Abstract
An understanding of the antigen presentation mechanisms that mediate induction of protective immune responses against malaria is essential for the development of successful immunization approaches. Here we show that dendritic cells presenting Plasmodium yoelii sporozoite antigens are able to activate specific CD4+ and CD8+ T cells and initiate protective immune responses against malaria in mice.
The intracellular parasite Plasmodium is the causative agent of malaria, one of the most prevalent human infectious diseases in the world, with high mortality rates during childhood and complications in infected adults. After injection by mosquito of Plasmodium sporozoites into the vertebrate host, the sporozoites must infect hepatocytes before developing into the pathogenic erythrocytic stages. Thus, protection induced against malaria liver stages diminishes or abrogates further development of the disease (15). In areas where malaria is endemic, individuals generate partial protective immunity against malaria only after several years of frequent exposure to the parasite. However, complete protection against malaria can be induced by a few inoculations of irradiated Plasmodium sporozoites (3, 16). Due to the lack of an in vitro system to generate large amounts of sporozoites, broad use of an irradiated sporozoite-based vaccine against malaria is not feasible. Therefore, an understanding of the mechanisms that mediate induction of protective immune responses against preerythrocytic forms of the parasite is important for the development of alternative immunization approaches.
Activation of T cells by antigen-presenting cells (APCs) is required to initiate specific immune responses. Dendritic cells (DCs) are a unique type of APC because of their ability to induce primary immune responses by efficient activation of naïve T cells (11). DCs reside in tissues as immature cells with high phagocytic capacity. Following activation and antigen capture, they migrate to the lymphoid organs, where they prime antigen-specific CD4+ and CD8+ T cells (1). Since the APCs that mediate protective immune responses to malaria have not yet been identified, we investigated the capacity of DCs to present Plasmodium sporozoite antigens to T cells and induce inhibition of liver-stage development of the parasite.
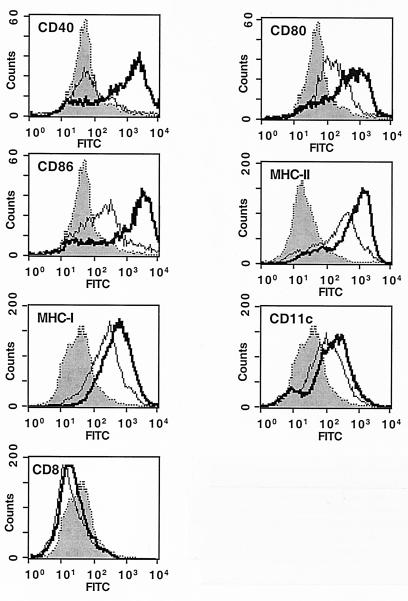
The ability of DCs to initiate protective antimalaria immune responses was examined by adoptive transfer to naïve mice of DCs presenting sporozoite-derived antigens. The activation of specific CD4+ and CD8+ T cells and the degree of protection against sporozoite infection were measured in these mice. Primary cultures of immature DCs from BALB/c mice (haplotype H-2Kd) were obtained by in vitro differentiation of bone marrow-derived precursors during 10 days (29), using the supernatant of the myeloma cell line Ag8653 expressing mouse recombinant granulocyte-macrophage colony-stimulating factor as a source of this cytokine (24). Analysis of the bone marrow-derived cell population was performed using fluorescence-activated cell sorting, which showed that these cells expressed the distinctive DC surface marker CD11c, as well as major histocompatibility complex (MHC) class I and II molecules. Activation of these cells by the addition of lipopolysaccharide (LPS) (1 μg/ml) for 24 h induced the characteristic DC increase of the costimulatory molecules CD40, CD80, and CD86 and of MHC class I and II molecules on the plasma membrane (29) (Fig. 1).
FIG. 1.
Characterization of DC cultures by fluorescence-activated cell sorting analysis. Control or LPS-activated bone marrow-derived cell cultures were incubated with antibodies against different DC markers. Thin lines, nonactivated DCs; thick lines, LPS-activated DCs; shading, irrelevant IgG control staining. FITC, fluorescein isothiocyanate.
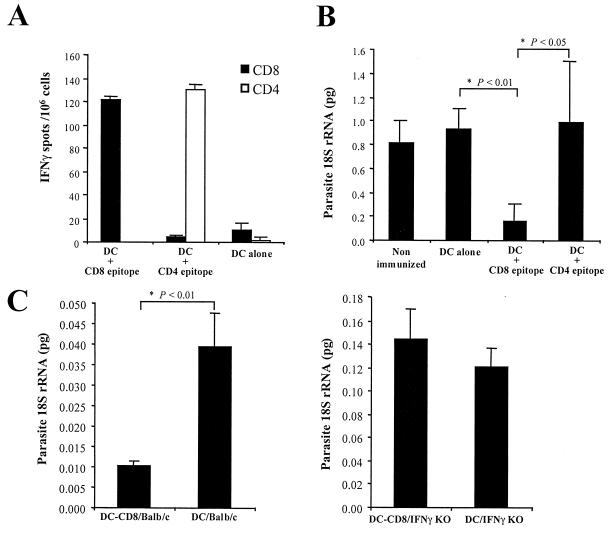
Peptides containing the identified H-2Kd-specific CD4+ (YNRNIVNRLLGDALNGKPEEK) (8) and CD8+ (SYVPSAEQI) (20) epitopes from the circumsporozoite protein (CS) of the rodent malaria species P. yoelii were incubated for 1 h at a concentration of 30 μg/ml with the mature DCs. The peptides comprising both epitopes are identical in P. yoelii nonlethal (17X) and lethal (YM) strains. Peptides were allowed to bind to the MHC molecules of DCs that were activated by incubation with LPS (1 μg/ml) for 24 h. DCs were washed and transferred to naïve BALB/c mice (7.5 × 105 DCs/mouse) by intravenous injection. Twelve days after transfer of DCs, activation of specific anti-CS CD4+ and CD8+ T cells was detected using an ELISPOT assay for the production of gamma interferon (IFN-γ) (13, 19). For the ELISPOT assay, T cells were enriched from spleen suspensions by the elimination of plastic adherent and immunoglobin G (IgG)- and IgM-reactive cells. The numbers of antigen-specific T cells are calculated by subtracting the mean spot numbers in control wells without peptide (mean value of 273 spots per 106 cells) from the mean spot numbers in parallel cultures where APCs (A20.2J cells) were preincubated with the corresponding peptides. High numbers of specific CD4+ and CD8+ T cells were found in mice injected with DCs pulsed with the corresponding CS epitopes (Fig. 2A). Transfer of the same number of control DCs did not induce a specific T-cell response. These results indicate that DCs presenting sporozoite epitopes are able to initiate specific antimalaria immune responses in naïve mice.
FIG. 2.
DCs presenting CS-derived CD4 and CD8 epitopes initiate specific antimalaria immune responses. (A) Results of ELISPOT showing the numbers of IFN-γ-secreting CD4+ (white bars) and CD8+ (black bars) T cells detected in the spleens of two mice immunized by intravenous inoculation of DCs preincubated with the specific CD4 or CD8 peptides. T cells from nonimmunized mice did not induce any spots in the cultures. The results are expressed as averages ± standard deviations for duplicate cultures. (B) Inhibition of parasite development in the livers of groups of three mice nonimmunized or immunized with DCs incubated with corresponding peptides or untreated, as detected by competitive RT-PCR. Results are expressed as averages ± standard deviations for three mice analyzed individually. Statistically significant differences between groups were determined by analysis of variance (ANOVA). (C) Inhibition of parasite development in the livers of groups of four BALB/c or IFN-γ knockout BALB/c mice immunized with DCs alone or DCs preincubated with the specific CD8 peptide, as detected by competitive RT-PCR. Results are expressed as averages ± standard deviations for four mice analyzed individually. Statistically significant differences among groups were determined by ANOVA.
The degree of protection conferred by DCs presenting CS epitopes was measured 2 weeks after adoptive transfer. Mice were challenged with 105 P. yoelii sporozoites (nonlethal parasite line 17X) obtained by dissection of infected Anopheles stephensi mosquito salivary glands. The degree of liver infection was determined by competitive reverse transcription (RT)-PCR of parasite 18S rRNA in mouse livers 42 h after sporozoite inoculation (20). Mice immunized by adoptive transfer of DCs loaded with the CS-derived CD8+ epitope displayed a much lower level of parasite RNA in the liver than mice transferred with DCs alone or DCs loaded with the CD4+ epitope (Fig. 2B). Although similar numbers of IFN-γ-producing CD4+ and CD8+ T cells were induced in the mice by DCs loaded with the corresponding peptides (Fig. 2A), only activation of CD8+ T cells inhibited development of parasites in the liver (Fig. 2B).
In mouse models, protection induced by irradiated sporozoites is mediated by CD8+ T cells to liver-stage parasites and is dependent on IFN-γ (21, 22, 28). To study whether DC-induced protection is mediated by a similar mechanism, control DCs or DCs loaded with the CS-derived CD8+ epitope were transferred to groups of four IFN-γ knockout mice [C129S7(B6)-IFNg; Jackson] and the corresponding background control mouse strain. Ten days later, protection against challenge with live sporozoites was evaluated as described above. In contrast to normal mice, transfer of DCs loaded with the CS-derived CD8+ epitope to IFN-γ knockout mice did not inhibit the development of P. yoelii parasites in the liver (Fig. 2C). These results indicate that, as previously shown for immunization with irradiated sporozoites, the protection induced by transfer of DCs is also mediated by IFN-γ. Increased replication of P. yoelii in the liver of IFN-γ knockout mice was observed (Fig. 2C), confirming the inhibitory effect of IFN-γ on Plasmodium development in the liver (12).
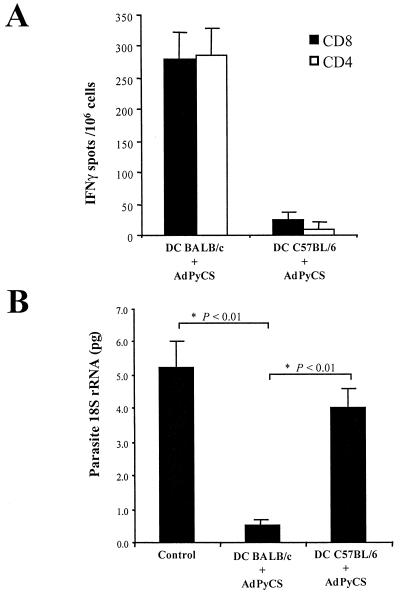
During the course of natural infections, correct processing of antigens and loading of resulting peptides onto MHC molecules of APCs is required to obtain T-cell activation. To test the ability of DCs to process and present sporozoite CS protein epitopes, we infected DCs with recombinant adenovirus expressing the CS protein of P. yoelii (AdPyCS) (18). DCs derived from BALB/c mice were infected with AdPyCS (multiplicity of infection, 200) for 24 h before being transferred to naïve BALB/c mice (5 × 105 DCs/mouse). Transfer of infected DCs resulted in the activation of specific anti-CS CD4+ and CD8+ T cells (Fig. 3A) and in the inhibition of development of parasites in the liver (Fig. 3B), as determined 2 weeks later.
FIG. 3.
DCs process CS protein and present CD4 and CD8 epitopes to T cells. (A) Results of ELISPOT showing the numbers of IFN-γ-secreting CD4+ (white bars) and CD8+ (black bars) T cells detected in the spleens of two mice immunized by intravenous inoculation of DCs infected with AdPyCS derived from BALB/c or C57BL/6 mice. The numbers of antigen-specific T cells were calculated as described in the text. The results are expressed as averages ± standard deviations for duplicate cultures. (B) Inhibition of parasite development in the livers of groups of three mice nonimmunized or immunized with DCs derived from BALB/c or C57BL/6 mice and infected with AdPyCS, as detected by competitive RT-PCR. Results are expressed as averages ± standard deviations for three mice analyzed individually. Statistically significant differences between groups were determined by analysis of variance.
Transfer of the same number of AdPyCS-infected DCs derived from C57BL/6 mice (haplotype H-2Kb) into BALB/c mice (haplotype H-2Kd) did not result in activation of T cells (Fig. 3A) or protection against sporozoite challenge (Fig. 3B). Taken together these results indicate that the immune response generated in syngeneic mice is induced after specific processing and presentation of CS protein by DCs. The lack of activation of an immune response by allogenic DCs indicates that this response cannot be attributed to mouse infection with residual adenovirus particles or to uptake of CS antigens from the infected DCs by APCs of the recipient mice.
Protection against liver-stage malaria is mainly dependent on the induction of effective T-cell immune responses that eliminate infected hepatocytes (10, 21, 22). Therefore, the development of antimalaria vaccines against this stage is based on the activation of cytotoxic T-cell responses (6, 14, 23). Nonreplicating vaccines, such as inactivated pathogens, can elicit effective antibody responses; however, the induction of effector CD8+ T-cell responses has been difficult to achieve. Recombinant vaccines, including genetically modified viral vectors and DNA vaccines, can overcome this problem, as they can induce both effective antibody and CD8+-mediated responses in vivo (17, 25, 26). These responses can be enhanced by targeting expression of recombinant antigens to APCs, and in particular to DCs (2, 4, 5, 7, 9), or by coexpression of granulocyte-macrophage colony-stimulating factor (27, 30), a cytokine that enhances the maturation of DC precursors. These observations reveal the importance of DCs as mediators of protective immune responses that are triggered by these vaccines. Our results show that DCs can initiate CD8+-mediated immune responses against malaria liver stages and should be targeted to obtain effective antimalaria vaccines.
Acknowledgments
We thank Ruth S. Nussenzweig and Moriya Tsuji for critically revising the manuscript; James M. Wilson for providing AdPyCS; and Ivette Caro and Sandy Nosseir for mosquito salivary gland dissection.
O.B.-R. was supported by the Starr Foundation. A.R. was supported by the American Liver Foundation.
REFERENCES
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y J, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Chattergoon M A, Kim J J, Yang J S, Robinson T M, Lee D J, Dentchev T, Wilson D M, Ayyavoo V, Weiner D B. Targeted antigen delivery to antigen-presenting cells including dendritic cells by engineered fas-mediated apoptosis. Nat Biotechnol. 2000;18:974–979. doi: 10.1038/79470. [DOI] [PubMed] [Google Scholar]
- 3.Clyde D F, Most H, McCarthy V C, Vanderberg J P. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973;266:169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 5.Deliyannis G, Boyle J S, Brady J L, Brown L E, Lew A M. A fusion DNA vaccine that targets antigen-presenting cells increases protection from viral challenge. Proc Natl Acad Sci USA. 2000;97:6676–6680. doi: 10.1073/pnas.120162497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doolan D L, Hedstrom R C, Gardner M J, Sedegah M, Wang H, Gramzinski R A, Margalith M, Hobart P, Hoffman S L. DNA vaccination as an approach to malaria control: current status and strategies. Curr Top Microbiol Immunol. 1998;226:37–56. doi: 10.1007/978-3-642-80475-5_3. [DOI] [PubMed] [Google Scholar]
- 7.Gentschev I, Dietrich G, Spreng S, Kolb-Maurer A, Daniels J, Hess J, Kaufmann S H, Goebel W. Delivery of protein antigens and DNA by virulence-attenuated strains of salmonella typhimurium and listeria monocytogenes. J Biotechnol. 2000;83:19–26. doi: 10.1016/s0168-1656(00)00293-5. [DOI] [PubMed] [Google Scholar]
- 8.Grillot D, Michel M, Muller I, Tougne C, Renia L, Mazier D, Corradin G, Lambert P H, Louis J A, Del Guidice G. Immune responses to defined epitopes of the circumsporozoite protein of the murine malaria parasite, Plasmodium yoelii. Eur J Immunol. 1990;20:1215–1222. doi: 10.1002/eji.1830200604. [DOI] [PubMed] [Google Scholar]
- 9.Haicheur N, Bismuth E, Bosset S, Adotevi O, Warnier G, Lacabanne V, Regnault A, Desaymard C, Amigorena S, Ricciardi-Castagnoli P, Goud B, Fridman W H, Johannes L, Tartour E. The B subunit of Shiga toxin fused to a tumor antigen elicits CTL and targets dendritic cells to allow MHC class I-restricted presentation of peptides derived from exogenous antigens. J Immunol. 2000;165:3301–3308. doi: 10.4049/jimmunol.165.6.3301. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman S L, Isenbarger D, Long G W, Sedegah M, Szarfman A, Waters L, Hollingdale M R, van der Meide P H, Finbloom D S, Ballou W R. Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science. 1989;244:1078–1081. doi: 10.1126/science.2524877. [DOI] [PubMed] [Google Scholar]
- 11.Mellman I, Turley S J, Steinman R M. Antigen processing for amateurs and professionals. Trends Cell Biol. 1998;8:231–237. doi: 10.1016/s0962-8924(98)01276-8. [DOI] [PubMed] [Google Scholar]
- 12.Mellouk S, Green S J, Nacy C A, Hoffman S L. IFN-gamma inhibits development of Plasmodium berghei exoerythrocytic stages in hepatocytes by an L-arginine-dependent effector mechanism. J Immunol. 1991;146:3971–3976. [PubMed] [Google Scholar]
- 13.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 14.Nardin E, Zavala F, Nussenzweig V, Nussenzweig R S. Pre-erythrocytic malaria vaccine: mechanisms of protective immunity and human vaccine trials. Parassitologia. 1999;41:397–402. [PubMed] [Google Scholar]
- 15.Nussenzweig R S, Long C A. Malaria vaccines: multiple targets. Science. 1994;265:1381–1383. doi: 10.1126/science.8073276. [DOI] [PubMed] [Google Scholar]
- 16.Nussenzweig R S, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 17.Ramshaw I A, Ramsay A J. The prime-boost strategy: exciting prospects for improved vaccination. Immunol Today. 2000;21:163–165. doi: 10.1016/s0167-5699(00)01612-1. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues E G, Zavala F, Eichinger D, Wilson J M, Tsuji M. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J Immunol. 1997;158:1268–1274. [PubMed] [Google Scholar]
- 19.Rodrigues M, Li S, Murata K, Rodriguez D, Rodriguez J R, Bacik I, Bennink J R, Yewdell J W, Garcia-Sastre A, Nussenzweig R S, et al. Influenza and vaccinia viruses expressing malaria CD8+ T and B cell epitopes. Comparison of their immunogenicity and capacity to induce protective immunity. J Immunol. 1994;153:4636–4648. [PubMed] [Google Scholar]
- 20.Rodrigues M M, Cordey A S, Arreaza G, Corradin G, Romero P, Maryanski J L, Nussenzweig R S, Zavala F. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991;3:579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 21.Romero P, Maryanski J L, Corradin G, Nussenzweig R S, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 22.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 23.Seder R A, Hill A V. Vaccines against intracellular infections requiring cellular immunity. Nature. 2000;406:793–798. doi: 10.1038/35021239. [DOI] [PubMed] [Google Scholar]
- 24.Stockinger B, Zal T, Zal A, Gray D. B cells solicit their own help from T cells. J Exp Med. 1996;183:891–899. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang D C, DeVit M, Johnston S A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 26.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 27.Weiss W R, Ishii K J, Hedstrom R C, Sedegah M, Ichino M, Barnhart K, Klinman D M, Hoffman S L. A plasmid encoding murine granulocyte-macrophage colony-stimulating factor increases protection conferred by a malaria DNA vaccine. J Immunol. 1998;161:2325–2332. [PubMed] [Google Scholar]
- 28.Weiss W R, Sedegah M, Beaudoin R L, Miller L H, Good M F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci USA. 1988;85:573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann V S, Davoust J, Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiang Z, Ertl H C. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]