Abstract
A new mechanism expanding mycoplasmal surface diversity is described. Exposure of surface epitopes on a constitutively expressed membrane protein (P56) of Mycoplasma hominis was subject to high-frequency phase variation due to phase-variable expression of the P120 antigen and its selective masking of P56 epitopes. Phase-variable masking may confer previously unrealized adaptive capabilities on mycoplasmas.
Many species of mycoplasma are causative agents of infectious diseases in plants and animals, including humans. Due to the lack of cell walls and surface appendages common to other eubacteria, the membrane proteins of many mycoplasmas are directly involved in mycoplasma-host interaction and play crucial roles in mycoplasma pathogenesis (14, 21). A common strategy utilized by pathogenic mycoplasmas for adaptation in a host appears to be the rapid diversification of surface protein expression and structure (22, 26), as recently reviewed in reference 14. Such changes lead to a dynamic phenotypic variation that may contribute to successful infection. Differential expression of mycoplasma surface proteins, which to date has been found to occur by promoter mutation (5, 27), DNA inversion (1, 17), or frameshift mutation (20, 29), has been widely implicated in phenotypic variation of mycoplasma populations. In addition, it has been indirectly suggested that variable expression of particular surface proteins may affect other membrane molecules (4, 16, 19).
We have used Mycoplasma hominis, a human pathogen associated with clinically diverse diseases (including urogenital infections, postpartum fever, and arthritis) (8), as a model system with which to study adaptive mechanisms of pathogenic mycoplasmas in the human host. Several surface lipoproteins of M. hominis, including the Vaa adhesin, P120, and Lmp, have been characterized in previous studies by us and others. Vaa is subject to size, phase, and antigenic variations in clonal populations and among strains of M. hominis (2, 6, 28, 29). We have shown in clinical isolate 1620 of M. hominis that (i) size variation of Vaa is caused by gain or loss of the tandem repetitive sequences in the central region of this protein; (ii) C-terminal sequence divergence of Vaa leads to antigenic variation among strains, as shown also by others in diverse isolates (2, 6); and (iii) single-nucleotide insertions or deletions in a polyadenine tract within the 5′ end of the vaa coding region create reversible frameshift mutations causing variable expression of Vaa, with concurrent alterations of M. hominis adherence to human cells in vitro (29). The abundant P120 surface lipoprotein contains a hypervariable N-terminal region, two central semivariable domains, and a highly conserved C-terminal region (3, 12). The hypervariable domain of P120 is exposed on the mycoplasma surface and is the target of a strong humoral response in the human host, while the conserved C-terminal region is not. The role of P120 has not been determined. Lmp antigens, encoded by a multiple-gene family, are also surface exposed and size variable among strains (9, 10). Lmp antigens contain multiple internal repeats, with primary sequences that are highly conserved among Lmp members. Deletion of repeats in Lmp antigens causes aggregation of mycoplasmas in broth culture, suggesting that this protein too may contribute to the surface properties of M. hominis (7).
In this report, we define a previously unknown, abundant surface protein (designated P56) in M. hominis isolate 1630 and provide direct evidence that phase-variable expression of the P120 lipoprotein affects the surface display of epitopes on the P56 protein, despite constitutive expression of P56 in this isolate. Specifically, (i) P56 exposure on the mycoplasma surface is subject to phase variation, as detected by colony immunoblotting with antibody (Ab) to this protein; (ii) the P56 product was constantly expressed in clonal lineages of isolate 1630, despite its variable surface exposure to Ab; and (iii) high-frequency phase variation in the expression of P120 appears directly and specifically to mask or unmask P56 epitopes, but not those of other surface antigens, thereby leading to selective phase variation in the surface phenotype of P56.
M. hominis isolates 1620 and 1630 were provided by Lyn D. Olson and Michael Barile, Center for Biologics Evaluation and Research, Food and Drug Administration, Bethesda, Md. These two isolates were recovered successively from a knee joint of a single patient who developed M. hominis-associated arthritis (13, 18). These isolates and M. hominis type strain PG21 (28) were propagated in our laboratory for two to three passages in modified Hayflick medium (25) supplemented with 20% heat-inactivated horse serum and 0.25% arginine. Clonal lineages of isolate 1630 were derived as previously described (15). Monoclonal Ab (MAb) 26.7D, which is specific for the hypervariable region of the P120 lipoprotein of M. hominis (3), was provided by Gunna Christiansen, University of Aarhus, Aarhus, Denmark. Extraction of M. hominis membrane proteins with Triton X-114 (TX-114) was performed as described elsewhere (24). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (11), Western immunoblotting, and colony immunoblotting (using mycoplasma colonies grown for 2 to 3 days on agar plates) were performed as described previously (28, 29).
P56 is a novel protein expressed in isolate 1630.
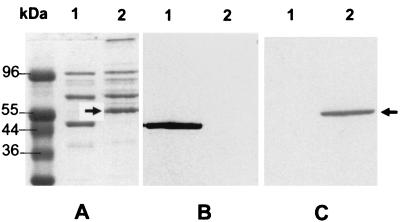
In the process of characterizing variable proteins in successive M. hominis isolates 1620 and 1630, we observed that isolate 1630 expressed a previously unobserved, abundant membrane protein with a molecular mass of 56 kDa that partitioned in the TX-114 phase (Fig. 1A). This protein was designated P56 and was shown to be metabolically labeled by [35S]cysteine (data not shown), consistent with the characteristics of mycoplasma lipoproteins (24). P56 appeared to be absent from isolate 1620 and the type strain PG21 as measured by these methods. This apparent strain difference in P56 could arise from its inability to be expressed or from a negative state of phase variation in the 1620 population propagated (although there is no evidence supporting the latter possibility). Assorted MAbs recognizing different M. hominis surface antigens (28) did not react with the P56 protein (Fig. 1B and data not shown). To generate specific Ab to P56, TX-114-fractionated membrane proteins of isolate 1630 were separated by SDS-PAGE and bands were visualized by negative staining with sodium acetate (23). The P56 band, well isolated from other proteins, was excised from the gel and electroeluted (S&S Elutrap; Schleicher & Schuell). Analysis of the eluate by SDS-PAGE revealed only the single 56-kDa protein (data not shown). The eluted protein was emulsified with incomplete Freund adjuvant and used to inoculate BALB/c mice as described previously (23). Western immunoblotting of membrane proteins from isolates 1620 and 1630 with preimmune or hyperimmune mouse serum demonstrated that the antiserum was highly specific for the P56 protein of isolate 1630 (Fig. 1C). The Ab to P56 failed to detect any proteins in isolate 1620 or in strain PG21. This suggested that P56 is not antigenically related to Vaa or other membrane proteins of M. hominis and that it may be restricted in its strain distribution.
FIG. 1.
SDS-PAGE and immunoblotting analysis of TX-114-partitioned membrane proteins of M. hominis isolates 1620 (lane 1) and 1630 (lane 2). Panel A shows a gel stained with Coomassie brilliant blue. Panels B and C show Western blots of the same samples immunostained, respectively, with MAb H3 (28), which is specific for a conserved epitope on Vaa (B), or specific polyclonal antiserum to P56 (C). Protein molecular size markers are indicated at the left of panel A. The position of P56 is indicated by the arrows.
P56 is expressed on the surface of M. hominis and undergoes phase variation in surface display.
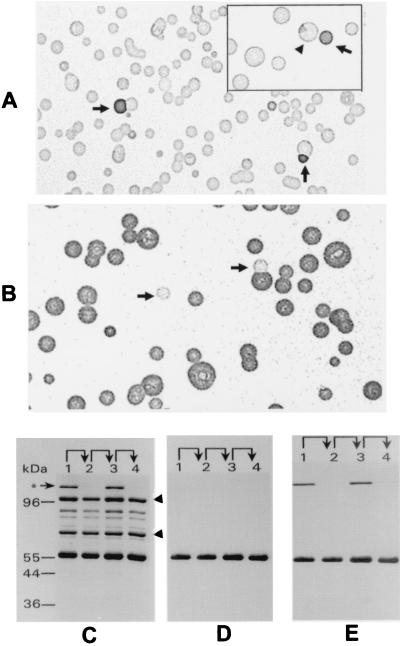
To determine whether P56 is exposed on the surface of M. hominis, mycoplasma colony lifts (24) from a clonal isolate (CL3.8) of isolate 1630 were immunostained with Ab to P56. Surprisingly, colonies of this clonal population were not uniformly stained with the Ab (Fig. 2A). Most colonies of CL3.8 were only weakly stained with Ab to P56 and were subsequently designated P56− in this study. In contrast, a minority of the colonies were strongly stained (designated P56+) or showed sectored staining patterns. To further examine variation of this surface phenotype, a single P56+ colony was selected from the P56− population, replated on mycoplasma agar plates, and immunoblotted with Ab to P56 (Fig. 2B). In this case, the majority of the colonies were P56+; however, a few were P56− or showed a sectored staining pattern. Isolation and replating of variants oscillating in their staining patterns with Ab to P56 established lineages that defined phase variation of P56 in clonal populations of M. hominis. These results indicated that P56 is localized on the surface of mycoplasma and that its surface display (measured by specific Ab binding) is subject to variation in clonal populations. This phase variation occurred at a frequency of approximately 10−2.
FIG. 2.
Phase variation of the P56 phenotype, detected by colony immunoblotting and protein profiles. Panel A shows the presence of P56+ variants (indicated by arrows) in a clonal P56− population. A sectored colony is shown (arrowhead) in the insert. Panel B shows revertant P56− variants (indicated by arrows) derived from a propagated P56+ colony that was selected from the population represented in panel A. TX-114 phase membrane proteins of a successive lineage (P56− [lane 1]→P56+ [lane 2]→P56− [lane 3]→P56+ [lane 4]) were analyzed by SDS-PAGE and Western immunoblotting. Panel C shows a gel stained with Coomassie brilliant blue. The arrowheads indicate the positions of the P100 protein (100 kDa) and the G5 protein antigen (70 kDa). Panel D represents a Western blot immunostained with Ab to P56. The blot shown in panel D was subsequently immunostained with MAb 26.7D to P120 (panel E). The asterisk indicates the position of P120. Protein molecular size markers are shown at the left of panel C.
Phase variation of P56 surface epitope display is not related to differential expression of this protein.
To determine the molecular basis of P56 phenotypic variation detected by colony immunoblotting, membrane proteins from successive clonal variants in a lineage of isolate 1630 oscillating in the staining patterns with Ab to P56 were analyzed by SDS-PAGE and Western blotting. After staining with Coomassie brilliant blue, the P56 band in each variant was quantified digitally with an IS-1000 digital imaging system (Alpha Innotech Corporation) using the G5 antigen (29) and a 100-kDa membrane protein (P100) as invariant internal standards in this lineage. As shown in Fig. 2C, the P56 product was constantly expressed, with similar relative abundance in each variant, despite marked switching in the surface display of epitopes recognized by cognate Ab (Fig. 2A and B). Western immunoblotting of these switching variants with Ab to P56 confirmed this observation (Fig. 2D). These findings demonstrated that phase variation of P56 is associated with the altered exposure of P56 surface epitopes rather than variable expression of the protein. This raised the possibility that P56 interacts in some way with another phase-variable component on the mycoplasma surface.
Variable expression of P120 selectively affects the surface display of P56.
To determine if phase variation of P56 is related to the expression profiles of other membrane proteins, successive clonal variants oscillating in P56 surface display were further examined by SDS-PAGE and Western blotting. This revealed a striking inverse correlation between the expression of a 120-kDa protein and the variable P56 phenotype. The 120-kDa protein was produced only in P56− variants and not in P56+ variants (Fig. 2C). Moreover, expression of other membrane proteins in these variants did not change. These observations provided a strong rationale for further examining the relationship between variable expression of the 120-kDa protein and the surface display of P56.
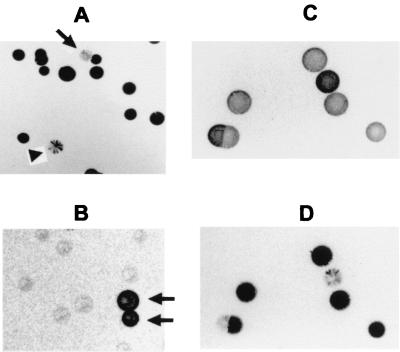
Based on its partitioning property in TX-114 and its size, this 120-kDa protein was suspected to be the previously characterized P120 surface lipoprotein (3). To assess this possibility, the blot shown in Fig. 2D was subsequently immunostained with MAb 26.7D, which is specific for P120 (3). The MAb specifically stained the 120-kDa protein present in P56− variants but did not detect any protein bands in P56+ variants (Fig. 2E), confirming that the phase-variable protein is, indeed, the P120 identified previously. Three lineages of clonal variants oscillating in patterns of staining with Ab to P56 were independently derived from isolate 1630. In each lineage, the expression status of P120 was always inversely correlated with the surface display of P56 epitopes. One lineage is depicted in Fig. 2C to E. To further examine the precise, inverse relationship between the phenotypes, colony blots of P56− or P56+ variants were immunostained with MAb 26.7D, showing the striking variation of P120 in clonal populations (Fig. 3A and B). In these clonal lineages and in otherwise unselected populations of M. hominis, a switching frequency of 10−2 was observed for P120 expression. As expected, most of the colonies in the P56+ population were only weakly stained with MAb 26.7D, whereas the majority of the colonies in the P56− population were strongly stained with this MAb. Furthermore, sectored colonies were observed in both populations. Immunostaining of replicate colony blots with MAb 26.7D or the anti-P56 Ab demonstrated a consistent, reciprocal staining pattern, even on sectored colonies (Fig. 3C and D). Together, these results establish that (i) P120 undergoes high-frequency phase variation by differential expression in clonal populations and (ii) on-off switching in the expression of P120 precisely correlates with changes in the surface accessibility of P56 epitopes, despite continuous expression of the P56 product. These results strongly suggest that phase variation governing the surface display of P56 in isolate 1630 results from a mechanism involving masking by the P120 lipoprotein. Masking of P56 by P120 appeared to be selective for P56 because the expression of P120 did not affect the surface exposure of other known membrane proteins, including the invariant G5 protein (data not shown) or the Vaa adhesin, previously shown in strain 1620 to phase vary independently of P120 expression (29). Vaa is not expressed in the 1630 lineages examined in the current study.
FIG. 3.
Reciprocal phase variation of P120 and surface masking of P56. Panels A and B are colony blots of the two clonal populations shown, respectively, in Fig. 2A and B that were immunostained with MAb 26.7D to P120. Phase variants in the predominant clonal populations are indicated by arrows. A sectored colony is indicated by the arrowhead in panel A. Panels C and D are replicate colony blots made from the same plate of a clonal population of 1630 that were immunostained, respectively, with anti-P56 Ab (C) or MAb 26.7D (D), showing the reciprocal staining patterns obtained with these two Abs.
Less well-defined examples of surface masking have been observed in Mycoplasma fermentans (19) and Mycoplasma hyorhinis (4). The masking component affecting the surface display of P29 lipoprotein epitopes of M. fermentans has not been identified, nor have the cryptic antigen targets that are selectively shielded by elongated versions of Vlp surface lipoproteins of M. hyorhinis been defined. We show in this report that high-frequency variation in the expression of the P120 surface protein in M. hominis can selectively affect the surface accessibility of P56 epitopes to cognate Abs, thereby providing direct evidence that variable expression of one surface protein can govern the phase-variable display of another surface molecule. In M. hyorhinis, surface masking by size-variable lipoproteins confers resistance to growth-inhibiting serum Ab (4). The functional consequence of masking of P56 by P120 is not known, nor is the biological role of either component established. Selective masking of P56 by P120 raises the possibility that these two surface proteins specifically interact as nearest neighbors. In this context, it is noteworthy that masking of P56 epitopes by P120 was not complete, since P56− colonies were still weakly stained by the anti-P56 Ab. In contrast, mycoplasma colonies of strain 1620, which does not express P56, did not react at all with the anti-P56 Ab in the colony immunoblotting assay. Two possibilities might explain the partial nature of masking: (i) only part of the P56 protein is masked by P120, leaving specific epitopes accessible to selected Ab populations in the polyclonal antiserum to P56, or (ii) only a portion of the P56 proteins present on the surface is masked by P120. The relative abundance of P120 and P56 remains to be formally determined.
P120 is encoded by a single-copy p120 gene on the M. hominis chromosome (3). Comparison of p120 alleles in different strains has revealed that P120 contains a hypervariable N-terminal region and a highly conserved C-terminal region (12). The hypervariable region of P120 is exposed on the mycoplasma surface and is immunodominant in the human host, while the highly conserved C-terminal region of P120 is apparently not a target of the humoral immune response. Variable expression among M. hominis strains and antigenic variation related to the hypervariable region of P120 have been documented (12). However, high-frequency phase variation, requiring analysis of reversible switching in clonal populations, has not been previously demonstrated. Clonal variants oscillating in the expression of the P120 product described in this study formally define high-frequency phase variation in the expression of the P120 product in clonal populations. The genetic basis for differential expression of P120 in clonal variants remains to be determined. Interestingly, a long, homopolymeric tract of thymidine residues flanking the 5′ end of the p120 gene has been reported (3). Whether this is subject to mutations affecting transcription is not known.
Isolates 1620 and 1630 were obtained successively from the knee joint of a single patient (13, 18). The occurrence of P56 in isolate 1630 is interesting in light of its apparent absence from other strains. Whether this is a fortuitous manifestation of phase variation or reflects some other event in the propagating population in this host niche remains to be determined. Nevertheless, the findings we report here define a complex system generating surface diversity in a human mycoplasma pathogen. Interplay of multiple mechanisms (differential expression and surface masking) affecting the variation of surface proteins may confer great flexibility on mycoplasmas in adapting to host niches.
Acknowledgments
We thank Gunna Christiansen for providing MAbs and Lyn Olson and Michael Barile for providing M. hominis isolates.
This study was supported in part by DHHS grants AR42537 (K.S.W.) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and AI32219 (K.S.W.) and T32 AI07276 (Q.Z.) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Bhugra B, Voelker L R, Zou N, Yu H, Dybvig K. Mechanism of antigenic variation in Mycoplasma pulmonis: interwoven, site-specific DNA inversions. Mol Microbiol. 1996;18:703–714. doi: 10.1111/j.1365-2958.1995.mmi_18040703.x. [DOI] [PubMed] [Google Scholar]
- 2.Boesen T, Emmersen J, Jensen L T, Ladefoged S A, Thorsen P, Birkelund S, Christiansen G. The Mycoplasma hominis vaa gene displays a mosiac gene structure. Mol Microbiol. 1998;1:97–110. doi: 10.1046/j.1365-2958.1998.00906.x. [DOI] [PubMed] [Google Scholar]
- 3.Christiansen G, Mathiesen S L, Nyvold C, Birkelund S. Analysis of a Mycoplasma hominis membrane protein, P120. FEMS Microbiol Lett. 1994;121:121–128. doi: 10.1111/j.1574-6968.1994.tb07085.x. [DOI] [PubMed] [Google Scholar]
- 4.Citti C, Kim M F, Wise K S. Elongated versions of Vlp surface lipoproteins protect Mycoplasma hyorhinis escape variants from growth-inhibiting host antibodies. Infect Immun. 1997;65:1773–1785. doi: 10.1128/iai.65.5.1773-1785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Citti C, Wise K S. Mycoplasma hyorhinis vlp gene transcription: critical role in phase variation and expression of surface lipoproteins. Mol Microbiol. 1995;18:649–660. doi: 10.1111/j.1365-2958.1995.mmi_18040649.x. [DOI] [PubMed] [Google Scholar]
- 6.Henrich B, Kitzerow A, Feldmann R C, Schaal H, Hadding U. Repetitive elements of the Mycoplasma hominis adhesin p50 can be differentiated by monoclonal antibodies. Infect Immun. 1996;64:4027–4034. doi: 10.1128/iai.64.10.4027-4034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen L T, Ladefoged S, Birkelund S, Christiansen G. Selection of Mycoplasma hominis PG21 deletion mutants by cultivation in the presence of monoclonal antibody 552. Infect Immun. 1995;63:3336–3347. doi: 10.1128/iai.63.9.3336-3347.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krause D C, Taylor-Robinson D. Mycoplasmas which infect humans. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 417–444. [Google Scholar]
- 9.Ladefoged S A, Birkelund S, Hauge S, Brock B, Jensen L T, Christiansen G. A 135-kilodalton surface antigen of Mycoplasma hominis PG21 contains multiple directly repeated sequences. Infect Immun. 1995;63:212–223. doi: 10.1128/iai.63.1.212-223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladefoged S A, Jensen L T, Brock B, Birkelund S, Christiansen G. Analysis of 0.5-kilobase-pair repeats in the Mycoplasma hominis lmp gene system and identification of gene products. J Bacteriol. 1996;178:2775–2784. doi: 10.1128/jb.178.10.2775-2784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Nyvold C, Birkelund S, Christiansen G. The Mycoplasma hominis P120 membrane protein contains a 216 amino acid hypervariable domain that is recognized by the human humoral immune response. Microbiology. 1997;143:675–688. doi: 10.1099/00221287-143-2-675. [DOI] [PubMed] [Google Scholar]
- 13.Olson L D, Shane S W, Karpas A A, Cunningham T M, Probst P S, Barile M F. Monoclonal antibodies to surface antigens of a pathogenic Mycoplasma hominis strain. Infect Immun. 1991;59:1683–1689. doi: 10.1128/iai.59.5.1683-1689.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosengarten R, Wise K S. Phenotypic switching in mycoplasmas: phase variation of diverse surface lipoproteins. Science. 1990;247:315–318. doi: 10.1126/science.1688663. [DOI] [PubMed] [Google Scholar]
- 16.Rosengarten R, Yogev D. Variant colony surface antigenic phenotypes within mycoplasma strain populations: implications for species identification and strain standardization. J Clin Microbiol. 1996;34:149–158. doi: 10.1128/jcm.34.1.149-158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmons W L, Zuhua C, Glass J I, Simecka J W, Cassell G H, Watson H L. Sequence analysis of the chromosomal region around and within the V-1-encoding gene of Mycoplasma pulmonis: evidence for DNA inversion as a mechanism for V-1 variation. Infect Immun. 1996;64:472–479. doi: 10.1128/iai.64.2.472-479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sneller M, Wellborne F, Barile M F, Plotz P. Prosthetic joint infection with Mycoplasma hominis. J Infect Dis. 1986;153:174–175. doi: 10.1093/infdis/153.1.174. [DOI] [PubMed] [Google Scholar]
- 19.Theiss P, Karpas A, Wise K S. Antigenic topology of the P29 surface lipoprotein of Mycoplasma fermentans: differential display of epitopes results in high-frequency phase variation. Infect Immun. 1996;64:1800–1809. doi: 10.1128/iai.64.5.1800-1809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theiss P, Wise K S. Localized frameshift mutation generates selective, high-frequency phase variation in a surface lipoprotein encoded by a mycoplasma ABC transporter operon. J Bacteriol. 1997;179:4013–4022. doi: 10.1128/jb.179.12.4013-4022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tryon V V, Baseman J B. Pathogenic determinants and mechanisms. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 457–471. [Google Scholar]
- 22.Wise K S. Adaptive surface variation in mycoplasmas. Trends Microbiol. 1993;1:59–63. doi: 10.1016/0966-842X(93)90034-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise K S, Kim M F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987;169:5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise K S, Kim M F, Watson-McKown R. Variant membrane proteins. In: Razin S, Tully J G, editors. Molecular and diagnostic procedures in mycoplasmology. New York, N.Y: Academic Press, Inc.; 1995. pp. 227–241. [Google Scholar]
- 25.Wise K S, Watson R K. Mycoplasma hyorhinis GDL surface protein antigen p120 defined by monoclonal antibody. Infect Immun. 1983;41:1332–1339. doi: 10.1128/iai.41.3.1332-1339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise K S, Yogev D, Rosengarten R. Antigenic variation. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 473–490. [Google Scholar]
- 27.Yogev D, Rosengarten R, Watson-McKown R, Wise K S. Molecular basis of Mycoplasma surface antigenic variation: a novel set of divergent genes undergo spontaneous mutation of periodic coding regions and 5′ regulatory sequences. EMBO J. 1991;10:4069–4079. doi: 10.1002/j.1460-2075.1991.tb04983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Wise K S. Molecular basis of size and antigenic variation of a Mycoplasma hominis adhesin encoded by divergent vaa genes. Infect Immun. 1996;64:2737–2744. doi: 10.1128/iai.64.7.2737-2744.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Q, Wise K S. Localized reversible frameshift mutation in an adhesin gene confers a phase-variable adherence phenotype in mycoplasma. Mol Microbiol. 1997;25:859–869. doi: 10.1111/j.1365-2958.1997.mmi509.x. [DOI] [PubMed] [Google Scholar]