Abstract
The effect of low-dose antigen exposure on the development of immunity to Helicobacter pylori infection was studied in outbred mice. Animals that were primed with a subinfectious number of H. pylori bacteria exhibited significantly lower bacterial loads after challenge with an infectious dose of pathogen (versus controls, P < 0.05).
The vast majority of individuals who are colonized with Helicobacter pylori remain infected despite the development of vigorous immune responses to the bacterium (5). For this reason, it is generally assumed that host immune responses are ineffective in clearing the infection. Nevertheless, studies on H. pylori seroprevalence in humans showed that up to 1.8% of individuals who were initially identified as being H. pylori positive by serology subsequently became negative upon retesting (15, 23, 24). It was suggested that this seroreversion in H. pylori-infected individuals was due to spontaneous elimination of the bacterium by the host (24). In such instances, however, the eradication of H. pylori as a consequence of antimicrobial treatment for unrelated infections could not be excluded.
The most compelling evidence for spontaneous elimination of H. pylori infection has come from nonhuman primate studies (6, 7). It was shown that certain monkeys developed transient infections after inoculation with H. pylori strains and that, in the early stages of colonization, individual monkeys displayed varying susceptibilities to different H. pylori strains (6, 7).
The type of host T helper (Th) cell phenotype induced during infection largely determines the outcome (protection or pathogenesis) of host immune responses. The Th response phenotype occurring in the host can be influenced by a multitude of factors, including the local cytokine environment and the size of the antigen or inoculum dose (1, 3, 4, 12, 20). In the Leishmania mouse infection model, it was shown that BALB/c mice that were exposed to a low-dose inoculum of Leishmania major parasites became resistant to infection despite the innate susceptibility of this mouse strain to L. major infection (3). This resistance was accompanied by a switch of the default Th response of BALB/c animals from a T helper 2 (Th2) type towards a dominant Th1 phenotype, thus mimicking the responses that spontaneously occur in Leishmania-resistant C57BL/6 mice (17, 18).
The aim of the present study was to determine whether exposure to a low-dose inoculum of H. pylori might confer immunity to infection in susceptible mice. To ensure diversity of host immune responses, we used an outbred mouse strain which had previously been shown to be highly susceptible to infection with the mouse-adapted H. pylori SS1 isolate (9, 16). Thus, 6-week-old specific-pathogen-free outbred Swiss mice (Centre d'Elevage R. Janvier, Le-Genest-St-Isle, France) were divided into three groups. Animals either were left untreated (“naive” animals; n = 5) or were administered intragastrically a single low-dose inoculum containing 15 CFU of H. pylori SS1 prepared in peptone trypsin broth (n = 19) or broth medium alone (n = 10). The minimum infectious dose of H. pylori SS1 for Swiss mice was previously determined to be equivalent to 102 CFU (9). One month later, H. pylori serum antibody levels in the mice were determined and compared to those in animals that had received no treatment (10). All animals were immunoglobulin G (IgG) seronegative for H. pylori (results not shown). Based on previous work (9), it was possible to conclude that the primed mice had not become infected with H. pylori. In addition, the serological data confirmed that the animals were free of intestinal Helicobacter spp. and therefore had not previously been exposed to Helicobacter antigens.
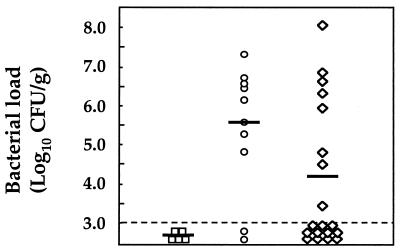
Mice primed with a low-dose inoculum (“primed/challenged” group) and those that had received peptone trypsin broth (“naive/challenged” group) were subsequently administered a challenge dose containing 104 CFU of H. pylori SS1. The animals were sacrificed 2 months postchallenge. Gastric tissue samples taken from the animals were homogenized, and the bacterial loads were determined by quantitative culture as previously described (9). At this time point, primed/challenged mice had significantly reduced gastric H. pylori loads compared to those from the naive/challenged group (4.2 log CFU/g versus 5.5 log CFU/g; Mann-Whitney test, P < 0.05; Fig. 1). This is a particularly striking result given the outbred genetic background of the animals. Indeed, it has been shown that outbred mice do not respond to low doses of antigen in a genetically restricted manner (8, 22). In addition, 62% (11/19) of the primed/challenged mice had undetectable levels of bacteria in their gastric mucosa following challenge versus 2 out of 10 of the naive/challenged mice (Fisher's exact test, P = 0.11). Although not statistically significant, the absence of detectable infection in these mice is worthy of note because other workers have been unable to achieve sterilizing immunity (as defined by culture negativity) against H. pylori infection in mice vaccinated with highly immunogenic H. pylori antigen-adjuvant preparations (11, 13, 14).
FIG. 1.
H. pylori bacterial loads determined from the gastric biopsies of mice. Control animals (“naive,” squares) were left untreated throughout, while the other groups were given either peptone trypsin broth (“naive/challenged,” circles) or a low-dose H. pylori inoculum (“primed/challenged,” diamonds) prior to challenge with an infectious pathogen dose. Each point corresponds to the mean value for a mouse, determined in duplicate. Horizontal bars represent the geometric means for each group of mice. The sensitivity of detection of the assay is indicated by a dashed horizontal line.
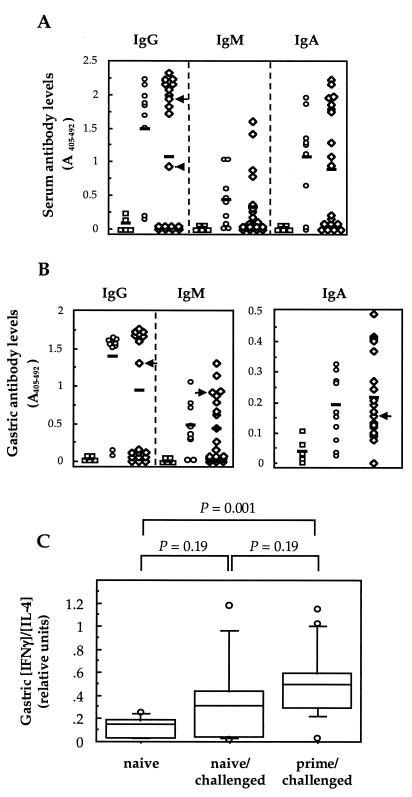
The results of H. pylori-specific serum and gastric antibody determinations were highly predictive of the culture status of the animals (Fig. 2). All culture-positive mice had increased levels of serum (IgG and IgA) and gastric (IgG and IgM) antibodies (Fig. 2A and B). Conversely, only 2 of the 11 culture-negative mice from the primed/challenged group had raised levels of H. pylori-specific serum IgG antibodies, while one of these mice also had increased levels of specific IgG, IgM, and IgA antibodies in its gastric contents (Fig. 2A and B). It is possible that the infection had been suppressed below the level of detection in these two antibody-positive animals. Nevertheless, the data strongly confirmed the absence of H. pylori infection in the majority of culture-negative mice in the primed/challenged group. Thus, despite repeated exposure of the primed/challenged mice to Helicobacter antigens, the animals did not exhibit any significant humoral response. Previous work with different infection models also showed a downregulation of humoral responses following low-dose antigen administration (3, 4, 18, 20). In the Leishmania infection model, this was associated with a switch of the default Th2 response of the host towards a Th1-dominant phenotype (3, 18).
FIG. 2.
Immune responses in mice after H. pylori challenge. H. pylori-specific serum (A) and gastric (B) IgG, IgM, and IgA antibody levels are shown. The groups of animals are the same as those described in the Fig. 1 legend. Each point corresponds to the mean value for a mouse, determined in triplicate. Individual values were considered to be significantly increased when they were greater than the mean ± 2 SD of readings for the respective antibody classes, determined from the naive group of mice. Horizontal bars represent the means for each group of mice. Mean serum IgG and IgA levels were significantly raised in naive/challenged and prime/challenged groups (versus controls, t test results were as follows: for IgG, P < 0.001 and P = 0.02; and for IgA, P = 0.004 and P = 0.02, respectively), whereas antibody levels were not statistically different between the two challenged groups (P > 0.05). Mean gastric IgG, IgM, and IgA levels were significantly raised in both challenged groups (versus controls: for IgG, P < 0.001 and P = 0.02; for IgM, P = 0.006 and P = 0.04; and for IgA, P = 0.02 and P = 0.008, respectively), but did not differ between them (P > 0.05). Arrows indicate two culture-negative mice from the primed/challenge group which had significantly raised levels of serum IgG antibodies (A) and one of these animals who also had raised gastric IgG, IgA, and IgM levels (B). (C) Gastric IFN-γ and IL-4 levels were determined directly on saponized extracts of stomach biopsy samples from mice and are expressed as ratios of the relative amounts of each cytokine (in picograms). The horizontal lines in the box plots correspond to the 10, 25, 50, 75, and 90 percentile values for each group of animals. Outlying values are indicated by symbols. P values were determined by the Mann-Whitney test.
To determine the types of Th responses induced following exposure to a low-dose Helicobacter inoculum, cytokine assays were performed on saponized gastric extracts from the animals using a modification of the technique of Bergquist et al. (2). Gamma interferon (IFN-γ) and interleukin-4 (IL-4) levels in gastric tissue samples were assayed by a double sandwich enzyme-linked immunosorbent assay technique (Pharmingen Inc., San Diego, Calif.). Primed/challenged mice were found to have significantly higher gastric IFN-γ/IL-4 ratios than naive animals (Mann-Whitney test, P = 0.001), yet the gastric cytokine levels in primed/challenged mice did not differ significantly from those in naive/challenged animals (P = 0.19). Therefore the shift in the immune responses of primed/challenged mice towards those of a predominantly cell-mediated Th1 phenotype could not per se account for the immunity observed in the animals. This finding would be consistent with the work of several investigators implicating both Th1 and Th2 responses in immune clearance of Helicobacter infection following oral immunization (11, 21). Others, however, reported that Th2-type responses alone were responsible for protection against Helicobacter infection (19). Clearly, further studies are warranted to better define the type of immune responses associated with immunity to H. pylori infection, particularly those involved in bacterial elimination following infection.
Acknowledgments
We are grateful to A. Labigne, in whose unit the work was undertaken, for her support. A. Labigne and P. J. Jenks are thanked for critical reading of the manuscript.
Financial support was provided in part by Acambis, Inc. (Cambridge, Mass.) and Aventis Pasteur Vaccins (Lyon, France). F.J.R was the recipient of a “Poste vert” postdoctoral position from the Institut de la Santé et de la Recherche Médicale, sponsored by the Conseil Régional d'Ile-de-France.
REFERENCES
- 1.Bancroft A J, Else K J, Grencis R K. Low-level infection with Trichuris muris significantly affects the polarization of the CD4 response. Eur J Immunol. 1994;24:3113–3118. doi: 10.1002/eji.1830241230. [DOI] [PubMed] [Google Scholar]
- 2.Bergquist C, Mattsson-Rydberg A, Lönroth H, Svennerholm A-M. Development of a new method for the determination of immune responses in the human stomach. J Immunol Methods. 2000;234:51–59. doi: 10.1016/s0022-1759(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 3.Bretscher P A, Wei G, Menon J N, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 4.Constant S L, Bottomly K. Induction of TH1 and TH2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree J E. Mucosal immune responses to Helicobacter pylori. Eur J Gastroenterol Hepatol. 1993;5(Suppl. 2):S30–S32. [Google Scholar]
- 6.Dubois A, Berg D E, Incecik E T, Fiala N, Heman-Ackah L M, Del Valle J, Yang M, Wirth H-P, Pérez-Pérez G I, Blaser M J. Host specificity of Helicobacter pylori strains and host responses in experimentally challenged nonhuman primates. Gastroenterology. 1999;116:90–96. doi: 10.1016/s0016-5085(99)70232-5. [DOI] [PubMed] [Google Scholar]
- 7.Dubois A, Berg D E, Incecik E T, Fiala N, Heman-Ackah L M, Pérez-Pérez G I, Blaser M J. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect Immun. 1996;64:2885–2891. doi: 10.1128/iai.64.8.2885-2891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fayolle C, O'Callaghan D, Martineau P, Charbit A, Clément J M, Hofnung M, Leclerc C. Genetic control of antibody responses induced against an antigen delivered by recombinant attenuated Salmonella typhimurium. Infect Immun. 1994;62:4310–4319. doi: 10.1128/iai.62.10.4310-4319.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrero R L, Thiberge J-M, Huerre M, Labigne A. Immune responses of specific-pathogen-free mice to chronic Helicobacter pylori (strain SS1) infection. Infect Immun. 1998;66:1349–1355. doi: 10.1128/iai.66.4.1349-1355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrero R L, Thiberge J-M, Labigne A. Local immunoglobulin G antibodies in the stomach may contribute to immunity against Helicobacter infection in mice. Gastroenterology. 1997;113:185–194. doi: 10.1016/s0016-5085(97)70094-5. [DOI] [PubMed] [Google Scholar]
- 11.Guy B, Hessler C, Fourage S, Haensler J, Vialon-Lafay E, Rokbi B, Quentin-Millet M-J. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine. 1998;16:850–856. doi: 10.1016/s0264-410x(97)00258-2. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Pando R, Pavön L, Arriaga K, Orozco H, Madrid-Marina V, Rook G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun. 1997;65:3317–3327. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keenan J, Oliaro J, Domigan N, Potter H, Aitken G, Allardyce R, Roake J. Immune response to an 18-kilodalton outer membrane antigen identifies lipoprotein 20 as a Helicobacter pylori vaccine candidate. Infect Immun. 2000;68:3337–3343. doi: 10.1128/iai.68.6.3337-3343.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleanthous H, Myers G A, Georgakopoulos K M, Tibbitts T J, Ingrassia J W, Gray H L, Ding R, Zhang Z-Z, Lei W, Nichols R, Lee C K, Ermak T H, Monath T P. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect Immun. 1998;66:2879–2886. doi: 10.1128/iai.66.6.2879-2886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumagai T, Malaty H M, Graham D Y, Hosogaya S, Misawa K, Furihata K, Ota H, Sei C, Tanaka E, Akamatsu T, Shimizu T, Kiyosawa K, Katsuyama T. Acquisition versus loss of Helicobacter pylori infection in Japan: results from an 8-year birth cohort study. J Infect Dis. 1998;178:717–721. doi: 10.1086/515376. [DOI] [PubMed] [Google Scholar]
- 16.Lee A, O'Rourke J, De Ungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 17.Menon J N, Bretscher P A. Characterization of the immunological memory state generated in mice susceptible to Leishmania major following exposure to low doses of L. major and resulting in resistance to a normally pathogenic challenge. Eur J Immunol. 1996;26:243–249. doi: 10.1002/eji.1830260138. [DOI] [PubMed] [Google Scholar]
- 18.Menon J N, Bretscher P A. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur J Immunol. 1998;28:4020–4028. doi: 10.1002/(SICI)1521-4141(199812)28:12<4020::AID-IMMU4020>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn S. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 20.Power C A, Wei G, Bretscher P A. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect Immun. 1998;66:5743–5750. doi: 10.1128/iai.66.12.5743-5750.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radcliff F J, Ramsay A J, Lee A. A mixed Th1/Th2 response may be necessary for effective immunity against Helicobacter. Immun Cell Biol. 1997;S1:A90. [Google Scholar]
- 22.Vaz N M, Levine B B. Immune responses of inbred mice to repeated low doses of antigen: relationship to histocompatibility (H-2) type. Science. 1970;168:852–854. doi: 10.1126/science.168.3933.852. [DOI] [PubMed] [Google Scholar]
- 23.Veldhuyzen van Zanten S J O, Pollak P T, Best L M, Bezanson G S, Marrie T. Increasing prevalence of Helicobacter pylori infection with age: continuous risk of infection in adults rather than cohort effect. J Infect Dis. 1994;169:434–437. doi: 10.1093/infdis/169.2.434. [DOI] [PubMed] [Google Scholar]
- 24.Xia H H, Talley N J. Natural acquisition and spontaneous elimination of Helicobacter pylori infection: clinical implications. Am J Gastroenterol. 1997;92:1780–1787. [PubMed] [Google Scholar]