Abstract
Patients with tuberculosis had higher expression of monocyte urokinase receptor (uPAR) and CD11b than controls. In vitro, lipoarabinomannan and lipopolysaccharide (LPS) from Escherichia coli shared the ability to enhance uPAR and CD11b expression on monocytes and granulocytes. In healthy volunteers, LPS induced increases in monocyte and granulocyte uPAR and CD11b.
The receptor for urokinase plasminogen activator (uPAR; also called CD87) can act as an adhesion receptor, and in vitro chemotaxis of monocytes and granulocytes at least in part depends on the expression of uPAR (2, 5). uPAR is a glycosylphosphatidylinositol-linked receptor without a transmembrane domain; it therefore lacks a direct link to the cell interior. However, uPAR can form functional complexes with complement receptor 3 (CR3, [CD11b/CD18]) on monocytes (13, 14) and granulocytes (4, 16), thus enabling signal transduction for both the monocytes and the granulocytes and facilitating the adhesive capacity of the granulocytes. In addition, uPAR gene-deficient mice demonstrated a reduced β-integrin-mediated leukocyte recruitment to inflamed areas in vivo (10). Hence, the ability of uPAR to interact with CR3 seems important for adhesion and migration of monocytes and granulocytes. Recently, it was reported that intravenous injection of lipopolysaccharide (LPS) into healthy humans was associated with an upregulation of monocyte uPAR expression, while the effect on granulocyte uPAR expression was inconsistent (3). Thus far, the expression of the uPAR-CR3 complex during infections in vivo has not been studied. Therefore, we found it of interest to measure cellular uPAR and CD11b expression in patients with tuberculosis (TB). In addition, the capacity of lipoarabinomannan (LAM; derived from Mycobacterium tuberculosis) to influence cellular uPAR and CD11b expression was compared with the effect of LPS, and the model of human experimental endotoxemia was used to further study the kinetics of uPAR and CD11b expression during inflammation in vivo.
All studies were approved by the institutional research and ethics committees, and written informed consent was obtained from all subjects. Blood was obtained from eight patients (six male and two female) with active, culture-proven TB attending the Academic Medical Center (n = 5), the Sint Lucas Hospital (n = 2), and the Municipal Health Center (n = 1) in Amsterdam, The Netherlands. The age (mean ± standard error) of TB patients was (32 ± 4 years) and did not differ from that of healthy controls (29 ± 2 years; four male and four female). Four of the patients had pulmonary TB, and four had extrapulmonary TB. Extrapulmonary sites included pleural (n = 2), soft tissue (n = 1), and gastrointestinal tract (n = 1) sites. Three TB patients were human immunodeficiency virus seropositive and were treated with antiretroviral therapy. None of the TB patients took immunosuppressive drugs. Six patients had fever (rectal temperature ≳ 38°C). Blood for fluorescence-activated cell sorter analysis was drawn prior to administration of antituberculous medication. On the same day a patient was analyzed, blood was also obtained from a healthy control. After collection, blood was immediately prepared for fluorescence-activated cell sorter analysis (Calibrite; Becton Dickinson, San Jose, Calif.). For in vitro experiments, blood was collected aseptically from six healthy subjects and diluted 1:1 with RPMI 1640 (BioWhittaker, Verviers, Belgium), to which LAM (1 μg/ml, prepared from M. tuberculosis strain H37Rv, provided by J. T. Belisle [Fort Collins, Colo.]) or LPS (10 ng/ml, from Escherichia coli serotype O111; B4; Sigma, [St Louis, Mo.]) was added, and incubated at 37°C for 6 h. The LAM preparation contained 21.6 pg of LPS per mg of LAM as determined by the Limulus test. LPS potentially contaminating the LAM dose used (1 μg of LAM may contain 21.6 pg of LPS) was insufficient to influence uPAR or CD11b expression (data not shown). In addition, eight healthy male subjects, ages 24 ± 2 years (mean ± standard error), received a bolus intravenous injection of LPS (from E. coli, lot G; U.S. Pharmacopeia, Rockville, Md.) at a dose of 4 ng/kg of body weight. Venous blood samples were obtained directly before and 6 h after injection of LPS. (The timing of blood sampling was based on a previous study, in which the effect of intravenous LPS on cellular uPAR expression in humans in vivo was determined [3]). Blood was put on ice immediately, and erythrocytes were lysed with bicarbonate-buffered ammonium chloride solution (pH 7.4). After centrifugation. 106 leukocytes were resuspended in phosphate-buffered saline containing EDTA (100 mM), sodium azide (0.1%), and bovine serum albumin (5%). Blood of patients was incubated with fluorescein isothiocyanate-labeled mouse anti-human uPAR monoclonal antibody (clone VIM-5; Instruchemie, Hilversum, The Netherlands) or with mouse anti-human CD11b (Central Laboratory of The Netherlands Red Cross Blood Transfusion Service, CLB, Amsterdam, The Netherlands), followed by a fluorescein isothiocyanate-labeled F(ab′)2 goat-anti-mouse antibody (Zymed, San Francisco, Calif.). A murine isotype-matched antibody was used to control for aspecific staining (immunoglobulin G1; Becton Dickinson & Co., Rutherford, NJ.). Blood samples from the in vitro experiments and blood samples from the volunteers receiving LPS were incubated with phycoerythrin-labeled mouse anti-human CD11b or mouse anti-human uPAR (both from Pharmingen, San Diego, Calif.). Aspecific staining was controlled for by incubation of cells with phycoerythrin-labeled mouse immunoglobulin G1 (Coulter Immunotech. Marseilles, France). Data are presented as the difference between mean cell fluorescence intensities of specifically and nonspecifically stained cells. Data were analyzed using the Wilcoxon test. A P value of <0.05 was considered statistically significant.
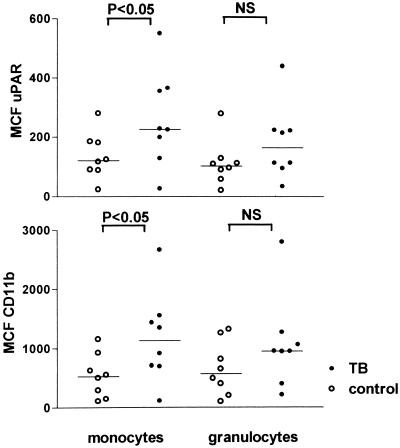
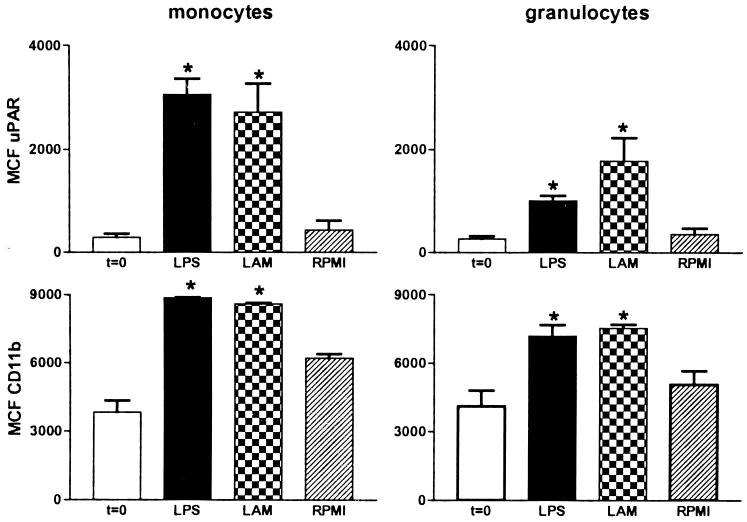
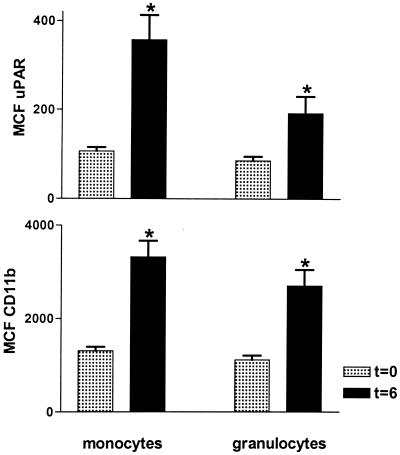
Patients with active TB had increased expression of both uPAR and CD11b on circulating monocytes compared to healthy controls (P < 0.05) (Fig. 1). Although granulocyte uPAR and granulocyte CD11b expression tended to be higher in patients than in controls, the differences did not reach statistical significance conceivably due to the relatively low number of patients that was studied. No differences were observed between patients with pulmonary TB and patients with extrapulmonary TB (data not shown). Next we investigated whether LAM, a cell wall component of M. tuberculosis, can influence uPAR or CD11b expression. For this purpose we incubated whole blood with LAM for 6 h. LAM significantly enhanced expression of uPAR and CD11b on both monocytes and granulocytes, although granulocyte upregulation was less prominent. Stimulation of whole blood with E. coli LPS yielded similar results, confirming our previous findings (Fig. 2). These findings are in line with other reports revealing that in vitro LPS and LAM effects on many different inflammatory reactions are very similar (7, 11). Having established that LPS can upregulate cellular uPAR and CD11b, we next used the human experimental endotoxemia model to study uPAR and CD11b expression during inflammation in vivo. Administration of LPS resulted in a strong upregulation of monocyte uPAR and CD11b expression (P < 0.05 for both), and a more modest increase in granulocyte uPAR and CD11b expression, at 6 h postinjection (P < 0.05) (Fig. 3).
FIG. 1.
Expression of uPAR and CD11b on peripheral blood monocytes and granulocytes of patients with active TB and healthy controls. Horizontal lines represent medians. MCF, mean channel fluorescence; NS, not significant.
FIG. 2.
Both LAM and LPS upregulate the expression of uPAR and CD11b on monocytes and granulocytes in whole blood in vitro. Whole blood was stimulated for 6 h with LPS (10 ng/ml) or LAM (1 μg/ml). RPMI, 6-h incubation without stimulus; t = 0, expression before incubation; MCF, mean channel fluorescence. Data are means ± standard errors for six different donors. ∗, P < 0.05 versus value obtained from RPMI.
FIG. 3.
Intravenous injection of LPS results in an upregulation of uPAR and CD11b on peripheral blood monocytes and granulocytes. Healthy subjects received an intravenous injection with LPS (4 ng/kg) at time zero. MCF, mean channel fluorescence. Data are means ± standard errors for eight subjects before (time zero) (t = 0) and 6 h after LPS administration (t = 6). ∗, P < 0.05 versus value obtained at time zero.
CR3 has been implicated in the capacity of mononuclear phagocytes to bind and phagocytose mycobacteria, and therefore it may play a role in early host defense against mycobacterial infection (6, 12, 15). Our findings of enhanced CD11b expression on circulating granulocytes and monocytes of patients with TB extends a previous report demonstrating that CD11b expression is increased on alveolar macrophages of patients with TB (8). Although in our hands LAM potently enhanced the expression of cellular CD11b in whole blood, THP-1 cells, derived from a monocyte/macrophage cell line, did not respond with an upregulation of CR3 upon stimulation with M. tuberculosis in vitro (9). It is conceivable that differences in culture systems (cell line versus mature blood cells in their natural environment) are at least in part responsible for this discrepancy.
The present study does not provide insight into the mechanisms underlying the effects of LAM on uPAR expression. It was previously shown that although recombinant tumor necrosis factor is able to enhance uPAR expression on monocytes, endogenously produced tumor necrosis factor is not required for LPS-induced uPAR expression (3). It seems unlikely that the enhanced expression of cell-associated uPAR represents a selective response. Indeed, the concurrent upregulation of CD11b points toward a more general response of activation. More extensive evaluation of different cellular activation markers, especially in response to LAM, is warranted to further investigate this issue. Very little is known about the expression of uPAR on cells in vivo. We have thus far evaluated the expression of uPAR only on circulating leukocytes of patients with TB. Investigations on the expression of cell-associated uPAR in patients with other infections are warranted to determine whether the response observed in TB patients also occurs as a consequence of bacterial infection. Intraperitoneal administration of LPS to mice was found in one study to increase uPAR mRNA in most tissues examined, the most profound upregulation being detected after 1 to 3 h (1). Expression of uPAR protein was not examined in that study. To the best of our knowledge, our study is the first to show enhanced expression of monocyte uPAR during human infection. In a previous study, investigators found an upregulation of uPAR on monocytes of healthy humans injected with LPS, whereas the increase in uPAR expression on granulocytes was inconsistent (3). These earlier findings conflict somewhat with the findings of upregulation of uPAR on monocytes and, albeit to a lesser extent, granulocytes in this study. This conflict may be explained by the fact that different staining antibodies were used in the two studies, or the fact that a slightly smaller study population was used in the earlier study, or both. M. tuberculosis, LAM, and LPS may influence the function of monocytes by stimulating the surface expression of the adhesion-mediating receptors uPAR and CD11b. This effect is shared by both gram-positive and gram-negative bacterial stimuli (3). Our results further suggest that monocytes are more sensitive than granulocytes in terms of uPAR upregulation upon stimulation.
Acknowledgments
This work was supported by grants from the “Mr. Willem Bakhuys Roozeboom” Foundation to N. P. Juffermans.
We thank J. T. Belisle (Fort Collins, Colo.) for providing LAM.
REFERENCES
- 1.Almus-Jacobs F, Varki N, Sawdey M S, Loskutoff D J. Endotoxin stimulates expression of the murine urokinase receptor gene in vivo. Am J Pathol. 1995;147:688–698. [PMC free article] [PubMed] [Google Scholar]
- 2.Blasi F. uPA, uPAR, PAI-1: key intersection of proteolytic, adhesive and chemotactic highways? Immunol Today. 1997;18:415–417. doi: 10.1016/s0167-5699(97)01121-3. [DOI] [PubMed] [Google Scholar]
- 3.Dekkers P E P, ten Hove T, te Velde A A, van Deventer S J H, van der Poll T. Upregulation of monocyte urokinase plasminogen activator receptor during human endotoxemia. Infect Immun. 2000;68:2156–2160. doi: 10.1128/iai.68.4.2156-2160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gyetko M R, Sitrin R G, Fuller J A, Todd III R F, Petty H, Standiford T J. Function of the urokinase receptor (CD87) in neutrophil chemotaxis. J Leukoc Biol. 1995;58:533–538. doi: 10.1002/jlb.58.5.533. [DOI] [PubMed] [Google Scholar]
- 5.Gyetko M R, Todd III R F, Wilkinson C C, Sitrin R C. The urokinase receptor is required for human monocyte chemotaxis in vitro. J Clin Investig. 1994;93:1380–1387. doi: 10.1172/JCI117114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hetland G, Wiker H G. Antigen 85C on Mycobacterium bovis BCG and M. tuberculosis promotes monocyte-CR3-mediated uptake of microbeads coated with mycobacterial products. Immunology. 1994;82:445–449. [PMC free article] [PubMed] [Google Scholar]
- 7.Juffermans N P, Verbon A, van Deventer S J H, van Deutekom H, Belisle J T, Ellis M E, Speelman P, van der Poll T. Elevated chemokine concentrations in sera of human immunodeficiency virus (HIV)-seropositive and HIV-seronegative patients with tuberculosis: a possible role for mycobacterial lipoarabinomannan. Infect Immun. 1999;67:4295–4297. doi: 10.1128/iai.67.8.4295-4297.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo H P, Ho T C, Wang C H, Yu C T, Lin H C. Increased production of hydrogen peroxide and expression of CD11b/CD18 on alveolar macrophages in patients with active pulmonary tuberculosis. Tuber Lung Dis. 1996;77:468–475. doi: 10.1016/s0962-8479(96)90122-7. [DOI] [PubMed] [Google Scholar]
- 9.López Ramírez G M, Rom W N, Ciotoli C, Talbot A, Martiniuk F, Cronstein B, Reibman J. Mycobacterium tuberculosis alters expression of adhesion molecules on monocytic cells. Infect Immun. 1994;62:2515–2520. doi: 10.1128/iai.62.6.2515-2520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May A E, Kanse S M, Lund L R, Gisler R H, Imhof B A, Preissner K T. Urokinase receptor (CD87) regulates leukocyte recruitment via β2 integrins in vivo. J Exp Med. 1998;188:1029–1037. doi: 10.1084/jem.188.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savedra R, Delude R L, Ingalls R R, Fenton M J, Golenbock D T. Mycobacterial lipoarabinomannan recognition requires a receptor that shares components of the endotoxin signaling system. J Immunol. 1996;157:2549–2554. [PubMed] [Google Scholar]
- 12.Schlesinger L S, Horwitz M A. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-γ activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991;147:1983–1994. [PubMed] [Google Scholar]
- 13.Simon D I, Rao N K, Xu H, Wei Y, Majdic O, Ronne E, Kobzik L, Chapman H A. Mac-1 (CD11b/CD18) and the urokinase receptor (CD87) form a functional unit on monocytic cells. Blood. 1996;88:3185–3194. [PubMed] [Google Scholar]
- 14.Sitrin R G, Todd III R F, Petty H R, Brock T G, Shollenberger S B, Albrecht E, Gyetko M R. The urokinase receptor (CD87) facilitates CD11b/CD18-mediated adhesion of human monocytes. J Clin Investig. 1996;97:1942–1951. doi: 10.1172/JCI118626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokes R W, Thorson L M, Speert D P. Nonopsonic and opsonic association of Mycobacterium tuberculosis with resident alveolar macrophages is inefficient. J Immunol. 1998;160:5514–5521. [PubMed] [Google Scholar]
- 16.Xue W, Kindzelskii A L, Todd III R F, Petty H R. Physical association of complement receptor type 3 and urokinase-type plasminogen activator receptor in neutrophil membranes. J Immunol. 1994;152:4630–4640. [PubMed] [Google Scholar]