Abstract
Interstitial lung diseases (ILD) are part of a large heterogeneous group of diseases that differ in many ways (in their cause, clinical presentation, and response to therapy, etc.), but there are similar pathophysiological mechanisms involved in the development of the inflammation and/or fibrosis of the lungs. Currently, several criteria for pulmonary fibrosis (PF) and progressive pulmonary fibrosis (PPF) are proposed, and the information on the prevalence and characteristics of these conditions is limited. The aim of this study was to evaluate the spectrum of PF and PPF according to the registry of patients with ILD in eastern Siberia. Materials and methods: The study included patients with ILD from all of the medical institutions in the Irkutsk region (eastern Siberia). Each case of ILD (n = 270) was reviewed by a multidisciplinary discussion panel. The ILD patient registry included information on the clinical findings, history, pulmonary function tests, high-resolution computed tomography (HRCT), and histological findings. The follow-up period for the patients varied from 1 to 5 years. Results: Pulmonary fibrosis was detected by HRCT in 104 patients with ILD (38.5%). PF was present in 100% of the patients with IPF and SS-ILD, in 90.9% of the patients with CHP, in 71.4% of the patients with NSIP, and in 60% of the patients with RA-ILD. Sixty-two patients met the criteria for PPF (23.0% of the entire ILD cohort and 59.6% of the patients with PF). PPF occurred most often in the patients with IPF, CHP, IPAF, and SSc-ILD: 100%, 72.7%, 40%, and 38.5% of them, respectively. The variables associated with fibrosis progression included Velcro crackles (OR 18.3, p < 0.001) and late diagnosis (OR 4.1, p < 0.001). Conclusion: Pulmonary fibrosis and progressive pulmonary fibrosis are common in patients with ILD. The high mortality rate of PPF dictates the need for the active, early detection of a progressive fibrosing course of a wide range of ILD and suggests that further studies assessing the effectiveness of the interventions might be warranted.
Keywords: interstitial lung diseases, pulmonary fibrosis, progressive pulmonary fibrosis, idiopathic pulmonary fibrosis
1. Introduction
Interstitial lung diseases (ILD) are a heterogeneous group of diffuse parenchymal processes in the lungs [1,2,3,4]. Currently, ILD are represented by more than 200 different entities that are heterogeneous in etiology, radiological and histological patterns, and prognosis, but there are similar pathophysiological pathways for the development of pulmonary inflammation and/or fibrosis [5,6,7].
Pulmonary fibrosis occurs in many ILD, where idiopathic pulmonary fibrosis (IPF) is a classic variant of progressive fibrosing ILD that is characterized by a rapidly progressive disease and a high mortality rate [8,9,10]. At the same time, other common forms of ILD, including idiopathic nonspecific interstitial pneumonia (iNSIP), chronic hypersensitivity pneumonitis (CHP), and connective tissue disease-associated ILD (CTD-ILD) [9,11], may also have signs of pulmonary fibrosis (PF), which as in the case of IPF can worsen or progress [12].
In progressive fibrosing ILD, high-resolution computed tomography (HRCT) reveals an increase in the amount of fibrotic changes in the lung parenchyma, a decrease in the lung function during serial observation, the worsening of symptoms, a decrease in the quality of life, and early mortality, despite conventional therapy with glucocorticosteroids and/or immunosuppressants [7,9,10]. In this regard, such variants of the course of ILD are increasingly described in the general terminology as progressive fibrosing ILD or progressive pulmonary fibrosis (PPF) [7,10,11]. The definitions of the progression of pulmonary fibrosis vary significantly, and so far, there are no universally accepted criteria [2,13,14].
A retrospective analysis of the data from a number of studies showed that in patients with IPF with a relative decrease in the forced vital capacity (FVC) of 10–15%, the risk of death was more than two times higher than it was in patients with an absolute decrease in the FVC of <5% (hazard ratio (HR) 2.20) [15]. Other studies have shown that even in the absence of a decrease in the FVC, an increase in the size of the area of the fibrotic changes according to the HRCT data is also a prognostic factor [16,17,18]. The worsening of symptoms alone or in combination with a decrease in the FVC or the progression of fibrosis in the HRCT also does not rule out the progression of ILD, but it requires further research to confirm it as a reliable predictor of the PPF outcome [1].
Relatively recently, an effective pharmacotherapy for the treatment of IPF has emerged: antifibrotics pirfenidone and nintedanib have been shown to slow the decline in lung function and may prevent the exacerbations of IPF [19]. In addition, antifibrotic agents have been shown to be effective in other ILD: nintedanib was shown to slow down the decline in the lung function in ILD associated with systemic sclerosis [14] and the progressive fibrosing ILD of various etiologies [11]. Based on these encouraging results, it is expected that a significant proportion of ILD patients with PPF may benefit from antifibrotic drugs.
Currently, there is only a little bit of information on the prevalence of PF and PPF in patients with various ILD. The current literature data are based on the results of a survey of doctors, according to which approximately 13–40% of the patients with ILD develop PPF [20,21,22,23,24]. The overall estimated prevalence of PPF, according to some studies, ranges widely from 2.2 to 20 per 100.000 people in Europe [25,26,27], and it occurs in 28 per 100.000 people in the United States [28], which most likely reflects the geographic and methodological heterogeneity used to calculate the rates rather than true differences the prevalence [29].
Therefore, we conducted a study to evaluate the spectrum of PF and PPF in a large cohort of patients with ILD based on the eastern Siberia registry. Our secondary aims included the characteristics of these conditions, including the underlying disease etiology, and also the potential factors associated with progression.
2. Materials and Methods
2.1. Study Population
Our cohort study was based on a prospective registry of patients with ILD in eastern Siberia. All of the consecutive patients over 18 years old with established or suspected diagnosis of ILD were prospectively registered in the database between December 2018 and December 2021. All of the referred ILD patients were reviewed in a multidisciplinary discussion (MDD). The multidisciplinary panel included pulmonologists, radiologists, pathologist, and whenever they were needed, rheumatologists or occupational physicians. All of the physicians involved in the multidisciplinary discussions were experienced in the management of patients with ILD. A final diagnosis was proposed when the team reached an agreement.
The study was approved by the Institutional Review Board of Irkutsk Scientific Research Branch of Russian Medical Academy of Continuing Professional Education (Protocol No. 9 of 29 November 2018). Written informed consent for participation in the study was obtained from every patient.
2.2. Data Collection
Data on the patient demographics, smoking habits, symptoms at the time of diagnosis, radiologic findings, pulmonary function tests, histopathologic findings, treatment regimen and duration, progression of the disease, and survival were collected by reviewing the medical records.
All of the patients underwent HRCT on 1.0–1.5-mm-thick overlapping sections using a high-spatial-frequency reconstruction algorithm, which were taken during a single breath hold using various computed tomography scanners. The HRCT images were evaluated by 2 experienced radiologists.
The PFTs were performed according to the recommendations of the Global Lung Function Initiative. The static lung volumes were measured using the plethysmography method.
The follow-up period ranged from 1 to 5 years. The frequency of the examinations was assessed individually depending on the specific form of the disease, the severity of the course, and the maintenance therapy.
2.3. Definitions
Pulmonary fibrosis was defined as the presence of traction bronchiectasis, reticulations with/without honeycombing with features of fibrosis affecting more than 10% of lung volume on HRCT confirmed by central review [11].
Progressive pulmonary fibrosis was defined as the patient meeting at least one the following criteria within 24 months before the screening despite treatment with corticosteroids and immunosuppressants:
(1) A relative decline in the FVC of at least 10% of the predicted value;
(2) A relative decline in the FVC of 5% to <10% of the predicted value and the worsening of the respiratory symptoms, or increased extent of fibrosis on HRCT;
(3) The worsening of the respiratory symptoms and increased extent of fibrosis on HRCT.
IPF was diagnosed according to the guideline criteria available at the time of diagnosis [30]. A diagnosis of idiopathic interstitial pneumonia (IIP) required chest HRCT with an appropriate pattern of a specific type of IIP or confirmation by surgical lung biopsy [31]. CHP was diagnosed based on the clinical history, radiographic pattern, and if it was applicable, a pathological confirmation [32]. CTD-ILD required the confirmation of an underlying CTD with clinical and immunological patterns according to the currently proposed diagnostic criteria [33,34,35]. Interstitial pneumonia with autoimmune features (IPAF) was diagnosed according to the proposed research criteria [36]. The patients with an ILD that was secondary to other causes (e.g., pneumoconiosis, etc.) were included in the analysis and grouped into a category labelled “Other” ILD.
2.4. Statistical Analysis
For the statistical analysis, the Statistica 12.0 program (Statsoft, Inc., Tulsa, OK, USA) was used. The data were expressed as mean and standard deviation (M ± SD) or median, lower, and upper quartiles (Me (Q1;Q3)), depending on the distribution. The normality of the distribution was checked using the Shapiro–Wilk test. A chi-square statistic test or Fisher’s exact test was used for the categorical data, and an unpaired Student’s t test or a Mann–Whitney test was used for continuous data. Logistic regression analyses were used to investigate the risk factors for the PPF. The adjusted odds ratio (OR) values and 95% confidence intervals (CI) are reported. A p value of less than 0.05 was considered to be statistically significant (two-tailed).
3. Results
In total, 293 patients were referred for MDD between December 2018 and December 2021, and after excluding 23 patients with infectious, oncological, and cardiogenic etiologies or insufficient data, 270 patients were enrolled in the registry. The mean age at ILD diagnosis was 58.5 (46;67) years, and women slightly predominated the group (58.5%). One hundred and six patients had ILD with a known etiology, 66 patients had IIP, 74 patients had sarcoidosis, and 24 patients had another ILD. The patients with IIP were statistically significantly older than the patients from the other groups were, with mean age 67 (61;75) years (p < 0.001). The functional status was significantly worse in the ILD patients with a known etiology and IIP: FVC 74.4 ± 21.9% and 78.2 ± 21.9%, respectively, while the FVC in patients with sarcoidosis was significantly higher—92.5 ± 20.7%.
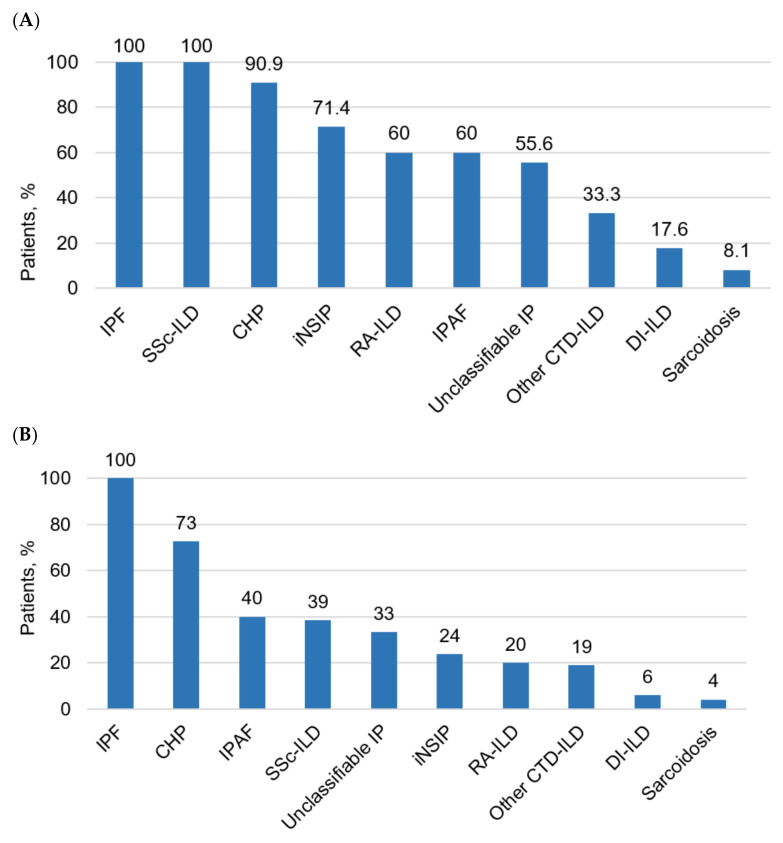
Signs of PF were detected by HRCT in 104 patients (38.5%). The largest number of patients with PF was observed among the ILD patients with a known etiology (n = 58; 54.7%) and in the patients with IIP (n = 40; 60.6%) with an absolute predominance of patients with IPF. Sixty-two patients met the criteria of PPF (23.0%) in entire ILD cohort and 59.6% of the patients had PF. PPF occurred most often in the patients with IPF, CHP, IPAF, and SSc-ILD: 100%, 72.7%, 40%, and 38.5%, respectively (Figure 1).
Figure 1.
Proportion of patients with pulmonary fibrosis (A) and progressive pulmonary fibrosis (B) among different ILD. IPF—Idiopathic pulmonary fibrosis; SSc-ILD—interstitial lung disease associated with systemic sclerosis; CHP—chronic hypersensitivity pneumonitis; iNSIP—idiopathic nonspecific interstitial pneumonia; RA-ILD—rheumatoid arthritis-associated interstitial lung disease; IPAF—interstitial pneumonia with autoimmune features; IP—interstitial pneumonia; CTD-ILD—connective tissue disease-related interstitial lung disease; DI-ILD—drug-induced interstitial lung disease.
Among the patients with ILD, the highest proportion of PF was observed in the patients with IPF, SSc-ILD, CHP, iNSIP, and RA-ILD: 100%, 100%, 90.9%, 71.4%, and 60.0% of the cases, respectively (Table 1). In terms of sarcoidosis, only 8.1% of the patients had signs of pulmonary fibrosis, whereas in the patients with organizing pneumonia, no cases of PF were observed (Table 1).
Table 1.
Spectrum of interstitial lung diseases according to the ILD registry.
| ILD Variants | ILD without PF, n (%) |
ILD with PF, n (%) |
ILD with PPF, n (%) |
|---|---|---|---|
| All ILD | 166 (61.5) | 104 (38.5) | 62 (23.0) |
| ILD with known etiology | 38 (40.9) | 55 (59.1) | 30 (32.3) |
| CHP | 2 (9.1) | 20 (90.9) | 16 (72.7) |
| CTD-ILD | 22 (40.7) | 32 (59.3) | 13 (24.1) |
| SSc-ILD | 0 | 13 (100.0) | 5 (38.5) |
| RA-ILD | 8 (40.0) | 12 (60.0) | 4 (20.0) |
| Other autoimmune ILD # | 14 (66.7) | 7 (33.3) | 4 (19.0) |
| DI-ILD | 14 (82.4) | 3 (17.6) | 1 (5.9) |
| IIP (total) | 24 (38.1) | 39 (61.9) | 26 (41.3) |
| IPF | 0 | 16 (100.0) | 16 (100.0) |
| iNSIP | 6 (28.6) | 15 (71.4) | 5 (23.8) |
| Unclassifiable IP | 4 (44.4) | 5 (55.6) | 3 (33.3) |
| IPAF | 2 (40.0) | 3 (60.0) | 2 (40.0) |
| COP | 12 (100.0) | 0 | 0 |
| Sarcoidosis | 68 (91.9) | 6 (8.1) | 3 (4.1) |
| Rare ILD | 22 (100.0) | 0 | 0 |
| ICEP | 9 (100.0) | 0 | 0 |
| LCH | 5 (100.0) | 0 | 0 |
| LAM | 5 (100.0) | 0 | 0 |
| PAP | 3 (100.0) | 0 | 0 |
| Other ILD * | 14 (77.8) | 4 (22.2) | 3 (16.7) |
ILD—interstitial lung diseases; PF—pulmonary fibrosis; PPF—progressive pulmonary fibrosis; CHP—chronic hypersensitive pneumonitis; SSc—systemic sclerosis; RA—rheumatoid arthritis; DI-ILD—drug-induced ILD; IIP—idiopathic interstitial pneumonia; IPF—idiopathic pulmonary fibrosis; iNSIP—idiopathic nonspecific interstitial pneumonia; IP—interstitial pneumonia; IPAF—interstitial pneumonia with autoimmune features; COP—cryptogenic organizing pneumonia; ICEP—idiopathic chronic eosinophilic pneumonia; LCH—langerhans cell histiocytosis; LAM—lymphangioleiomyomatosis; PAP—pulmonary alveolar proteinosis; # ILD, associated with ankylosing spondylitis, mixed connective tissue disease, systemic lupus erythematosus, Sjogren ‘s disease, dermatopolymiositis, and vasculitis. * other ILD included pneumoconiosis, ILD associated with HIV, lymphocytic interstitial pneumonia in patient with general variable immune insufficiency, ILD associated with nonspecific ulcerative colitis, lymphoid interstitial pneumonia, respiratory bronchiolitis-associated interstitial lung disease, pulmonary amyloidosis, and pulmonary alveolar microlithiasis.
The ILD patients with PF were older than the ILD patients without PF: 61.4 ± 13.6 years vs. 53.1 ± 15.2 years (p < 0.001). In general, there were no significant differences in the age, gender, symptom severity, and lung function between the PF and PPF patient groups. In the group of patients with PF, smoking was more common (46.1% vs. 31.9%; p = 0.018), and a history and contact with harmful occupational or domestic factors was also more common (34.6% vs. 22.9%; p = 0.03). In the patients with PF, clubbing was observed six times more (22.1% vs. 3.6%; respectively, p < 0.001), as well as Velcro crackles (80.8% vs. 18.6%; p < 0.001). In the ILD patients with PF, the symptoms were also significantly more common: dyspnea (96.2% vs. 68.1%; p < 0.001), a cough (93.3% vs. 72.3%; p < 0.001), weakness (78.8% vs. 63.2%; p = 0.007), and weight loss (31.7% vs. 16.8%; p = 0.007) (Table 2). The patients with PF had significantly lower PFT parameters: FVC 69 (58;87)% vs. 88 (73;101)%, predicted at p < 0.001.
Table 2.
Clinical, functional, and HRCT characteristics of ILD patients without PF and with PF and PPF.
| Value | ILD without PF | ILD with PF | ILD with PPF | p # | p ## |
|---|---|---|---|---|---|
| Number, n | 166 | 104 | 62 | ||
| Age, years | 55 (42;65) | 64 (55;70) | 64 (56;73) | <0.001 | 0.71 |
| Male, n (%) | 59 (35.5) | 53 (51.0) | 36 (58.1) | 0.01 | 0.32 |
| Symptoms | |||||
| Dyspnea, n (%) | 113 (68.1) | 100 (96.2) | 62 (100.0) | <0.001 | 0.96 |
| mMRC dyspnea, points | 2 (1;2) | 3 (2;3) | 3 (2;3) | <0.001 | 0.79 |
| Cough, n (%) | 120 (72.3) | 97 (93.3) | 59 (95.2) | <0.001 | 0.91 |
| Weight loss, n (%) | 28 (16.8) | 33 (31.7) | 27 (43.5) | 0.004 | 0.10 |
| Clubbing, n (%) | 6 (3.6) | 23 (22.1) | 20 (32.2) | <0.001 | 0.10 |
| Velcro crackles, n (%) | 31 (18.6) | 84 (80.8) | 53 (85.5) | <0.001 | 0.13 |
| Functional characteristics | |||||
| SpO2, % | 97 (95;98) | 95 (92;97) | 94 (92;96) | <0.001 | 0.31 |
| FVC, % predicted | 88 (73;101) | 69 (58;87) | 63 (52;76) | <0.001 | 0.08 |
| HRCT patterns | |||||
| Honeycombing, n (%) | 0 | 57 (54.8) | 37 (59.7) | <0.001 | 0.74 |
| Traction bronchiectasis, n (%) | 0 | 69 (63.5) | 43 (69.4) | <0.001 | 0.67 |
| Reticular changes, n (%) | 75 (45.2) | 99 (95.2) | 59 (95.2) | <0.001 | 0.96 |
| Ground-glass opacity, n (%) | 58 (34.9) | 40 (38.5) | 23 (37.1) | 0.56 | 0.93 |
| Consolidation, n (%) | 51 (30.7) | 13 (12.5) | 9 (14.5) | <0.001 | 0.89 |
| Basal predominance, n (%) | 28 (16.9) | 63 (60.6) | 39 (62.9) | <0.001 | 0.88 |
| Course and outcome | |||||
| Time from the onset of symptoms to diagnosis, months | 6 (2;24) | 24 (7;48) | 30 (12;40) | <0.001 | 0.88 |
| Time from the first symptom to death, months | 47 (26;65) | 48 (27;72) | 40 (21;62) | 0.62 | 0.55 |
| Death, n (%) | 19 (11.4) | 47 (45.2) | 37 (59.8) | <0.001 | 0.05 |
ILD—interstitial lung diseases; PF—pulmonary fibrosis; PPF—progressive pulmonary fibrosis; mMRC—modified Medical Research Council; SpO2—oxygen saturation of the blood; FVC—forced vital capacity; HRCT—high-resolution computed tomography. # p values compare ILD without PF and with PF. ## p values compare ILD with PF and with PPF.
The mortality rate from all of the causes during the observation period among the ILD patients with PF was significantly higher than it was in the patients without PF (45.2 and 11.4%, respectively; p < 0.001).
The clinical, functional, and HRCT characteristics of the ILD patients with PPF are presented in Table 2. Significant differences between the patients with various ILD in the PPF group were observed in clubbing (p = 0.05) and honeycombing (p < 0.001), which were more common in the patients with IPF (Table 3). In addition, the IPF patients were characterized by had the highest mortality rate (100%) and the shortest time interval from the first symptoms onset to death, 35.5 (24;49) months (p = 0.01), compared with those of other PPF patients (Table 3).
Table 3.
Clinical, functional, and HRCT characteristics of ILD patients with PPF.
| Value | IPF | CHP | CTD-ILD | Other ILD | p |
|---|---|---|---|---|---|
| Number, n | 16 | 16 | 13 | 10 | |
| Symptoms | |||||
| Dyspnea, n (%) | 16 (100.0) | 16 (100.0) | 13 (100.0) | 10 (100.0) | - |
| mMRC dyspnea, points | 3.7 ± 0.4 | 3.4 ± 0.6 | 3.1 ± 0.5 | 3.4 ± 0.5 | 0.06 |
| Cough, n (%) | 16 (100.0) | 16 (100.0) | 13 (100.0) | 10 (100.0) | - |
| Weight loss, n (%) | 13 (81.2) | 11 (68.7) | 7 (53.8) | 7 (70.0) | 0.3 |
| Clubbing, n (%) | 10 (62.5) | 9 (56.2) | 3 (23.1) | 4 (40.0) | 0.05 |
| Velcro crackles, n (%) | 16 (100.0) | 16 (100.0) | 11 (84.6) | 8 (80.0) | 0.12 |
| Functional characteristics | |||||
| SpO2, % | 84.2 ± 5.5 | 84 ± 6.8 | 90.1 ± 5.3 | 86.5 ± 6.7 | 0.05 |
| FVC, % predicted | 54.7 ± 8.6 | 49.7 ± 15.6 | 59.9 ± 17.2 | 61.6 ± 11.9 | 0.13 |
| HRCT patterns | |||||
| Honeycombing, n (%) | 13 (81.2) | 13 (81.2) | 6 (46.1) | 7 (70.0) | 0.19 |
| Traction bronchiectasis, n (%) | 16 (100.0) | 8 (50.0) | 11 (84.6) | 9 (90.0) | <0.001 |
| Reticular changes, n (%) | 16 (100.0) | 18 (100.0) | 13 (100.0) | 10 (100.0) | - |
| Ground-glass opacity, n (%) | 1 (6.2) | 6 (37.5) | 5 (38.5) | 3 (40.0) | 0.25 |
| Consolidation, n (%) | 0 | 0 | 1 (7.7) | 2 (20.0) | 0.04 |
| Basal predominance, n (%) | 14 (87.5) | 9 (56.2) | 8 (61.5) | 3 (30.0) | 0.04 |
| Course and outcome | |||||
| Time from the onset of symptoms to diagnosis, months. | 16 (6;24) | 12 (9.5;48) | 31 (9;84) | 30 (12;48) | 0.1 |
| Time from the first symptom to death, months. | 35 (24;49) | 55 (23;90) | 54 (27;57) | 83 (36;99) | 0.01 |
| Death, n (%) | 16 (100.0) | 6 (37.5) | 5 (38.5) | 7 (70.0) | 0.007 |
IPF—idiopathic pulmonary fibrosis; CHP—chronic hypersensitive pneumonitis; ILD—interstitial lung diseases; CTD-ILD—connective tissue disease-related interstitial lung disease; VAS—visual analogue scale; mMRC—modified Medical Research Council; SpO2—oxygen saturation of the blood; FVC—forced vital capacity; HRCT—high-resolution computed tomography.
The heterogeneity of the PPF cohort is noteworthy. Thus, IPF and CHP with PPF do not statistically significantly differ in terms of parameters such as the severity of dyspnea (p = 0.31) and a cough (p = 0.27), SpO2 (p = 0.98), FVC (p = 0.58), honeycombing (p = 0.3), and the time from the appearance of the first symptom to diagnosis (p = 0.50) (Table 3). However, the time from the onset of the disease to death in the patients with CHP and PPF was longer than it was in IPF (p = 0.042).
Compared to IPF, CTD-ILD with PPF were characterized by a more favorable course in terms of the parameters such as the severity of dyspnea (p = 0.009) and a cough (p = 0.009), the presence of clubbing (p = 0.03), SpO2 (p = 0.009), and mortality (p = 0.005).
Risk factors for PPF among the patients with ILD based on the logistic regression analysis are presented in Table 4. The greatest contribution was made by the factors such as Velcro crackles, odds ratio (OR) 18.3 (p < 0.001), and a late diagnosis, OR 4.1 (p < 0.001) (Table 4).
Table 4.
Risk factors of the PPF in ILD.
| Feature | OR | 95% CI | p |
|---|---|---|---|
| Velcro crackles | 18.3 | 9.8–34.2 | <0.001 |
| Time to diagnosis ≥ 12 months | 4.1 | 2.4–7.1 | <0.001 |
| Dyspnea at the onset of the disease | 2.2 | 1.3–3.8 | 0.002 |
| Weight loss | 2.2 | 1.2–3.9 | 0.006 |
| Age ≥ 65 years | 2.1 | 1.2–3.3 | 0.005 |
| Male gender | 2.1 | 1.2–3.3 | 0.004 |
| Current smoking | 1.8 | 1.1–3.0 | 0.01 |
4. Discussion
In this study, we evaluated the actual prevalence of PF and PPF in a large cohort of ILD patients using objective criteria based on PFT and HRCT. Pulmonary fibrosis was detected in 104 of ILD patients (38.5%). The most frequent PF diagnoses were IPF (100%), SSc-ILD (100%), CHP (90.9%), iNSIP (71.4%), and RA-ILD (60%). Sixty-two patients met the criteria for PPF (23.0% of the entire ILD cohort and 59.6% of the patients with PF). PPF occurred most often in the patients with IPF (100%), CHP (72.7%), IPAF (40%), and SSc-ILD (38.5%), respectively.
The literature currently presents several studies on the prevalence of fibrosing ILD, which ranges from 16.34 per 100.000 of the population [25] to 30.3 per 100.000 among men and 27.5 per 100.000 among women [28]. The prevalence of pulmonary fibrosis according to our registry was 21.8 per 100.000 population, while the prevalence of PPF is 13.0 per 100.000 population, which is comparable with the epidemiological data presented in the literature.
Most often, PF and PPF in ILD were observed in the patients with IIP (60.6% and 39.4%, respectively) and somewhat less often in the patients with a known etiology of ILD (54.7% and 31.1%, respectively), while only rare cases of PF and PPF were recorded among the patients with sarcoidosis (8.1% and 4.1%, respectively). However, when we were evaluating various ILD forms, it was found that with the exclusion of IPF, which is characterized by a 100% progressive fibrosing course, PPF was most often presented in CHP (72.7%). The data of the INBUILD study also indicate the high prevalence of CHP among PPF [11]. In addition, the results of a comparative study of patients with IPF and CHP with PPF demonstrate a comparable profile of the disease progression and the survival of patients with these ILD [37].
In a recently published study based on the data from Canadian Registry for Pulmonary Fibrosis from between 2015 and 2020, Hambly et al. reported that 1376 out of 2746 patients (50%) met the PPF criteria [38]. PPF occurred in 427 (59%) patients with IPF, 125 patients (58%) with fibrotic CHP, 281 patients (51%) with unclassifiable ILD (U-ILD), and 402 patients (45%) with CTD-ILD. Compared with IPF, the time to progression was similar in the patients with CHP, but it was delayed in the patients with U-ILD and CTD-ILD.
The logistic regression analysis of our data showed that bilateral Velcro crackles was the most significant risk factor for PPF: OR 18.3 (95% CI 9.8–34.2, p < 0.001). In a study of 132 patients with suspected ILD, bilateral Velcro crackles were noted in 63% of the patients, and they were associated with an HRCT pattern of usual interstitial pneumonia [39]. Thus, in the patients with ILD, Velcro crackles should be considered as an alarming factor for the progressive fibrosing course of the disease. In addition to Velcro crackles, the significant risk factors for PPF were a late diagnosis of ILD, OR 4.1 (95% CI 2.4–7.1, p < 0.001), as well as dyspnea at the onset of the disease, weight loss, being male, being over 65 years old, and a having history of smoking.
In our study the proportion of progressive autoimmune ILD was 24.1%, among which more than 2/3 of the patients are represented, as in the INBUILD study [11], by two diseases—SSc and RA. Similar data were observed in the study by Atienza-Mateo et al. Within the group of 111 CTD-ILD patients, most of the cases had a diagnosis of RA (27.0%), SSc (26.1%), or anti-synthetase syndrome (17.1%) [40]. There were significantly smaller proportions of the patients with iNSIP (8.2% of all patients with PPF) and unclassifiable IIP (4.9%) than those in the INBUILD study.
It should be noted that IPF, as a prototype of PPF, was characterized by the most unfavorable prognosis among all of the patients with PF. Thus, the median time from the first symptom to death was 35.5 months, which corresponds with the literature data [41,42]. The high mortality rate among the patients with PPF requires the more active monitoring of patients with fibrosing ILD in order to timely determine the disease progression and the necessary change in the therapeutic tactics.
Our study had some limitations. Firstly, our study is based on data from only one regional registry. The patients in our registry were recruited from several tertiary care centers, and thus, it is possible that referral bias could also lead to an overestimation of the prevalence of PF and PPF. Secondly, we used the INBILD criteria, and the evidence of progression was only assessed up to 24 months after the diagnosis, and so extending follow-up over time could lead to an increased prevalence of meeting the PPF criteria over time. Thirdly, in our study, among the patients with PF, the proportion of patients with IPF was only 10%. This fact is rather difficult to explain, and we can only speculate that the geographic location, the relatively young age of the population of our region, and the features of the industrial infrastructure, etc., contribute to this. On the other hand, a relatively low proportion of patients with IPF among the ILD cases was observed in certain regions, for example, in a study from India, the overall proportion of IPF was 13.7% [43], and in a study from the greater Paris region, it was 11.6% [25]. Fourthly, in our study we did not assess the effect of the antifibrotic therapy on the outcomes because the study began before the approval of antifibrotics for PPF, but this factor should be taken into account in future studies.
5. Conclusions
Pulmonary fibrosis and progressive pulmonary fibrosis are common in patients with ILD. The high mortality rate of PPF dictates the need for the active, early detection of a progressive fibrosing course of a wide range of ILD and suggests that further studies assessing the effectiveness of interventions might be warranted.
Author Contributions
All the authors made a significant contribution to the research, analytical work, and article drafting, read and approved the final version before publication. Data collection, creation, and maintenance of the ILD register, interpretation and statistical analysis of the results, writing—original draft preparation, M.S.N.; data collection, methodology, writing—original draft preparation, I.N.T.; conceptualization, methodology, writing review and editing, supervision, B.A.C.; formal analysis, writing review and editing, S.N.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of Irkutsk State Medical Academy of Postgraduate Education—Branch Campus of the Federal State Budgetary Educational Institution of Further Professional Education «Russian Medical Academy of Continuing Professional Education» of the Ministry of Healthcare of the Russian Federation (protocol No. 9, dated 29 November 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bowman W.S., Echt G.A., Oldham J.M. Biomarkers in Progressive Fibrosing Interstitial Lung Disease: Optimizing Diagnosis, Prognosis, and Treatment Response. Front. Med. (Lausanne) 2021;10:680997. doi: 10.3389/fmed.2021.680997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryerson C.J., Collard H.R. Update on the diagnosis and classification of ILD. Curr. Opin. Pulm. Med. 2013;19:453–459. doi: 10.1097/MCP.0b013e328363f48d. [DOI] [PubMed] [Google Scholar]
- 3.Travis W.D., Costabel U., Hansell D.M., King T.E., Jr., Lynch D.A., Nicholson A.G., Valeyre D. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valeyre D., Duchemann B., Nunes H., Uzunhan Y., Annesi-Maesano I. Interstitial lung diseases. Respiratory Epidemiology. ERS Monogr. 2014;65:14–17. [Google Scholar]
- 5.Disayabutr S., Calfee C.S., Collard H.R., Wolters P.J. Interstitial lung diseases in the hospitalized patient. BMC Med. 2015;13:245. doi: 10.1186/s12916-015-0487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasakova M., Poletti V. Fibrosing interstitial lung diseases involve different pathogenic pathways with similar outcomes. Sarcoidosis Vasc. Diffuse Lung Dis. 2015;32:246–250. [PubMed] [Google Scholar]
- 7.Kolb M., Vasakova M. The natural history of progressive fibrosing interstitial lung diseases. Respir. Res. 2019;20:57. doi: 10.1186/s12931-019-1022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan S.D., Shlobin O.A., Weir N., Ahmad S., Kaldjob J.M., Battle E., du Bois R.M. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest. 2011;140:221–229. doi: 10.1378/chest.10-2572. [DOI] [PubMed] [Google Scholar]
- 9.Wells A.U., Brown K.K., Flaherty K.R., Kolb M., Thannickal V.J. What’s in a name? That which we call IPF, by any other name would act the same. Eur. Respir. J. 2018;51:1800692. doi: 10.1183/13993003.00692-2018. [DOI] [PubMed] [Google Scholar]
- 10.Cottin V., Wollin L., Fischer A., Quaresma M., Stowasser S., Harari S. Fibrosing interstitial lung diseases: Knowns and unknowns. Eur. Respir. Rev. 2019;28:180100. doi: 10.1183/16000617.0100-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty K.R., Wells A.U., Cottin V., Devaraj A., Walsh S.L., Inoue Y., Brown K.K. Nintedanib in progressive fibrosing interstitial lung diseases. N. Engl. J. Med. 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 12.Olson A., Hartmann N., Patnaik P., Wallace L., Schlenker-Herceg R., Nasser M., Cottin V. Estimation of the Prevalence of Progressive Fibrosing Interstitial Lung Diseases: Systematic Literature Review and Data from a Physician Survey. Adv. Ther. 2021;38:854–867. doi: 10.1007/s12325-020-01578-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spagnolo P., Distler O., Ryerson C.J., Tzouvelekis A., Lee J.S., Bonella F., Matteson E.L. Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs) Ann. Rheum. Dis. 2021;80:143–150. doi: 10.1136/annrheumdis-2020-217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Distler O., Highland K.B., Gahlemann M., Azuma A., Fischer A., Mayes M.D., Raghu G., Sauter W., Girard M., Alves M., et al. SENSCIS Trial Investigators. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N. Engl. J. Med. 2019;380:2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 15.Paterniti M.O., Bi Y., Rekic D., Wang Y., Karimi-Shah B.A., Chowdhury B.A. Acute Exacerbation and Decline in Forced Vital Capacity Are Associated with Increased Mortality in Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2017;14:1395–1402. doi: 10.1513/AnnalsATS.201606-458OC. [DOI] [PubMed] [Google Scholar]
- 16.Oda K., Ishimoto H., Yatera K., Naito K., Ogoshi T., Yamasaki K., Mukae H. High-resolution CT scoring system-based grading scale predicts the clinical outcomes in patients with idiopathic pulmonary fibrosis. Respir. Res. 2014;15:1–9. doi: 10.1186/1465-9921-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S.M., Seo J.B., Oh S.Y., Kim T.H., Song J.W., Kim N. Prediction of survival by texture-based automated quantitative assessment of regional disease patterns on CT in idiopathic pulmonary fibrosis. Eur. Radiol. 2018;28:1293–1300. doi: 10.1007/s00330-017-5028-0. [DOI] [PubMed] [Google Scholar]
- 18.Jacob J., Bartholmai B.J., van Moorsel C.H.M., Rajagopalan S., Devaraj A., van Es H.W., Wells A.U. Longitudinal prediction of outcome in idiopathic pulmonary fibrosis using automated CT analysis. Eur. Respir. J. 2019;54:1802341. doi: 10.1183/13993003.02341-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finnerty J.P., Ponnuswamy A., Dutta P., Abdelaziz A., Kamil H. Efficacy of antifibrotic drugs, nintedanib and pirfenidone, in treatment of progressive pulmonary fibrosis in both idiopathic pulmonary fibrosis (IPF) and non-IPF: A systematic review and meta-analysis. BMC Pulm. Med. 2021;21:411. doi: 10.1186/s12890-021-01783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson A.L., Patnaik P., Hartmann N., Bohn R.L., Garry E.M., Wallace L. Prevalence and Incidence of Chronic Fibrosing Interstitial Lung Diseases with a Progressive Phenotype in the United States Estimated in a Large Claims Database Analysis. Adv. Ther. 2021;38:4100–4114. doi: 10.1007/s12325-021-01786-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baughman R.P., Lower E.E. Frequency of acute worsening events in fibrotic pulmonary sarcoidosis patients. Respir. Med. 2013;107:2009–2013. doi: 10.1016/j.rmed.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Wijsenbeek M., Kreuter M., Olson A., Fischer A., Bendstrup E., Wells C.D., Cottin V. Progressive fibrosing interstitial lung diseases: Current practice in diagnosis and management. Curr. Med. Res. Opin. 2019;35:2015–2024. doi: 10.1080/03007995.2019.1647040. [DOI] [PubMed] [Google Scholar]
- 23.Zamora-Legoff J.A., Krause M.L., Crowson C.S., Ryu J.H., Matteson E.L. Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. 2017;69:542–549. doi: 10.1002/art.39971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann-Vold A.M., Aalokken T.M., Lund M.B., Garen T., Midtvedt Ø., Brunborg C., Molberg Ø. Predictive value of serial high-resolution computed tomography analyses and concurrent lung function tests in systemic sclerosis. Arthritis Rheumatol. 2015;67:2205–2212. doi: 10.1002/art.39166. [DOI] [PubMed] [Google Scholar]
- 25.Duchemann B., Annesi-Maesano I., de Naurois J.C., Sanyal S., Brillet P.Y., Brauner M., Nunes H. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of Greater Paris. Eur. Respir. J. 2017;50:1602419. doi: 10.1183/13993003.02419-2016. [DOI] [PubMed] [Google Scholar]
- 26.Karakatsani A., Papakosta D., Rapti A., Antoniou K.M., Dimadi M., Markopoulou A., Bouros D. Epidemiology of interstitial lung diseases in Greece. Respir. Med. 2009;103:1122–1129. doi: 10.1016/j.rmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Thomeer M., Demedts M., Vandeurzen K. VRGT Working Group on Interstitial Lung Diseases. Registration of interstitial lung diseases by 20 centres of respiratory medicine in Flanders. Acta Clin. Belg. 2001;56:163–172. doi: 10.1179/acb.2001.026. [DOI] [PubMed] [Google Scholar]
- 28.Coultas D.B., Zumwalt R.E., Black W.C., Sobonya R.E. The epidemiology of interstitial lung diseases. Am. J. Respir. Crit. Care Med. 1994;150:967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 29.Spagnolo P., du Bois R.M., Cottin V. Rare lung disease and orphan drug development. Lancet Respir. Med. 2013;1:479–487. doi: 10.1016/S2213-2600(13)70085-7. [DOI] [PubMed] [Google Scholar]
- 30.Raghu G., Remy-Jardin M., Myers J.L., Richeldi L., Ryerson C.J., Lederer D.J., Wilson K.C. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 31.Travis W.D., Hunninghake G., King T.E., Jr., Lynch D.A., Colby T.V., Galvin J.R., Wells A. Idiopathic nonspecific interstitial pneumonia: Report of an American Thoracic Society project. Am. J. Respir. Crit. Care Med. 2008;177:1338–1347. doi: 10.1164/rccm.200611-1685OC. [DOI] [PubMed] [Google Scholar]
- 32.Fernández Pérez E.R., Travis W.D., Lynch D.A., Brown K., Johannson K., Selman M., Frazer-Green L. Executive Summary: Diagnosis and Evaluation of Hypersensitivity Pneumonitis: CHEST Guideline and Expert Panel Report. Chest. 2021;160:595–615. doi: 10.1016/j.chest.2021.03.067. [DOI] [PubMed] [Google Scholar]
- 33.Demoruelle M.K., Mittoo S., Solomon J.J. Connective tissue disease-related interstitial lung disease. Best Pract. Res. Clin. Rheumatol. 2016;30:39–52. doi: 10.1016/j.berh.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Arnett F.C., Edworthy S.M., Bloch D.A., Mcshane D.J., Fries J.F., Cooper N.S., Hunder G.G. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumol. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 35.American Rheumatism Association Diagnostic and Therapeutic Criteria Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee: Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheumol. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 36.Fischer A., Antoniou K.M., Brown K.K., Cadranel J., Corte T.J., Du Bois R.M., Cottin V. An official European Respiratory Society/American Thoracic Society research statement: Interstitial pneumonia with autoimmune features. Eur. Respir. J. 2015;46:976. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 37.Alberti M.L., Malet Ruiz J.M., Fernandez M.E., Fassola L., Caro F., Roldán I.B., Paulin F. Comparative survival analysis between idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. Pulmonology. 2020;26:3–9. doi: 10.1016/j.pulmoe.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Hambly N., Farooqi M.M., Dvorkin-Gheva A., Donohoe K., Garlick K., Scallan C., Kolb M. Prevalence and characteristics of progressive fibrosing interstitial lung disease in a prospective registry. Eur. Respir. J. 2022;60:2102571. doi: 10.1183/13993003.02571-2021. [DOI] [PubMed] [Google Scholar]
- 39.Sellarés J., Hernández-González F., Lucena C.M., Paradela M., Brito-Zerón P., Prieto-González S., Xaubet A. Auscultation of Velcro Crackles is Associated With Usual Interstitial Pneumonia. Medicine. 2016;95:e2573. doi: 10.1097/MD.0000000000002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atienza-Mateo B., Remuzgo-Martínez S., Mora Cuesta V.M., Iturbe-Fernández D., Fernández-Rozas S., Prieto-Peña D., Cifrián J.M. The Spectrum of Interstitial Lung Disease Associated with Autoimmune Diseases: Data of a 3.6-Year Prospective Study from a Referral Center of Interstitial Lung Disease and Lung Transplantation. J. Clin. Med. 2020;26:1606. doi: 10.3390/jcm9061606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cottin V., Hirani N.A., Hotchkin D.L., Nambiar A.M., Ogura T., Otaola M., Wells A.U. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur. Respir. Rev. 2018;27:180076. doi: 10.1183/16000617.0076-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strongman H., Kausar I., Maher T.M. Incidence, prevalence, and survival of patients with idiopathic pulmonary fibrosis in the UK. Adv. Ther. 2018;35:724–736. doi: 10.1007/s12325-018-0693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh S., Collins B.F., Sharma B.B., Joshi J.M., Talwar D., Katiyar S., Raghu G. Interstitial Lung Disease in India. Results of a Prospective Registry. Am. J. Respir. Crit. Care Med. 2017;195:801–813. doi: 10.1164/rccm.201607-1484OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.