Abstract
The goal of this review is to provide an overview of the current findings on the major carotenoids and their content in pumpkin products and by-products. The content of total carotenoids and the composition of carotenoids in pumpkins depend mainly on the species and cultivar, pedoclimatic conditions, the part of the plant (pulp, peel or seed), extraction procedures and the type of solvent used for extraction. The major carotenoids identified in pumpkins were β-carotene, α-carotene, lutein and zeaxanthin. β-Carotene is the major carotenoid in most pumpkin species. The number and content of total carotenoids are higher when minor carotenoids and ester forms are considered. The use of carotenoids in the development of functional foods has been the topic of many versatile studies in recent years, as they add significant value to foods associated with numerous health benefits. In view of this, pumpkin and pumpkin by-products can serve as a valuable source of carotenoids.
Keywords: carotenoids, seed, peel, pulp, individual carotenoids, functional value
1. Introduction
The pumpkin (Cucurbita L.) is a squash fruit vegetable belonging to the Cucurbitaceae family, also known as the gourd family, with 130 genera and 800 species. Cucurbita is native to Latin America and has been cultivated in Europe for over 500 years. The genus Cucurbita includes five domesticated species (Cucurbita argyrosperma, Cucurbita ficifolia, Cucurbita maxima, Cucurbita moschata and Cucurbita pepo), of which Cucurbita moschata, Cucurbita maxima and Cucurbita pepo are the most economically important species cultivated worldwide [1] and are widely used in the food industry for the commercial production of pumpkin pie, flour, seed oil, seeds as snacks, bread, cookies, desserts, cereals, ice cream, pancakes, puddings, pumpkin butter, salads, soups and stuffings [2]. In addition to the different pumpkin species, there are also numerous varieties that differ from the same species in chemical composition, color, shape and, due to agroclimatic conditions, agrotechnical measures. However, with some differences between varieties, all parts of the pumpkin plant, i.e., fruits, flowers, leaves, roots, stems and seeds, are edible.
Currently, the world production of pumpkin (including squash and gourds) amounts to approximately 27 million tons, of which China is the main producer with 5.5 million tons. In Europe, the current production of pumpkin for consumption is more than 4 million tons [3]. The huge global production of pumpkin and its consumption, e.g., in cooked, baked, and processed forms, is probably related to the growing interest of consumers in consuming a wide range of nutrients and phytochemicals through an adequate and balanced diet. As a result of this great use of pumpkin, its by-products are produced. In addition, it is important to emphasize that the cultivation and processing of pumpkin seeds produce by-products in the form of peel and pulp. On the other hand, when pumpkin is used for cooking, the peel and seeds are by-products. A good example of the problem of pumpkin by-products is the production of pumpkin seeds. For example, the production of pumpkin seeds from C. maxima produces about 49 times the amount of by-products (pulp and peel) than the number of seeds produced [4]. In general, the processing of pumpkin produces about 72–76% pulp, 2.6–16% peels and 3.1–4.4% seeds [5].
The by-product fractions are underutilized and are usually used for animal feed enrichment [6], but due to the phytochemical densities, these fractions have economic potential and could be explored for various other applications, including those with therapeutic and pharmacological benefits for human health, such as antibacterial, antiparasitic, antioxidant and pro-oxidant, carcinopreventive, antidiabetic, analgesic and anti-inflammatory [7,8,9,10,11,12].
Pumpkin by-products could also be used as a source of valuable components in cosmetic and food industries for the fortification of various products, as biodegradable food packaging and as carriers in encapsulation processes [2,13,14,15,16,17,18]. From a nutritional point of view, pumpkin fruits, i.e., pulp, peel and seeds, contain carbohydrates, proteins, lipids, fiber and minerals [19,20]. For example, partially defatted pumpkin seed flour obtained from Cucurbita maxima, which is a by-product of pumpkin seed oil production, is considered a good source of proteins (42.75%), lipids (12.28%) and total carbohydrates (37.4%), of which 26.64% is crude fiber [21]. This partially defatted pumpkin seed flour also contains high amounts of minerals such as potassium (1290 mg/100 g), magnesium (693 mg/100 g), iron (87.8 mg/100 g), zinc (11.5 mg/100 g) and copper (2.49 mg/100 g). Pumpkin fruits are also rich in phenolic compounds, fatty acids, essential amino acids, vitamins, terpenoids, saponins, sterols, tocopherols and carotenoids [9,19,22,23,24]. Their content in pumpkin seeds is highly variable and depends mainly on the pumpkin species and varieties, as well as on the fractions excreted.
Nowadays, by-products of cucurbits have also become very attractive as health-promoting ingredients and natural pigments with multiple uses due to the presence of carotenoids [25]. In the human body, carotenoids maintain chemical reactivity by scavenging free radicals and active atomic oxygen, which is particularly important for hard-working people. Carotenoids provide significant added value to foods associated with antioxidant and anti-cancer activity, photoprotection, cardiovascular disease protection and anti-inflammatory effects. Therefore, the use of carotenoids in the development of nutritionally enhanced functional foods and nutraceuticals from waste and by-products is one of the major challenges for many researchers today. In this context, the present work describes the potential of pumpkins, their waste fractions and pumpkin products to be used as a source of carotenoids. Since carotenoids have an unsaturated chemical structure and are, therefore, susceptible to reactions such as oxidation and isomerization, it is necessary to consider their stability during the processing and storage of pumpkin products. Here, the special focus is on the content and carotenoid profile of pumpkin products and pumpkin by-products.
2. Carotenoids: Sources, Diversity and Chemical Structure
Carotenoids are lipid-soluble natural pigments of the tetraterpenoid group that can be biosynthesized in plants, yeasts, fungi, algae and photosynthetic bacteria. They are responsible for the yellow, orange and red colors of various fruits and vegetables as well as processed foods of plant origin. Carotenoids consist of a 40-carbon skeleton of isoprene units covalently linked together, resulting in multiple conjugated double bonds. The structure may be cyclized at one or both ends with various degrees of hydrogenation and has oxygen-containing functional groups. Lycopene and α-carotene, for example, are acyclized and cyclized carotenoids, respectively. The most characteristic feature of carotenoids is the long series of conjugated double bonds that form the central part of the molecule. This gives them their shape, chemical reactivity and light-absorbing properties. Most naturally occurring carotenoids have the trans configuration, but they are isomerized into the cis configuration by conjugation during processing or by exposure to certain environmental conditions (light and heat). The isomeric form of carotenoids affects their functions in biological tissues, such as bioavailability, vitamin A activity, stability to electrophiles and specificity to cleaving enzymes [26].
Carotenoids are classified into two main groups: carotenes and xanthophylls. The carotenes are hydrocarbons (they consist only of carbon and hydrogen) and the xanthophylls contain oxygen as a functional group in their structure. Although more than 700 natural carotenoids have been identified, α- and β-carotene, lycopene, lutein, zeaxanthin and β-cryptoxanthin (PubChem) are the most widely distributed (Table 1).
Table 1.
Chemical structure of carotenoids found in Cucurbita spp.
| Compound Trivial Name | Semisystematic Name | Empirical Formula | Structure |
|---|---|---|---|
| α-carotene | (6′R)-β,ε-carotene | C40H56 |
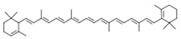
|
| β-carotene | β,β-Carotene | C40H56 |
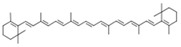
|
| Lutein | (3R,3′R,6′R)-β,ε-carotene-3,3′-diol | C40H56O2 |
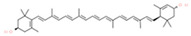
|
| Zeaxanthin | (3R,3′R)-β,β-carotene-3,3′-diol | C40H56O2 |
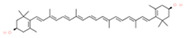
|
| β-Cryptoxanthin | (3R)-β,β-caroten-3-ol | C40H56O |
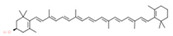
|
Pumpkin contains large amounts of pro-vitamin A carotenoids, which give it different colors due to the presence of components such as lutein (bright-yellow color) and β-carotene (orange color). β-Carotene contains 100% of pro-vitamin A activity and, after conversion to vitamin A in the body, is responsible for night vision, growth and the development and maintenance of epithelial tissue and also has an immunological function. Although lycopene is one of the most studied carotenoids, it is present in pumpkins only in low concentrations. In addition, xanthophylls such as lutein, zeaxanthin and cryptoxanthin, as well as carotenes (α-carotene, β-carotene and lycopene), have been detected by many researchers in varying concentrations, depending mainly on the pumpkin variety and the way the pumpkins are treated [27].
2.1. Carotenoids in Cucurbita spp. Varieties
Numerous scientific papers have demonstrated a great diversity of carotenoids in different pumpkin varieties. Both the total content of carotenoids (Table 2) and the content of individual carotenoids (Table 3) are highly variable and depend on the variety and species (genetic characteristics), pedoclimatic conditions and agrotechnical measures in the cultivation of the pumpkin, the part of the plant (pulp, peel or seed) and extraction procedures (technique, type of solvent, mass ratio, etc.). For example, in the work of de Carvalho [28], it was shown that the mean total carotenoid content in two landrace samples of raw pumpkin pulp (C. moschata) is different, being 404.98 μg/g in sample A and 234.21 μg/g in sample B, respectively. Hussain et al. [29] investigated the total carotenoid content in three fractions of pumpkin (peel, pulp, and seed powder). The highest content of total carotenoids was found in pumpkin pulp powder (35.2 mg/100 g), while the lowest content of total carotenoids was found in pumpkin seed powder (8.2 mg/100 g). According to Kreck et al. [30], the total carotenoid content of pumpkin peel and pulp depends on the variety. Their results showed that the cultivar ‘Butternut’ had lower concentrations of total carotenoids in pulp (44 mg/kg) and peel (12 mg/kg) than the cultivar ‘Hokkaido’ pulp (218 mg/kg) and peel (1048 mg/kg). Total carotenoids in C. maxima seed oil were 107.5 and 32.5 μg/kg, at 440 and 460 nm, respectively [31]. In the peel and pulp of C. maxima, C. moschata and C. andreana, the total carotenoid content ranged from 0.2 to 19.57 mg/100 g, depending on the number of varieties of the three Cucurbita spp. evaluated [4]. In the lipid fraction of pumpkin seeds of ‘Nova caravela’, ‘Mini Paulisa’, ‘Menina Brasileira’ (C. moschata) and ‘Moranga de Mesa’ (C. maxima) cultivars, the most appreciated by consumers, the values of total carotenoids ranged from 7.67 to 26.80 µg/g [32]. In the oils obtained by the cold pressing of C. pepo seeds, the amounts of total carotenoids ranged from 6.95 to 7.60 μmol/kg depending on the growing region [33]. The total carotenoid content, expressed as β-carotene, in oils of C. maxima, ‘Moranga de Mesa’ variety, obtained from a mixture of peels and seeds by ultrasound-assisted extraction (UAE) and Soxhlet extraction, is high, ranging from 225.43 to 296.94 mg/100 g of oil, depending on the method and temperature [34]. Compared to the oil obtained from the mixture of peels and seeds, the seed oil extracted with UAE at 75 °C contained an extremely low mass fraction of β-carotene (1.5 mg per 100 g of oil). In another study, the effects of different extraction conditions of carotenoids from pumpkin pulp were investigated. Carotenoid extraction was optimized for temperature (15, 30 and 45 °C), extraction time (8, 12 and 16 h), and solid-to-solvent ratio (1:50, 1:100 and 1:150). The authors observed that nonpolar solvents were a critical factor for the extraction of nonpolar carotenoids such as lycopene and β-carotene from pumpkin pulp [35]. It was also found that the yield of carotenoids was increased when the ratio of solid to solvent was increased up to 1:150. In another study, Portillo-Lopez et al. [36] used canola, corn and soybean oils at different solid-to-solvent ratios (1:10, 2:10 and 3:10) as alternative green solvents for the extraction of carotenoids from pumpkin pulp (C. argyrosperma). Before extraction, the pumpkin pulp slices were dried at 60 °C, ground and sieved (0.425 mm) to obtain a fine powder. The obtained results showed that the type of solvent and the solid–liquid ratio significantly affected the carotenoid yield. The highest carotenoid content was obtained with canola oil due to its physical (e.g., boiling point, surface tension and viscosity) and molecular properties (e.g., dipole moment, electronic polarizability, ability to release and accept hydrogen bonds and ability to release and accept electron pairs). It was also found that the solid-to-solvent ratio of 1:10 g/mL gave the highest yield of carotenoids. Sharma and Bhat [37] used corn oil as a green solvent and three different extraction techniques (ultrasound-assisted extraction, microwave-assisted extraction and conventional extraction) for carotenoid extraction from the pulp and peel of two pumpkin (C. maxima) cultivars. A higher carotenoid content was found in the samples after ultrasound-assisted extraction than in the samples after microwave-assisted extraction and conventional extraction with organic solvents (hexane and isopropyl alcohol). In addition, it was found that the carotenoid content varied depending on the plant part, and the peel powder had a higher content of carotenoids than the pulp powder. Several studies have also reported the recovery of carotenoids (lycopene and β-carotene) from food waste and by-products (pomegranate waste and mango pulp) using vegetable oils as solvents [38,39].
Table 2.
Total content of carotenoids in pumpkin products and by-products.
| Cucurbita Species | Variety | Products/By-Products | Extraction Solvent | Total Carotenoid Content (µg/g) | Reference |
|---|---|---|---|---|---|
| C. moschata | Duches A | Pulp | Acetone/ petroleum ether |
404.98 | [28] |
| Duches B | 234.21 | ||||
| C. maxima | - | Seed oil | n-Hexane | 0.107 | [31] |
| C. maxima | Db1# | Peel and pulp |
n-Hexane/ Acetone/ Ethanol |
195.7 | [4] |
| C. moschata | Nova Caravela | Lipid fraction from seeds | 7.67 | [32] | |
| Mini Paulista | 26.80 | ||||
| Menina Brasileira | Chloroform/ Methanol |
26.03 | |||
| C. maxima | Moranga de Mesa | Water | 11.53 | ||
| C. pepo | - | Seed oil | - | 69.5–76.0 | [33] |
| C. maxima | Rouge | Pulp Peel |
Acetone/hexane | 683 1751 |
[30] |
| C. moschata | - | Pulp | Virgin coconut oil | 171.96 | [35] |
| C. moschata | Duch. cv. Miben | Peel | Ethanol/petroleum ether | 344.9 | [40] |
| C. moschata | Pulp | Acetone/Ethanol | 367 | [41] | |
| C. maxima | - | Pulp | Petroleum ether | 21.20 | [42] |
| C. maxima | - | Oil from seed/peel mixture | Ethanol | 2254–2960 | [34] |
| Seed oil | 15 | ||||
| C. maxima | - | Pulp | Acetone | 675.62 | [43] |
| C. moschata | Butternut Waltham Muscade de Provence Llena de Napoles Butternut Rugosa |
Pulp |
Acetone |
143.8 3797.2 |
[44] |
| 881.8 | |||||
| 338.7 | |||||
| C. moschata | - | Pulp | Hexane/Acetone/Ethanol | 10.09 | [45] |
| C. moschata | Pluto | Pulp | Acetone | 34.54 | [46] |
| C. maxima | - | Peel powder Pulp powder Seed powder |
Acetone/n-Hexane | 237 352 82 |
[29] |
| C. moschata | - | Pulp | Acetone | 196,7 | [47] |
| C. maxima | - | Pulp | Acetone | 41.1 | [48] |
| C. maxima | Justynka | Pulp | Methanol/Ethyl acetate/light petroleum | 487.4 | [49] |
| C. argyrosperma | Pulp powder | Canola oil | 613.9 | [36] | |
| C. maxima | Gold Nugget Amoro F1 |
Peel powder Pulp powder Peel powder Pulp powder |
Hexane/Isopropanol | 192.1 150.1 162.1 123.3 |
[37] |
| C. maxima | Amazonka Ambar Atlantic Giant Bambino |
Pumpkin flower | Acetone/ n-Hexane |
401.7 273.8 309.5 390.1 |
[50] |
| C pepo | Miranda | Pumpkin flower | Acetone/ n-Hexane |
458.2 |
[50] |
| C. moschata | Muscade de Provence Butternut squash Rouge vif d’Etampes |
Pumpkin flower | Acetone/ n-Hexane |
213.6 152.7 184.4 |
[50] |
Table 3.
Content of major carotenoids in pumpkin products and by-products.
| Cucurbita Species | Variety | Products /By-Products | Carotenoid/(µg/g) | Reference | |||
|---|---|---|---|---|---|---|---|
| β-Carotene | α-Carotene | Lutein | Zeaxanthin | ||||
| - | - | Seed oil for dietary use Seed oil for therapeutic use |
- | - | 93–121 * 102–106 * |
98–116 * |
[51] |
|
109–113 * | |||||||
| C. pepo | - | Seed oil | 5.4–6.0 * | - | 0.2–0.3 * | 26.5–29.2 | [33] |
| C. moschata | Ariel Pluto |
Pulp | 2.04 3.67 |
- | - |
- |
[46] |
| C. maxima | Berrettina | Seed oil | 2.5 * | 8 * | [52] | ||
| C. pepo | Herakles | Seed oil | 17–23 | 63–88 | [53] | ||
| - | Pulp Peel Seed |
1.48 39.48 17.46 |
- | - | |||
| C. pepo | - | [54] | |||||
| Pulp | 5.70 | - | - | ||||
| C. moschata | Peel | 68.30 | - | - | - | [54] | |
| Seed | 7.15 | - | - | ||||
| Pulp | 17.04 | - | - | ||||
| C. maxima | Peel | 123.19 | - | - | - | [54] | |
| Seed | 31.40 | - | - | ||||
| C. pepo | Oleifera | Seed oil | 1.2–2.1 | 0.1–0.2 | 1.2–2.7 | [55] | |
| C. moschata | - | Juice | 1.27 | 1.23 | 1.35 | [56] | |
| C. moschata | Menina Brasileira | Puree | 19.45 | 12.60 | 0.59 | [57] | |
| C. maxima | Exposiçăo | Puree | 13.38 | 0.43 | 10.43 | - | [57] |
| C. maxima | Hokkaido | Pumpkin-enriched grits extrudates | 2.1 | 0.03–0.18 | 36.2–108.9 | [58] | |
| - | Momordica balsamina L. | Pumpkin leaves | 90 | - | 341.3 | 13 | [59] |
* (µg/mL).
2.2. Overview of the Individual Carotenoid Characteristics for Cucurbita spp. Varieties
In addition to the determination of the total carotenoid, the results of the analyses of the individual carotenoids are also described in the present work (Table 3). The composition of carotenoids in pumpkins can be influenced by some factors such as differences in ripening stage, pedoclimatic conditions and harvest and postharvest treatment. Fruit ripening is generally associated with increased carotenogenesis and, consequently, an increased concentration of carotenoids during ripening. Normally, the biosynthesis of carotenoids decreases at lower temperatures. Four carotenoids are predominant in pumpkins. β-Carotene is the major carotenoid in most species. The number of total carotenoids may be higher when minor carotenoids and ester forms are considered. Other carotenoids such as α-carotene, lutein, and zeaxanthin are also abundant. Procida et al. [51] showed that commercial pumpkin seed oil from C. pepo contains 93–121 mg/kg lutein and 98–116 mg/kg zeaxanthin. Although the total amount of lutein and zeaxanthin is very similar, the availability of the two carotenoids seems to be different. It was found that after hydrolysis of the oil to obtain the free, esterified and total content of lutein and zeaxanthin, the ratio between the free and esterified fractions is significantly different. For example, zeaxanthin is present mainly in esterified form in all samples, while lutein is found in esterified form in only three samples. A study by Akin et al. [33] revealed that C. pepo cold-pressed oil contains low quantities of lutein, β-carotene, β-cryptoxanthin and zeaxanthin. Using high-performance liquid chromatography analysis with diode array detector, electrospray ionization and tandem mass spectrometry (HPLC-DAD-ESI/MS/MS), 0.02–0.03 mg/100 g lutein, 0.54–0.6 mg/100 g β-carotene, 0.43–0.49 mg/100 g cryptoxanthin and 2.65–2.91 mg/100 g zeaxanthin were found.
Other studies indicated that oils extracted from seeds can also be used as an excellent source of carotenoids. For example, seed oil extracted from C. maxima, variety ‘Berrettina’, dried at 60 °C for 24 h in a hot air oven and analyzed by HPLC with diode array and mass spectrometry detection (HPLC-DAD-MS) contained two important carotenoids, namely, lutein and β-carotene at concentrations of 8 mg/L and 2.5 mg/L, respectively [52]. The oil obtained by the cold pressing and aqueous enzymatic extraction of C. pepo seeds also contained lutein and β-carotene [53]. Their mass fraction varied between 30.1 and 38.7% and 25.3 and 28.1% for lutein and β-carotene, respectively, depending on the extraction methods used. The (9Z)-β-carotene, α-carotene and traces of zeaxanthin were also identified. The carotenoids were also analyzed in roasted and unroasted seeds, and it was found that the roasting method had a great influence on the carotenoid content, i.e., the total carotenoid content increased from 0.29 to 0.54 mg/100 g, from unroasted to roasted [55]. The major carotenoid is lutein, which accounted for about 44% of the total carotenoid content and increased to 50% after roasting. β-Carotene is the second most abundant carotenoid, and its content in unroasted and roasted pumpkin seeds is 43 and 39%, respectively. The content of cryptoxanthin in unroasted seeds is twice as high as in roasted seeds.
In addition to seeds, the other pumpkin fractions, such as peel and pulp, also contained high amounts of carotenoids. Thus, β-carotene and β-cryptoxanthin are found in the peel, seeds and pulp of C. pepo, C. moschata and C. maxima [54]. Measurement by HPLC with UV/VIS detection showed that the peels contained more β-carotene than the other parts. The values ranged from 39.48 to 123.19 mg/kg, depending on the species. The seed fraction of C. maxima also contained a high amount of β-carotene (31.40 mg/kg). β-Cryptoxanthin was detected only in the pulp of C. maxima (0.65 mg/kg), in the peels of C. pepo, C. moschata and C. maxima (0.13–6.52 mg/kg), and in the seeds of C. maxima (0.21 mg/kg) and C. pepo (0.16 mg/kg). In the work of de Carvalho et al. [28], HPLC analysis was used to determine α- and β-carotenes and their isomers, (9Z)- and (13Z)-β-carotene, in the pulp of two landrace samples (A and B) of raw C. moschata to verify its production potential. The results showed that (all-E)-β-carotene was the most abundant in both landrace materials, containing 244.22 and 141.95 μg/g in samples A and B, respectively. α-carotene is found in amounts ranging from 67.06 μg/g (sample A) to 72.99 μg/g (sample B). The levels of (9Z)-β-carotene and (13Z)-β-carotene were relatively negligible compared to the levels of α- and β-carotene in landrace samples A and B, respectively. The values for (9Z)-β-carotene were 2.34 μg/g (A) and 0.97 μg/g (B), and those for (13Z)-β-carotene were 3.67 (A) to 1.84 μg/g (B). In addition to the various plant parts, carotenoids were also determined in various pumpkin products such as pumpkin juice, pumpkin puree and similar products. Suo et al. [56] investigated the carotenoid content in pumpkin juice and the effect of sonication of pumpkin juice on carotenoid content. The results showed that sonication led to a significant increase in the content of carotenoids in the sonicated juice (22.83–32.28% in β-carotene, 23.57–32.52% in α-carotene, 22.55–32.33% in β-cryptoxanthin, 23.70–32.59% in zeaxanthin and 24.14–34.48% in lycopene). Provesi et al. [57] investigated by HPLC the concentration of the main carotenoids in the pumpkin puree of two pumpkin cultivars, C. moschata ‘Menina Brasileira’ and C. maxima ‘Exposição’ and reported that the most abundant carotenoids in the pumpkin puree of C. moschata ‘Menina Brasileira’ were (all-E)-β-carotene and α-carotene, with lesser amounts of ψ-carotene, violaxanthin and lutein. The most abundant carotenoid in the samples of C. maxima ‘Exposição’ was (all-E)-β-carotene, followed by violaxanthin and lutein. The retentions of carotenes after heat treatment, namely, α-carotene and (all-E)-β-carotene in C. moschata ‘Menina Brasileira’ squashes and (all-E)-β-carotene in C. maxima ‘Exposição’ squashes, were high (>75%). Norshazila et al. [35] reported a significantly higher total carotenoid content at 30 °C than at 45 °C. According to Oliveira et al. [60], the degradation of carotenoids occurs at temperatures close to 40 °C. Moreover, Das and Bera [61] found that the maximum β-carotene extraction yield was at 40 °C and decreased with a further increase in temperature. Kurz et al. [62] noted the presence of relatively high levels of β-carotene and lutein in eight investigated pumpkin varieties of three pumpkin species (Cucurbita maxima Duch., Cucurbita pepo L. and Cucurbita moschata Duch.)
Carotenoids were also determined in pumpkin-enriched corn grits extrudates [58]. The peeled, oven-dried at 60 °C, ground and powdered pumpkins with mass fractions of 4, 6 and 8% were added to corn grits with and without the addition of ascorbic acid. The results showed that the content of lutein and zeaxanthin in the extruded samples is higher than in the raw material. The amount of lutein ranges from 3.16 to 7.93 mg/100 g, depending on whether ascorbic acid and pumpkin powder were added to the raw material or not. After extrusion, the amounts of lutein range from 4.79–12.29 mg/100 g to 3.62–10.89 mg/100 g, depending on the temperatures used. Zeaxanthin was found in amounts of 1.74–8.93 mg/100 g in the raw material and 2.94–10.53 and 2.83–9.39 mg/100 g in the extruded samples. α-Carotene showed low amounts at 0.03–0.18 mg/100 g, and the lowest values were obtained in the extruded samples, indicating that a high temperature has a decreasing effect in the case of this carotenoid. Moreover, (9Z)-β-carotene and (13Z)-β-carotene were found in low amounts, i.e., 0.03–0.33 mg/100 g and 0.023–0.14 mg/100 g, respectively. In recent years, edible flowers have become a culinary trend. Edible flowers that are now increasingly available on the market are squash blossoms. Biezanowska-Kopec et al. [50] studied the nutrient composition and antioxidant activity of squash flowers from different squash species (C. maxima, C. moschata and C. pepo) and cultivars ‘Amazonka’, ‘Ambar’, ‘Atlantic Giant’, ‘Bambino’, ‘Butternut’, ‘Muscade de Provence’, ‘Rouge vif d’Etampes’ and ‘Miranda’. The highest carotenoid content (45.82 mg/100 g) was found in the ‘Miranda’ cultivar (C. pepo), while the lowest content (15.27 mg/100 g) was found in the ‘Butternut’ cultivar (C. moschata). In addition to the pumpkin flowers, the pumpkin leaves can also be used for cooking. Mashiani et al. [59] studied the carotenoid profiles of African pumpkin (Momordica balsamina L.) leaves subjected to different cooking techniques. The results showed that the different cooking techniques increased the content of lutein, carotene and zeaxanthin in the pumpkin leaves.
3. Conclusions
The most studied Cucurbita species are Cucurbita pepo, C. moschata and C. Maxima. Pumpkin processing generated large amounts of by-products. Pumpkin by-products are still underutilized. The available research reports have pointed out how four carotenoids are predominant in pumpkins. β-Carotene is the major carotenoid in most species. Pumpkin seeds are mainly used for the production of pumpkin seed oil, which is used in cooking, but recently, it has also been used in the pharmaceutical and cosmetic industries. Lutein and zeaxanthin are the most abundant carotenoids in seed oil, while β-carotene is the most abundant carotenoid in pumpkin peel and pulp. The recovery of carotenoids from pumpkin by-products through sustainable green technologies could be used in the development of functional food products and pharmaceuticals to prevent chronic diseases and improve health. While the demand for carotenoids is increasing in the global carotenoid market, the amount of natural carotenoids extracted from plants, animals and microorganisms is very small. Future trends are aimed at better understanding for more efficient and environmentally friendly extraction methods of carotenoids that comply with green extraction principles and utilize non-conventional sustainable technologies such as supercritical fluid extraction and extraction using ultrasound, pulsed electric fields, microwaves and enzymatic treatments. In addition, the use of environmentally friendly bio-based solvents as a green alternative to petroleum-derived solvents such as hexane is a key requirement for sustainable development. In addition, hybrid technologies that merge different energy sources will also be a novelty in this field. These new findings will provide new opportunities for large-scale production of new natural ingredients and products in the food, feed, cosmetic and pharmaceutical industries based on pumpkin by-products.
Author Contributions
Conceptualization, S.R.B. and A.N.G.; writing—original draft preparation, A.N.G. and S.R.B.; writing—review and editing, M.B.S. and R.M.; visualization, supervision, J.Š.Ž. and M.B.; project administration, M.B.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by means of the Croatian Science Foundation project IP-2019-04-9750.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Loy J. Morpho-physiological aspects of productivity and quality in squash and pumpkins (Cucurbita spp.) Crit. Rev. Plant Sci. 2004;23:337–363. doi: 10.1080/07352680490490733. [DOI] [Google Scholar]
- 2.Kaur S., Panghal A., Garg M.K., Mann S., Khatkar S.K., Sharma P., Chhikara N. Functional and nutraceutical properties of pumpkin—A review. Nutr. Food Sci. 2020;50:384–401. doi: 10.1108/NFS-05-2019-0143. [DOI] [Google Scholar]
- 3.FAOSTAT. [(accessed on 5 October 2020)]. Available online: www.fao.org/faostat.
- 4.Chen Q., Lyu Y., Bi J., Wu X., Jin X., Qiao Y., Hou H., Lyu C. Quality assessment and variety classification of seed-used pumpkin by-products: Potential values to deep processing. Food Sci. Nutr. 2019;7:4095–4104. doi: 10.1002/fsn3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rico X., Gullon B., Alonso J.L., Yanez R. Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Food Res. Int. 2020;132:109086. doi: 10.1016/j.foodres.2020.109086. [DOI] [PubMed] [Google Scholar]
- 6.Valdez-Arjona L.P., Ramírez-Mella M. Pumpkin waste as livestock feed: Impact on nutrition and animal health and on quality of meat, milk, and egg. Animals. 2019;9:769. doi: 10.3390/ani9100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saavedra M.J., Aires A., Dias C., Almeida J.A., De Vasconcelos M.C.B.M., Santos P., Rosa E.A. Evaluation of the potential of squash pumpkin by-products (seeds and shell) as sources of antioxidant and bioactive compounds. J. Food Sci. Technol. 2015;52:1008–1015. doi: 10.1007/s13197-013-1089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu C., Shi H., Li Q.H. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006;61:73–80. doi: 10.1007/s11130-006-0016-6. [DOI] [PubMed] [Google Scholar]
- 9.Patel S., Rauf A. Edible seeds from Cucurbitaceae family as potential functional foods: Immense promises, few concerns. Biomed. Pharmacother. 2017;91:330–337. doi: 10.1016/j.biopha.2017.04.090. [DOI] [PubMed] [Google Scholar]
- 10.Salehi B., Capanoglu E., Adrar N., Catalkaya G., Shaheen S., Jaffer M., Giri L., Suyal R., Jugran A.K., Calina D., et al. Cucurbita plants: A key emphasis to its pharmacological potential. Molecules. 2019;24:1854. doi: 10.3390/molecules24101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolnik A., Beata O. Vegetables from the Cucurbitaceae family and their products: Positive effect on human health. Nutrition. 2020;70:110788. doi: 10.1016/j.nut.2020.110788. [DOI] [PubMed] [Google Scholar]
- 12.Bulut S., Popovic S., Hromis N., Suput D., Lazic V., Kocic-Tanackov S., Dimic G., Kravic S. Antibacterial activity of biopolymer composite materials obtained from pumpkin oil cake and winter savory or basil essential oil against various pathogenic bacteria. J. Food Nutr. Res. 2020;59:250–258. [Google Scholar]
- 13.Yildiz E., Ozcan T. Functional and textural properties of vegetable-fibre enriched yoghurt. Int. J. Dairy Technol. 2019;72:199–207. doi: 10.1111/1471-0307.12566. [DOI] [Google Scholar]
- 14.Norfezah N.M., Hardacre A., Brennan C.S. Comparison of waste pumpkin material and its potential use in extruded snack foods. Food Sci. Technol. Int. 2011;17:367–373. doi: 10.1177/1082013210382484. [DOI] [PubMed] [Google Scholar]
- 15.Lemus-Mondaca R., Marin J., Rivas J., Sanhueza L., Soto Y., Vera N., Puente-Díaz L. Pumpkin seeds (Cucurbita maxima). A review of functional attributes and by-products. Rev. Chil. Nutr. 2019;46:783–791. doi: 10.4067/S0717-75182019000600783. [DOI] [Google Scholar]
- 16.Lalnunthari C., Devi L.M., Amami E., Badwaik L.S. Valorisation of pumpkin seeds and peels into biodegradable packaging films. Food Bioprod. Process. 2019;118:58–66. doi: 10.1016/j.fbp.2019.08.015. [DOI] [Google Scholar]
- 17.Öztürk T., Turhan S. Physicochemical properties of pumpkin (Cucurbita pepo L.) seed kernel flour and its utilization in beef meatballs as a fat replacer and functional ingredient. J. Food Process. Preserv. 2020;44:e14695. doi: 10.1111/jfpp.14695. [DOI] [Google Scholar]
- 18.Čakarević J., Šeregelj V., Tumbas Šaponjac V., Ćetković G., Čanadanović Brunet J., Popović S., Kostić M.H., Popović L. Encapsulation of beetroot juice: A study on the application of pumpkin oil cake protein as new carrier agent. J. Microencapsul. 2020;37:121–133. doi: 10.1080/02652048.2019.1705408. [DOI] [PubMed] [Google Scholar]
- 19.Salehi B., Sharifi-Rad J., Capanoglu E., Adrar N., Catalkaya G., Shaheen S., Jaffer M., Giri L., Suyal R., Jugran A.K., et al. Cucurbita plants: From farm to industry. Appl. Sci. 2019;9:3387. doi: 10.3390/app9163387. [DOI] [Google Scholar]
- 20.Pereira A.M., Krumreich F.D., Ramos A.H., Richter Krolow A.C., Santos R.B., Gularte M.A. Physicochemical characterization, carotenoid content and protein digestibility of pumpkin access flours for food application. Food Sci. Technol. 2020;40:691–698. doi: 10.1590/fst.38819. [DOI] [Google Scholar]
- 21.Apostoli L., Berca L., Mosoiu C., Badea M., Bungau S., Oprea O.B., Cioca G. Partially defatted pumpkin (Cucurbita maxima) seeds—A rich source of nutrients for use in food products. Rev. Chem. 2018;6:1398–2904. doi: 10.37358/RC.18.6.6332. [DOI] [Google Scholar]
- 22.Karrar E., Sheth S., Navicha S.S., Navicha W.B., Wei W., Hassanin H., Abdalla M., Wang X. A potential new source: Nutritional and antioxidant properties of edible oils from cucurbit seeds and their impact on human health. J. Food Biochem. 2018;43:e12733. doi: 10.1111/jfbc.12733. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira D.F., Barina J.S., Binello A., Veselov V.V., Cravotto G. Highly efficient pumpkin-seed extraction with the simultaneous recovery of lipophilic andhydrophilic compounds. Food Bioprod. Process. 2019;117:224–230. doi: 10.1016/j.fbp.2019.07.014. [DOI] [Google Scholar]
- 24.Kulczyński B., Gramza-Michałowska A. The profile of carotenoids and other bioactive molecules in various pumpkin fruits (Cucuriba maxima Duchesne) cultivars. Molecules. 2019;24:3212. doi: 10.3390/molecules24183212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melendez-Martinez A.J., Mandić A.I., Bantis F., Bohm V., Borge G.I.A., Brnčić M., Bysted A., Cano M.P., Dias M.G., Elgersma A., et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022;62:1999–2049. doi: 10.1080/10408398.2020.1867959. [DOI] [PubMed] [Google Scholar]
- 26.Melendez-Martinez A., Stinco C., Liu C., Wang X. A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chem. 2013;138:1341–1350. doi: 10.1016/j.foodchem.2012.10.067. [DOI] [PubMed] [Google Scholar]
- 27.Murkovic M., Mulleder U.M., Neunteuf H. Carotenoid Content in Different Varieties of Pumpkins. J. Food Compos. Anal. 2002;15:633–638. doi: 10.1006/jfca.2002.1052. [DOI] [Google Scholar]
- 28.De Carvalho L.M.J., Barros Gomes P., De Oliveira Godoy R.L., Pacheco S., Fernandes do Monte P.H., De Carvalho J.L.V., Nutti M.R., Lima Neves A.C., Rodrigues Alves Vieira A.C., Ramalho Ramos S.R. Total carotenoid content, α-carotene and β-carotene, of landrace pumpkins (Cucurbita moschata Duch): A preliminary study. Food Res. Int. 2012;47:337–340. doi: 10.1016/j.foodres.2011.07.040. [DOI] [Google Scholar]
- 29.Hussain A., Kausar T., Din A., Murtaza A., Jamil M.A., Noreen S., Rehman R., Shabbir H., Ramzan M.A. Determination of total phenolic, flavonoid, carotenoid, and mineral contents in peel, flesh, and seeds of pumpkin (Cucurbita maxima) J. Food Process. Preserv. 2021;45:e15542. doi: 10.1111/jfpp.15542. [DOI] [Google Scholar]
- 30.Kreck M., Kürbel P., Ludwig M., Paschold P.J., Dietrich H. Identification and quantification of carotenoids in pumpkin cultivars (Cucurbita maxima L.) and their juices by liquid chromatography with ultraviolet-diode array detection. J. Appl. Bot. Food Qual. 2006;80:93–99. [Google Scholar]
- 31.Siano F., Straccia M.C., Paolucci M., Fasulo G., Boscaino F., Volpe M.G. Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils. J. Sci. Food Agric. 2016;96:1730–1735. doi: 10.1002/jsfa.7279. [DOI] [PubMed] [Google Scholar]
- 32.Médici Veronezi C., Jorge N. Bioactive compounds in lipid fractions of pumpkin (Cucurbita sp.) seeds for use in food. J. Food Sci. 2012;77:C653–C657. doi: 10.1111/j.1750-3841.2012.02736.x. [DOI] [PubMed] [Google Scholar]
- 33.Akin G., Arslan F.N., Karuk Elmas S.N., Yilmaz I. Cold-pressed pumpkin seed (Cucurbita pepo L.) oils from the central Anatolia region of Turkey: Characterization of phytosterols, squalene, tocols, phenolic acids, carotenoids and fatty acid bioactive compounds. Grasas Aceites. 2018;69:e232. doi: 10.3989/gya.0668171. [DOI] [Google Scholar]
- 34.Massa T.B., Stevanato N., Cardozo-Filho L., da Silva C. Pumpkin (Cucurbita maxima) by-products: Obtaining seed oil enriched with active compounds from the peel by ultrasonic-assisted extraction. J. Food Process Eng. 2019;42:e13125. doi: 10.1111/jfpe.13125. [DOI] [Google Scholar]
- 35.Norshazila S., Koy C.N., Rashidi O., Ho L.H., Azrina I., Nurul Zaizuliana R.A. The effect of time, temperature and solid to solvent ratio on pumpkin carotenoids extracted using food grade solvents. Sains Malays. 2017;46:231–237. [Google Scholar]
- 36.Portillo-Lopez R., Morales-Contreras B.E., Lozano-Guzman E., Basilio-Heredia J., Muy-Rangel M.D., Ochoa-Martinez L.A., Rosas-Flores W., Morales-Castro J. Vegetable oils as green solvents for carotenoid extraction from pumpkin (Cucurbita argyrosperma Huber) by-products: Optimization of extraction parameters. J. Food Sci. 2021;86:3122–3136. doi: 10.1111/1750-3841.15815. [DOI] [PubMed] [Google Scholar]
- 37.Sharma M., Bhat R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil) Foods. 2021;10:787. doi: 10.3390/foods10040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goula A.M., Ververi M., Adamopoulou A., Kaderides K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochemistry. 2017;34:821–830. doi: 10.1016/j.ultsonch.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 39.Baria B., Upadhyay N., Singh A.K., Malhotra R.K. Optimization of ‘green’ extraction of carotenoids from mango pulp using split plot design and its characterization. LWT Food Sci. Technol. 2019;104:186–194. doi: 10.1016/j.lwt.2019.01.044. [DOI] [Google Scholar]
- 40.Song J., Yang Q., Huang W., Xiao Y., Li D., Liu C. Optimization of trans lutein from pumpkin (Cucurbita moschata) peel by ultrasound assisted extraction. Food Bioprod. Process. 2018;107:104–112. doi: 10.1016/j.fbp.2017.10.008. [DOI] [Google Scholar]
- 41.Priori D., Valduga E., Villela J.C.B., Mistura C.C., Vizzotto M., Valgas R.A. Characterization of bioactive compounds, antioxidant activity and minerals in landraces of pumpkin (Cucurbita moschata) cultivated in Southern Brazil. Food Sci. Technol. 2017;37:33–40. doi: 10.1590/1678-457x.05016. [DOI] [Google Scholar]
- 42.Kandlakunta B., Rajendran A., Thingnganing L. Carotene content of some common (cereals, pulses, vegetables, spices and condiments) and unconventional sources of plant origin. Food Chem. 2008;106:85–89. doi: 10.1016/j.foodchem.2007.05.071. [DOI] [Google Scholar]
- 43.Wang S.M., Yu D.J., Song K.B. Physicochemical property of pumpkin slices dehydrated with red algae extract. J. Korean Soc. Appl. Biol. Chem. 2011;54:921–925. doi: 10.1007/BF03253181. [DOI] [Google Scholar]
- 44.Nawirska A., Figiel A., Kucharska A.Z., Sokół-Łetowska A., Biesiada A. Drying kinetics and quality parameters of pumpkin slices dehydrated using different methods. J. Food Eng. 2009;94:14–20. doi: 10.1016/j.jfoodeng.2009.02.025. [DOI] [Google Scholar]
- 45.Dirim S., Çalıskan G. Determination of the effect of freeze drying processon the production of pumpkin (Cucurbita moschata) puree powder and the powder properties. J. Food. 2012;37:203–210. [Google Scholar]
- 46.Armesto J., Rocchetti G., Senizza B., Pateiro M., Barba F.J., Domınguez R., Lucini L., Lorenzo J.M. Nutritional characterization of Butternut squash (Cucurbita moschata D.): Effect of variety (Ariel vs. Pluto) and farming type (conventional vs. organic) Food Res. Int. 2020;132:109052. doi: 10.1016/j.foodres.2020.109052. [DOI] [PubMed] [Google Scholar]
- 47.Köprüalan O., Altay O., Bodruk A., Kaymak-Ertekin F. Effect of hybrid drying method on physical, textural and antioxidant properties of pumpkin chips. J. Food Meas. Charact. 2021;15:2995–3004. doi: 10.1007/s11694-021-00866-1. [DOI] [Google Scholar]
- 48.Marquez-Cardozo C.J., Caballero-Gutierrez B.L., Ciro-Velazquez H.J., Restrepo-Molina D.A. Effect of pretreatment and temperature on the drying kinetics and physicochemical and techno-functional characteristics of pumpkin (Cucurbita maxima) Heliyon. 2021;7:e06802. doi: 10.1016/j.heliyon.2021.e06802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ouyang M., Huang Y., Wang Y., Luo F., Liao L. Stability of carotenoids and carotenoid esters in pumpkin (Cucurbita maxima) slices during hot air drying. Food Chem. 2022;367:130710. doi: 10.1016/j.foodchem.2021.130710. [DOI] [PubMed] [Google Scholar]
- 50.Biezanowska-Kopec R., Ambroszczyk A.M., Piątkowska E., Leszczynska T. Nutritional Value and Antioxidant Activity of Fresh Pumpkin Flowers (Cucurbita sp.) Grown in Poland. Appl. Sci. 2022;12:6673. doi: 10.3390/app12136673. [DOI] [Google Scholar]
- 51.Procida G., Stancher B., Catenia F., Zacchignaa M. Chemical composition and functional characterisation of commercial pumpkin seed oil. J. Sci. Food Agric. 2013;93:1035–1041. doi: 10.1002/jsfa.5843. [DOI] [PubMed] [Google Scholar]
- 52.Montesano D., Blasi F., Simonetti M.S., Santini A., Cossignani L. Chemical and nutritional characterization of seed oil from Cucurbita maxima L. (var. Berrettina) pumpkin. Foods. 2018;7:30. doi: 10.3390/foods7030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konopka I., Roszkowska B., Czaplicki S., Tańska M. Optimization of Pumpkin Oil Recovery by Using Aqueous Enzymatic Extraction and Comparison of the Quality of the Obtained Oil with the Quality of Cold-Pressed Oil. Food Technol. Biotechnol. 2016;54:412–420. doi: 10.17113/ftb.54.04.16.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim M.Y., Kim E.J., Kim Y.N., Choi C., Lee B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012;6:21–27. doi: 10.4162/nrp.2012.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raczyk M., Siger A., Radziejewska-Kubzdela E., Ratusz K., Rudzińska M. Roasting pumpkin seeds and changes in the composition and oxidative stability of cold-presses oils. Acta Sci. Pol. Technol. Aliment. 2017;16:293–301. doi: 10.17306/J.AFS.0494. [DOI] [PubMed] [Google Scholar]
- 56.Suo G., Zhou C., Su W., Hu X. Effects of ultrasonic treatment on color, carotenoid content, enzyme activity, rheological properties, and microstructure of pumpkin juice during storage. Ultrason. Sonochemistry. 2022;84:105974. doi: 10.1016/j.ultsonch.2022.105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Provesi J.G., Dias C.O., Amante E.R. Changes in carotenoids during processing and storage of pumpkin puree. Food Chem. 2011;128:195–202. doi: 10.1016/j.foodchem.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 58.Obradović V., Babić J., Šubarić D., Jozinović A., Ačkar D., Klarić I. Influence of dried Hokkaido pumpkin and ascorbic acid addition on chemical properties and colour of corn extrudates. Food Chem. 2015;183:136–143. doi: 10.1016/j.foodchem.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 59.Mashiane P., Shoko T., Manhivi V., Slabbert R., Sultanbawa Y., Sivakumar D. A Comparison of Bioactive Metabolites, Antinutrients, and Bioactivities of African Pumpkin Leaves (Momordica balsamina L.) Cooked by Different Culinary Techniques. Molecules. 2022;27:1901. doi: 10.3390/molecules27061901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliveira R.G.A., Carvalho M.J.L., Nutti R.M., Carvalho L.V.J., Fukuda W.G. Assessment and degradation study of total carotenoid and ß-carotene in bitter yellow cassava (Manihot esculenta crantz) varieties. Afr. J. Food Sci. 2010;4:148–155. [Google Scholar]
- 61.Das S., Bera D. Mathematical model study on solvent extraction of carotene from carrot. Int. J. Res. Eng. Technol. 2013;2:343–349. [Google Scholar]
- 62.Kurz C., Carle R., Schieber A. HPLC-DAD-MSn characterisation of carotenoids from apricots and pumpkins for the evaluation of fruit product authenticity. Food Chem. 2008;110:522–530. doi: 10.1016/j.foodchem.2008.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


