Abstract
The localization and accessibility of the group B streptococcus (GBS) surface immunogenic protein (Sip) at the surface of intact GBS cells were studied by flow cytometric assay and immunogold electron microscopy. Antibodies present in pooled sera collected from mice after immunization with purified recombinant Sip efficiently recognized native Sip at the surfaces of the different GBS strains tested, which included representatives of all nine serotypes. Examination of GBS cells by immunogold electron microscopy revealed that the Sip-specific antibodies attached preferentially to polar sites and the septal region. This result confirmed that Sip is exposed at the intact-cell surface, but it also suggests that its distribution is restricted to certain regions of the cell.
Group B streptococcus (GBS) is the leading cause of life-threatening bacterial infections in newborns (11). GBS has also emerged as an important pathogen among adults, especially the elderly or patients with chronic diseases such as diabetes, cirrhosis, malignancies, and immunodeficiencies (11). Nine serotypes of GBS, based on the capsular polysaccharide, have been recognized to date (7). The major invasive disease-causing serotypes are Ia, Ib, II, and III (11), but a recent population-based surveillance study has indicated an increasing importance of serotype V strains (1). Efforts are under way to develop multivalent vaccines based on the capsular antigens (6, 14, 15). Some GBS surface proteins, such as the R protein, the α and β subunits of the C protein, and the Rib protein (5, 10, 12), have also been investigated as potential vaccine candidates. Unfortunately, these proteins were not found to be present in all clinical isolates (4, 12).
Recently, we identified a protein called Sip, for surface immunogenic protein (2). Comparison of the predicted amino acid sequences of Sip proteins from six serologically distinct strains clearly indicated that this protein is highly conserved. Immunoblot assays using a Sip-specific monoclonal antibody also indicated that a protein band with an approximate molecular mass of 53 kDa was present in every GBS strain tested, which included representative isolates of all serotypes (2). In addition, the immune response induced after immunization with recombinant Sip (rSip) efficiently protected mice against experimental infection with GBS strains representing serotypes Ia/c, Ib, II/R, III, V, and VI (2).
A cell wall anchoring motif (LPXTG) in the C-terminal region of Sip was not identified (2). However, analysis of the sequence revealed a 25-amino-acid signal peptide at the N terminus of Sip, which is an indication that this protein could be exported outside the cell, where it could be associated with the cell wall of the bacteria (2). In this study, mouse polyclonal anti-Sip sera and a monoclonal Sip-specific antibody were thus used to localize and evaluate the accessibility of Sip epitopes on the surfaces of different GBS strains.
A collection of 11 strains of GBS representing the nine capsular serotypes and a bovine isolate were used in this study. These strains were obtained from the American Type Culture Collection (Manassas, Va.), the Children's Hospital of Eastern Ontario (Ottawa, Ontario, Canada), and the National Center for Streptococcus, Provincial Laboratory of Public Health for Northern Alberta (Edmonton, Alberta, Canada).
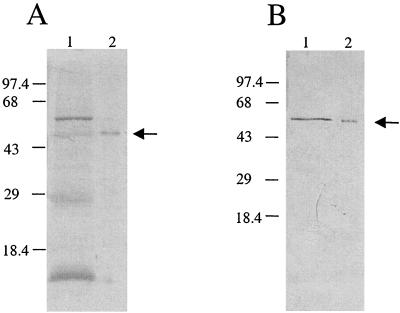
rSip was produced and purified as described previously (2). The purity of rSip was estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to be >90% (Fig. 1A, lane 2), and the protein concentration was determined by the bicinchoninic acid assay in accordance with the manufacturer's (Pierce Chemical Company, Rockford, Ill.) instructions. To generate Sip-specific antisera, female CD-1 mice (Charles River Laboratories, Montreal, Quebec, Canada), 4 to 6 weeks old, were injected subcutaneously three times at 3-week intervals with 20 μg of purified rSip mixed with 20 μg of QuilA adjuvant (Cedarlane Laboratories, Hornby, Ontario, Canada). Individual mouse sera were collected 3 weeks after the third immunization and pooled. Immunoblots clearly indicated that antibodies in the pooled sera recognized purified rSip (Fig. 1B, lane 2). In addition, the pooled sera also reacted with a protein band with an approximate molecular mass of 53 kDa that was present in a GBS whole-cell preparation (Fig. 1B, lane 1). This protein band had been previously shown, by using Sip-specific monoclonal antibody 5A12, to correspond to Sip (2). Sera collected from mice injected with 20 μg of QuilA adjuvant were pooled and used as negative controls for cytofluorimetric and electron microscopic assays.
FIG. 1.
Silver-stained sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis gel (A) and corresponding immunoblot (B) showing the reactivity of Sip-specific mouse sera with a GBS whole-cell preparation from strain C388/90 (serotype Ia/c) (lane 1) and purified rSip (lane 2). Arrows indicate the location of Sip. Size standards are marked on the left in kilodaltons.
A flow cytometric assay was used to study the attachment of Sip-specific antibodies at the intact bacterial surface. Bacterial cells were grown to early exponential phase in Todd-Hewitt broth (THB; Difco Laboratories, Detroit, Mich.), and the optical density at 600 nm was adjusted with THB to 0.15 (corresponding to ≈108 CFU/ml). Ten microliters of mouse Sip-specific or control sera was added to 1 ml of the bacterial suspension. The tubes containing the bacterial and serum suspensions were incubated for 2 h at 4°C under gentle rotation. Samples were washed three times in blocking buffer (phosphate-buffered saline [PBS] containing 2% [wt/vol] bovine serum albumin [Sigma Chemical Co., St. Louis, Mo.]), and then 1 ml of fluorescein-conjugated goat anti-mouse immunoglobulin G (IgG) plus IgM (Jackson ImmunoResearch Laboratories, Mississauga, Ontario, Canada) diluted in blocking buffer was added. After a further incubation for 60 min at room temperature, samples were washed three times in blocking buffer and fixed with 0.3% formaldehyde in PBS buffer for 18 h at 4°C. Cells were washed two times in PBS buffer and resuspended in 0.5 ml of PBS buffer. Cells were kept in the dark at 4°C until analysis by flow cytometry (Epics XL; Beckman Coulter Inc., Fullerton, Calif.). Ten thousand GBS cells were analyzed in each sample. To study the influence of growth phase, bacteria were plated on tryptic soy agar plates containing 5% (vol/vol) sheep blood (Quelab Laboratories, Montreal, Quebec, Canada) and incubated at 37°C in an 8% CO2 atmosphere for 18 to 24 h. Bacterial cells were then harvested in THB, and the optical density at 600 nm was adjusted with THB to 0.15. Bacterial cells were labeled with mouse Sip-specific serum as described above.
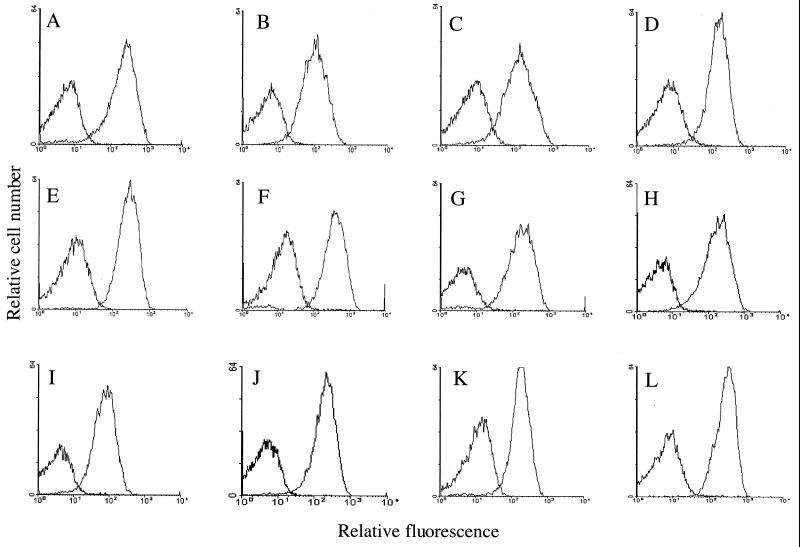
Figure 2 shows the attachment of the GBS-specific antibodies present in the sera from rSip-immunized mice to the surface of GBS serotype Ia/c strain C388/90 (Fig. 2A), the homologous strain from which the sip gene had been cloned, as well as 10 heterologous strains representing the nine capsular serotypes and a bovine isolate (Fig. 2B to K). Antibodies in the control sera did not show any binding to the 11 strains (left peak), while antibodies in the mouse Sip-specific sera clearly attached to the cells of all GBS isolates, as illustrated by the increased level of fluorescence (right peak). In addition, GBS cell populations showed a unimodal distribution of antibody-binding fluorescent signals. It was determined that more than 90% of the 10,000 GBS cells analyzed were labeled by Sip-specific antibodies. Similar results were obtained when each of these strains was incubated with rabbit anti-Sip serum or when the cells were grown to stationary phase instead of early exponential phase (Fig. 2L). The flow cytometric analyses thus revealed that Sip-specific antibodies efficiently recognized their corresponding surface-exposed epitopes on all of the GBS strains tested and that Sip is present throughout the bacterial life cycle.
FIG. 2.
Evaluation by flow cytometry of the accessibility to antibodies of Sip at the surface of GBS serotype Ia/c strain C388/90 (A), serotype Ib strain ATCC 12401 (B), serotype II/R strain NCS 246 (C), serotype III strain NCS 954 (D), serotype III strain ATCC 12403 (E), serotype IV strain NCS 97SR331 (F), serotype V strain NCS 535 (G), serotype VI strain NCS 9842LCDC (H), serotype VII strain NCS 7271 (I), serotype VIII strain NCS 970886 (J), bovine type strain ATCC 27956 (K), and serotype Ia/c strain C388/90 grown to exponential phase (L). GBS cells were incubated with either mouse anti-Sip or control sera, followed by the corresponding fluorescein isothiocyanate-conjugated secondary antibody. In each graph, the left peak represents the binding of control pooled sera while the right peak represents the binding of Sip-specific mouse pooled sera to intact GBS cells.
The attachment of Sip-specific antibodies at the surface of GBS cells was confirmed by immunogold electron microscopy, which was done as described by Díaz et al. (3), with some modifications. Briefly, bacteria were plated on tryptic soy agar plates containing 5% (vol/vol) sheep blood (Quelab Laboratories) and incubated at 37°C in an 8% CO2 atmosphere for 18 to 24 h. Bacterial cells were then harvested in 5 ml of PBS. Suspensions (250 μl) were centrifuged at 10,000 × g for 30 s, and the pellet was resuspended in 500 μl of Sip-specific or control mouse sera diluted 1/25 in blocking buffer (PBS containing 3% [wt/vol] bovine serum albumin) or in 1 ml of undiluted Sip-specific monoclonal antibody 5A12 (2). Samples were incubated for 1 h at room temperature with rotation. Samples were washed three times in PBS, and then 500 μl of colloidal gold particles (12 nm) conjugated to goat anti-mouse IgG (Jackson ImmunoResearch Laboratories) diluted in blocking buffer was added and the mixture was incubated for 1 h at room temperature with rotation. Samples were washed two times in ammonium acetate buffer (0.1 M, pH 7.0), and the bacteria were resuspended in 250 μl of the same buffer. Ten microliters of the bacterial suspension was placed on Formvar-coated grids and allowed to partially air dry. Samples were examined with an electron microscope at an accelerating voltage of 60 kV (Philips EM300). To study the influence of growth phase, bacteria were grown in THB as described for the flow-cytometric assay and labeling was done as described above.
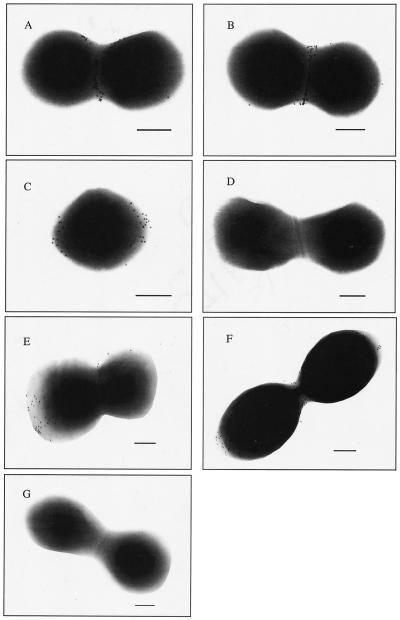
Cells of GBS strains C388/90 (serotype Ia/c) and NCS 954 (serotype III) incubated with mouse Sip-specific sera were labeled with gold particles in the septal region (Fig. 3A and B) and polar sites (Fig. 3C). Cells of GBS strain NCS 954 (serotype III) (Fig. 3D) or C388/90 (serotype Ia/c) (data not shown) incubated with mouse control pooled sera were not labeled with gold particles. To confirm the labeling observed with the polyclonal sera, GBS strains were incubated with Sip-specific monoclonal antibody 5A12. Similar labeling of cells of GBS strains C388/90 (serotype Ia/c) (Fig. 3E) and NCS 954 (serotype III) (data not shown) was obtained, confirming that Sip can be recognized by specific antibodies at the intact GBS cell surface. In addition, similar labeling of the septal region and polar sites of cells of GBS strains C388/90 (serotype Ia/c) (Fig. 3F) and NCS 954 (serotype III) (data not shown) was obtained when GBS cells were grown to early exponential phase. To confirm the Sip surface association, GBS cells were treated with trypsin (Roche Diagnostics, Laval, Quebec, Canada) at 1 mg/ml as described by Tamura et al. (13) and labeled with mouse Sip-specific serum as described above. Cells of GBS strain C388/90 (serotype Ia/c) (Fig. 3G) or NCS 954 (serotype III) (data not shown) treated with trypsin and incubated with Sip-specific mouse sera were not labeled with gold particles, confirming the surface association of Sip on GBS cells. Taken together, these results clearly demonstrated that the Sip-specific antibody efficiently recognized Sip at the surface of the two GBS strains tested and that Sip is present throughout the bacterial life cycle.
FIG. 3.
Transmission electron micrographs of whole cells of GBS serotype Ia/c strain C388/90 (A and E), strain C388/90 grown to early exponential phase (F), trypsin-treated strain C388/90 (G), and serotype III strain NCS 954 (B, C, and D). GBS cells were successively probed with mouse anti-Sip (A, B, C, F, and G) or control (D) pooled sera or monoclonal antibody 5A12 (E) and a gold-conjugated secondary antibody. Bars, 200 nm.
Even though a cell wall anchoring motif was not found in the C-terminal region, our results confirm that Sip is exposed at the intact GBS cell surface, where it is accessible to specific antibodies. This is not surprising, since Sip was found in the GBS culture supernatant, suggesting that this protein or a portion of it could be secreted (2). In addition, the identification of a signal peptide at the N terminus of Sip is an indication that this is exported outside the cell, where it is associated with the cell wall of the bacteria (2). The mechanism that mediates this cell-surface association has yet to be identified.
Since Sip-specific antibodies efficiently recognized surface-exposed epitopes on GBS cells, antibodies against these particular epitopes could play a role in antibody-mediated protective immunity. This immunity seems to play an important role in the prevention of GBS infection. Indeed, Lancefield et al. (8) demonstrated that specific antibodies directed to either polysaccharide or protein antigens of a single strain can protect against infection mediated by streptococci producing these particular antigens. Available evidence also indicates that antibodies directed against GBS surface proteins conferring protective immunity are sufficient to prevent lethal experimental GBS infection (9, 10, 12). Finally, we recently reported that passive administration of rabbit anti-Sip serum to pregnant mice or immunization of female mice before pregnancy with purified rSip conferred protective immunity to GBS infection on their offspring. Thus, the observed protection clearly involved the transfer of functional antibodies from pregnant mice to their pups (D. Martin, M. Boyer, J. Hamel, S. Rioux, F. Couture, and B. R. Brodeur, Abstr. 100th Gen. Meet. Am. Soc. Microbiol. 2000, abstr. E-33, p. 300). Further studies are required to determine the particular surface epitopes that could play a role in antibody-mediated protective immunity.
In conclusion, the present report confirms that Sip is exposed at the surface of intact GBS cells of every serotype tested. Most importantly, the surface exposure of Sip is not hindered by other surface antigens. These observations further intensify the interest in Sip as a potential vaccine candidate.
Acknowledgments
We are grateful to Maurice Dufour for his expertise in flow cytometry.
This work was financially supported by a grant from Biochem Pharma. S.R. is the recipient of a fellowship from the Medical Research Council of Canada and the Pharmaceutical Manufacturers Association of Canada.
REFERENCES
- 1.Blumberg H M, Stephens D S, Modansky M, Erwin M, Elliot J, Facklam R R, Schuchat A, Baughman W, Farley M M. Invasive group B streptococcal disease: the emergence of serotype V. J Infect Dis. 1996;173:365–373. doi: 10.1093/infdis/173.2.365. [DOI] [PubMed] [Google Scholar]
- 2.Brodeur B R, Boyer M, Charlebois I, Hamel J, Couture F, Rioux C R, Martin D. Identification of group B streptococcal Sip protein, which elicits cross-protective immunity. Infect Immun. 2000;68:5610–5618. doi: 10.1128/iai.68.10.5610-5618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Díaz E, García E, Ascaso C, Méndez E, López R, García J L. Subcellular localization of the major pneumococcal autolysin: a peculiar mechanism of secretion in Escherichia coli. J Biol Chem. 1989;264:1238–1244. [PubMed] [Google Scholar]
- 4.Ferrieri P, Flores A E. Surface protein expression in group B streptococcal invasive isolates. Adv Exp Med Biol. 1997;418:635–637. doi: 10.1007/978-1-4899-1825-3_148. [DOI] [PubMed] [Google Scholar]
- 5.Flores A E, Ferrieri P. Molecular species of R-protein antigens produced by clinical isolates of group B streptococci. J Clin Microbiol. 1989;27:1050–1054. doi: 10.1128/jcm.27.5.1050-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasper D L, Paoletti L C, Wessels M R, Guttormsen H-K, Carey V J. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Investig. 1996;98:2308–2314. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kogan G, Uhrin D, Brisson J R, Paoletti L C, Blodgett A E, Kasper D L, Jennings H J. Structural and immunochemical characterization of the type VIII group B streptococcus capsular polysaccharide. J Biol Chem. 1996;271:8786–8790. doi: 10.1074/jbc.271.15.8786. [DOI] [PubMed] [Google Scholar]
- 8.Lancefield R C, McCarty M, Everly W N. Multiple mouse-protective antibodies directed against group B streptococci. J Exp Med. 1975;142:165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson C, Stålhammar-Carlemalm M, Lindahl G. Protection against experimental infection with group B streptococcus by immunization with a bivalent protein vaccine. Vaccine. 1999;17:454–458. doi: 10.1016/s0264-410x(98)00218-7. [DOI] [PubMed] [Google Scholar]
- 10.Michel J L, Madoff L C, Kling D E, Kasper D L, Ausubel F M. Cloned alpha and beta C-protein antigens of group B streptococci elicit protective immunity. Infect Immun. 1991;59:2023–2028. doi: 10.1128/iai.59.6.2023-2028.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513. doi: 10.1128/cmr.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stålhammar-Carlemalm M, Stenberg L, Lindahl G. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med. 1993;177:1593–1603. doi: 10.1084/jem.177.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura G S, Kuypers J M, Smith S, Raff H, Rubens C E. Adherence of group B streptococci to cultured epithelial cells: roles of environmental factors and bacterial surface components. Infect Immun. 1994;62:2450–2458. doi: 10.1128/iai.62.6.2450-2458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wessels M R, Paoletti L C, Rodewald A K, Michon F, Difabio J, Jennings H J, Kasper D L. Stimulation of protective antibodies against type Ia and Ib group B streptococci by a type Ia polysaccharide-tetanus toxoid conjugate vaccine. Infect Immun. 1993;61:4760–4766. doi: 10.1128/iai.61.11.4760-4766.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wessels M R, Paoletti L C, Pinel J, Kasper D L. Immunogenicity and protective activity in animals of a type V group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Infect Dis. 1995;171:879–884. doi: 10.1093/infdis/171.4.879. [DOI] [PubMed] [Google Scholar]