Abstract
Fusarium species are the most destructive phytopathogenic and toxin-producing fungi, causing serious diseases in almost all economically important plants. Sporulation is an essential part of the life cycle of Fusarium. Fusarium most frequently produces three different types of asexual spores, i.e., macroconidia, chlamydospores, and microconidia. It also produces meiotic spores, but fewer than 20% of Fusaria have a known sexual cycle. Therefore, the asexual spores of the Fusarium species play an important role in their propagation and infection. This review places special emphasis on current developments in artificial anti-sporulation techniques as well as features of Fusarium’s asexual sporulation regulation, such as temperature, light, pH, host tissue, and nutrients. This description of sporulation regulation aspects and artificial anti-sporulation strategies will help to shed light on the ways to effectively control Fusarium diseases by inhibiting the production of spores, which eventually improves the production of food plants.
Keywords: Fusarium, sporulation, anti-sporulation, spore inhibition
1. Introduction
Fusarium species are the most important phytopathogenic and toxic fungi distributed worldwide, and their spores act as infective propagules that initiate infection [1,2]. Fusaria are soil-born filamentous fungi, belonging to the class Ascomycetes and family Hypocreaceae. The genus Fusarium, which was characterized for the first time by Link in 1809, consists of hundreds of species, many of which are found in the soil, and some of them are associated with plants [3,4]. This fungus is found in tropical, subtropical, and also in temperate regions [5]. Fusarium produces white-, pink-, red-, purple-, salmon-, or grey-colored colonies with velvet to cottony surfaces. The capacity of this fungus to grow on a variety of substrates and its highly effective spore dispersal ability account for its extensive dissemination [6,7]. Therefore, understanding the strategies for the regulation and inhibition of the sporulation of Fusarium species is important for controlling their propagation and infection.
Some Fusaria are harmful to agricultural products, animals, and humans, because many of them are phytopathogenic and produce mycotoxins on plants that can adversely affect humans and animals if they enter the food chain [8,9,10]. Fusarium produces a variety of noxious secondary metabolites, such as fumonisins, zearalenone, and trichothecenes, that infect agricultural commodities and create a risk for human health and consumption [11]. The genome of Fusarium verticillioides, Fusarium graminearum, and Fusarium oxysporum f.sp. lycopersici contains about 46 secondary metabolite biosynthesis gene clusters that encode these mycotoxins [12]. There are more than 145 distinct Fusarium species, of which about one-seventh produce toxins [13]. As plant pathogens, Fusarium species result in significant economic damages and harvest losses [14].
Fusarium is one of the most economically destructive plant pathogens, causing major diseases in nearly all economically important plants and resulting in billion dollars of losses in the field of agriculture worldwide [15]. It is also capable of infecting crops in the moderate climate zones of the world. Fusarium produces mycotoxins such as Trichothecenes that can act as a source of infection in plant diseases [16,17,18,19,20,21]. As Fusarium is a soil-born plant pathogenic fungus, it can survive for a long time in soil by decomposing plant debris, the infected soil then moves from one place to another through animals or agriculture tools and can spread the pathogen to new areas [22]. Wilts, blights, rots, and cankers are diseases caused by Fusarium in many ornamentals, field crops, and forest trees [23]. The famous Panama disease of banana, also known as Fusarium wilt, was also caused by these fungi, which itself is one of the most destructive diseases of plants [5]. It is known from the literature that Fusarium diseases can survive in a variety of environmental conditions, but dry and warm weather is the most favorable condition for Fusarium wilt in chickpea [22,24].
Several cultural, physical, and chemical control strategies have been developed to control Fusarium. However, these strategies have little influence on Fusarium diseases, because Fusaria produce highly resistant chlamydospores, volatile inhibitors, and antibiotics [25]. Therefore, pathogenomics and biological control agents have gained great interest for the control of Fusarium. Various transgenic approaches have enabled the identification of genes, regulators, and transcription factors that are associated with virulence and pathogenicity. Fungal genes involved in pathogenicity may be employed as molecular tools for fungicide development or to develop transgenics [26,27].
2. Sporulation in Fusarium
Spores and fruiting bodies are the two most important morphological characters used by mycologists to categorize fungi into genera and to differentiate closely related species [28]. In fungi, asexual propagules are produced throughout the life cycle, typically requiring less investment for each propagule than for sexual spores, and dispersal is their sole function [29]. Fusarium species generate sexual spores and three different types of asexual spores. Less than 20% of Fusarium species, however, reproduce sexually, and not all Fusarium species produce all forms of spores [30]. As long as a food source is present, asexual spores are continuously produced in Fusarium and other fungi.
2.1. Sexual Spores
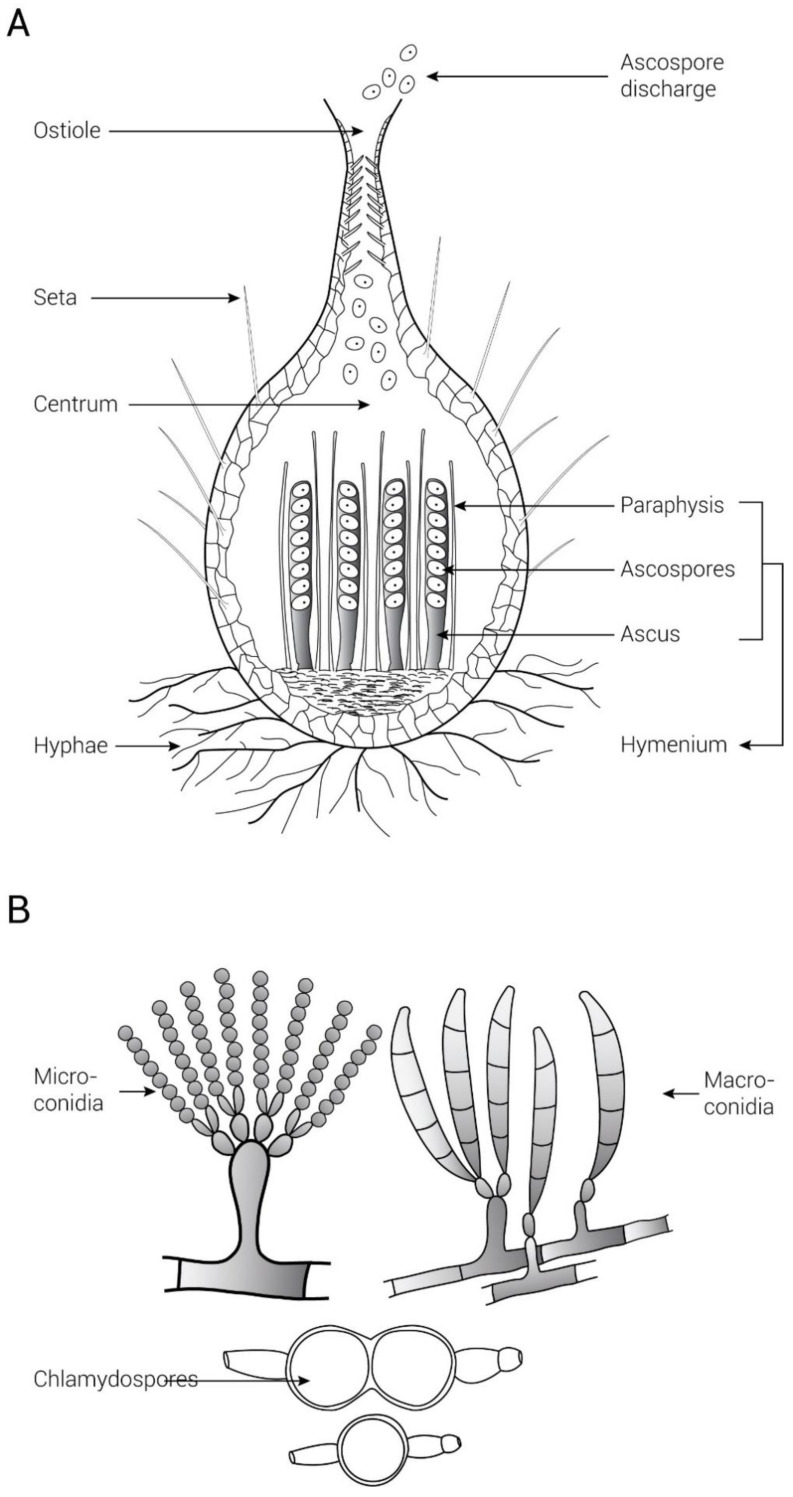
Some Fusarium species generate sexual spores (Figure 1). The role of sexual development in Fusarium spp. Is diverse. In F. graminearum, ascospore is an important primary inoculum that causes head blight disease of wheat and barley [31]. Furthermore, for disease production, sexual development was shown to be essential in F. graminearum, as it undergoes both sexual and asexual stages. In Fusarim solani f.sp. pisi, ascospores are not essential as propagules for dispersal [32]. As we know, the majority of Fusarium species produce fruiting structures in a culture (laboratory), but in the field, sexual development is rare. This is why out of the 12 species with Gibberella teleomorphs, fruiting is common only in G. zeae, whereas G. fujikuroi occasionally produce fruiting bodies in the field. The remaining species (G. baccata, G. ircinate, G. coronicola, G. avenaceae, G. moniliformis, G. nygamai, G. pulicaris, G. intermedia, G. subglutinans, and G. thapsina) have rarely or never produced fruiting structures in nature [33]. However, the majority of these species develop perithecia in the laboratory on natural or artificial substrates [3].
Figure 1.
Sexual (A) and asexual (B) spores of Fusarium.
2.2. Asexual Spores
Fusarium species can produce three different forms of asexual spores (mitotic), including macroconidia, chlamydospores, and microconidia (Figure 1). These asexual spores are the most efficient means of reproduction and dispersal, and they also act as the main source of plant infection. These contagious propagules are crucial components of the disease cycle. They are also important for survival and protection in harsh environmental conditions [3,34,35]. Furthermore, chlamydospores play a vital role in the survival of Fusarium wilt diseases and thus cause more severe disease symptoms [36,37]. Chlamydospores are thick-walled cells that arise from mycelial hyphae, and conidia are produced in sporodochia, which are clusters of conidia-producing cells in a slimy mass [29]. Likewise, macroconidia are fusiform to sickle-shaped, multi-celled by transverse septa, with a foot-shaped basal cell. Microconidia can be globose, oval, and reni-form to fusiform, and they are often single-celled, though they can also be three- to five-celled. Only a few species generate microconidia in chains, but most do so in solitary or slimy heads [38]. Although Fusarium produces both sexual and asexual spores, asexual reproduction is more common. In this review, we focus on asexual reproduction.
2.3. Genetic Pathway Responsible for Spore Formation
With the advancement in molecular techniques, several genes in Fusarium that are involved in sporulation have been characterized. For example, in F. graminearum, several genes were identified and expressed that are reported to be involved in spore formation processes [39]. Similarly, in Fusarium and Aspergillus, mycotoxin production and sporulation are both regulated by G protein-signaling (RGS) pathways. Further, it was revealed that a number of genes were identified that are involved in the process of sporulation, altering several signal transduction pathway steps [40,41,42]. Furthermore, several regulators are also involved with asexual reproduction in F. graminearum. For instance, several genes required for conidiation are regulated by transcriptional factor AbaA, suggesting that AbaA is essential for asexual sporulation [43]. Meanwhile, in F. graminearum, WetA is required for conidiogenesis and maturation of the conidia [44].
Moreover, FgFlbA (RGS proteins) is required for conidiation in Fusarium, as it induces conidiation in F. graminearum [45]. For F. graminearum to produce asexual spores, a number of other proteins are also required. However, the appropriate expression of HEX1, which encodes the hexagonal peroxisome protein, is essential for controlling conidiogenesis [46]. Similarly, the autophagy-related lipase Atg15 is also essential for morphogenesis and conidia formation [47]. In addition, Mes1 (methyl salicylate esterase), a homologue of MeSA, is necessary for conidiogenesis in F. graminearum [48]. The deletion of velvet genes veA and velB showed increased conidial production [49,50,51]. Additional proteins involved in conidiation include Mid1 (mating-induced death), HDF1 (histone deacetylase), CATs (carnitine acetyltransferases; CAT1 and CAT2), Acl (ATP citrate lyase), and Top1 (topoisomerase I) [52,53,54,55,56]. In a similar way, the actin binding protein and Fimbrin are also key factors in the conidiation process, as they increase the production of conidia in F. graminearum [57].
3. Growth Conditions and Environmental Factors Affecting Sporulation
Sporulation is mostly induced or stimulated by endogenous and environmental factors [40,58]. Environmental conditions that trigger sporulation include nutrient depletion, osmotic stress, oxidative stress, carbon and nitrogen status, calcium signaling, pH, aerial stimuli, desiccation, changes in CO2 partial pressure, secondary metabolites produced by competing organisms, and light. Similarly, endogenous factors such as conidiogenone, sporogen PF-1, and volatile organic compounds also stimulate conidiation [59,60,61]. However, various fungal species have diverse responses to these stimuli.
3.1. Temperature
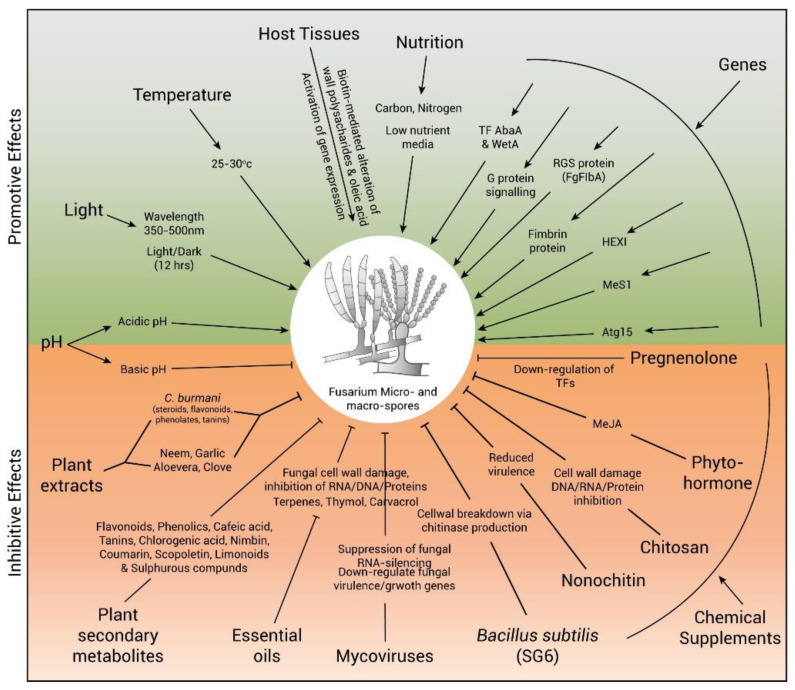
Temperature is an essential component that affects the growth and sporulation of Fusarium as well as the host’s susceptibility to diseases (Figure 2) [62,63,64]. Generally, Fusarium species can be grown in several temperature ranges [64,65,66,67]. However, the optimum temperature for growth and sporulation of Fusarium is 25–30 °C [68]. The optimal temperature for maximum growth and sporulation of F. oxysporum f.sp. ricini was shown to be 27 ± 2 °C on potato dextrose agar media [69].
Figure 2.
Factors affecting induction and inhibition of sporulation.
3.2. Light
Light is considered to be one of the most important factors for spore formation (Figure 2). However, in some species of Basidiomycetes, Myxomycetes, Ascomycetes, and Zygomycetes, near-UV light irradiation successfully induced sporulation [70,71,72,73]. Excessive irradiation can inhibit sporulation. However, a wavelength ranging from 350–500 nm proved to be effective in enhancing sporulation [74,75,76]. For efficient sporulation, 12 h light and 12 h darkness is the best method available [77,78]. Under 12 h light and 12 h dark conditions, F. solani developed concentric sporulation ring patterns, but this pattern was lost when the fungus was exposed to continuous light or darkness [79]. Numerous other fungi, including Fusarium, were stimulated to sporulate by near-UV light, either on their own or in combination with cool white light [78,80]. Light also stimulated the production of metabolites in Fusarium, and in several other species, it also promoted the production of conidia and sexual fruiting bodies [81]. Several light-sensing proteins, such as White Collar-1 and the Vivid protein, and a few transcription factors, such as BLR-1 (blue-light regulator) and BLR-2, have been found to be involved in fungal sporulation [82,83,84,85,86,87,88,89]. Some Fusarium species also conidiate in the dark, rather than under continuous illumination, such as Fusarium fujikuroi [90].
3.3. PH
Fusarium mostly need various pH values for growth and sporulation (Figure 2) [91,92]. An acidic pH is most suitable for the growth and sporulation of F. oxysporum and F. solani [93]. G. fujikuroi and F. oxysporum were shown to grow and sporulate at 5–5.5 pH [94,95]. The best pH for the growth and sporulation of F. oxysporum was proved to be 5.5 to 7 [91,92,96].
3.4. Host Tissue
In pathogenic fungi, host tissues may also be used to stimulate sporulation (Figure 2). Banana petioles were shown to increase the sporulation of endophytic fungi isolated from wild banana (Musa acuminata) leaves [97]. The leaves of Rhododendron pulchrum cv. Ohmurasaki were autoclaved and used to enhance the sporulation of Guignardia endophyllicola [98]. Similarly, the leaves of Dianthus caryophyllus were also reported to be effective for conidiation in Fusarium and Pestalotiopsis species [99,100,101]. Though some plant tissues were also observed to be effective in inducing sporulation, such as in some Botryosphaeriaceae spp., pycnidia were stimulated using autoclaved pine needle [70]. Autoclaved corn hulls promoted macroconidia and mycelial growth of F. graminearum. Wheat bran and carnation leaves induced mycelial growth and macroconidia in F. graminearum and F. proliferatum [102].
Biotin also plays important role in the sporulation process. Due to its presence in plant tissue, it might change the formation of the cell walls and oleic acid, altering the expression of the genes related to sporulation [103,104,105,106,107]. Similarly, in mulberry leaves, biotin enhanced the sporulation of Colletotrichum dematium [108].
3.5. Nutrition
Some nutritional factors such as microelements, carbon, and nitrogen sources also influence sporulation (Figure 2) [109]. Therefore, several fungi need a particular amount of carbon and nitrogen for sporulation [110,111]. Moreover, sporulation is induced with reduced mycelial growth, and it is inhibited under factors that promote rapid mycelial growth [70]. Hence, food shortage or low nutrient media enhance sporulation [112,113]. Synthetic nutrient-poor agar medium, water agar media, and half- or ¼-strength potato dextrose agar (PDA) are some low-nutrient media that induce sporulation [114]. The polysaccharides starch and inulin were shown to induce sporulation in F. oxysporum.
For fungal isolation and culture, PDA is the most commonly used medium. Similarly, potato sucrose agar, Czapek yeast autolysate agar, yeast extract-phosphate medium, cornmeal agar, malt dextrose agar, V8 vegetable juice agar, potato carrot agar, and malt extract agar are also widely used mediums [115]. These media promoted the growth of many endophytic and pathogenic fungi, but they were not very effective in enhancing the sporulation of sterile isolates [116,117]. Furthermore PDA, MEA, and oatmeal agar were shown to be the best mediums for the induction of sporulation in Fusarium [93,118,119]. Similarly, MB and PDB media also promoted sporulation in Fusarium [93].
4. Artificial Control of Sporulation in Fusarium
Fusarium diseases are a major interruption to food production and are very difficult to control [120]. Farmers still use synthetic fungicides to control Fusarium disease. There are several other reasons to completely stop or minimize the use of synthetic chemicals, aside from their negative impact on the environment.
4.1. Biological Control Agents
Nowadays, botanical fungicides are used instead of synthetic fungicides for safety considerations. The botanical fungicides are developed from the extracts of higher plants, and these plant extracts contain antifungal and anti-microbial compounds that act as an anti-sporulation agent to control fungal diseases (Figure 2). In Indonesia, 37,000 plant species have been identified, but only 1% of them have been used as botanical fungicides [121]. Several tropical plant extracts possess antifungal activities that control plant pathogens [122,123,124,125]. Four species of plants, namely, Eugenia aromatica, Piper bettle, Alpinia galanga, and Sphaeranthus indicus, have been used as antifungal agents to control F. oxysporum f.sp. vanilae [126]. Similarly, the extracts of 14 tropical plants inhibited the growth of F. oxysporum f.sp. capsici, which causes Fusarium wilt in paprika [125]. Plant extracts of garlic, ginger, onion, neem, vinca, Indian pennywort, wild sage, marigold, and goat weed showed a complete inhibition of sporulation against Fusarium moniliforme [127]. Pea seed extract was used to inhibit the sporulation of Fusarium oxysporum f.sp. pisi race2 [128]. Chinese gall was found to be effective in inhibiting the sporulation of Fusarium graminearum, and tillecur and white mustard seed flour were found to be best in inhibiting conidia in in vitro conditions [129]. Higher plants also produced secondary metabolites such as phenolic acid, cafeic acid, chlorogenic acid, and scopoletin, which are toxic to pathogens [130]. Aloe vera and clove plant extracts significantly inhibited the growth and spore formation of F. oxysporum f.sp. lycopersici [131]. Clove contains eugenol, and Aloe vera contains phenolic compounds as an antifungal agent [132,133].
4.1.1. Leaf Extracts
The leaf extract of Pometia pinnata has been used to efficiently suppress potato late blight [134]. The leaf extract of Cinnamomum burmanni has been used to prevent the development of Fusarium wilt on tomato. It reduced the growth, biomass, and spore formation of F. oxysporum f.sp. lycopersici. The leaf extract of C. burmanni contains steroid, flavonoid, phenolate, and tannin, which are responsible for antifungal activity [135]. Similarly, leaf extracts of neem (Azadirachta indica) contain a highly toxic compound that showed a complete inhibition of sporulation and mycelial growth in F. oxysporum [136,137,138,139,140,141,142,143]. Neem contains antifungal compounds such as limonoids, protomeliacins, gedunin, azadirone, amino acids, vilasinin, salanin, nimbin, azadirachtin, coumarin., polysaccharides, sulphurous compounds, dihydrochalcone, glycosides, tannins, and flavonoids. These antifungal compounds are toxic and prevent the growth of pathogenic fungi [144,145,146,147,148,149]. The foliar spray of aqueous extract of neem showed antifungal activity against powdery mildew of balsam [150]. Neem was found to be best against tomato seedlings’ damping-off developed by F. oxysporum f.sp. lycopersici [151]. The leaf extract of Parthenium hysterophorus significantly suppressed the growth and spore formation of F. oxysporum, causing mung bean wilting [152].
4.1.2. Essential Oils
Essential oil is also used as an antifungal agent against pathogenic fungi and is one of the most promising natural products for fungal inhibition. The main components of essential oil are carvacrol, thymol, and terpenes/terpenoids, which act as antifungal agents. The cell wall, cytoplasm, and mitochondria are the main targets for antifungal agents [153,154]. The antifungal agents can deactivate the fungus by disrupting the cell membrane and inhibiting the cell wall formation, the action of mitochondrial dehydrogenases, and efflux pumps. Because of their low molecular weight and high lipophilic nature, terpenes are capable of damaging the cell wall and cell membrane of fungi and also inhibiting its sporulation [154]. Similarly, the essential oil of Litsea cubeba contains citral, which acts as an antifungal agent against F. moniliforme and F. solani, affecting their cell wall and membrane, and it also inhibits DNA, RNA, and protein biosynthesis [155,156]. Garlic oil was shown to inhibit the mycelial growth and sporulation in F. oxysporum, which causes wilting in chili [137]. Other researchers have also used garlic against many diseases and reported that garlic contains a sulphur-containing antibiotic that is toxic to plant pathogens [157,158,159,160]. Garlic also contains allicin, which is the main antifungal compound [161,162]. Mint oil and clove oil reduced spore formation and the growth of F. oxysporum f.sp. lycopersici [131]. Rosmarinus officinalis essential oil reduced the sporulation of F. verticillioides [163].
4.1.3. Mycovirus
Mycoviruses have also been used as natural enemies for the management of pathogenic fungi (Figure 2) [164,165,166,167]. These can trigger targets and in some cases suppress RNA silencing, which is the antiviral response of the fungus. Viruses defend themselves from the antiviral response of the fungus by suppressing RNA silencing. Mycoviruses regulate gene expression of the host fungus and also downregulate genes involved in virulence and growth. Wu et al. (2017) used the Sclerotinia sclerotiorum 4 (SsMYR4) infection to downregulate the critical cellular activities and singling pathways of the host [168]. Moreover, the F. graminearum virus China 9 (FgV-ch9) and the F. graminearum viruses FgV1 and FgV2 induced hypovirulence in pathogenic fungi such as F. graminearum [169,170]. Thus, the relation of F. graminearum isolate china 9 with dsRNA mycovirus (Fgv-ch9) showed a significant reduction in conidiation [171]. In 2018, Lemus-Minor et al. used F. oxysporum f.sp. dianthi virus 1 (FodV1) to induce hypovirulence in F. oxysporum. This resulted in reduced mycelial growth, conidiation, and virulence on carnation plants, suggesting it functions as a biocontrol agent for Fusarium wilt of carnation [171].
4.1.4. Rhizospheric Bacteria
Some rhizospheric bacterial species are employed as biological control agents, shielding plants from soil-borne diseases and promoting plant growth (Figure 2). Streptomyces albospinus CT205 and Bacillus sp. str. SV101 and SV104 have been used as biocontrol agents to inhibit Fusarium wilt [172,173]. In 2014, Zhao et al. used Bacillus subtilis SG6, which inhibits the growth and sporulation of Fusarium graminearum, to break down the cell wall of F. graminearum by producing chitinase [174]. Paenibacillus polymyxa NSY50 inhibited the growth of F. oxysporum in the rhizosphere of cucumber and thus protected the plant from pathogen invasion [175].
4.2. Chemical Supplements
Some chemical supplements are also used to control pathogenic fungi (Figure 2). Chitosan is known to inhibit spore formation and to act as antifungal agent. The fungal cell membrane is the primary target of chitosan [176]. The interaction of negatively charged phospholipid of the fungal cell membrane and positively charged chitosan increases membrane permeability that results in the leakage of cellular contents, which ultimately results in cell death [177]. They also function as chelating agents and bind to trace elements, hence rendering the vital nutrients inaccessible for the normal growth of fungi. Chitosan also punctures the fungal cell wall and binds to its DNA to inhibit the synthesis of mRNA [178,179]. Its inhibitory effect was proved with soil-borne phytopathogenic fungi, including Fusarium wilt pathogens [180,181,182]. It also inhibited the growth and sporulation of F. solani and F. oxysporum f.sp. cubense race 4 (FocR4) [183,184]. Nano chitin whisker also significantly inhibited the mycelial growth and conidiation of Fusarium species [185].
Potassium phosphonate inhibited the production of microconidia in F. oxysporum [186]. Similarly, pregnenolone inhibited sporulation in Fusarium graminearum. Pregnenolone might be targeted to the transcriptional factors required for sporulation [187]. Sulfamethoxazole and the indole alkaloid gramine are two natural compounds that decreased disease symptoms caused by F. graminearum in Arabidopsis and wheat [188].
Methyl jasmonate is a signaling molecule that modulates plant defense responses. It stimulates phenolic acids, flavonoids, and phytoalexins responsible for the plant’s defense against pathogens. [189,190,191,192]. Methyl jasmonate induced a defensin-like protein in Pganax notoginsen (PnDEFL1), which showed resistance to F. solani in transgenic tobacco [193]. Methyl jasmonate had an inhibitory effect on the sporulation and mycelial growth of F. solani. Radial growth and sporulation were significantly inhibited in F. oxysporum and F. solani by using different concentrations of salicylic acid [184,194]. Coumarin also inhibited the sporulation of Fusarium oxysporum f.sp. niveum by suppressing activities of pathogenesis-related enzymes [195].
4.3. Transgenic Approaches to Control of Sporulation in Fusarium
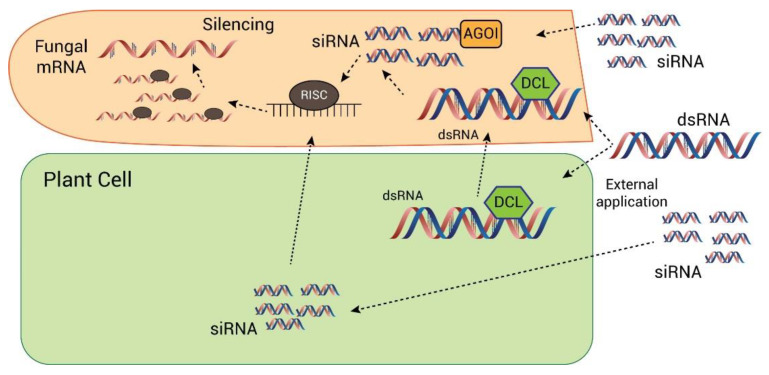
For transgenic approaches to sporulation control in Fusarium, RNA interference (RNAi) is frequently used as a tool to regulate gene expression and provide protection against viruses and pathogens [196,197,198,199]. It was first reported in 1990 by Napoli and Jorgensen [200]. RNAi is activated in the presence of double-stranded RNA (dsRNA) in the host plant and degrades the double-stranded RNA molecule into single-stranded RNA molecules, hence causing silence or knockdown of the targeted gene of the pathogen. This artificial manipulation of gene silencing is used in both transgenic and non-transgenic plants and can be used to control Fusarium growth and sporulation by silencing the genes responsible for conidiation (Figure 3). Generally, there are two ways to perform RNA interference.
Figure 3.
Long double-stranded RNAs (dsRNAs) are transferred to the plants. These are cut into small interfering RNAs (siRNAs) by fungal DCL proteins or plant Dicer-like proteins. The siRNA molecules bind to the complementary sequence of the target mRNA, resulting in its degradation.
4.3.1. Host-Delivered RNAi or Host-Induced Gene Silencing
Host-delivered RNAi (HD-RNAi) uses the host plant as a delivery system and silences the targeted gene of the pathogen [196,201]. In this approach, the siRNA or dsRNA is transformed to the host plant, thus targeting the gene of the pathogen. When this transgenic plant becomes infected, and the pathogen starts feeding from the host, the small interfering RNA (siRNA) and dsRNA molecules from the plant are transferred to the pathogen cells, hence activating an RNAi response in the pathogen and silencing the targeted gene of the pathogen [202]. This strategy was used on various Fusarium species. It was tested in tobacco against F. verticillioides. When the pathogen started feeding off transgenic tobacco plants, GUS-RNAi expressed and significantly silenced the GUS gene in the pathogen [203]. This technology was also used against F. graminearum in Arabidopsis and barley and significantly silenced three fungal cytochrome P450 lanosterol C-14 α-demethylase (CYP51) genes and also increased resistance against pathogens [204]. The silencing of the Cmk1 gene (Colletotrichum lagenarium MAP kinase) in C. lagenarium showed a reduction in conidiation [205]. Similarly, Fmk1, Hog1, and Pbs2 are mitogen-activated protein kinase genes responsible for fungal growth, development, sporulation, and virulence, and so the silencing of these genes in F. oxysporum showed reduced growth, sporulation, and pathogenicity [206]. However, silencing of the Hog1 gene in F. graminearum showed significantly reduced conidiation [207]. Furthermore, the silencing of FOW2 and chsV (class V chitin synthase) in F. oxysporum and F. solani showed reduced mycelial growth and sporulation, which confirmed their involvement in pathogenicity [208]. In F. oxysporum f.sp cubense, the SGE1 gene (Six gene expression 1) is involved in pathogenicity and virulence. Therefore, silencing of this gene showed reduced sporulation and pathogenicity [209]. Moreover, the ODC gene (ornithine decarboxylase) in F. oxysporum is important for fungal growth and causes Fusarium wilt in tomato. Hence, the silencing of this gene showed resistance to Fusarium wilt in tomato [210].
4.3.2. Spray-Induced Gene Silencing
The exogenous application of dsRNA and siRNA is another very promising approach to gene silencing [211,212]. The siRNA and dsRNA target the essential pathogen gene on the plant surface. They can also be sprayed on a wounded surface of the plant, and then this siRNA or dsRNA is taken up by the plants and transferred through the vascular system of fungi. This is an environmentally friendly strategy and is easily accepted by the public and biosafety authorities, and it is optimized faster than HIGS [213]. Koch et al. used this method on Fusarium and sprayed barley leaves with CYP3-dsRNA to check the growth of F. graminearum, and they found that the growth and conidiation of F. graminearum was inhibited by CYP3-dsRNA [214]. Myo5 dsRNA was sprayed on a wounded surface of the plant and silenced the Myo5 gene in the fungus. Myo5 has five segments, Myo5-3, Myo5-4, Myo5-5, Myo5-7, and Myo5-8, and all of these were significantly silenced by dsRNAs. As a result, both the sexual and asexual reproduction of F. asiaticum were significantly reduced. Meanwhile, Myo5-8 significantly reduced the growth of F. asiaticum, F. tricinctum, F. graminearum, and F. oxysporum f.sp. lycopersici [215].
5. Future Perspectives in Sporulation Control in Fusarium
Fusarium is one of the most harmful plant pathogens that causes wilt diseases of crops. Fusarium spores are easily spread in the field, causing invasive and disseminated infections. Fusarium sporulation is mostly induced or stimulated by endogenous and environmental factors. Several strategies have been developed to control the production of spores. In particular, various biocontrol agents and chemicals were used to control Fusarium sporulation, but most of these experiments were performed under in vitro conditions, so they should be validated under field conditions. We expect that more efficient biocontrol agents and chemicals will be identified from further field experiments. The management of Fusarium diseases by gene silencing was also considered to be a powerful method to control the sporulation of Fusarium, and more studies should be carried out in the future to characterize and identify the genes that are involved in sporulation. Currently, two genes in the ergosterol synthetic pathway that are relevant to the sporulation of Fusarium were identified by our team (unpublished). The control of the sporulation of Fusarium and then the control of the spread of wilt diseases will eventually become a new approach to increase crop yield and quality.
Acknowledgments
We acknowledge financial support from the Key Scientific Research Projects of Higher Education Institutions in Henan Province (21A180006).
Author Contributions
Conceptualization, H.C. and M.A.; methodology, A.H., A.A. and H.L.; validation, H.C., A.H. and A.A.; resources, M.A., A.A. and A.H.; writing—original draft preparation, M.A., A.H., A.A., H.L. and H.C.; writing—review and editing, H.L., A.H., A.A. and H.C.; visualization, A.H., H.L. and H.C.; supervision, H.C. and A.H.; project administration, H.L. and H.C.; funding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was funded by the Key Scientific Research Projects of Higher Education Institutions in Henan Province (21A180006).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bottalico A., Perrone G. Toxigenic Fusarium Species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe, Mycotoxins in Plant Disease. Eur. J. Plant Pathol. 2002;108:611–624. doi: 10.1023/A:1020635214971. [DOI] [Google Scholar]
- 2.McMullen M., Bergstrom G., De Wolf E., Dill-Macky R., Hershman D., Shaner G., Sanford D.V. A unified effort to fight an enemy of wheat and barley: Fusarium Head Blight. Plant Dis. 2012;96:1712–1855. doi: 10.1094/PDIS-03-12-0291-FE. [DOI] [PubMed] [Google Scholar]
- 3.Leslie J., Summerell B. Fusarium laboratory workshops-A recent history. Mycotoxin Res. 2006;22:73. doi: 10.1007/BF02956766. [DOI] [PubMed] [Google Scholar]
- 4.Babadoost M. Fusarium: Historical and Continued Importance. Books on Demand; Balikesir, Turkey: 2017. [DOI] [Google Scholar]
- 5.Early R. Pathogen control in primary production: Crop foods. Foodborne Pathog. 2009;2009:205–279. [Google Scholar]
- 6.Mui-Yun W. Fusarium oxysporum f. sp. lycopersici (Sacc.): PP728 Soil-Borne Plant Pathogen Class Project. North Carolina State University; Raleigh, NC, USA: 2003. [Google Scholar]
- 7.Nelson P.E., Dignani M.C., Anaissie E.J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 1994;7:479–504. doi: 10.1128/CMR.7.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arif M., Pani D.R., Zaidi N.W., Singh S.U. PCR-based identification and characterization of Fusarium sp. associated with mango malformation. Biotechnol. Res. Int. 2011;6:141649. doi: 10.4061/2011/141649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balali G., Iranpour M. Identification and genetic variation of Fusarium species in Isfahan, Iran, using pectic Zymogram technique. Iran J. Sci. Technol. 2006;30:91–102. [Google Scholar]
- 10.Wang H., Xiao M., Kong F., Chen S., Dou H.T., Sorrell T., Li R.Y., Xu Y.C. Accurate and practical identification of 20 Fusarium species by seven-locus sequence analysis and reverse line blot hybridization, and an in vitro antifungal susceptibility study. J. Clin. Microbiol. 2011;49:1890–1898. doi: 10.1128/JCM.02415-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhat R., Rai R.V., Karim A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010;9:57–81. doi: 10.1111/j.1541-4337.2009.00094.x. [DOI] [PubMed] [Google Scholar]
- 12.Ma L.J., Van Der Does H.C., Borkovich K.A., Coleman J.J., Daboussi M.J., Di Pietro A., Dufresne M., Freitag M., Grabherr M., Henrissat B.J. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature. 2010;464:367–373. doi: 10.1038/nature08850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss M.O., Thrane U. Fusarium taxonomy with relation to trichothecene formation. Toxicol. Lett. 2004;153:23–28. doi: 10.1016/j.toxlet.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Windels C.E. Economic and social impacts of Fusarium head blight: Changing farms and rural communities in the Northern Great Plains. Phytopathology. 2000;90:17–21. doi: 10.1094/PHYTO.2000.90.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Aoki T., O’Donnell K., Geiser D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. Gen. Plant Pathol. 2014;80:189–201. doi: 10.1007/s10327-014-0509-3. [DOI] [Google Scholar]
- 16.Asam S., Habler K., Rychlik M. Fusarium Mycotoxins in Food, Chemical Contaminants and Residues in Food. Elsevier; Amsterdam, The Netherlands: 2017. pp. 295–336. [Google Scholar]
- 17.Bai G.H., Desjardins A., Plattner R.D. Deoxynivalenol-nonproducing Fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia. 2002;153:91–98. doi: 10.1023/A:1014419323550. [DOI] [PubMed] [Google Scholar]
- 18.Desmond O.J., Manners J.M., Stephens A.E., Maclean D.J., Schenk P.M., Gardiner D.M., Munn A.L., Kazan K. The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol. Plant Pathol. 2008;9:435–445. doi: 10.1111/j.1364-3703.2008.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desjardins A.E., Proctor R., Bai G., McCormick S., Shaner G., Buechley G., Hohn T. Reduced virulence of trichothecene-nonproducing mutants of Gibberella zeae in wheat field tests. Mol. Plant Microbe Interact. 1996;9:775–781. doi: 10.1094/MPMI-9-0775. [DOI] [Google Scholar]
- 20.Ilgen P., Maier F., Schäfer W. Trichothecenes and lipases are host-induced and secreted virulence factors of Fusarium graminearum. Cereal Res. Commun. 2008;36:421–428. doi: 10.1556/CRC.36.2008.Suppl.B.35. [DOI] [Google Scholar]
- 21.Proctor R.H., Hohn T.M., McCormick S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant Microbe Interact. 1995;8:593–601. doi: 10.1094/MPMI-8-0593. [DOI] [PubMed] [Google Scholar]
- 22.Muimba-Kankolongo A. Food Crop Production by Smallholder Farmers in Southern Africa: Challenges and Opportunities for Improvement. Elsevier; Amsterdam, The Netherlands: 2018. p. 368. [Google Scholar]
- 23.Woloshuk C.P., Shim W.B. Aflatoxins, fumonisins, and trichothecenes: A convergence of knowledge. FEMS Microbiol. Rev. 2013;37:94–109. doi: 10.1111/1574-6976.12009. [DOI] [PubMed] [Google Scholar]
- 24.Knights E., Hobson K. Chickpea Overview, Reference Module in Food Science. Elsevier; Amsterdam, The Netherlands: 2016. pp. 316–323. [DOI] [Google Scholar]
- 25.Shanmugam V., Chugh P., Sharma P. Cold-tolerant Trichoderma species for the managementof Fusarium wilt of tomato plants. Ann. Microbiol. 2015;65:543–551. doi: 10.1007/s13213-014-0890-3. [DOI] [Google Scholar]
- 26.Rampersad S.N. Pathogenomics and management of Fusarium diseases in plants. Pathogen. 2020;9:340. doi: 10.3390/pathogens9050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman J.J., Rounsley S.D., Rodriguez-Carres M., Kuo A., Wasmann C.C., Grimwood J., Schmutz J., Taga M., White G.J., Zhou S., et al. The genome of Nectria haematococca: Contribution of supernumerary chromosomes to gene xxpansion. PLoS Genet. 2009;5:e1000618. doi: 10.1371/journal.pgen.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyde K.D., Abd-Elsalam K., Cai L.J.M. Morphology: Still essential in a molecular world. Mycotaxon. 2010;114:439–451. doi: 10.5248/114.439. [DOI] [Google Scholar]
- 29.Deacon J. Fungal Spores, Spore Dormancy, and Spore Dispersal. Fungal Biology. 4th ed. Blackwell Publishing; Oxford, UK: 2006. pp. 184–212. [Google Scholar]
- 30.Ma L.J., Geiser D.M., Proctor R.H., Rooney A.P., O’Donnell K., Trail F., Gardiner D.M., Manners J.M., Kazan K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013;67:399–416. doi: 10.1146/annurev-micro-092412-155650. [DOI] [PubMed] [Google Scholar]
- 31.Desjardins A.E., Plattner R.D., Shaner G., Brown D.W., Buechley G., Proctor R.H., Turgeon G. Proceedings of the 2006 National Fusarium Head Blight Forum. University of Kentucky; Lexington, KY, USA: 2006. Field release of Gibberella zeae genetically modified to lack ascospores; pp. 39–44. [Google Scholar]
- 32.VanEtten H.D. Identification of additional habitats of Nectria haematococca mating population VI. Phytopathology. 1978;68:6. doi: 10.1094/Phyto-68-1552. [DOI] [Google Scholar]
- 33.Desjardins A.E. Gibberella from A (venaceae) to Z (eae) Annu. Rev. Phytopathol. 2003;41:177–198. doi: 10.1146/annurev.phyto.41.011703.115501. [DOI] [PubMed] [Google Scholar]
- 34.Agrios G.N. Plant Pathology. 5th ed. Elsevier Academic Press; Amsterdam, The Netherlands: 2005. [Google Scholar]
- 35.Adams T.H., Wieser J.K., Yu J.H.J.M. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 1998;62:35–54. doi: 10.1128/MMBR.62.1.35-54.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon T.R. Fusarium oxysporum and the Fusarium wilt syndrome. Annu. Rev. Phytopathol. 2017;55:23–39. doi: 10.1146/annurev-phyto-080615-095919. [DOI] [PubMed] [Google Scholar]
- 37.Srinivas C., Devi D., Murthy K., Mohan C., Lakshmeesha T., Singh B., Kalagatur N., Niranjana S., Hashem A., Alqarawi A. Fusarium oxysporum f. sp. lycopersici causal agent of vascular wilt disease of tomato: Biology to diversity—A review. Saudi J. Biol. Sci. 2019;26:1315–1324. doi: 10.1016/j.sjbs.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okungbowa F., Shittu H. Fusarium Wilts: An Overview. Environ. Res. J. 2014;6:83–102. [Google Scholar]
- 39.Xu J.R. MAP kinases in fungal pathogens. Fungal Genet. Biol. 2000;31:137–152. doi: 10.1006/fgbi.2000.1237. [DOI] [PubMed] [Google Scholar]
- 40.Roncal T., Ugalde U. Conidiation induction in Penicillium. Res. Microbiol. 2003;154:539–546. doi: 10.1016/S0923-2508(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 41.Xu J.W., Zhao W., Xu Y.N., Zhong J.J. Isolation and analysis of differentially expressed genes during asexual sporulation in liquid static culture of Ganoderma lucidum by suppression subtractive hybridization. Mol. Biol. Rep. 2012;39:3603–3610. doi: 10.1007/s11033-011-1134-2. [DOI] [PubMed] [Google Scholar]
- 42.Zhou G., Wang J., Qiu L., Feng M.G. A Group III histidine kindase (mhk1) pstream of high-osmolarity glycerol pathway regulates sporulation, multi-stress tolerance and virulence of Metarhizium robertsii, a fungal entomopathogen. Environ. Biol. 2012;4:817–829. doi: 10.1111/j.1462-2920.2011.02643.x. [DOI] [PubMed] [Google Scholar]
- 43.Son H., Kim M.G., Min K., Seo Y.S., Lim J.Y., Choi G.J., Kim J.C., Chae S.K., Lee Y.W. AbaA regulates conidiogenesis in the ascomycete fungus Fusarium graminearum. PLoS ONE. 2013;8:e72915. doi: 10.1371/journal.pone.0072915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Son H., Kim M.G., Min K., Lim J.Y., Choi G.J., Kim J.C., Chae S.K., Lee Y. WetA is required for conidiogenesis and conidium maturation in the ascomycete fungus Fusarium graminearum. Eukaryot. Cell. 2014;13:87–98. doi: 10.1128/EC.00220-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park A.R., Cho A.R., Seo J.A., Min K., Son H., Lee J., Choi G.J., Kim J.C., Lee Y.W. Functional analyses of regulators of G protein signaling in Gibberella zeae. Fungal Genet. Biol. 2012;49:511–520. doi: 10.1016/j.fgb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Son M., Lee K.M., Yu J., Kang M., Park J.M., Kwon S.J., Kim K.H. The HEX1 gene of Fusarium graminearum is required for fungal asexual reproduction and pathogenesis and for efficient viral RNA accumulation of Fusarium graminearum virus 1. J. Virol. 2013;87:10356–10367. doi: 10.1128/JVI.01026-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen L.N., Bormann J., Le G.T.T., Stärkel C., Olsson S., Nosanchuk J.D., Giese H., Schäfer W. Autophagy-related lipase FgATG15 of Fusarium graminearum is important for lipid turnover and plant infection. Fungal Genet. Biol. 2011;48:217–224. doi: 10.1016/j.fgb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Rittenour W.R., Harris S.D. Characterization of Fusarium graminearum Mes1 reveals roles in cell-surface organization and virulence. Fungal Genet. Biol. 2008;45:933–946. doi: 10.1016/j.fgb.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Lee J., Myong K., Kim J.E., Kim H.K., Yun S.H., Lee Y.W. FgVelB globally regulates sexual reproduction, mycotoxin production and pathogenicity in the cereal pathogen Fusarium graminearum. J. Microbiol. 2012;158:1723–1733. doi: 10.1099/mic.0.059188-0. [DOI] [PubMed] [Google Scholar]
- 50.Jiang J., Liu X., Yin Y., Ma Z. Involvement of a velvet protein FgVeA in the regulation of asexual development, lipid and secondary metabolisms and virulence in Fusarium graminearum. PLoS ONE. 2011;6:e28291. doi: 10.1371/journal.pone.0028291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang J., Yun Y., Liu Y., Ma Z. FgVELB is associated with vegetative differentiation, secondary metabolism and virulence in Fusarium graminearum. Fungal Genet. Biol. 2012;49:653–662. doi: 10.1016/j.fgb.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Son H., Min K., Lee J., Choi G.J., Kim J.C., Lee Y.W. Mitochondrial carnitine-dependent acetyl coenzyme A transport is required for normal sexual and asexual development of the ascomycete Gibberella zeae. Eukaryot. Cell. 2012;11:1143–1153. doi: 10.1128/EC.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baldwin T.K., Urban M., Brown N., Hammond-Kosack K.E. A role for topoisomerase I in Fusarium graminearum and F. culmorum pathogenesis and sporulation. Mol. Plant Microbe Interact. 2010;23:566–577. doi: 10.1094/MPMI-23-5-0566. [DOI] [PubMed] [Google Scholar]
- 54.Cavinder B., Hamam A., Lew R.R., Trail F. Mid1, a mechanosensitive calcium ion channel, affects growth, development, and ascospore discharge in the filamentous fungus Gibberella zeae. Eukaryot. Cell. 2011;10:832–841. doi: 10.1128/EC.00235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han Y.K., Lee T., Han K.H., Yun S.H., Lee Y.W. Functional analysis of the homoserine O-acetyltransferase gene and its identification as a selectable marker in Gibberella zeae. Curr. Genet. 2004;46:205–212. doi: 10.1007/s00294-004-0528-2. [DOI] [PubMed] [Google Scholar]
- 56.Li Y., Wang C., Liu W., Wang G., Kang Z., Kistler H.C., Xu J.R. The HDF1 histone deacetylase gene is important for conidiation, sexual reproduction, and pathogenesis in Fusarium graminearum. Mol. Plant-Microbe Interact. 2011;24:487–496. doi: 10.1094/MPMI-10-10-0233. [DOI] [PubMed] [Google Scholar]
- 57.Zheng Z., Gao T., Zsinhang Y., Hou Y., Wang J., Zhou M. FgFim, a key protein regulating resistance to the fungicide JS 399-19, asexual and sexual development, stress responses and virulence in Fusarium graminearum. Mol. Plant Pathol. 2014;15:488–499. doi: 10.1111/mpp.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steyaert J.M., Weld R.J., Mendoza-Mendoza A., Stewart A. Reproduction without sex: Conidiation in the filamentous fungus Trichoderma. Microbiology. 2010;156:2887–2900. doi: 10.1099/mic.0.041715-0. [DOI] [PubMed] [Google Scholar]
- 59.Katayama M., Yanagi M., Marumo S. Isolation of sporogen-PF 1, a blue light-induced sporogenic substance, from Penicillium funiculosum. Agric. Biol. Chem. 1989;53:3379–3380. doi: 10.1271/bbb1961.53.3379. [DOI] [Google Scholar]
- 60.Roncal T., Cordobés S., Sterner O., Ugalde U. Conidiation in Penicillium cyclopium is induced by conidiogenone, an endogenous diterpene. Eukaryot. Cell. 2002;1:823–829. doi: 10.1128/EC.1.5.823-829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoppacher N., Kluger B., Zeilinger S., Krska R., Schuhmacher R. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods. 2010;81:187–193. doi: 10.1016/j.mimet.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Bosland P., Williams P., Morrison R. Influence of soil temperature on the expression of yellows and wilt of crucifers by Fusarium oxysporum. Plant Dis. 1988;72:777–780. doi: 10.1094/PD-72-0777. [DOI] [Google Scholar]
- 63.Frans M., Aerts R., Van Laethem S., Ceusters J. Environmental effects on growth and sporulation of Fusarium spp. causing internal fruit rot in bell pepper. Eur. J. Plant Pathol. 2017;149:875–883. [Google Scholar]
- 64.Rossi V., Scandolara A., Battilani P. Effect of environmental conditions on spore production by Fusarium verticillioides, the causal agent of maize ear rot. Eur. J. Plant Pathol. 2009;123:159–169. doi: 10.1007/s10658-008-9351-9. [DOI] [Google Scholar]
- 65.Marin S., Sanchis V., Magan N.J. Water activity, temperature, and pH effects on growth of Fusarium moniliforme and Fusarium proliferatum isolates from maize. Can. J. Microbiol. 1995;41:1063–1070. doi: 10.1139/m95-149. [DOI] [PubMed] [Google Scholar]
- 66.Doohan F., Brennan J., Cooke B. Influence of Climatic Factors on Fusarium Species Pathogenic to Cereals, Epidemiology of Mycotoxin Producing Fungi. Springer; Dordrecht, Netherlands: 2003. pp. 755–768. [Google Scholar]
- 67.Tonapi V.A., Mundada R.R., Navi S.S., Reddy R.K., Thakur R.P., Bandyopadhyay R., Varanavasiappan S., Seetharama N. Effect of temperature and humidity regimes on grain mold sporulation and seed quality in sorghum (Sorghum bicolor (L.) Moench) Arch. Phytopathol. Plant Prot. 2007;40:113–127. doi: 10.1080/03235400500355626. [DOI] [Google Scholar]
- 68.Daami-Remadi M., Jabnoun-Khiareddine H., Ayed F., El Mahjoub M. Effect of temperature on aggressivity of Tunisian Fusarium species causing potato (Solanum tuberosum L.) tuber dry rot. J. Agron. 2006;5:350–355. doi: 10.3923/ja.2006.350.355. [DOI] [Google Scholar]
- 69.Desai A., Dange S., Patel D.S., Patel D.B. Variability in Fusarium oxysporum f. sp. ricini causing wilt of castor. Mycol. Plant Pathol. 2003;33:37–41. [Google Scholar]
- 70.Crous P.W., Slippers B., Wingfield M.J., Rheeder J., Marasas W.F., Philips A.J., Alves A., Burgess T., Barber P., Groenewald J.Z. Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 2006;55:235–253. doi: 10.3114/sim.55.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Idnurm A., Rodríguez-Romero J., Corrochano L.M., Sanz C., Iturriaga E.A., Eslava A.P., Heitman J. The Phycomyces madA gene encodes a blue-light photoreceptor for phototropism and other light responses. Proc. Natl. Acad. Sci. USA. 2006;103:4546–4551. doi: 10.1073/pnas.0600633103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu L., Li F., Xie H., Liu X. A novel method for promoting conidial production by a nematophagous fungus, Pochonia chlamydosporia AS6.8. World J. Microbiol. Biotechnol. 2009;25:1989–1994. doi: 10.1007/s11274-009-0099-y. [DOI] [Google Scholar]
- 73.Starostzik C., Marwan W. A photoreceptor with characteristics of phytochrome triggers sporulation in the true slime mould Physarum polycephalum. FEBS Lett. 1995;370:146–148. doi: 10.1016/0014-5793(95)00820-Y. [DOI] [PubMed] [Google Scholar]
- 74.Dahlberg K.R., Etten J. Physiology and biochemistry of fungal sporulation. Ann. Rev. Phytopathol. 1982;20:281–301. doi: 10.1146/annurev.py.20.090182.001433. [DOI] [Google Scholar]
- 75.Rakoczy L. Influence of monochromatic light on the fructification of Physarum nudum. Acta Soc. Bot. Pol. 1963;11:559–562. [Google Scholar]
- 76.Rakoczy L. Action spectrum in sporulation of slime-mold Physarum nudum Macbr. Acta Soc. Bot. Pol. 1965;34:97–112. doi: 10.5586/asbp.1965.006. [DOI] [Google Scholar]
- 77.Tisch D., Schmoll M. Light regulation of metabolic pathways in fungi. Appl. Microbiol. Biotechnol. 2010;85:1259–1277. doi: 10.1007/s00253-009-2320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leach C.M. Sporulation of diverse species of fungi under near-ultraviolet radiation. Can. J. Bot. 1962;40:151–161. doi: 10.1139/b62-016. [DOI] [Google Scholar]
- 79.Das J., Busse H. Light-driven diurnal zonation in the filamentous fungus Fusarium solani. Int. J. Dev. Biol. 1990;34:319–322. [PubMed] [Google Scholar]
- 80.Leach C., Tulloch M. Induction of sporulation of fungi isolated from Dactylis glomerata seed by exposure to near-ultraviolet radiation. Ann. Appl. Biol. 1972;72:155–159. doi: 10.1111/j.1744-7348.1972.tb01280.x. [DOI] [Google Scholar]
- 81.Avalos J., Estrada A.F. Regulation by light in Fusarium. Fungal Genet. Biol. 2010;47:930–938. doi: 10.1016/j.fgb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 82.Corrochano L.M. Fungal photoreceptors: Sensory molecules for fungal development and behaviour. Photochem. Photobiol. Sci. 2007;6:725–736. doi: 10.1039/b702155k. [DOI] [PubMed] [Google Scholar]
- 83.Rodriguez-Romero J., Hedtke M., Kastner C., Müller S., Fischer R. Fungi, hidden in soil or up in the air: Light makes a difference. Annu. Rev. Microbiol. 2010;64:585–610. doi: 10.1146/annurev.micro.112408.134000. [DOI] [PubMed] [Google Scholar]
- 84.Ruger-Herreros C., Rodríguez-Romero J., Fernández-Barranco R., Olmedo M., Fischer R., Corrochano L.M., Canovas D. Regulation of conidiation by light in Aspergillus nidulans. Genetics. 2011;188:809–822. doi: 10.1534/genetics.111.130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Froehlich A.C., Liu Y., Loros J.J., Dunlap J.C. White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science. 2002;297:815–819. doi: 10.1126/science.1073681. [DOI] [PubMed] [Google Scholar]
- 86.He Q., Cheng P., Yang Y., Wang L., Gardner K.H., Liu Y. White collar-1, a DNA binding transcription factor and a light sensor. Science. 2002;297:840–843. doi: 10.1126/science.1072795. [DOI] [PubMed] [Google Scholar]
- 87.Schafmeier T., Diernfellner A.C. Light input and processing in the circadian clock of Neurospora. FEBS Lett. 2011;585:1467–1473. doi: 10.1016/j.febslet.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 88.Idnurm A., Verma S., Corrochano L.M. A glimpse into the basis of vision in the kingdom Mycota. Fungal Genet. Biol. 2010;47:881–892. doi: 10.1016/j.fgb.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sánchez-Arreguín A., Pérez-Martínez A.S., Herrera-Estrella A. Proteomic analysis of Trichoderma atroviride reveals independent roles for transcription factors BLR-1 and BLR-2 in light and darkness. Eukaryot. Cell. 2012;11:30–41. doi: 10.1128/EC.05263-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Estrada A.F., Avalos J. The White-Collar protein WcoA of Fusarium fujikuroi is not essential for photocarotenogenesis, but is involved in the regulation of secondary metabolism and conidiation. Fungal Genet. Biol. 2008;45:705–718. doi: 10.1016/j.fgb.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 91.Souramma V., Singh J. Effect of temperature and pH on growth and sporulation of wilt causing pathogen in linseed. J. Oilseeds Res. 2004;21:206–207. [Google Scholar]
- 92.Groenewald S. Biology, Pathogenicity and Diversity of Fusarium oxysporum Fsp Cubense. University of Pretoria; Pretoria, South Africa: 2005. p. 316. [Google Scholar]
- 93.Gupta V., Misra A., Gaur R. Growth characteristics of Fusarium spp. causing wilt disease in Psidium guajava L. in India. J. Plant Prot. Res. 2010;50:453–462. doi: 10.2478/v10045-010-0076-3. [DOI] [Google Scholar]
- 94.Ahamad S., Agarwal D., Narain U., Chauhan S. Effect of temperature, pH, light and incubation periods on growth, sporulation, biomass and gibberellic acid production. Ann. Plant Prot. Sci. 2002;10:343–348. [Google Scholar]
- 95.Sharma R., Singh B., Thakur M., Thapak S.K. Effect of media, temperature, pH and Light on the growth and sporulation of Fusarium oxysporum f. sp. lini (Bolley) Snyder and Hensan. Ann. Plant Prot. Sci. 2005;13:172–174. [Google Scholar]
- 96.Kishore R., Pandey M., Dubey M.K., Kumar Y. Effect of Temperature and pH on Growth and Sporulation of Fusarium Oxysporum f. sp. Lini (Bolley) Snyder and Hensan Causing Linseed Wilt. Progress. Agric. 2009;9:147–149. [Google Scholar]
- 97.Photita W., Lumyong S., Lumyong P., Hyde K.D. Endophytic fungi of wild banana (Musa acuminata) at doi Suthep Pui National Park, Thailand. Mycol. Res. 2001;105:1508–1513. doi: 10.1017/S0953756201004968. [DOI] [Google Scholar]
- 98.Okane I., Nakagiri A., Ito T. Identity of Guignardia sp. inhabiting ericaceous plants. Can. J. Bot. 2001;79:101–109. [Google Scholar]
- 99.Fisher N.L., Burgess L., Toussoun T., Nelson P.E. Carnation leaves as a substrate and for preserving cultures of Fusarium species. Phytopathology. 1982;72:151–153. doi: 10.1094/Phyto-72-151. [DOI] [Google Scholar]
- 100.Liu A.R., Chen S.C., Wu S.Y., Xu T., Guo L.D., Jeewon R., Wei J.G. Cultural studies coupled with DNA based sequence analyses and its implication on pigmentation as a phylogenetic marker in Pestalotiopsis taxonomy. Mol. Phylogenet. Evol. 2010;57:528–535. doi: 10.1016/j.ympev.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 101.Wei J., Xu T., Guo L.D. Morphological stability and taxonomical significance of the genus Pestalotiopsis. J. Laiyang Agric. Coll. 2006;23:280–284. [Google Scholar]
- 102.Hassan Y.I., Bullerman L.B. Wheat bran as an alternative substrate for macroconidia formation by some Fusarium species. J. Microbiol. Methods. 2009;77:134–136. doi: 10.1016/j.mimet.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 103.Leaver F., Leal J., Brewer C. Nutritional studies on Piricularia oryzae. J. Bacteriol. 1947;54:401–408. doi: 10.1128/jb.54.4.401-408.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Misawa T. Nutritional factors for phytopathogenic fungi on culture media. Jpn. J. Phytopathol. 1965;31:27–34. doi: 10.3186/jjphytopath.31.Special1_27. [DOI] [Google Scholar]
- 105.Su Y., Qi Y., Cai L. Induction of sporulation in plant pathogenic fungi. Fungal Biol. 2012;3:195–200. [Google Scholar]
- 106.Timberlake W.E. Developmental gene regulation in Aspergillus nidulans. Dev. Biol. 1980;78:497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- 107.Yamaguchi H. Mycelial development and chemical alteration of Candida albicans from biotin insufficiency. Sabouraudia. 1974;12:320–328. doi: 10.1080/00362177485380461. [DOI] [PubMed] [Google Scholar]
- 108.Yoshida S., Shirata A. Biotin induces sporulation of mulberry anthracnose fungus, Colletotrichum dematium. J. Gen. Plant Pathol. 2000;66:117–122. doi: 10.1007/PL00012931. [DOI] [Google Scholar]
- 109.Timnick M.B., Lilly V.G., Barnett H. The effect of nutrition on the sporulation of Melanconium fuligineum in culture. Mycologia. 1951;43:625–634. doi: 10.1080/00275514.1951.12024159. [DOI] [Google Scholar]
- 110.Engelkes C., Nuclo R., Fravel D. Effect of carbon, nitrogen, and C: N ratio on growth, sporulation, and biocontrol efficacy of Talaromyces flavus. J. Phytopathol. 1997;87:500–505. doi: 10.1094/PHYTO.1997.87.5.500. [DOI] [PubMed] [Google Scholar]
- 111.Gao L., Sun M.H., Liu X.Z., Che Y.S. Effects of carbon concentration and carbon to nitrogen ratio on the growth and sporulation of several biocontrol fungi. Mycol. Res. 2007;111:87–92. doi: 10.1016/j.mycres.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 112.Wulandari N., To-Anun C., Hyde K., Duong L., De Gruyter J., Meffert J., Groenewald J., Crous P. Phyllosticta citriasiana sp. nov., the cause of Citrus tan spot of Citrus maxima in Asia. Fungal Divers. 2009;34:23–39. [Google Scholar]
- 113.Braun U., Crous P.W., Groenewald J.Z., Scheuer C. Pseudovirgaria, a fungicolous hyphomycete genus. IMA Fungus. 2011;2:65–69. doi: 10.5598/imafungus.2011.02.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masangkay R.F., Paulitz T.C., Hallett S.G., Watson A.K. Characterization of sporulation of Alternaria alternata f. sp. sphenocleae. Biocontrol Sci. Technol. 2000;10:385–397. doi: 10.1080/09583150050114981. [DOI] [Google Scholar]
- 115.Booth C. Chapter II Fungal Culture Media, Methods in Microbiology. Academic Press Inc.; London, UK: 1971. pp. 49–94. [Google Scholar]
- 116.Guo L.D. A method to promote sporulation in palm endophytic fungi. Fungal Divers. 1998;1:109–113. [Google Scholar]
- 117.Li W.C., Zhou J., Guo S.Y., Guo L.D. Endophytic fungi associated with lichens in Baihua mountain of Beijing, China. Fungal Divers. 2007;25:69–80. [Google Scholar]
- 118.Kishore C., Kulkarni S. Evaluation of Plant Extracts and Biocontrol Agents Against Fusarium Oxysporum f. sp. Gerberae Causing Wilt of Gerbera. J. Plant Dis. Sci. 2008;3:108–110. [Google Scholar]
- 119.Mezzomo R., Rolim J.M., Poletto T., De Oliveira M.B., Lazarotto M., Muñiz M.F. Mycelial growth and sporulation of Fusarium spp. Pathogenic to Ilex paraguariensis in different culture media and under exposure to different light levels. Sci. Agrar. 2018;19:14–19. doi: 10.5380/rsa.v19i1.55844. [DOI] [Google Scholar]
- 120.Arie T. Fusarium diseases of cultivated plants, control, diagnosis, and molecular and genetic studies. J. Pestic. Sci. 2019;44:275–281. doi: 10.1584/jpestics.J19-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Djunaedy A. Biopesticides as control of plant pests (opt) which are environmentally friendly. Embryo. 2009;6:88–95. [Google Scholar]
- 122.Astiti N.P.A., Suprapta D.N. Antifungal activity of teak (Tectona grandis Lf) leaf extract against Arthrinium phaeospermum (corda) MB Ellis, the cause of wood decay on Albizia falcataria (L) ISSAAS. 2012;18:62–69. [Google Scholar]
- 123.Suprapta D., Sudana M., Alit-Susanta W., Sudiarta P. Plant extracts to control cocoa black pod disease caused by Phytophthora palmivora. J. Int. Soc. Southeast Asian Agric. Sci. 2008;13:1–30. [Google Scholar]
- 124.Suprapta D., Sudarma M., Arya N., Ohsawa K. Plant Extracts to Control Wilt Desease in Banana Seedlings. J. Int. Soc. Southeast Asian Agric. Sci. 2005;11:84–90. [Google Scholar]
- 125.Suprapta D.N., Khalimi K. Anti-fungal activities of selected tropical plants from Bali Island. J. Phytopharm. 2012;2:265–270. [Google Scholar]
- 126.Suprapta D.N., Khalimi K. Efficacy of plant extract formulations to suppress stem rot disease on vanilla seedlings. J. Int. Soc. Southeast Asian Agric. Sci. 2009;15:34–41. [Google Scholar]
- 127.Begum S., Devi R.T., Singh N.I. Evaluation of fungicides, biocontrol agents and botanicals for management of damping-off in cabbage seedlings caused by Fursarium moniliforme sheld. J. Appl. Nat. Sci. 2015;7:106–110. doi: 10.31018/jans.v7i1.572. [DOI] [Google Scholar]
- 128.Pfleger F., Harman G. Fungal antisporulant activity of a complex lipid fraction extracted from pea seeds. Can. J. Bot. 1975;53:1625–1629. doi: 10.1139/b75-192. [DOI] [Google Scholar]
- 129.Drakopoulos D., Luz C., Torrijos R., Meca G., Weber P., Bänziger I., Voegele R.T., Six J., Vogelgsang S. Use of botanicals to suppress different stages of the life cycle of Fusarium graminearum. Phytopathology. 2019;109:2116–2123. doi: 10.1094/PHYTO-06-19-0205-R. [DOI] [PubMed] [Google Scholar]
- 130.Sinaga S. Principle of Plant Diseases. Jakarta; Penebar, Swadaya: 2006. [Google Scholar]
- 131.Selim E.M., Ammar M., Amer G., Awad H. Effect of some plant extracts, plant oils and Trichoderma spp. on tomato Fusarium wilt disease. Menoufia J. Plant Prot. 2020;5:155–167. doi: 10.21608/mjapam.2020.138483. [DOI] [Google Scholar]
- 132.Danish P., Ali Q., Hafeez M., Malik A. Antifungal and antibacterial activity of aloe vera plant extract. Biol. Clin. Sci. Res. J. 2020;2020:4. doi: 10.54112/bcsrj.v2020i1.4. [DOI] [Google Scholar]
- 133.Castellanos L.M., Olivas N.A., Ayala-Soto J., De La O Contreras C.M., Ortega M.Z., Salas F.S., Hernández-Ochoa L. In vitro and in vivo antifungal activity of clove (Eugenia caryophyllata) and pepper (Piper nigrum L.) essential oils and functional extracts against Fusarium oxysporum and Aspergillus niger in tomato (Solanum lycopersicum L.) Int. J. Microbiol. 2020;8:1702037. doi: 10.1155/2020/1702037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suprapta D., Swari I.G., Arya N., Ohsawa K. Pometia pinnata leaves extract to control light blight disease in potato. J. Int. Soc. Southeast Asian Agric. Sci. 2002;8:31–36. [Google Scholar]
- 135.Darmadi A.A.K., Suprapta D.N., Temaja I., Swantara I.M.D., Sudirman J., Indonesia D.B. Leaf extract of Cinnamomum burmanni blume effectively suppress the growth of Fusarium oxysporum f. sp. lycopersici the cause of Fusarium wilt disease on tomato. J. Biol. Agric. Healthc. 2015;5:131–137. [Google Scholar]
- 136.Shivpuri A., Sharma O., Jhamaria S. Fungitoxic properties of plant extracts against pathogenic fungi. J. Mycol. Plant Pathol. 1997;27:29–31. [Google Scholar]
- 137.Singh J.K., Kumar M., Kumar S., Kumar A., Mehta N. Inhibitory effect of botanicals on growth and sporulation of Fusarium oxysporum inciting wilt of Chilli (Capsicum annuum L.) J. Pharmacogn. Phytochem. 2017;6:2199–2204. [Google Scholar]
- 138.Hassanein N., Zeid M.A., Youssef K., Mahmoud D. Efficacy of leaf extracts of neem (Azadirachta indica) and chinaberry (Melia azedrach) against early blight and wilt diseases of tomato. Aust. J. Basic Appl. Sci. 2008;2:763–772. [Google Scholar]
- 139.Enespa D.S., Dwivedi S. Effectiveness of some antagonistic fungi and botanicals against Fusarium solani and Fusarium oxysporum f. sp. lycopersici infecting brinjal and tomato plants. Asian. J. Plant Pathol. 2014;8:18–25. [Google Scholar]
- 140.El-Ghany A., Roushdy M., Mohamed A. Efficacy of certain plant extracts as safe fungicides against phytopathogenic and mycotoxigenic fungi. J. Agric. Biol. Sci. 2015;1:71–75. [Google Scholar]
- 141.Rai V.R., Lokesh S., Khan A. Occurrence and Management of some Seedborne Fungal Pathogens of Maize and Sorghum in vitro. Seed Res. New Delhi. 2002;30:112–117. [Google Scholar]
- 142.Ramaiah A.K., Garampalli R.K.H. In vitro antifungal activity of some plant extracts against Fusarium oxysporum f. sp. lycopersici. Asian J. Plant Sci. Res. 2015;5:22–27. [Google Scholar]
- 143.Yelmame M., Mehta B., Deshmukh A., Patil V. Evaluation of some organic extracts in in vitro to control Fusarium solani causing chilli wilt. Int. J. Pharma Bio. Sci. 2010;1:B551 ref 8.. [Google Scholar]
- 144.Arora R., Singh S., Sharma R. Botanical Medicine in Clinical Practice. CABI; Wallingford, UK: 2008. Neem Leaves: Indian Herbal Medicine; pp. 85–98. [Google Scholar]
- 145.Atawodi S.E., Atawodi J.C. Azadirachta indica (neem): A plant of multiple biological and pharmacological activities. Phytochem. Rev. 2009;8:601–620. doi: 10.1007/s11101-009-9144-6. [DOI] [Google Scholar]
- 146.Brahmachari G. Neem—An omnipotent plant: A retrospection. Chembiochem. 2004;5:408–421. doi: 10.1002/cbic.200300749. [DOI] [PubMed] [Google Scholar]
- 147.Girish K., Shankara B.S. Neem–a green treasure. Electron. J. Biol. 2008;4:102–111. [Google Scholar]
- 148.Sarkar K., Bose A., Laskar S., Choudhuri S.K., Dey S., Roychowdhury P.K., Baral R. Antibody response against neem leaf preparation recognizes carcinoembryonic antigen. Int. Immunopharmacol. 2007;7:306–312. doi: 10.1016/j.intimp.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 149.Tiwari R., Verma A.K., Chakraborty S., Dhama K., Singh S.V. Neem (Azadirachta indica) and its potential for safeguarding health of animals and humans: A review. J. Biol. Sci. 2014;14:110. doi: 10.3923/jbs.2014.110.123. [DOI] [Google Scholar]
- 150.Singh U., Maurya S., Singh A., Gohain L. Foliar spray of aqueous extract of neem (Azadirachta indica) cake to control balsam (Impatiens balsaminia) powdery mildew. Arch. Phytopathol. Plant Prot. 2010;43:1056–1063. doi: 10.1080/03235400802285315. [DOI] [Google Scholar]
- 151.Mangi A.H., Jiskani A.M., Khaskhell M.I., Jiskani M.M., Poussio G.B., Qambrani R.A., Mahar M.A. Evaluation of Neem Products Against Damping of Disease of Tomato. Pak. J. Phytopathol. 2021;33:37–45. doi: 10.33866/phytopathol.033.01.0607. [DOI] [Google Scholar]
- 152.Vani M.S., Kumar S., Gulya R. In vitro evaluation of fungicides and plant extracts against Fusarium oxysporum causing wilt of mungbean. J. Pharm. Innov. 2019;8:297–302. [Google Scholar]
- 153.Chen Y., Zeng H., Tian J., Ban X., Ma B., Wang Y. Antifungal mechanism of essential oil from Anethum graveolens seeds against Candida albicans. J. Med. Microbiol. 2013;62:1175–1183. doi: 10.1099/jmm.0.055467-0. [DOI] [PubMed] [Google Scholar]
- 154.Nazzaro F., Fratianni F., Coppola R., De Feo V. Essential oils and antifungal activity. Pharm. J. 2017;10:86. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gogoi P., Baruah P., Nath S.C. Antifungal Activity of the Essential Oil of Litsea cubeba. Pers. J. Essent. Oil Res. 1997;9:213–215. doi: 10.1080/10412905.1997.9699462. [DOI] [Google Scholar]
- 156.Xia Z., Yang J., Li P. Study on antifungal mechanism of Litsea cubeba oil in Candida albicans. Bull. Hunan Med. Univ. 1995;20:107–108. [Google Scholar]
- 157.Ark P.A., Thompson J.P. Control of certain diseases of plants with antibiotics from garlic (Allium sativum L.) Plant Dis. Rep. 1959;43:276–282. [Google Scholar]
- 158.Council N.R. Regulating Pesticides in Food: The Delaney Paradox. National Academies Press; Cambridge, MA, USA: 1987. [PubMed] [Google Scholar]
- 159.Perello A.E., Noll U., Slusarenko A.J. In vitro efficacy of garlic extracts to control fungal pathogens of wheat. J. Med. Plant Res. 2013;7:1809–1817. [Google Scholar]
- 160.Wilson C., Solar J., El Ghaouth A., Wisniewski M. Rapid evaluation of plant extracts and essential oils for antifungal activity against Botrytis cinerea. Plant Dis. 1997;81:204–210. doi: 10.1094/PDIS.1997.81.2.204. [DOI] [PubMed] [Google Scholar]
- 161.Cavallito C.J., Bailey J.H. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944;66:1950–1951. doi: 10.1021/ja01239a048. [DOI] [Google Scholar]
- 162.Muhsin T.M., Al-Zubaidy S.R., Ali E.T. Effect of garlic bulb extract on the growth and enzymatic activities of rhizosphere and rhizoplane fungi. Mycopathologia. 2001;152:143–146. doi: 10.1023/A:1013184613159. [DOI] [PubMed] [Google Scholar]
- 163.Achimón F., Brito V.D., Pizzolitto R.P., Sanchez A.R., Gómez E.A., Zygadlo J.A. Chemical composition and antifungal properties of commercial essential oils against the maize phytopathogenic fungus Fusarium verticillioides. Rev. Argent. Microbiol. 2021;53:292–303. doi: 10.1016/j.ram.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 164.Ghabrial S.A., Caston J.R., Jiang D., Nibert M.L., Suzuki N. 50-plus years of fungal viruses. J. Virol. 2015;479–480:356–368. doi: 10.1016/j.virol.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 165.Ghabrial S.A., Suzuki N. Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 2009;47:353–384. doi: 10.1146/annurev-phyto-080508-081932. [DOI] [PubMed] [Google Scholar]
- 166.Pearson M.N., Beever R.E., Boine B., Arthur K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 2009;10:115–128. doi: 10.1111/j.1364-3703.2008.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xie J., Jiang D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 2014;52:45–68. doi: 10.1146/annurev-phyto-102313-050222. [DOI] [PubMed] [Google Scholar]
- 168.Wu S., Cheng J., Fu Y., Chen T., Jiang D., Ghabrial S.A., Xie J. Virus-mediated suppression of host non-self recognition facilitates horizontal transmission of heterologous viruses. PLoS Pathog. 2017;3:e1006234. doi: 10.1371/journal.ppat.1006234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Darissa O., Adam G., Schäfer W. A dsRNA mycovirus causes hypovirulence of Fusarium graminearum to wheat and maize. Eur. J. Plant Pathol. 2012;134:181–189. doi: 10.1007/s10658-012-9977-5. [DOI] [Google Scholar]
- 170.Lee K.M., Cho W.K., Yu J., Son M., Choi H., Min K., Lee Y.W., Kim K.H. A comparison of transcriptional patterns and mycological phenotypes following infection of Fusarium graminearum by four mycoviruses. PLoS ONE. 2014;9:e100989. doi: 10.1371/journal.pone.0100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lemus-Minor C.G., Cañizares M.C., García-Pedrajas M.D., Pérez-Artés E.J. Fusarium oxysporum f. sp. dianthi virus 1 accumulation is correlated with changes in virulence and other phenotypic traits of its fungal host. Phytopathology. 2018;108:957–963. doi: 10.1094/PHYTO-06-17-0200-R. [DOI] [PubMed] [Google Scholar]
- 172.Aydi Ben Abdallah R., Jabnoun-Khiareddine H., Nefzi A., Mokni-Tlili S., Daami-Remadi M. Biocontrol of Fusarium wilt and growth promotion of tomato plants using endophytic bacteria isolated from Solanum elaeagnifolium stems. J. Phytopathol. 2016;164:811–824. doi: 10.1111/jph.12501. [DOI] [Google Scholar]
- 173.Wang S., Liang Y., Shen T., Yang H., Shen B. Biological characteristics of Streptomyces albospinus CT205 and its biocontrol potential against cucumber Fusarium wilt. Biocontrol Sci. Technol. 2016;26:951–963. doi: 10.1080/09583157.2016.1172203. [DOI] [Google Scholar]
- 174.Zhao Y., Selvaraj J.N., Xing F., Zhou L., Wang Y., Song H., Tan X., Sun L., Sangare L., Folly Y.M.E. Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS ONE. 2014;9:e92486. doi: 10.1371/journal.pone.0092486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shi L., Du N., Shu S., Sun J., Li S., Guo S. Paenibacillus polymyxa NSY50 suppresses Fusarium wilt in cucumbers by regulating the rhizospheric microbial community. Sci. Rep. 2017;7:1–13. doi: 10.1038/srep41234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.García-Rincón J., Vega-Pérez J., Guerra-Sanchez M.G., Hernandez-Lauzardo A.N., Peña-Díaz A., Valle M.G.V.-D. Effect of chitosan on growth and plasma membrane properties of Rhizopus stolonifer (Ehrenb.: Fr.) Vuill. Biochem. Physiol. 2010;97:275–278. doi: 10.1016/j.pestbp.2010.03.008. [DOI] [Google Scholar]
- 177.Liu H., Du Y., Wang X., Sun L.J. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004;95:147–155. doi: 10.1016/j.ijfoodmicro.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 178.Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 179.Roller S., Covill N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999;47:67–77. doi: 10.1016/S0168-1605(99)00006-9. [DOI] [PubMed] [Google Scholar]
- 180.Guo Z., Chen R., Xing R., Liu S., Yu H., Wang P., Li C., Li P. Novel derivatives of chitosan and their antifungal activities in vitro. Carbohydr. Res. 2006;341:351–354. doi: 10.1016/j.carres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 181.Tikhonov V., Stepnova E., Babak V., Yamskov I., Palma-Guerrero J., Jasson H., lopez-Lorca l.V., Salinas J., Gerasimenko D.V., Avdienko I.D., et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its n-/2 (3)-(Dodec-2-Enyl) Succinoyl/-derivatives. Carbohydr. Polym. 2006;64:66–72. doi: 10.1016/j.carbpol.2005.10.021. [DOI] [Google Scholar]
- 182.Xu J., Zhao X., Han X., Du Y. Antifungal activity of oligochitosan against Phytophthora capsici and other plant pathogenic fungi in vitro. Pestic. Biochem. Physiol. 2007;87:220–228. doi: 10.1016/j.pestbp.2006.07.013. [DOI] [Google Scholar]
- 183.Anirban B. Fungicidal potential of chitosan against phytopathogenic Fusarium solani. J. Exp. Biol. Agric. Sci. 2013;1:258–263. [Google Scholar]
- 184.Ramteke P.K. Effect of resistance inducers on in vitro inhibition of mycelial growth and sporulation of Fusarium solani causing root rot of fenugreek. Plant Pathol. Quar. 2019;9:198–209. doi: 10.5943/ppq/9/1/18. [DOI] [Google Scholar]
- 185.Liang R., Li X., Yuan W., Jin S., Hou S., Wang M., Wang H. Antifungal activity of nanochitin whisker against crown rot diseases of wheat. J. Agric. Food Chem. 2018;66:9907–9913. doi: 10.1021/acs.jafc.8b02718. [DOI] [PubMed] [Google Scholar]
- 186.Davis A.J., Grant B.R. The effect of phosphonate on the sporulation of Fusarium oxysporum f.sp. cubense. Australas. Plant Pathol. 1996;25:31–35. doi: 10.1071/AP96007. [DOI] [Google Scholar]
- 187.Lin H., Travisano M., Kazlauskas R.J. The Fungus Trichoderma Regulates Submerged Conidiation Using the Steroid Pregnenolone. ACS Chem. Biol. 2016;11:2568–2575. doi: 10.1021/acschembio.6b00376. [DOI] [PubMed] [Google Scholar]
- 188.Schreiber K.J., Nasmith C.G., Allard G., Singh J., Subramaniam R., Desveaux D. Found in translation: High-throughput chemical screening in Arabidopsis thaliana identifies small molecules that reduce Fusarium head blight disease in wheat. Mol. Plant-Microbe Interact. 2011;24:640–648. doi: 10.1094/MPMI-09-10-0210. [DOI] [PubMed] [Google Scholar]
- 189.Faurie B., Cluzet S., Corio-Costet M.F., Mérillon J.M. Methyl jasmonate/ethephon cotreatment synergistically induces stilbene production in" Vitis vinifera" cell suspensions but fails to trigger resistance to Erysiphe necator. J. Int. Sci. Vigne. Vin. 2009;43:99–110. doi: 10.20870/oeno-one.2009.43.2.800. [DOI] [Google Scholar]
- 190.Konan Y.K.F., Kouassi K.M., Kouakou K.L., Koffi E., Kouassi K.N., Sekou D., Kone M., Kouakou T.H. Effect of methyl jasmonate on phytoalexins biosynthesis and induced disease resistance to Fusarium oxysporum f. sp. Vasinfectum in cotton (Gossypium hirsutum L.) Int. J. Agron. 2014;11:806439. [Google Scholar]
- 191.Wasternack C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Larronde F., Gaudillère J.P., Krisa S., Decendit A., Deffieux G., Mérillon J.M. Airborne methyl jasmonate induces stilbene accumulation in leaves and berries of grapevine plants. Am. J. Enol. Vitic. 2003;54:63–66. doi: 10.5344/ajev.2003.54.1.63. [DOI] [Google Scholar]
- 193.Wang Q., Qiu B., Li S., Zhang Y., Cui X., Ge F., Liu D. A methyl jasmonate induced defensin like protein from Panax notoginseng confers resistance against Fusarium solani in transgenic tobacco. Biol. Plant. 2019;63:797–807. doi: 10.32615/bp.2019.123. [DOI] [Google Scholar]
- 194.Abdel-Monaim M.F., Abdel-Gaid M.A.W., Armanious A.H. Effect of chemical inducers on root rot and wilt diseases, yield and quality of tomato. Int. J. Agric. Sci. 2012;2:211–220. [Google Scholar]
- 195.Wu H.S., Raza W., Liu D.Y., Wu C.L., Mao Z.S., Xu Y.C., Shen Q.-R.J. Allelopathic impact of artificially applied coumarin on Fusarium oxysporum f. sp. niveum. World J. Microbiol. Biotechnol. 2008;24:1297–1304. doi: 10.1007/s11274-007-9602-5. [DOI] [Google Scholar]
- 196.Ali I., Husnain T., Riazuddin S. RNA interference: The story of gene silencing in plants and humans. Biotechnol. Adv. 2008;26:202–209. doi: 10.1016/j.biotechadv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 197.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 198.Jinek M., Doudna J.A. A three-dimensional view of the molecular machinery of RNA interference. Nature. 2009;457:405–412. doi: 10.1038/nature07755. [DOI] [PubMed] [Google Scholar]
- 199.Watson J.M., Fusaro A.F., Wang M., Waterhouse P.M. RNA silencing platforms in plants. FEMS. Lett. 2005;579:5982–5987. doi: 10.1016/j.febslet.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 200.Napoli C., Lemieux C., Jorgensen R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell. 1990;2:279–289. doi: 10.2307/3869076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fairbairn D.J., Cavallaro A.S., Bernard M., Mahalinga-Iyer J., Graham M.W., Botella J.R. Host-delivered RNAi: An effective strategy to silence genes in plant parasitic nematodes. Planta. 2007;226:1525–1533. doi: 10.1007/s00425-007-0588-x. [DOI] [PubMed] [Google Scholar]
- 202.Hu Z., Parekh U., Maruta N., Trusov Y., Botella J.R. Down-regulation of Fusarium oxysporum endogenous genes by host-delivered RNA interference enhances disease resistance. Front. Chem. 2015;3:1. doi: 10.3389/fchem.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tinoco M.L.P., Dias B., Dall’Astta R.C., Pamphile J.A., Aragão F. In vivo trans-specific gene silencing in fungal cells by in planta expression of a double-stranded RNA. BioMed Central. 2010;8:1–11. doi: 10.1186/1741-7007-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Koch A., Kumar N., Weber L., Keller H., Imani J., Kogel K.H. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase–encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA. 2013;110:19324–19329. doi: 10.1073/pnas.1306373110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Takano Y., Kikuchi T., Kubo Y., Hamer J.E., Mise K., Furusawa I. The Colletotrichum lagenarium MAP kinase gene CMK1 regulates diverse aspects of fungal pathogenesis. Mol. Plant-Microbe Interact. 2000;13:374–383. doi: 10.1094/MPMI.2000.13.4.374. [DOI] [PubMed] [Google Scholar]
- 206.Pareek M., Rajam M.V. RNAi-mediated silencing of MAP kinase signalling genes (Fmk1, Hog1, and Pbs2) in Fusarium oxysporum reduces pathogenesis on tomato plants. Fungal Biol. 2017;121:775–784. doi: 10.1016/j.funbio.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 207.Zheng D., Zhang S., Zhou X., Wang C., Xiang P., Zheng Q., Xu J.R. The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum. PLoS ONE. 2012;7:e49495. doi: 10.1371/journal.pone.0049495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shanmugam V., Sharma V., Bharti P., Jyoti P., Yadav S.K., Aggarwal R., Jain S. RNAi induced silencing of pathogenicity genes of Fusarium spp. for vascular wilt management in tomato. Ann. Microbiol. 2017;67:359–369. doi: 10.1007/s13213-017-1265-3. [DOI] [Google Scholar]
- 209.Fernandes J., Angelo P., Cruz J., Santos J., Sousa N.R., Silva G. Post-transcriptional silencing of the SGE1 gene induced by a dsRNA hairpin in Fusarium oxysporum f. sp cubense, the causal agent of Panama disease. Genet. Mol. Res. 2016;15:1–142. doi: 10.4238/gmr.15027941. [DOI] [PubMed] [Google Scholar]
- 210.Singh N., Mukherjee S.K., Rajam M.V. Silencing of the ornithine decarboxylase gene of Fusarium oxysporum f. sp. lycopersici by host-induced RNAi confers resistance to Fusarium wilt in tomato. Plant Mol. Biol. Rep. 2020;38:419–429. doi: 10.1007/s11105-020-01205-2. [DOI] [Google Scholar]
- 211.Yin G., Sun Z., Liu N., Zhang L., Song Y., Zhu C., Wen F. Production of double-stranded RNA for interference with TMV infection utilizing a bacterial prokaryotic expression system. Appl. Microbiol. Biotechnol. 2009;84:323–333. doi: 10.1007/s00253-009-1967-y. [DOI] [PubMed] [Google Scholar]
- 212.Dalakouras A., Wassenegger M., McMillan J.N., Cardoza V., Maegele I., Dadami E., Runne M., Krczal G., Wassenegger M. Induction of silencing in plants by high-pressure spraying of in vitro-synthesized small RNAs. Front. Plant Sci. 2016;7:1327. doi: 10.3389/fpls.2016.01327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gebremichael D.E., Haile Z.M., Negrini F., Sabbadini S., Capriotti L., Mezzetti B., Baraldi E. RNA interference strategies for future management of plant pathogenic fungi: Prospects and challenges. Plants. 2021;10:650. doi: 10.3390/plants10040650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Koch A., Biedenkopf D., Furch A., Weber L., Rossbach O., Abdellatef E., Linicus L., Johannsmeier J., Jelonek L., Goesmann A., et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog. 2016;12:e1005901. doi: 10.1371/journal.ppat.1005901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Song X.S., Gu K.X., Duan X.X., Xiao X.M., Hou Y.P., Duan Y.B., Wang J.X., Yu N., Zhou M.G. Secondary amplification of siRNA machinery limits the application of spray-induced gene silencing. Mole. Plant Pathol. 2018;19:2543–2560. doi: 10.1111/mpp.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.