, 26, 28) and the dot locus (defect in organelle trafficking) (2, 4, 5). Avirulent mutant 25D was complemented by insertion of a plasmid carrying the locus icm, which restored the bacterial ability to grow in and kill macrophages (19). 25D was used in our study to compare the behavior of wild-type and nonreplicative L. pneumophila, since its interactions with monocytes were widely characterized earlier (16, 30).
Phagocytosis of L. pneumophila occurs through binding of complement receptors CR1 and CR3 (22) and other unknown receptors (9, 24). CR3-mediated entry of L. pneumophila in human monocytes activates tyrosine kinases and protein kinase C and induces actin polymerization at the site of infection, regardless of the virulence state of the bacteria (8). In contrast, interaction of wild-type, but not avirulent mutant, L. pneumophila with macrophages induces phosphorylation of a 76-kDa protein, suggesting that this signal might be relevant for pathogenicity (31). In this study, we further analyzed the signal transduction pathway occurring at the very early stages of L. pneumophila phagocytosis by U937 cells. Since phosphatidylinositol 3-kinases (PI3K) have been implicated in phagocytosis through Fc receptors (20) and macropinocytosis (1), we evaluated their role in L. pneumophila ingestion using wortmannin as an inhibitor of PI3K (3).
Parameters such as phagocytosis efficiency, ruffling, and actin polymerization were studied during infection with virulent wild-type JR32 and avirulent mutant 25D, both derived from Philadelphia-1 L. pneumophila (16, 26). As a comparison with conventional phagocytosis, we also determined the behavior of a serum-resistant strain of Escherichia coli Bi 1:61–42 serotype O9:K29H− (11). Bacteria were grown at 37°C for 2 days on ABCYE agar plates for L. pneumophila strains as described elsewhere (30) or on Luria-Bertani for Escherichia coli. Bacteria were suspended and diluted in RPMI at 37°C at 2 × 108 CFU/ml to infect the cells at a multiplicity of infection of 300:1 to 600:1 for L. pneumophila and 100:1 to 300:1 for E. coli. Variation of the multiplicity of infection on different days of experiments did not affect the number of bacteria bound to or internalized by the cells (data not shown). When required, bacteria were incubated for 30 min at 37°C in RPMI containing 10% nonimmune human serum from healthy volunteers as described elsewhere (14) to allow opsonization by serum components. U937 cells were plated at a concentration of 2 × 106 cell/ml and differentiated into adherent macrophage-like cells by incubation for 2 days with 10 ng of phorbol 12-myristate 13-acetate (Sigma Chemical Co., St. Louis, Mo.) per ml. For phagocytosis and binding assays, cells were plated in six-well culture dishes. Experiments were done in triplicate and repeated on different days three times for virulent L. pneumophila and E. coli, twice for opsonized avirulent L. pneumophila, and once for unopsonized avirulent L. pneumophila. For microscopy experiments, cells were plated on coverslip chambers as previously described (27). Before infection, cells were washed with RPMI at 37°C and incubated for 30 min with or without 100 nM wortmannin (Sigma) in RPMI in the absence of serum.
Effect of wortmannin on phagocytosis.
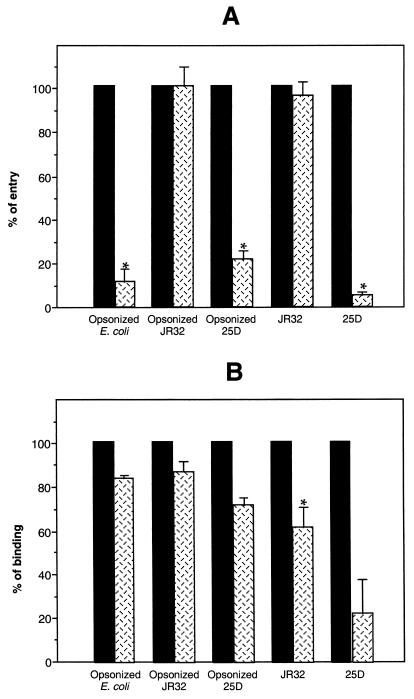
Opsonized or nonopsonized bacteria were layered on U937 cells by centrifugation at 800 × g for 10 min. Infected cells were washed three times with RPMI at 37°C to remove unbound bacteria. For binding assays, cells were lysed immediately by the addition of 1 ml of water at 4°C, followed by 5 min of incubation at −20°C. For phagocytosis assays, infected cells were incubated for an additional 2 h at 37°C in RPMI containing 100 μg of gentamicin per ml (to kill extracellular bacteria) and 100 nM wortmannin (if present during the initial infection). After three washes with RPMI at 37°C, cells were lysed as described above. Cell lysates were homogenized by vigorous pipetting, diluted, and plated on appropriate agar plates. Bacterial counts were determined after the plates had been incubated for 2 to 3 days at 37°C. As shown in Fig. 1A, ingestion of opsonized E. coli by conventional phagocytosis was strongly inhibited by wortmannin (7 × 107 versus 8.5 × 106 CFU/well). In contrast, phagocytosis of opsonized wild-type L. pneumophila JR32 was not affected by the drug (1.8 × 107 CFU/well). As with E. coli, phagocytosis of opsonized avirulent variant 25D was efficiently inhibited by wortmannin (2.7 × 107 versus 6.2 × 106 CFU/well). Nonopsonized wild-type JR32 was similarly internalized by U937 cells whether treated with wortmannin or not (1.7 × 107 CFU/well), whereas the entry of nonopsonized avirulent mutant 25D was strongly inhibited by wortmannin (1.9 × 105 versus 1.2 × 104 CFU/well). It should be noted that in the absence of wortmannin, phagocytosis of all strains (opsonized or nonopsonized) was to a similar extent, with the exception of nonopsonized 25D, for which internalization was less efficient but similarly affected by wortmannin (approximately a 1-log difference). However, this difference might result only from day-to-day variation, since experiments with different bacterial preparations were not always performed on the same day. All experiments to test for differences with or without wortmannin were performed in parallel. We determined that bacterial viability in medium containing 100 nM wortmannin was similar to that in drug-free medium and that 0.1 to 0.2% of the initial inoculum survived treatment with 100 μg of gentamicin per ml (data not shown).
FIG. 1.
Effect of wortmannin on bacterial phagocytosis and binding. Differentiated U937 cells were treated with 100 nM wortmannin ( ) or not treated (■) and infected with opsonized or nonopsonized L. pneumophila wild-type (JR32) or avirulent mutant (25D) or with opsonized E. coli. (A) Phagocytosis by wortmannin-treated cells was expressed as a percentage of phagocytosis by untreated cells. (B) Bacterial binding to wortmannin-treated cells was expressed as a percentage of bacterial binding to untreated cells. Values of phagocytosis and binding obtained in the presence of wortmannin were expressed as a percentage of the values obtained in absence of treatment (defined as 100%). Error bars represent standard errors. Asterisks represent values for treated cells statistically different from values for untreated cells with a value of P < 0.05 as determined with the Student t test.
) or not treated (■) and infected with opsonized or nonopsonized L. pneumophila wild-type (JR32) or avirulent mutant (25D) or with opsonized E. coli. (A) Phagocytosis by wortmannin-treated cells was expressed as a percentage of phagocytosis by untreated cells. (B) Bacterial binding to wortmannin-treated cells was expressed as a percentage of bacterial binding to untreated cells. Values of phagocytosis and binding obtained in the presence of wortmannin were expressed as a percentage of the values obtained in absence of treatment (defined as 100%). Error bars represent standard errors. Asterisks represent values for treated cells statistically different from values for untreated cells with a value of P < 0.05 as determined with the Student t test.
Since phagocytosis is dependent on the ability of the microorganism to bind to the cell surface, we also studied the effect of wortmannin on bacterial attachment to the cells. As shown in Fig. 1B, the binding of opsonized bacteria was not affected significantly by wortmannin (2.1 × 108 versus 1.8 × 108 CFU/well for wild-type JR32; 1.4 × 108 versus 9.6 × 107 CFU/well for avirulent mutant 25D; 8 × 107 versus 6.6 × 107 CFU/well for E. coli). Thus, the decrease in uptake of opsonized mutant 25D and opsonized E. coli was not due to an inability to bind wortmannin-treated cells. However, the drug appeared to decrease the binding ability of nonopsonized wild-type (5.8 × 107 versus 3.5 × 107 CFU/well) and mutant (3 × 108 versus 6.7 × 107 CFU/well) L. pneumophila. In the case of nonopsonized wild-type JR32, the decrease in binding is moderate and does not have a dramatic effect on phagocytosis (Fig. 1A). For nonopsonized mutant 25D, there was a large variation in the amount of binding, so the difference in binding is not statistically significant. The reduced binding may contribute to the reduction of phagocytosis of nonopsonized 25D shown in Fig. 1B, but phagocytosis of opsonized avirulent mutant 25D is decreased by wortmannin with no change in binding.
Attachment of opsonized bacteria to the host complement receptors CR1 and CR3 can be inhibited by antibodies specific for complement receptors (22). However, the same antibodies do not inhibit binding of nonopsonized bacteria (9, 24), suggesting the involvement of other unidentified receptors. The fact that entry of both opsonized and nonopsonized bacteria was similarly affected by wortmannin indicates that complement receptor-mediated phagocytosis may not be the discriminating factor necessary to generate a replicative phagosome and supports the idea of another receptor specifically involved in L. pneumophila entry.
In conclusion, we found that phagocytosis of wild-type L. pneumophila JR32, whether opsonized or not, was unaffected by treatment with wortmannin, whereas the phagocytosis of the avirulent mutant 25D, whether opsonized or not, and of opsonized E. coli was strongly inhibited by the PI3K inhibitor. These results suggest that the mechanisms leading to wild-type or mutant phagocytosis are different and that PI3K may play a role in bacterial uptake. These findings indicate that the icm genes of L. pneumophila encode some product(s) that specifically affects cellular signaling through PI3K. We suggest that PI3K is required for conventional phagocytosis and might be inactivated or bypassed by wild-type L. pneumophila, allowing the establishment of a specific intracellular niche.
Ruffling and actin polymerization induced by bacteria.
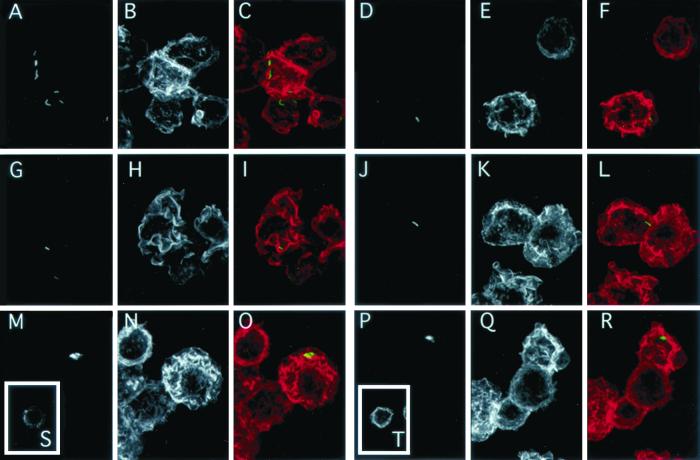
To determine if the differences in the internalization of wild-type and mutant L. pneumophila were due to variations in the ability to generate an appropriate cell membrane response, we first examined changes in morphology by differential interference contrast microscopy on live cells. Bacterial suspensions were directly applied to the cells at 37°C. After 2 to 4 min, the surfaces of differentiated U937 cells infected with either wild-type JR32 or avirulent mutant 25D exhibited extensive ruffling and pseudopod formation which were not affected by wortmannin treatment (data not shown). These results suggest that PI3K are not essential for these activities and are consistent with previous data showing that ruffling of macrophages was not affected by PI3K inhibitors (1). The fact that the kinetics and extent of the ruffling induced by wild-type and avirulent mutant L. pneumophila appeared to be very similar suggests that ruffling does not determine the intracellular pathway of L. pneumophila. It also suggests that the products of icm genes, which are important to target bacteria to a replicative phagosome, are not essential for the induction of ruffling. In the case of L. pneumophila, it is possible that other factors, such as lipopolysaccharide, which has been implicated in stimulation of ruffling (21), may be responsible for membrane activity in response to infection. We also evaluated by confocal microscopy the ability of L. pneumophila wild-type JR32 and avirulent mutant 25D to induce actin polymerization and the effect of wortmannin on these features. After 3 min of infection with nonopsonized bacteria, cells were quickly washed with RPMI at 37°C and fixed for 2.5 min at room temperature with 6.6% paraformaldehyde–0.5% glutaraldehyde diluted in medium 1 (150 mM NaCl, 20 mM Hepes, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2 [pH 7.4]). After fixation, all treatments were performed at room temperature. Cells were permeabilized with 250 μg of saponin per ml in medium 1 containing 20 mM glycine and 10% calf serum. Bacteria were labeled for 45 min using a fluorescein-conjugated rabbit anti-L. pneumophila antibody (m-Tech, Atlanta, Ga.) diluted 1/100 in medium 1. Cells were washed three times with medium 1, and actin was labeled for 45 min using rhodamine-conjugated phalloidin (Molecular Probes Inc., Eugene, Oreg.) diluted at 0.3 μg/ml in medium 1. Figure 2 shows that the contours of noninfected U937 cells were flat and displayed little actin polymerization (Fig. 2S). This morphology was not modified by wortmannin treatment (Fig. 2T). In contrast, cells infected with wild-type JR32 exhibited an intense network of membrane ruffles and projections which contained a high concentration of F-actin, as shown by phalloidin staining (Fig. 2A to C). Similar morphological modifications and actin polymerization were observed in response to infection with avirulent mutant 25D (Fig. 2G to 2I). Membrane movement and actin polymerization induced by JR32 or 25D were not affected by wortmannin (Fig. 2D to F and 2J to L), indicating that the products of icm genes are not required for induction of actin polymerization.
FIG. 2.
Induction of actin polymerization by L. pneumophila. Differentiated U937 cells were treated with 100 nM wortmannin (D to F, J to L, P to R, and T) or not treated (A to C, G to I, M to O, and S) and infected with L. pneumophila wild-type JR32 (A to F), avirulent mutant 25D (G and L), or heat-killed JR32 (M to R) or not infected (S and T). Four minutes after the addition of bacteria, the cells were fixed and permeabilized. Bacteria were labeled with a fluorescein-conjugated rabbit anti-L. pneumophila antibody (green). Actin was detected using rhodamine-phalloidin (red). Cells were observed with a confocal scanning laser microscope (model MRC600; Bio-Rad Microscience, Cambridge, Mass.) using an argon laser (514-nm excitation) and a 63× objective (numerical aperture, 1.4). Images were acquired as a succession of focal planes (0.6-μm step size) and were processed with Metamorph and Photoshop (Adobe) software. Background fluorescence intensity was determined as the average intensity of a blank field and was subtracted from original images. Confocal images are presented as a summation projection of these corrected images. Composite images (C, F, I, L, O, and R) were produced by overlaying the images of L. pneumophila (A, D, G, J, M, and P) and actin images (B, H, N, I, K, Q, S, and T respectively). Bar, 10 μm.
To determine if bacterial viability was required to induce actin polymerization, we incubated cells with heat-killed JR32 (boiled for 20 min). As with live bacteria, dead L. pneumophila also induced subcortical actin polymerization that followed the same kinetics (Fig. 2M to O), implying that the product(s) required for these effects is heat stable and does not need to be newly synthesized. The actin rearrangement was not inhibited by wortmannin (Fig. 2P to R), suggesting that PI3K activity is not essential for actin polymerization in response to heat-killed L. pneumophila. The lack of effect on actin polymerization is consistent with the lack of effect on ruffling and pseudopod extension. We noted that the profile of actin labeling obtained in our work differed from those observed in another recent study, in which actin polymerization occurred at discrete spots, concentrated at L. pneumophila infection sites (8). We suggest that this variation may be due to the different infection times after which the cells were fixed and observed (3 min in our experiments versus 15 min for their experiments). Our data on actin polymerization are in agreement with the results concerning phagocytosis of immunoglobulin G-coated particles via Fc receptors for which the early steps of phagosome formation were not affected by PI3K inhibitors (1). This work showed that pseudopods initiated and started engulfing immunoglobulin G-coated erythrocytes but never achieved the fusion event necessary for phagosome completion (1).
The kinetics observed in our experiments also suggest that a pivotal step leading to the specific phagosome formation by L. pneumophila occurs before its completion. Both the wild-type L. pneumophila and the avirulent mutant were able to bind to cells and to generate the membrane activity necessary to initiate the formation of a phagosome even after the wortmannin treatment. However, despite these similarities, the avirulent mutant 25D, lacking the icm locus, does not complete the entry process in wortmannin-treated cells and remains extracellular. These results suggest that a pivotal step in L. pneumophila survival and intracellular multiplication is an early event, occurring after binding but before internalization. They also suggest that this step requires one or more products encoded by the icm or dot genes. The establishment of a modulation very early during infection is supported by recent data from Wiater et al. showing that the intracellular fate of L. pneumophila is determined by the icm or dot gene products and that, as early as 30 min after infection, phagosomes containing avirulent mutants are fully fused with lysosomes (30). Moreover, it has been shown that expression of the dotA gene product is required before macrophage internalization for efficient formation of the replicative phagosome (25). All these results suggest that the phagosomal maturation processes that appear to be required for intracellular multiplication of microorganisms may be generated by specific interactions occurring at the onset of infection and may be determined by the pathogen's ability to interact with specific molecules at the host cell surface.
Acknowledgments
We thank Lawrence A. Wiater, Laura Hales, Philip Leopold, and Xiaohui Zha for helpful discussion and enthusiastic support during this work.
This work was supported by NIH grant DK27083 (F.R.M.).
REFERENCES
- 1.Akari N, Johnson M T, Swanson J A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews E L, Vogel J P, Isberg R R. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun. 1998;66:950–958. doi: 10.1128/iai.66.3.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcaro A, Wymann M P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-triphosphate in neutrophil responses. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger K H, Isberg R R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 5.Berger K H, Merriam J J, Isberg R R. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Mol Microbiol. 1994;14:809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 6.Brand B D, Sadosky A B, Shuman H A. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol Microbiol. 1994;14:797–808. doi: 10.1111/j.1365-2958.1994.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 7.Clemens D L, Horwitz M A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coxon P Y, Summersgill J T, Ramirez J A, Miller R D. Signal transduction during Legionella pneumophila entry into human monocytes. Infect Immun. 1998;66:2905–2913. doi: 10.1128/iai.66.6.2905-2913.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson F C, III, Tzianabos A O, Rodgers F G. Adherence of Legionella pneumophila to U937 cells, guinea-pig alveolar macrophages, and MRC-5 cells by a novel, complement-independent binding mechanism. Can J Microbiol. 1994;40:865–872. doi: 10.1139/m94-137. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz M A, Silverstein S C. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Investig. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz M A, Silverstein S C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Investig. 1980;65:82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horwitz M A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horwitz M A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwitz M A. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz M A, Maxfield F R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984;99:1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz M A. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987;166:1310–1328. doi: 10.1084/jem.166.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isberg R R, Rankin S, Roy C R, Swanson M S, Berger K H. Legionella pneumophila: factors involved in the route and response to an intracellular niche. Infect Agents Dis. 1993;2:220–223. [PubMed] [Google Scholar]
- 18.Marra A, Horwitz M A, Shuman H A. The HL-60 model for the interaction of human macrophages with the Legionnaires' disease bacterium. J Immunol. 1990;144:2738–2744. [PubMed] [Google Scholar]
- 19.Marra A, Blander S J, Horwitz M A, Shuman H A. Identification of a region of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci USA. 1992;89:9607–9611. doi: 10.1073/pnas.89.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ninomiya N, Hazeki K, Fukui Y, Seya T, Okada T, Hazeki O, Ui M. Involvement of phosphatidylinositol 3-kinase in Fcγ receptor signaling. J Biol Chem. 1994;269:22732–22737. [PubMed] [Google Scholar]
- 21.Paulsen D B, Mosier D A, Clinkenbeard K D, Confer A W. Direct effects of Pasteurella haemolytica lipopolysaccharide on bovine pulmonary endothelial cells in vitro. Am J Vet Res. 1989;50:1633–1637. [PubMed] [Google Scholar]
- 22.Payne N R, Horwitz M A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987;166:1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearlman E, Jiwa A H, Engleberg N C, Eiseinstein B I. Growth of Legionella pneumophila in a human macrophage-like (U937) cell line. Microb Pathog. 1988;5:87–95. doi: 10.1016/0882-4010(88)90011-3. [DOI] [PubMed] [Google Scholar]
- 24.Rodgers F G, Gibson F C., III Opsonin-independent adherence and intracellular development of Legionella pneumophila within U-937 cells. Can J Microbiol. 1993;39:718–722. doi: 10.1139/m93-103. [DOI] [PubMed] [Google Scholar]
- 25.Roy C R, Berger K H, Isberg R R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 26.Sadosky A B, Wiater L A, Shuman H A. Identification of Legionella pneumophila genes required for growth within and killing of macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salzman N H, Maxfield F R. Fusion accessibility of endocytic compartments along the recycling and lysosomal endocytic pathway in intact cells. J Cell Biol. 1989;109:2097–2104. doi: 10.1083/jcb.109.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segal G, Shuman H A. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect Immun. 1997;65:5057–5066. doi: 10.1128/iai.65.12.5057-5066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiater L A, Dunn K, Maxfield F R, Shuman H A. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect Immun. 1998;66:4450–4460. doi: 10.1128/iai.66.9.4450-4460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Klein T W, Shinomiya B, Nakano M, Friedman H. Infection of macrophages with Legionella pneumophila induces phosphorylation of a 76-kilodalton protein. Infect Immun. 1992;60:3452–3455. doi: 10.1128/iai.60.8.3452-3455.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]