Abstract
Bile duct tumor thrombus (BDTT) is an uncommon finding in hepatocellular carcinoma (HCC), potentially mimicking cholangiocarcinoma (CCA). Recent studies have suggested that HCC with BDTT could represent a prognostic factor. We report the case of a 47-year-old male patient admitted to the University Hospital of Bari with abdominal pain. Blood tests revealed the presence of an untreated hepatitis B virus infection (HBV), with normal liver function and without jaundice. Abdominal ultrasonography revealed a cirrhotic liver with a segmental dilatation of the third bile duct segment, confirmed by a CT scan and liver MRI, which also identified a heterologous mass. No other focal hepatic lesions were identified. A percutaneous ultrasound-guided needle biopsy was then performed, detecting a moderately differentiated HCC. Finally, the patient underwent a third hepatic segmentectomy, and the histopathological analysis confirmed the endobiliary localization of HCC. Subsequently, the patient experienced a nodular recurrence in the fourth hepatic segment, which was treated with ultrasound-guided percutaneous radiofrequency ablation (RFA). This case shows that HCC with BDTT can mimic different types of tumors. It also indicates the value of an early multidisciplinary patient assessment to obtain an accurate diagnosis of HCC with BDTT, which may have prognostic value that has not been recognized until now.
Keywords: HCC, hepatobiliary surgery, bile duct tumor thrombus, BDTT
1. Introduction
Colorectal liver metastases are the most frequent secondary liver tumor; on the other hand, hepatocellular carcinoma (HCC) is the most common primary liver cancer, and surgical resection is the mainstay treatment [1,2,3,4,5,6,7].
HCC with bile duct tumor thrombus (BDTT) is relatively uncommon, with an incidence of 0.5–12.9% [8].
HCC with BDTT was first described by Mallory et al. in 1947, and in 1975, by Lin et al. named BDTT “Icteric-Type Hepatocarcinoma” based on the often-associated symptom of jaundice, although it may not be obvious at first diagnosis [9].
Patients with a history of HBV or HCV infection have a higher risk of developing BDTT in the background of viral damage to the liver parenchyma. The way in which BDTT develops is controversial, but can be summarized into two main pathways: cancer cells can compose the thrombus or they can cause a cancerous thrombosis due to blood clots consequent an invasive hemorrhage of the bile duct wall [9].
The mechanisms of these two pathways are not yet known, but four hypotheses have been formulated. Firstly, cancer cells found inside the bile duct can be directly related to the primary tumor, which expands itself until invading the bile duct and creating a thrombosis [9]. Secondly, the primary tumor can invade microvessels and lymphatic vessels, invading the micro-circle of the bile duct entering the biliary system [9]. Thirdly, it can be hypothesized that the creation of an arteriovenous shunt to the bile duct system might be a way of tumor cells diffusion [9]. The last hypothesis pertains to the cells’ diffusion through the nerves that cover the wall of the bile duct, but there are not much evidences of this behavior [9]. In clinical practice, nowadays, the most reliable hypothesis is the first one, because even micro-BDTT is considered a feature of the invasiveness of HCC and it is related to a poorer prognosis. A new relationship between micro-BDTT and the inflammatory pathway has been described [10].
Despite the evolving technologies in imaging diagnosis and the support of Artificial Intelligence in Hepatobiliary and Pancreatic Surgery (HPB) [5,11,12,13,14], the diagnosis of HCC with BDTT is very challenging. Its treatment is still debated due to a complex pre- and postoperative patients’ management [15,16].
HCCs with BDTT are always associated with both parenchymal and intraductal lesions [17], but the primary tumor can generate a thrombus when the parenchymal lesion is still small and undetectable by preoperative imaging [9,17,18].
So the incidence of BDTT without macroscopic HCC as a specific subtype is not really evident in the literature, but there are no doubts that it might further jeopardize the diagnosis of HCC with BDTT [8].
Furthermore, another challenge concerning HCC with BDTT is represented by the clinically and radiologically mimetism with cholangiocarcinoma (CCA) [8,17].
Laboratory and clinical-anamnestic data can help the differential diagnosis: predisposing factors for cirrhosis such as hepatitis B (HBV) or C (HCV) virus and elevated serum α-fetoprotein (AFP) levels may suggest HCC with BDTT diagnosis [19].
CCAs may include cholestasis (such as primary sclerosing cholangitis, hepatolithiasis, or bile duct cysts) and chronic inflammation pathway (such as biliary parasitosis, viral hepatitis, or Non-Alcoholic SteatoHepatitis (NASH)) [20].
Carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA), as preoperative diagnostic biomarkers of CCAs, showed low sensitivity [21].
Integration with imaging features and, if necessary, with biopsy histological reports is still required to make the final diagnosis.
So considering the challenging clinical picture and the lack of evidences in literature, we aim to clearly describe the management of a HCC with BDTT compared with most relevant experiences already reported.
2. Case Report
A 47-year-old male patient was admitted to the University Hospital Policlinico of Bari (Italy) with abdominal pain in the right hypochondrium.
During the initial examination, the patient incidentally tested HBV-positive, in the absence of jaundice. Subsequent blood tests revealed an untreated HBV infection and normal liver and pancreatic function tests.
The laboratory findings were HBsAg 5000 IU/mL (normal value (NV) < 0.05 IU/mL); AFP 2157 ng/mL (NV 0–5 ng/mL); total bilirubin 0.9 mg/dL (NV 0.3–1 mg/dL), direct bilirubin 0.2 mg /dL (NV 0–0.4 mg/dL), indirect bilirubin 0.7 mg/dL (NV 0.1–1 mg/dL), γ-GT 44 U/l (NV < 50 U/L), and CA19–9 222 U/mL (NV 0–37 U/mL); the CEA level was undetectable.
An emergency abdominal ultrasound showed inhomogeneity hepatic echo structure. The bile duct of the third segment showed segmental dilation, furthermore the gallbladder appeared distended, without gallstones and regular wall.
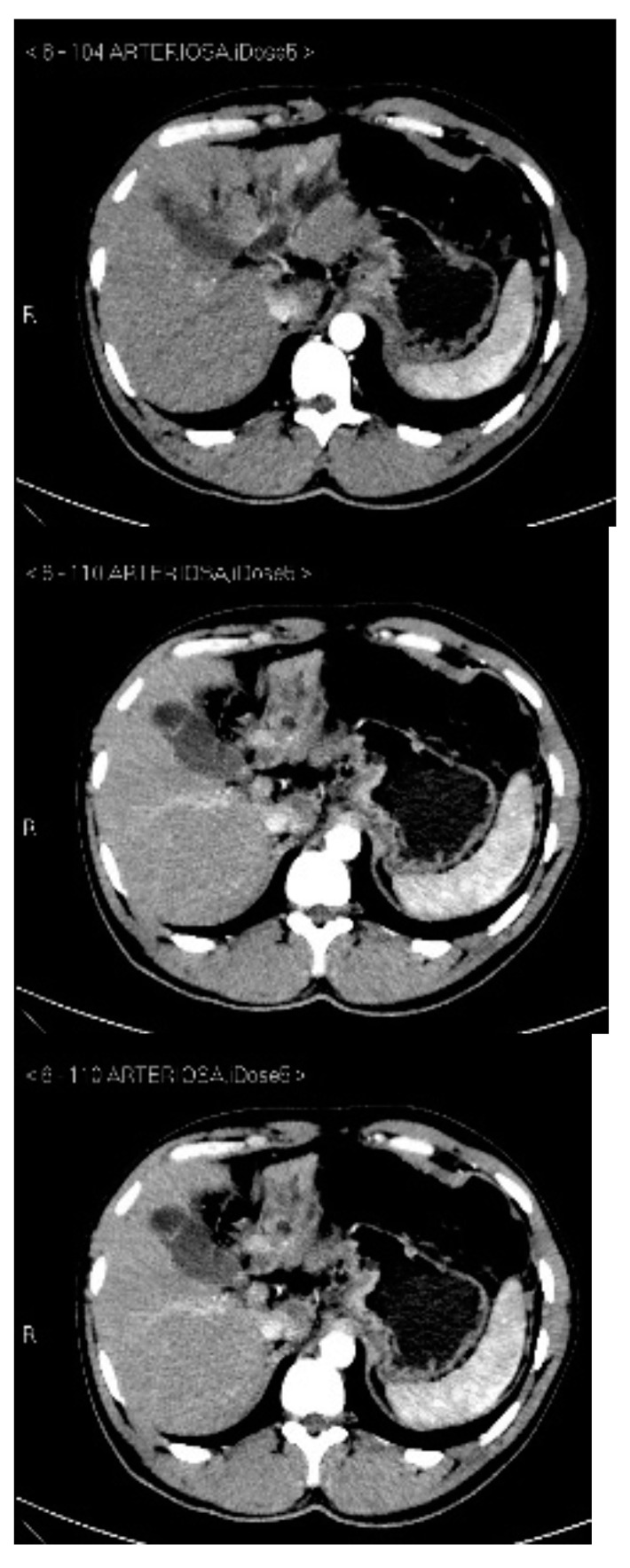
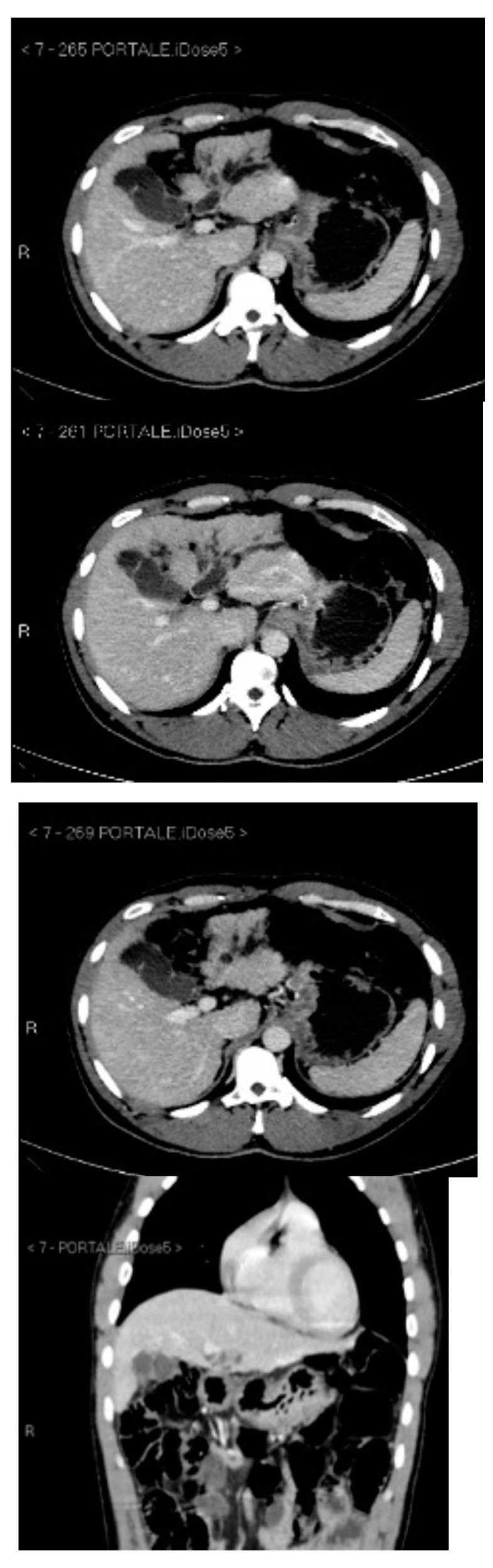
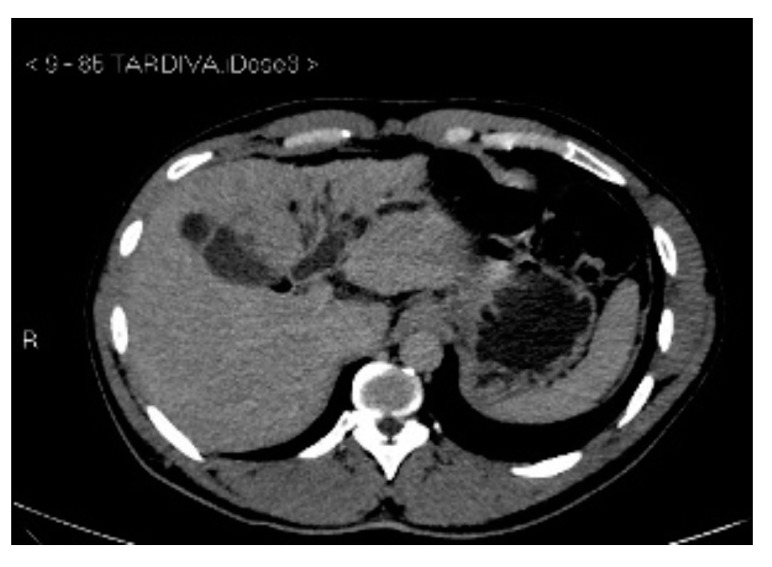
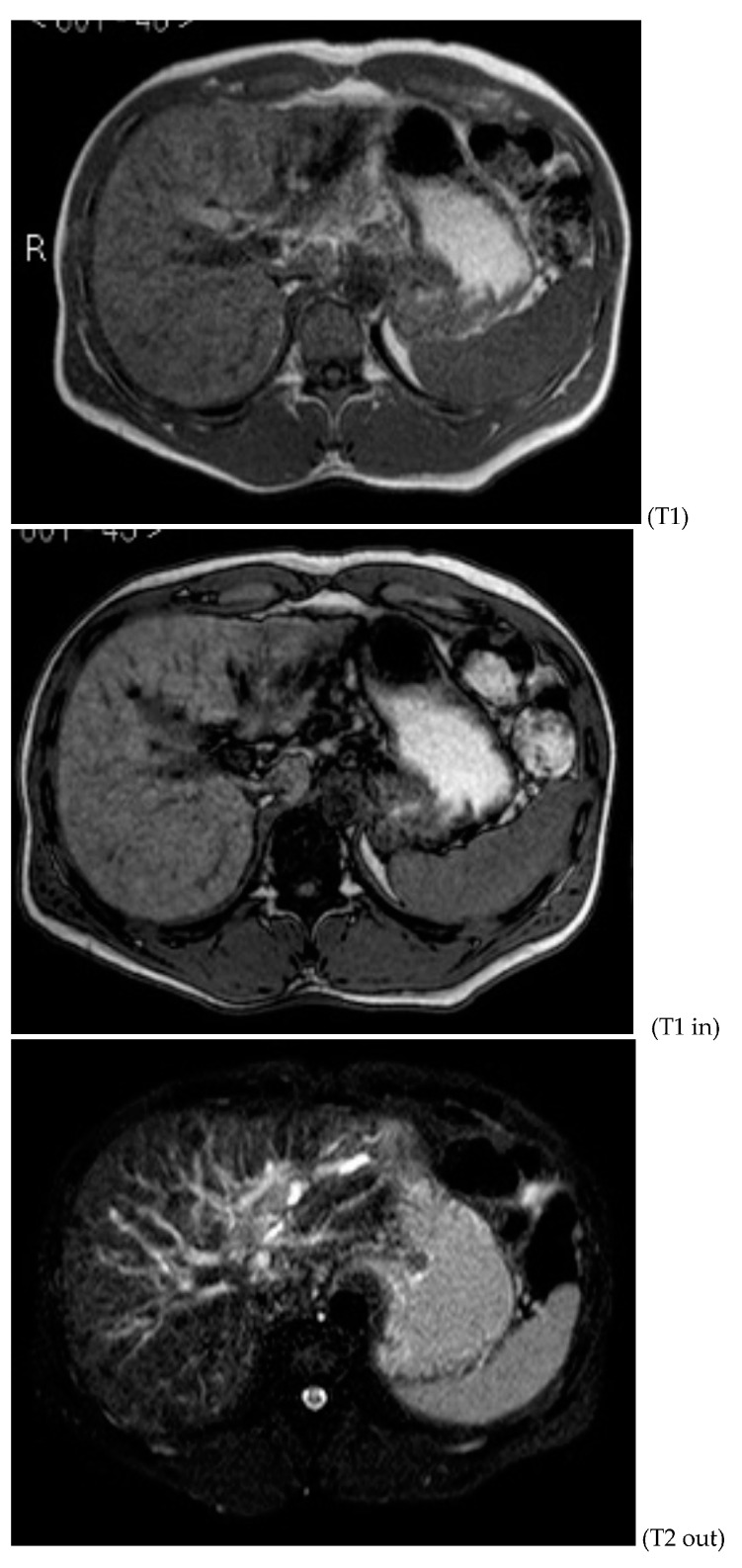
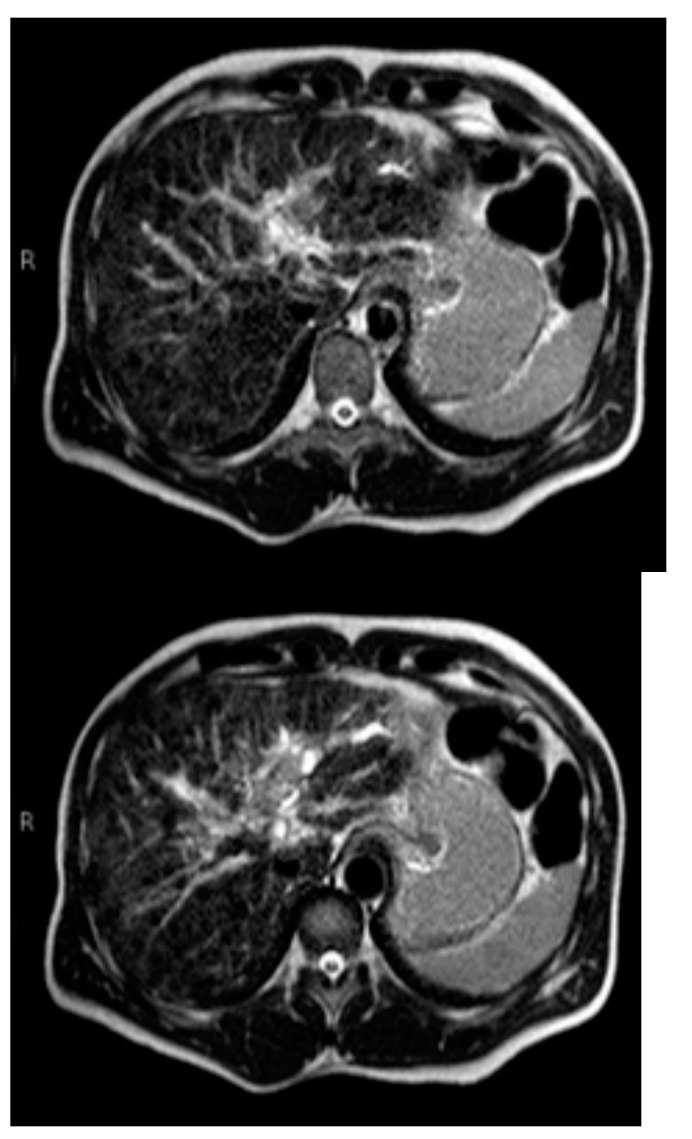
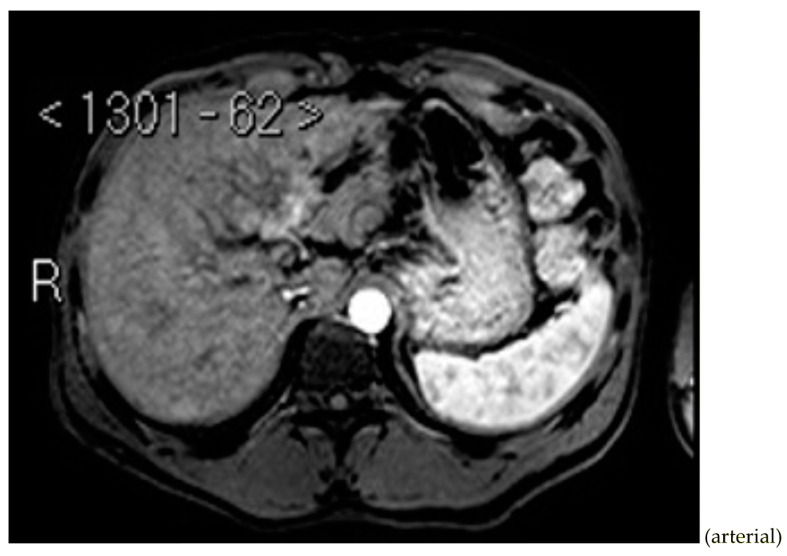
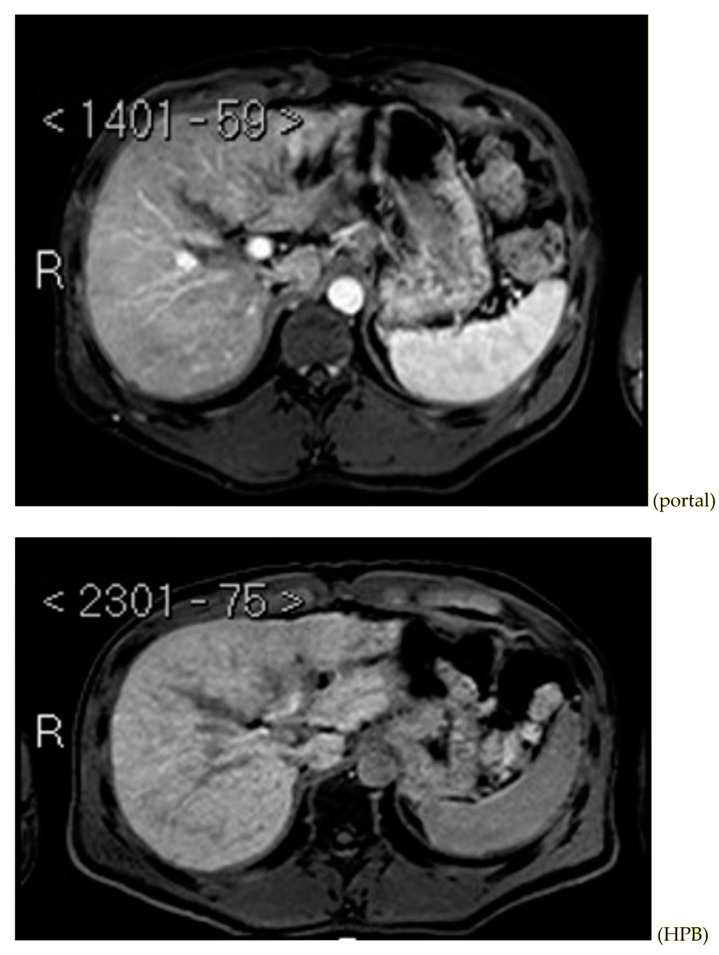
Subsequently, an upper-abdomen Computed Tomography (CT) scan and liver Magnetic Resonance Imaging (MRI) were performed (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7).
Figure 1.
CT arterial phase.
Figure 2.
CT portal phase.
Figure 3.
CT delayed phase.
Figure 4.
MRI T1, T1 in, T1 out, and DWI.
Figure 5.
MRI T2.
Figure 6.
MRI arterial, portal, and HPB phases.
Figure 7.
Cholangio-MRI.jpg.
A mass of 1.8 cm was detected within the biliary branch for the third hepatic segment, characterized by nodular impregnation in the arterial phase and irregular and partial washout in the portal venous phase. These findings were compatible with a heterologous lesion, although it was not possible to perform a diagnosis among HCC, intrahepatic CCA or other pathological lesions.
Consequently, in order to obtain a histological diagnosis, a percutaneous ultrasound-guided needle biopsy was performed.
The histological report revealed the presence of a moderately differentiated HCC (Edmondson grade II); immunohistochemistry results showed positive CK7 and CD34 staining and negative HEP par-1 staining consistent with the hypothesis.
Therefore, after a multidisciplinary team (MDT) discussion, third segment segmentectomy was performed through a minimally invasive approach, with intraoperative examination confirming endoluminal HCC with BDTT.
The histopathological analysis of the surgical specimen confirmed endobiliary metastasis from HCC. The postoperative histological report showed hepatic cirrhosis, end-stage HBV-related, and dysplastic nodules. The invasion of the major hepatic ducts was caused by carcinomatous proliferation with the morphological features of HCC.
The postoperative course was uneventful, and the patient was discharged on postoperative day 7.
No recurrence was evidenced until 6 months postoperatively, while a CT scan detected a nodule with HCC typical radiological characteristics (~1 cm in greatest diameter) in the fourth segment, with AFP serum level of 280 ng/mL. MDT team decide to perform ultrasound-guided percutaneous radiofrequency ablation (RFA) of the nodule. A CT scan carried out 1 month after ablation confirmed the nodule’s complete necrosis.
3. Discussion
Our experience showed how challenging is the diagnosis of HCC with BDTT and its impact on further management of the patients. Another key point is absence of a systematic classification that includes BDTT as a prognostic factor. These two points do not allow clinicians to appropriately relate BDTT to a stage of HCC; however, surgical treatment appears to be the first treatment option [22,23,24,25,26,27,28,29]. Considering the undeniable benefits of minimally invasive surgery, we have to underline that it offers a safer surgical approach for patients with a Performance Status (PS) of 1 or 2, allowing a shorter length of stay and faster recovery which as show a great impact also in patients undergoing surgical downstaging [22,23,24,25,26,27,28,29].
Furthermore, the COVID-19 pandemic era changed the surgical scenario of specialized surgical fields such as HPB surgery. A huge number of Hub and Spoke learning programs allowed the peripheral center to achieve high specialization in HPB [2]. During the pandemic, these programs always granted the standard of care for patients with consequent savings of time and money, avoiding the costs of health mobility [30].
Besides the diffusion of the newest surgical skills, it was necessary to support them with the earliest accurate diagnosis of cancer. This is probably the most important effort to improve patients’ survival. In oncological imaging, CT remains the main diagnostic tool for detection, follow-up and tumor staging [31].
Therefore, the achievement of the most precise treatment for each patient requires the diffusion of standardized diagnostic protocols [32,33].
To better understand the results of our case, we performed literature research through the main search engines (PubMed and Medline).
Regarding our literature review, we have extrapolated 20 articles with a total population of 890 patients [8,9,17,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] (Table 1 and Table 2).
Table 1.
Literature review: baseline characteristics.
| Author | Year | Study Type | N. of Cases | Age, Years | Sex, M/F | Symptoms | Jaundice | HBV Positive | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| Satoh et al. [31] | 2000 | Retrospective cohort study | 17 | 58.18 ± 8.94 | 15 (88.24)/2 (11.76) | Jaundice | 9 (52.94) | 5 (29.4) | Ultrasonography; CT |
| Shiomi et al. [38] | 2001 | Retrospective cohort study | 17 | 58.8 ± 2 | 15 (88.24)/2 (11.76) | Jaundice, abdominal pain, poor appetite, general fatigue, or fever | 10 (58.82) | 7 of 14 (50) | Ultrasonography; CT |
| Peng et al. [39] | 2004 | Retrospective cohort study | 8 | 51.75 ± 8.15 | 7 (87.5)/1 (12.5) | Jaundice | 8 (100) | 6 (75) | Ultrasonography; CT; MRI |
| Esaki et al. [40] | 2005 | Retrospective cohort study | 19 | 59.79 ± 11.26 | 19 (100)/0 (0) | Jaundice, fever, or abdominal pain | NA | 8 (42.10) | Ultrasonography; CT; MRI; angiography |
| Shao et al. [41] | 2011 | Retrospective cohort study | 27 | 47.1 ± 10.5 | 23 (85.18)/4 (14.81) | NA | NA | 8 (42.10) | Chest XR; abdominal ultrasonography; CT; CPRE |
| Yu et al. [42] | 2011 | Retrospective cohort study | 20 | 50.6 ± 2.4 | 17 (85)/3 (15) | Obstructive jaundice and upper abdominal pain | 14 (70) | 16 (80) | NA |
| Noda et al. [43] | 2011 | Retrospective cohort study | 22 | 45% were ≤60 y; 55% were >60 y |
21 (95)/1 (5) | NA | 8 (36.36) | 15 (68.18) | Ultrasonography; CT; angiography; ERCP or MR cholangiopancreatography |
| Moon et al. [44] | 2012 | Retrospective cohort study | 73 | 54.2 ± 11.1 | 52 (71.23)/21 (28.77) | Jaundice | 34 (46.60) | 59 (80.82) | CT; MRI |
| Oba et al. [45] | 2014 | Retrospective cohort study | 13 | 60.85 ± 8.64 | 12 (92.31)/1 (7.69) | NA | NA | 4 (30.77) | Ultrasonography; CT; MRI |
| Wong et al. [34] | 2014 | Retrospective cohort study | 37 | 56.75 ± 14.75 | 29 (78.38)/8 (21.62) | NA | NA | 30 (81.1) | CT; MRI |
| Rammohan et al. [46] | 2014 | Retrospective cohort study | 39 | 52.1 ± 10.9 | 28 (71.80)/11 (28.20) | Jaundice | 18 (46.10) | 7 (17.9) | Abdominal ultrasonography; abdominal CT |
| Ha et al. [35] | 2014 | Retrospective cohort study | 14 | 54.6 ± 5.6 | 10 (71.43)/4 (28.57) | Jaundice | 9 (64.29) | 11 (78.57) | NA |
| Kasai et al. [47] | 2015 | Retrospective cohort study | 44 | 64 ± 9.1 | 35 (79.5) /9 (20.5) | Jaundice | 27 (61.36) | 8 (18.2) | NA |
| Chotirosniramit et al. [48] | 2017 | Retrospective cohort study | 19 | 51.1 ± 11.5 | 15 (78.95)/4 (21.05) | Jaundice or cholangitis | 14 (73.68) | 16 (84.2) | Abdominal CT |
| Kim et al. [36] | 2018 | Retrospective cohort study | 257 | 61 ± 11.6 | 210 (81.71)/47 (18.29) | Jaundice | 120 (46.70) | 115 (44.75) | NA |
| Lin et al. [49] | 2019 | Retrospective cohort study | 49 | 55.51 ± 13.09 | 43 (87.75)/6 (12.25) | NA | NA | NA | Abdominal ultrasonography; abdominal CT; hepatic angiography; MR cholangiopancreatography |
| Zhou et al. [9] | 2020 | Retrospective cohort study | 7 | 66 ± 6.24 | 6 (85.71)/1 (14.28) | Jaundice | 7 (100) | 4 (57.14) | CT; MRI; |
| Zhou et al. [17] | 2020 | Retrospective cohort study | 58 | 49.84 ± 10.23 | 51 (87.93)/7 (2.07) | Jaundice and upper abdominal pain | 39 (67.24) | 42 (72.41) | CT |
| Sun et al. [50] | 2020 | Retrospective multicenter study | 120 | 50.55 ± 11.35 | 106 (88.33)/14 (11.67) | NA | NA | 95 (79.17) | NA |
| Wu et al. [8] | 2021 | Multicenter study | 30 | 48.5 ± 13.04 | 23 (76.67)/7 (23.33) | NA | NA | 29 (96.67) | CT; MRI |
| Conticchio et al. | 2022 | Case report | 1 | 47 | 1 (100)/0 (0) | Abdominal pain | 0 (0) | 1 (100) | Ultrasonography; CT; MRI |
Abbreviations: M, male; F, female; HBV, hepatitis B virus; CT, Computed Tomography; MRI, Magnetic Resonance Imaging; ERCP, Endoscopic Retrograde Cholangiopancreatography; NA, Not Available.
Table 2.
Literature review: pre-, intra-, and postoperative characteristics.
| Author | AFP (>400 ng/mL), n. |
AFP, Mean ± SD | Total Bilirubin, Mean ± SD | Tumor Size, cm | Surgical Procedure | Mortality, n | Overall Survival, % | ||
|---|---|---|---|---|---|---|---|---|---|
| 1-Year | 3-Year | 5-Year | |||||||
| Satoh et al. [31] | NA | NA | NA | NA | NA | 12 (70.59) | NA | NA | NA |
| Shiomi et al. [38] | NA | 73.87 ± 72.89 | 6.9 ± 1.8 mg/dL | 6.1 ± 1.2 | 1 right hepatic trisegmentectomy with caudate lobectomy; 5 right hepatic lobectomies with caudate lobectomy; 6 left hepatic lobectomies with caudate lobectomy; 1 right anterior segmentectomy; 1 right anterior segmentectomy with caudate lobectomy; 1 segmentectomy S5; 1 S4; 1 S1 | 11 (64.70) | NA | 47 | 28 |
| Peng et al. [39] | 7 (87.5) | NA | Ra 68.4–436.4 umol/L | Ra 2–9 | 3 hepatectomies with removal of the tumor thrombus; 1 hepatectomy combined with extrahepatic bile duct resection; 3 thrombectomies through a choledochotomy; 1 orthotopic liver transplantation | 7 (87.5) | 62.5 | 37.5 | NA |
| Esaki et al. [40] | NA | NA | 5 ± 7.3 mg/dL | NA | 7 left hepatectomies; 1 lateral segmentectomy; 6 right hepatectomies; 1 central bisegmentectomy; 3 medial segmentectomies; 1 anterior segmentectomy | NA | 79 | 45 | 33 |
| Shao et al. [41] | 16 (59.3) | NA | 116.4 ± 135.4 umol/L | NA | 1 right anterior resection; 2 right posterior resections; 4 right hepatectomies; 8 left hepatectomies; 1 left hepatectomy with caudate lobectomy; 3 left lateral resections; 2 left medial resections; 6 partial resections | 1 (3.70) | NA | NA | NA |
| Yu et al. [42] | 9 (45) | 2651.85 ± 6135.32 | 123.25 ± 142.06 mol/L | 3.65 ± 2.4 | 5 hepatectomies with thrombectomy; 7 hepatectomies with thrombectomy and T-tube drainage; 6 hepatectomies with resection of the common bile duct and hepaticojejunostomy; 2 liver transplantations | 6 (30) | 73.1 | 20.6 | NA |
| Noda et al. [43] | 12 (55) | NA | NA | 59% were ≤5 cm; 41% were >5 cm | 16 lobectomies; 6 surgically noncurative procedures | 0 (0) | 62 | 30 | 30 |
| Moon et al. [44] | NA | 25,280.10 ± 109,395.40 | 5.7 ± 5.9 mg/dL | 5.8 ± 3.7 | 25 right hemihepatectomies ± caudate lobectomy; 4 right trisectionectomies ± caudate lobectomy; 29 left hemihepatectomies ± caudate lobectomy; 1 posterior sectionectomies; 2 anterior sectionectomies; 4 lateral sectionectomies; 2 central bisectionectomies; 1 S5-S6 bisegmentectomy; 1 isolated caudate lobectomy; 4 nonsystematic hepatectomies; 2 partial hepatectomies; 1 partial caudate lobectomy; 1 S8 subsegmentectomy | 3 (4.11) | 76.5 | 41.4 | 32 |
| Oba et al. [45] | NA | 2193.25 ± 2815.06 | NA | 6.37 ± 4.01 | 4 left hepatectomies; 4 right hepatectomies; 1 right hepatectomy and segment 2/3 limited resection; 1 central bisegmentectomy; 1 right trisegmentectomy; 1 anterior segmentectomy; 1 posterior segmentectomy; 6 bile duct resections and bilioenteric anastomosis | 7 (53.85) | 92 | 77 | 48 |
| Wong et al. [34] | NA | 50 (Ra 2–63,320) | 29.05 ± 15 umol/L | 9.5 ± 6 | 34 major hepatectomies; 3 left lateral sectionectomies | 1 (2.7) | 69.4 | 54.3 | 38.5 |
| Rammohan et al. [46] | 28 (71.7) | NA | 6.1 ± 5.1 mg/dL | 5.6 ± 3.2 | 16 right hepatectomies with thrombectomy; 10 extended right hepatectomies with extrahepatic bile duct excision; 9 left hepatectomies; 2 extended left hepatectomies; 2 left lateral segmentectomies | 2 (5.1) | 82 | 48 | 10 |
| Ha et al. [35] | 14 (100) | 2043.1 ± 5528.6 | 5.1 ± 5.2 mg/dL | 3.9 ± 1.9 | 13 living-donor transplantations; 1 deceased-donor transplantation | 1 (7.14) | 92.9 | 57.1 | 50 |
| Kasai et al. [47] | NA | 5.31 ± 13.02 | 1.2 ± 0.8 mg/dL | 5.8 ± 3.5 | 41 bisectionectomies; 3 monosectionectomies; 7 combined BDRs | 2 (4.54) | NA | NA | 31 |
| Chotirosniramit et al. [48] | NA | 12,673.82 ± 12,499.87 | 11.3 ± 6.45 mg/dL | 8.2 ± 4.2 | 2 right trisectionectomies + bile duct resection + caudate resection; 1 left trisectionectomy + bile duct resection + caudate resection; 1 right hepatectomy + bile duct resection; 4 left hepatectomies + bile duct resection; 3 right hepatectomies; 4 left hepatectomies + CBD exploration to remove BDTT; 1 left hepatectomy; 2 CBD explorations to remove BDTT and palliative biliary drainage; 1 no operation; | 0 (0) | NA | 60 | NA |
| Kim et al. [36] | NA | 754.25 ± 451.5 | 2.85 ± 1.03 | NA | 121 right hemihepatectomies; 7 right trisectionectomy; 81 left hemihepatectomies; 2 left trisectionectomies; 5 posterior sectionectomies; 12 anterior sectionectomies; 8 left lateral sectionectomies; 3 left medial sectionectomies; 6 central bisectionectomies; 10 nonsystematic resections; 2 liver transplantations | NA | 74.5 | 52.9 | 43.6 |
| Lin et al. [49] | NA | NA | NA | NA | 25 radical resections; 7 thrombectomies through a choledochotomy; 17 palliative internal and external bile duct drainages | NA | 42.86 | 18.37 | 12.24 |
| Zhou et al. [9] | 2 (28.57) | 1497.03 ± 3503.20 | 91.36 ± 80.92 umol/L | NA | NA | NA | NA | NA | NA |
| Zhou et al. [17] | 39 (67.24) | NA | NA | 4.60 ± 1.02 | 36 simple hepatectomies; 11 hepatectomies plus bile duct excision | NA | NA | NA | NA |
| Sun et al. [50] | 46 (38.33) | NA | 149.4 ± 129.75 | 5.05 ± 2.75 | 19 right hepatectomies; 35 left hepatectomies; 6 left lateral sectionectomies; 13 right sectionectomies; 47 non-anatomic resections; | 3 (2.5) | NA | NA | NA |
| Wu et al. [8] | 27 (90) | NA | 15.9 ± 4.15 umol/L | 7.4 ± 3.05 | NA | NA | NA | NA | NA |
| Conticchio et al. | 1 (100) | 2157 | 0.9 mg/dL | 1.8 | Segmentectomy S3 | 0 (0) | NA | NA | NA |
Abbreviations: AFP, α-fetoprotein; NA, Not Available; Ra, Range; BDR, bile duct resection; CBD, common bile duct.
Concerning baseline characteristics, our patient is 47 years old, which is in line with the literature mean age (55.35 ± 9.49). As shown in Table 1, males are more frequently affected by this pathology than females (82.80% vs. 17.20%) [8,9,17,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50].
Jaundice is the most common presenting symptom, as reported by 13 of 20 articles. A total of 53.28% (317/595) of patients had jaundice as a presenting symptom (Table 1).
The 57.97% (200/345) patients showed a mean AFP value >400 ng/mL (Table 2).
A meta-analysis conducted by Navadgi et al. in 2016 compared clinic-pathological characteristics and survival outcomes between HCC patients who underwent hepatic resection, with and without BDTT, including 6,051 patients from 11 studies, mostly conducted in Asia [51].
Patients with HCC with BDTT had worse histological features compared to those without BDTT in terms of higher rates of macrovascular and lymphovascular invasion and poorer differentiation. However, in the BDTT group after hepatectomy, this meta-analysis revealed an inferior long-term survival rate, with no decrease in the 3-year survival rate [51].
Another retrospective analysis, conducted by Wong et al., compared outcomes between all 37 HCC patients with BDTT and 222 control patients who underwent hepatic resection between 1989 and 2012. Notably, it also revealed similar 5-year overall survival (OS) and disease-free survival (DFS) when matched for tumor stage and adverse prognostic factors, which seems to suggest that BDTT was not relevant for patients’ prognosis [34].
So BDTT is not included as a prognostic factor in the most common HCC staging systems, such as Barcelona Clinic Liver Cancer (BCLC) and American Joint Commission on Cancer (AJCC) [52,53].
Anyway, recent evidences are still debating on the topic.
A retrospective study by Lu et al. analyzed 622 HCC Chinese patients who underwent hepatic resections, considering that the BCLC staging system is mainly based on data from Western HCC populations. The most commonly underlying liver disease was HBV (77%). This study revealed that patients with HCC with BDTT had a worse OS at 1, 3, and 5 years compared to those without BDTT (77%, 42%, and 23% vs. 80%, 60%, and 48%, respectively), limited, however, to the early stages of the disease (BCLC 0 and BCLC A). After recategorizing HCC with BDTT 0-A as BLCL B, the modified BLCL staging system showed a better prediction of OS and mortality [54].
In our review, 485 (485/838 57.87%) patients had a positive HBV test [8,9,17,34,35,36,37,38,39,40,41,42,43,45,46,47,48,50] (Table 1).
1-, 3-, and 5-year OS are reported in Table 2 [34,35,36,38,40,42,43,44,45,46,47,48,49].
Huang et al. analyzed outcomes of 1021 patients with HCC underwent R0 resection at 9 hepatobiliary referral centers. A total of 177 (17.34%) presented BDTT and it seems to be an independent risk factor. Furthermore, HCC with BDTT without macrovascular invasion was classified as BCLC B and AJCC IIIA, whereas HCC with BDTT with macrovascular invasion was classified as BCLC C and AJCC IIIB [55].
In addition, an higher incidence of post-liver-transplant recurrence in HCC patients with BDTT has been reported in the literature, although not in large-volume studies [35].
These results appear consistent with our clinical experience.
A single lesion ≤ 2 cm in diameter, such as the one we have described, with preserved liver function, is currently staged as BCLC 0, whereas nodular recurrence, which occurred in our case report, appears to be more consistent with a worse prognostic pattern.
HCC with BDTT is both clinically and radiologically difficult to distinguish from other primary biliary cancers, especially CCA.
CCAs are divided by anatomical localization into three types: perihilar, intrahepatic, and peripheral. This classification has also a prognostic and therapeutic value [56].
Perihilar CCA or Klatskin tumor is the most common one. Its growth is more often of the “periductal-infiltrating” type. It tends to be diagnosed earlier, with a smaller size compared to the intrahepatic one, due to the earlier presentation of symptoms.
Intrahepatic CCA more often has “mass-forming” growth, well limited from the surrounding hepatic parenchyma [57].
It is the most common type in the absence of other tumors or cirrhosis, although it can coexist with such diseases.
Peripheral CCA has histological features similar to the perihilar type [56].
A misdiagnosis between HCC with BDTT and CCA is described with an incidence of 4–55% [17,36].
However, it is important to recognize both of them in order to define patient management.
CCA patients are usually not candidates for liver transplantation because, despite radical surgery, they recur in 60% of cases, mainly in the first 2 years [56,58].
Surgery for CCA, if resectable, varies based on the location. For example, perihilar CCAs are usually treated with the resection of the biliary convergence with the main biliary duct, with major hepatic resection and caudectomy, while peripheral CCAs are treated with pancreatic-duodenectomy [56].
Conversely, partial hepatic resection or hemihepatectomy with bile duct preservation is the main surgical option for HCC with BDTT [17,59]. Recent Asian studies have proposed a more aggressive surgical approach, including major liver resection combined with bile duct resection [36].
HCC with hilar bile duct tumor thrombus (HBDTT) is a common subtype of HCC with BDTT, and it shares some imaging features with perihilar CCA: hilar mass, obstructed hilar bile duct, and upstream bile duct dilatation [17].
However, some other features can help in the differential diagnosis.
HCC typically has an increased arterial blood supply, so it usually shows hyperattenuation in the arterial phase and hypoattenuation in the portal venous phase, compared to the hepatic parenchyma.
Most HBDTTs should show the same enhancement pattern. However, some HCCs can show iso- or hypoattenuation in the arterial phase, with the enhancement in the arterial phase inversely correlated with the degree of blood clots and necrosis. So, hypoattenuation in the portal venous phase seems to be the most important imaging feature to distinguish HCC with BDTT from perihilar CCA [17].
Furthermore, HBDTT rarely infiltrates into the bile duct wall, which, consequently, is often regular without relevant enhancement.
Conversely, perihilar CCA more frequently reproduces the “periductal-infiltrating” type, so it usually shows a narrowed hilar bile duct with irregular or even obliterated wall thickening, with typically progressively delayed enhancement.
Washout in the portal venous phase is also the main feature to distinguish HCC with BDTT from intrahepatic CCA, together with the presence of tortuous tumoral vessels [17].
4. Conclusions
In conclusion, the diagnosis of HCC with BDTT can be reasonably considered in the presence of lesions of both hepatic parenchyma and bile ducts with a cirrhotic underlined liver disease, especially if they show typical washout in the portal venous phase.
However, several factors can jeopardize the diagnosis.
Furthermore, as already pointed out, the primary parenchymal tumor can be undetectable by preoperative imaging once the thrombus appears. These findings support the value of an early multidisciplinary patient assessment to obtain an accurate diagnosis of HCC with BDTT, which may have prognostic value that has not been recognized until now.
Author Contributions
Conceptualization, R.M. and A.S.; methodology, M.R.; validation, V.F., M.T., and M.R.F.; investigation, M.C.B.; resources, R.I.; data curation, R.V.; writing—original draft preparation, M.C.; writing—review and editing, N.M., P.A.; visualization, R.C.; supervision, A.C.; project administration, N.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Data supporting the results of the study will be available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Peng Y., Chen K., Li B., Xu H., Wei Y., Liu F. Laparoscopic versus open liver resection for resectable HCC with BCLC stage B: A propensity score-matched analysis. Updates Surg. 2022;74:1291–1297. doi: 10.1007/s13304-022-01309-2. [DOI] [PubMed] [Google Scholar]
- 2.Ceccarelli G., Andolfi E., Fontani A., Calise F., Rocca A., Giuliani A. Robot-assisted liver surgery in a general surgery unit with a “Referral Centre Hub&Spoke Learning Program”. Early outcomes after our first 70 consecutive patients. Minerva Chir. 2018;73:460–468. doi: 10.23736/s0026-4733.18.07651-4. [DOI] [PubMed] [Google Scholar]
- 3.Rocca A., Cipriani F., Belli G., Berti S., Boggi U., Bottino V., Cillo U., Cescon M., Cimino M., Corcione F., et al. The Italian Consensus on minimally invasive simultaneous resections for synchronous liver metastasis and primary colorectal cancer: A Delphi methodology. Updates Surg. 2021;73:1247–1265. doi: 10.1007/s13304-021-01100-9. [DOI] [PubMed] [Google Scholar]
- 4.Rocca A., Scacchi A., Cappuccio M., Avella P., Bugiantella W., De Rosa M., Costa G., Polistena A., Codacci-Pisanelli M., Amato B., et al. Robotic surgery for colorectal liver metastases resection: A systematic review. Int. J. Med. Robot. 2021;17:e2330. doi: 10.1002/rcs.2330. [DOI] [PubMed] [Google Scholar]
- 5.Rocca A., Brunese M.C., Santone A., Avella P., Bianco P., Scacchi A., Scaglione M., Bellifemine F., Danzi R., Varriano G., et al. Early Diagnosis of Liver Metastases from Colorectal Cancer through CT Radiomics and Formal Methods: A Pilot Study. J. Clin. Med. 2021;11:31. doi: 10.3390/jcm11010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loffredo D., Marvaso A., Ceraso S., Cinelli N., Rocca A., Vitale M., Rossi M., Genovese E., Amato B., Cinelli M. Minimal invasive surgery in treatment of liver metastases from colorectal carcinomas: Case studies and survival rates. BMC Surg. 2013;13((Suppl. S2)):S45. doi: 10.1186/1471-2482-13-S2-S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y.W., Yong C.C., Lin C.C., Wang C.C., Chen C.L., Cheng Y.F., Wang J.H., Yen Y.H. Liver resection in elderly patients with hepatocellular carcinoma: Age does matter. Updates Surg. 2021;73:1371–1380. doi: 10.1007/s13304-021-01021-7. [DOI] [PubMed] [Google Scholar]
- 8.Wu J.Y., Huang L.M., Bai Y.N., Wei Y.G., Zhang Z.B., Yan M.L. Imaging Features of Hepatocellular Carcinoma with Bile Duct Tumor Thrombus: A Multicenter Study. Front. Oncol. 2021;11:723455. doi: 10.3389/fonc.2021.723455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou D., Hu G.F., Gao W.C., Zhang X.Y., Guan W.B., Wang J.D., Ma F. Hepatocellular carcinoma with tumor thrombus in bile duct: A proposal of new classification according to resectability of primary lesion. World J. Gastroenterol. 2020;26:7005–7021. doi: 10.3748/wjg.v26.i44.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J.Y., Sun J.X., Wu J.Y., Huang X.X., Bai Y.N., Zeng Y.Y., Zhang Z.B., Cheng S.Q., Yan M.L. A nomogram based on combining systemic and hepatic inflammation markers for predicting microscopic bile duct tumor thrombus in hepatocellular carcinoma. BMC Cancer. 2021;21:272. doi: 10.1186/s12885-021-07956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rompianesi G., Pegoraro F., Ceresa C.D., Montalti R., Troisi R.I. Artificial intelligence in the diagnosis and management of colorectal cancer liver metastases. World J. Gastroenterol. 2022;28:108–122. doi: 10.3748/wjg.v28.i1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viganò L., Jayakody Arachchige V.S., Fiz F. Is precision medicine for colorectal liver metastases still a utopia? New perspectives by modern biomarkers, radiomics, and artificial intelligence. World J. Gastroenterol. 2022;28:608–623. doi: 10.3748/wjg.v28.i6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji G.W., Wang K., Xia Y.X., Li X.C., Wang X.H. Application and challenge of radiomics technique in the era of precision medicine for hepatobiliary disease. Chin. J. Surg. 2020;58:749–753. doi: 10.3760/cma.j.cn112139-20200605-00439. [DOI] [PubMed] [Google Scholar]
- 14.Altini N., Prencipe B., Cascarano G.D., Brunetti A., Brunetti G., Triggiani V., Carnimeo L., Marino F., Guerriero A., Villani L., et al. Liver, kidney and spleen segmentation from CT scans and MRI with deep learning: A survey. Neurocomputing. 2022;490:30–53. doi: 10.1016/j.neucom.2021.08.157. [DOI] [Google Scholar]
- 15.Amato B., Compagna R., Rocca A., Bianco T., Milone M., Sivero L., Vigliotti G., Amato M., Danzi M., Aprea G., et al. Fondaparinux vs warfarin for the treatment of unsuspected pulmonary embolism in cancer patients. Drug Des. Devel. Ther. 2016;10:2041–2046. doi: 10.2147/dddt.s106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komici K., Cappuccio M., Scacchi A., Vaschetti R., Delli Carpini G., Picerno V., Avella P., Brunese M.C., Rengo G., Guerra G., et al. The Prevalence and the Impact of Frailty in Hepato-Biliary Pancreatic Cancers: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022;11:1116. doi: 10.3390/jcm11041116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X., Wang J., Tang M., Huang M., Xu L., Peng Z., Li Z.P., Feng S.T. Hepatocellular carcinoma with hilar bile duct tumor thrombus versus hilar Cholangiocarcinoma on enhanced computed tomography: A diagnostic challenge. BMC Cancer. 2020;20:54. doi: 10.1186/s12885-020-6539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocca A., Brunese M.C., Cappuccio M., Scacchi A., Martucci G., Buondonno A., Perrotta F.M., Quarto G., Avella P., Amato B. Impact of Physical Activity on Disability Risk in Elderly Patients Hospitalized for Mild Acute Diverticulitis and Diverticular Bleeding Undergone Conservative Management. Medicina. 2021;57:360. doi: 10.3390/medicina57040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim A.Y., Jeong W.K. Intraductal malignant tumors in the liver mimicking cholangiocarcinoma: Imaging features for differential diagnosis. Clin. Mol. Hepatol. 2016;22:192–197. doi: 10.3350/cmh.2016.22.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banales J.M., Marin J.J., Lamarca A., Rodrigues P.M., Khan S.A., Roberts L.R., Cardinale V., Carpino G., Andersen J.B., Braconi C., et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H.S., Han Y., Kang J.S., Kang Y.H., Lee M., Sohn H.J., Kim H., Kwon W., Jang J.Y. Serum carcinoembryonic antigen and carbohydrate antigen 19-9 as preoperative diagnostic biomarkers of extrahepatic bile duct cancer. BJS Open. 2021;5:zrab127. doi: 10.1093/bjsopen/zrab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bracale U., Podda M., Castiglioni S., Peltrini R., Sartori A., Arezzo A., Corcione F., Agresta F. Changes in surgicaL behaviOrs dUring the COVID-19 pandemic. The SICE CLOUD19 Study. Updates Surg. 2021;73:731–744. doi: 10.1007/s13304-021-01010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallo G., Picciariello A., Di Tanna G.L., Santoro G.A., Perinotti R., Grossi U. E-consensus on telemedicine in colorectal surgery: A RAND/UCLA-modified study. Updates Surg. 2022;74:163–170. doi: 10.1007/s13304-021-01139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldrighetti L., Boggi U., Falconi M., Giuliante F., Cipriani F., Ratti F., Torzilli G. Perspectives from Italy during the COVID-19 pandemic: Nationwide survey-based focus on minimally invasive HPB surgery. Updates Surg. 2020;72:241–247. doi: 10.1007/s13304-020-00815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavaliere D., Parini D., Marano L., Cipriani F., Di Marzo F., Macrì A., D’Ugo D., Roviello F., Gronchi A. Surgical management of oncologic patient during and after the COVID-19 outbreak: Practical recommendations from the Italian society of Surgical Oncology. Updates Surg. 2021;73:321–329. doi: 10.1007/s13304-020-00921-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuliani A., Avella P., Segreto A.L., Izzo M.L., Buondonno A., Coluzzi M., Cappuccio M., Brunese M.C., Vaschetti R., Scacchi A., et al. Postoperative Outcomes Analysis After Pancreatic Duct Occlusion: A Safe Option to Treat the Pancreatic Stump after Pancreaticoduodenectomy in Low-Volume Centers. Front. Surg. 2021;8:804675. doi: 10.3389/fsurg.2021.804675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luciani C., Scacchi A., Vaschetti R., Di Marzo G., Fatica I., Cappuccio M., Guerra G., Ceccarelli G., Avella P., Rocca A. The uniportal VATS in the treatment of stage II pleural empyema: A safe and effective approach for adults and elderly patients-a single-center experience and literature review. World J. Emerg. Surg. 2022;17:46. doi: 10.1186/s13017-022-00438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceccarelli G., Rocca A., De Rosa M., Fontani A., Ermili F., Andolfi E., Bugiantella W., Levi Sandri G.B. Minimally invasive robotic-assisted combined colorectal and liver excision surgery: Feasibility, safety and surgical technique in a pilot series. Updates Surg. 2021;73:1015–1022. doi: 10.1007/s13304-021-01009-3. [DOI] [PubMed] [Google Scholar]
- 29.Marte G., Scuderi V., Rocca A., Surfaro G., Migliaccio C., Ceriello A. Laparoscopic splenectomy: A single center experience. Unusual cases and expanded inclusion criteria for laparoscopic approach. Updates Surg. 2013;65:115–119. doi: 10.1007/s13304-013-0197-0. [DOI] [PubMed] [Google Scholar]
- 30.Buondonno A., Avella P., Cappuccio M., Scacchi A., Vaschetti R., Di Marzo G., Maida P., Luciani C., Amato B., Brunese M.C., et al. A Hub and Spoke Learning Program in Bariatric Surgery in a Small Region of Italy. Front. Surg. 2022;9:855527. doi: 10.3389/fsurg.2022.855527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Granata V., Fusco R., Bicchierai G., Cozzi D., Grazzini G., Danti G., De Muzio F., Maggialetti N., Smorchkova O., D’Elia M., et al. Diagnostic protocols in oncology: Workup and treatment planning. Part 1: The optimitation of CT protocol. Eur. Rev. Med. Pharmacol. Sci. 2021;25:6972–6994. doi: 10.26355/eurrev_202111_27246. [DOI] [PubMed] [Google Scholar]
- 32.Granata V., Bicchierai G., Fusco R., Cozzi D., Grazzini G., Danti G., De Muzio F., Maggialetti N., Smorchkova O., D’Elia M. Diagnostic protocols in oncology: Workup and treatment planning. Part 2: Abbreviated MR protocol. Eur. Rev. Med. Pharmacol. Sci. 2021;25:6499–6528. doi: 10.26355/eurrev_202111_27094. [DOI] [PubMed] [Google Scholar]
- 33.Bevilacqua V., Brunetti A., Trotta G.F., Dimauro G., Elez K., Alberotanza V., Scardapane A. A novel approach for Hepatocellular Carcinoma detection and classification based on triphasic CT Protocol; Proceedings of the 2017 IEEE Congress on Evolutionary Computation (CEC); Donostia, Spain. 5–8 June 2017; Manhattan, NY, USA: IEEE; 2017. [Google Scholar]
- 34.Wong T.C., Cheung T.T., Chok K.S., Chan A.C., Dai W.C., Chan S.C., Poon R.T., Fan S.T., Lo C.M. Outcomes of hepatectomy for hepatocellular carcinoma with bile duct tumour thrombus. HPB. 2015;17:401–408. doi: 10.1111/hpb.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ha T.Y., Hwang S., Moon D.B., Ahn C.S., Kim K.H., Song G.W., Jung D.H., Park G.C., Park H.W., Park Y.H., et al. Long-term survival analysis of liver transplantation for hepatocellular carcinoma with bile duct tumor thrombus. Transplant. Proc. 2014;46:774–777. doi: 10.1016/j.transproceed.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 36.Kim D.S., Kim B.W., Hatano E., Hwang S., Hasegawa K., Kudo A., Ariizumi S., Kaibori M., Fukumoto T., Baba H., et al. Surgical Outcomes of Hepatocellular Carcinoma with Bile Duct Tumor Thrombus: A Korea-Japan Multicenter Study. Ann. Surg. 2020;271:913–921. doi: 10.1097/SLA.0000000000003014. [DOI] [PubMed] [Google Scholar]
- 37.Satoh S., Ikai I., Honda G., Okabe H., Takeyama O., Yamamoto Y., Yamamoto N., Iimuro Y., Shimahara Y., Yamaoka Y. Clinicopathologic evaluation of hepatocellular carcinoma with bile duct thrombi. Surgery. 2000;128:779–783. doi: 10.1067/msy.2000.108659. [DOI] [PubMed] [Google Scholar]
- 38.Shiomi M., Kamiya J., Nagino M., Uesaka K., Sano T., Hayakawa N., Kanai M., Yamamoto H., Nimura Y. Hepatocellular carcinoma with biliary tumor thrombi: Aggressive operative approach after appropriate preoperative management. Surgery. 2001;129:692–698. doi: 10.1067/msy.2001.113889. [DOI] [PubMed] [Google Scholar]
- 39.Peng S.Y., Wang J.W., Liu Y.B., Cai X.J., Deng G.L., Xu B., Li H.J. Surgical intervention for obstructive jaundice due to biliary tumor thrombus in hepatocellular carcinoma. World J. Surg. 2004;28:43–46. doi: 10.1007/s00268-003-7079-4. [DOI] [PubMed] [Google Scholar]
- 40.Esaki M., Shimada K., Sano T., Sakamoto Y., Kosuge T., Ojima H. Surgical results for hepatocellular carcinoma with bile duct invasion: A clinicopathologic comparison between macroscopic and microscopic tumor thrombus. J. Surg. Oncol. 2005;90:226–232. doi: 10.1002/jso.20260. [DOI] [PubMed] [Google Scholar]
- 41.Shao W., Sui C., Liu Z., Yang J., Zhou Y. Surgical outcome of hepatocellular carcinoma patients with biliary tumor thrombi. World J. Surg. Oncol. 2011;9:2. doi: 10.1186/1477-7819-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu X.H., Xu L.B., Liu C., Zhang R., Wang J. Clinicopathological characteristics of 20 cases of hepatocellular carcinoma with bile duct tumor thrombi. Dig. Dis. Sci. 2011;56:252–259. doi: 10.1007/s10620-010-1256-8. [DOI] [PubMed] [Google Scholar]
- 43.Noda T., Nagano H., Tomimaru Y., Murakami M., Wada H., Kobayashi S., Marubashi S., Eguchi H., Takeda Y., Tanemura M., et al. Prognosis of hepatocellular carcinoma with biliary tumor thrombi after liver surgery. Surgery. 2011;149:371–377. doi: 10.1016/j.surg.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Moon D.B., Hwang S., Wang H.J., Yun S.S., Kim K.S., Lee Y.J., Kim K.H., Park Y.K., Xu W., Kim B.W., et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: A Korean multicenter study. World J. Surg. 2013;37:443–451. doi: 10.1007/s00268-012-1845-0. [DOI] [PubMed] [Google Scholar]
- 45.Oba A., Takahashi S., Kato Y., Gotohda N., Kinoshita T., Shibasaki H., Ikeda M., Konishi M. Usefulness of resection for hepatocellular carcinoma with macroscopic bile duct tumor thrombus. Anticancer Res. 2014;34:4367–4372. [PubMed] [Google Scholar]
- 46.Rammohan A., Sathyanesan J., Rajendran K., Pitchaimuthu A., Perumal S.K., Balaraman K., Ramasamy R., Palaniappan R., Govindan M. Bile duct thrombi in hepatocellular carcinoma: Is aggressive surgery worthwhile? HPB. 2015;17:508–513. doi: 10.1111/hpb.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasai Y., Hatano E., Seo S., Taura K., Yasuchika K., Uemoto S. Hepatocellular carcinoma with bile duct tumor thrombus: Surgical outcomes and the prognostic impact of concomitant major vascular invasion. World J. Surg. 2015;39:1485–1493. doi: 10.1007/s00268-015-2985-9. [DOI] [PubMed] [Google Scholar]
- 48.Chotirosniramit A., Liwattanakun A., Lapisatepun W., Ko-Iam W., Sandhu T., Junrungsee S. A single institution report of 19 hepatocellular carcinoma patients with bile duct tumor thrombus. J. Hepatocell. Carcinoma. 2017;4:41–47. doi: 10.2147/JHC.S126308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Z., Han M., Zhou Z. Prognosis for patients with hepatocellular carcinoma (HCC) with bile duct tumor thrombus (BDTT) after surgical treatment. Biosci. Trends. 2019;13:77–85. doi: 10.5582/bst.2018.01234. [DOI] [PubMed] [Google Scholar]
- 50.Sun J., Wu J., Shi J., Liu C., Wei Y., Zhou J., Zhang Z., Yan M., Cheng S. Thrombus-First Surgery for Hepatocellular Carcinoma with Bile Duct Tumor Thrombus. J. Gastrointest. Surg. 2021;25:1973–1979. doi: 10.1007/s11605-020-04813-1. [DOI] [PubMed] [Google Scholar]
- 51.Navadgi S., Chang C.C., Bartlett A., McCall J., Pandanaboyana S. Systematic review and meta-analysis of outcomes after liver resection in patients with hepatocellular carcinoma (HCC) with and without bile duct thrombus. HPB. 2016;18:312–316. doi: 10.1016/j.hpb.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado Á., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chun Y.S., Pawlik T.M., Vauthey J.N. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann. Surg. Oncol. 2018;4:845–847. doi: 10.1245/s10434-017-6025-x. [DOI] [PubMed] [Google Scholar]
- 54.Lu W.P., Tang H.W., Yang Z.Y., Jiang K., Chen Y.L., Lu S.C. A proposed modification for the Barcelona Clinic Liver Cancer staging system: Adding bile duct tumor thrombus status in patients with hepatocellular carcinoma. Am. J. Surg. 2020;220:965–971. doi: 10.1016/j.amjsurg.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Huang Q., Chen Y., Lin K., Sun C., Zheng S., Chen J., Wang Y., Zhou Y., Zhou W., Liu J., et al. Redefining Hepatocellular Carcinoma Staging Systems Based on the Bile Duct Invasion Status: A Multicenter Study. Front. Oncol. 2021;11:673285. doi: 10.3389/fonc.2021.673285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Associazione Italiana di Oncologia Medica (AIOM) Linee Guida “Tumori delle Vie Biliari”. 2019. [(accessed on 2 November 2022)]. Available online: https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Vie_biliari.pdf.
- 57.Machairas N., Kostakis I.D., Schizas D., Kykalos S., Nikiteas N., Sotiropoulos G.C. Meta-analysis of laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma. Updates Surg. 2021;73:59–68. doi: 10.1007/s13304-020-00930-3. [DOI] [PubMed] [Google Scholar]
- 58.Gruttadauria S., Barbara M., Liotta R. Liver transplantation for unresectable intrahepatic cholangiocarcinoma: An Italian experience. Updates Surg. 2021;4:1587–1588. doi: 10.1007/s13304-021-01064-w. [DOI] [PubMed] [Google Scholar]
- 59.Peng B.G., Liang L.J., Li S.Q., Zhou F., Hua Y.P., Luo S.M. Surgical treatment of hepatocellular carcinoma with bile duct tumor thrombi. World J. Gastroenterol. 2005;11:3966–3969. doi: 10.3748/wjg.v11.i25.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the results of the study will be available on request.