Abstract
Neutrophil extracellular traps (NETs) are an important mechanism for defense against pathogens. Their overproduction can be harmful since excessive NET formation promotes inflammation and tissue damage in several diseases. Nucleases are capable to degrade NET on basis of their DNA hydrolysis activity, including the CdcPDE, a nuclease isolated from Crotalus durissus collilineatus snake venom. Here, we report a new finding about CdcPDE activity, demonstrating its efficiency in degrading cell-free DNA from NETs, being a potential candidate to assist in therapies targeting inflammatory diseases.
Keywords: phosphodiesterase, snake venom, CdcPDE, svPDE
1. Introduction
Among the mechanisms involved in the immune system defense, neutrophil-derived extracellular traps (NETs) can be highlighted [1,2,3,4]. Indeed, NETs play an important role in controlling infections promoted by viruses, fungi, and bacteria [5]. NETs are composed of decondensed chromatin, and cytotoxic proteins, such as histones, neutrophil elastase, and myeloperoxidase [6,7]. Although they represent an important defense mechanism, NETs are highly encountered in the blood of patients during several inflammatory diseases, such as rheumatoid arthritis, psoriasis, systemic lupus erythematosus (SLE), COVID-19, and sepsis [8,9,10,11,12,13].
Venomous animal-derived toxins are known to display fabulous pharmacological properties, representing interesting lead compounds for the development of novel medicines. Indeed, some venom-derived drugs are already in the market, and several are under clinical trials [14]. The global pharmaceutical industry has benefited greatly from biodiversity-rich countries stimulating the bioprospection of novel biomolecules derived from venoms for novel drug design [15].
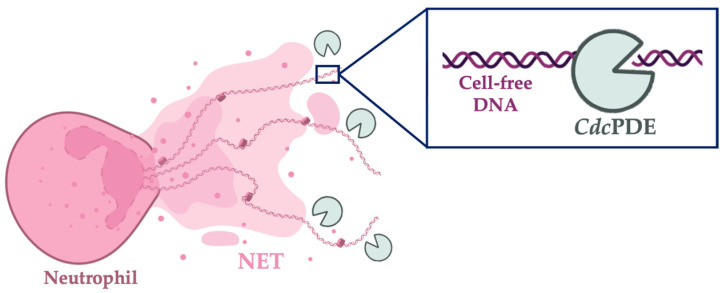
DNAses are potent agents capable of degrading NETs, being able to digest the DNA present in the mesh [16]. CdcPDE, a phosphodiesterase, was recently (2021) isolated from the Crotalus durissus collilineatus rattlesnake venom, and was completely characterized. Moreover, functional previous studies demonstrated that the CdcPDE was able to inhibit ADP-induced platelet aggregation and to cause a cytotoxic effect to human keratinocytes [17]. Knowing that CdcPDE is classified as a nuclease, able to degrade DNA (i.e., DNAse [18,19], this study aimed to verify if the rattlesnake-derived molecule could degrade cell-free DNA from NETs (Figure 1).
Figure 1.
The mechanism of CdcPDE-induced NETs’ degradation. A representative figure is used to illustrate the mechanism of NET degradation by a phosphodiesterase enzyme. The right panel shows the CdcPDE degrading the presented DNA-free in the neutrophils’ mesh. The figure was created with BioRender.com.
2. Results and Discussion
The isolation and characterization of CdcPDE was performed in our previous study, showing its low recovery (0.71%) [17]. CdcPDE at 20 µg/mL (0.2 mM) exhibited significantly lower levels of cell-free-DNA in comparison with undigested groups (Figure 2), indicating its ability of cell-free DNA degradation. Although the highest tested concentration (20 μg/mL) inhibited ~38%, the amount of the used CdcPDE was 25-times lower than the DNAse control (500 μg/mL). Unfortunately, we could not test CdcPDE using higher concentration due to its very low recovery, making a dose-response curse unfeasible. Indeed, to enable to carry out a dose-response curve, assays with inhibitors, and in vivo tests, heterologous expression will be necessary.
Figure 2.
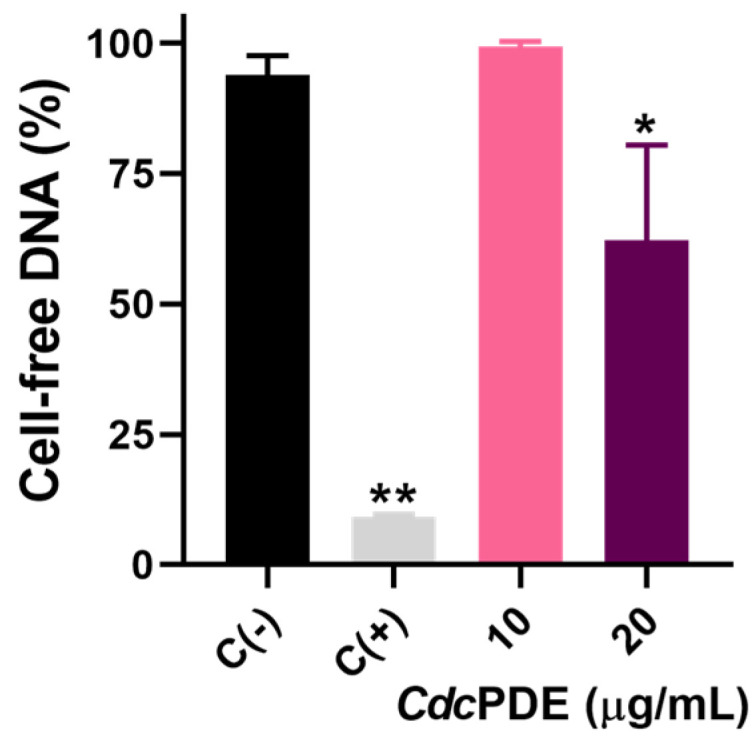
Cell-free DNA degradation assay. Isolated DNA were treated with PBS, DNase1 (500 μg/mL), or CdcPDE (10 or 20 μg/mL) for 30 min at 37 °C. Wells were stained with 0.2 μM SYTOX Green for 10 min. Fluorescence emission intensities were determined using 488-nm excitation and 525-nm emission. The results are presented as relative fluorescence units (RFU) and bars express percentages relative to the PBS group. C (−): wells treated with PBS. C (+): wells treated with DNAse (500 μg/mL). * p < 0.01 and ** p < 0.0001 when compared with C (−). Data (n = 3) are presented as mean ± SD, which were analyzed by one-way ANOVA and Tukey’s post-hoc test using GraphPad Prism 9 software.
Others substrates were tested in PDEs studies to characterize their enzymatic activity, such as bis (p-nitrophenyl) phosphate (0.4 mM [20], 1 mM [17] and 5 mM [21]), adenosine triphosphate (ATP, 0.05 mM), nicotinamide adenine dinucleotide (NAD, 0.05 mM), adenosine diphosphate (ADP, 0.05 mM), nicotinamide guanine dinucleotide (NGD, 0.05 mM), and adenosine monophosphate (AMP, 0.05 mM) [22], among others [18,19]. Although our study did not aim to characterize the enzymatic kinetics of CdcPDE, using λDNA as substrate, we observed that the amount of substrate used in the cited studies is much higher than in our study (6 × 10−13 mM), and even though the hydrolysis was observed. Therefore, our study indicates that CdcPDE may present a high specificity/affinity for the used substrate.
Only a few studies report that snake venoms can present DNAse activity [23,24]. Sittenfeld and colleagues (1991) tested DNAse activity of Bothrops asper, B. godmani, B. schlegelii, B. lateralis, B. nasutus, C. durissus, and Lachesis muta venoms, through radial diffusion in gel, and observed that all of them demonstrated DNAse activity [23]. Sales and Santoro (2008) have tested DNAse activity in 28 Brazilian venoms belonging to Bothrops, Crotalus, Lachesis, and Micrurus genera, observing that B. brazili presented the highest DNAse activity [24]. In contrast, here we pioneer demonstrated the DNAse activity of an isolated phosphodiesterase from the C. d. collilineatus venom. Moreover, to the best of our knowledge, this is the first study to test a snake venom-derived PDE using cell-free DNA from NETs.
Knowing that immune mechanisms participate on pathogenesis of several inflammatory diseases [25], the impairment of NETs degradation may promote endothelial damage, organ dysfunction, inflammation, and autoimmunity [26,27]. Supporting that, few studies showed that exogenous DNAse can improve the outcome of some diseases, such as sepsis and COVID-19 [28,29]. Therefore, attenuation of neutrophil-induced effects (i.e., NETs) may be a potent target for controlling diseases characterized by an influx of granulocytes and their activation, as was here demonstrated by CdcPDE biomolecule.
3. Conclusions
Our communication supports the use of NETs’ inhibitors by degrading DNA-free from NETs, as a strategy to ameliorate multi-organ damage during the clinical course of NETs-associated inflammatory diseases. Although further studies are needed, our study pioneering shows that a snake-venom derived PDE can degrade cell-free DNA, which may contribute to reduce the pathogenicity of inflammatory diseases.
4. Methods
CdcPDE was isolated from Crotalus durissus collilineatus in our previous study [17].
The DNAse activity was measured following the protocol of Colón and colleagues (2019) [29] with some modifications. Briefly, we diluted λDNA from the Quant-iT™ PicoGreen™ dsDNA Assay Kit (Cat. P11496, Lot. 2313058, ThermoFisher Scientific, Waltham, MA, USA) to a concentration of 20 µg/mL (1 µg = 0.03 pmol), the diluent was the Roswell Park Memorial Institute Medium (Ref. 15-040-CV, Lot. 17921005, Corning Inc., Corning, NY, USA). The λDNA solution was placed on a 96-well black plate with a clear bottom (Ref. 3603, Corning Inc., Corning, NY, USA), and after this, samples were treated with CdcPDE (10 or 20 μg/mL), Pulmozyme™ (500 μg/mL, Roche, Basel, Switzerland) that was the positive control of DNAse activity, and PBS (Cat. 14190144, Corning Inc., Corning, NY, USA) that was the negative control of DNAse activity. On the same plate, we placed the samples, Pulmozyme, or PBS with RPMI medium without the λDNA solution, this well was used to correct the amount of DNA contained in the samples. We incubated the plate for 2 h at 37 °C and immediately after, we used the SYTOX Green™ (Cat. S7020, ThermoFisher Scientific, Waltham, MA, USA) to stain the remaining DNA in the wells. After incubation for 5 min protected from the light, we used the Flexstation 3 (Molecular Devices, San Jose, CA, USA) to read the fluorescence at 488 nm and excitation at 525 nm. Our results are shown as the percentage of fluorescence at each well. The average of the wells containing only medium and the λDNA solution was considered 100% fluorescence.
Author Contributions
I.O., V.C. and F.V. performed the assays. M.P., E.A., F.C. and T.C. coordinate the study. I.F. and W.M. provided important contributions during the development of this work. All authors corrected the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
NETs can be harmful during inflammatory diseases, albeit can be prevented by nucleases. A nuclease from Crotalus durissus collilineatus snake venom, CdcPDE is capable of degrading DNA-NETs and may be a drug candidate to control NET-associated inflammatory diseases.
Funding Statement
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation, scholarship to I.O. 2020/13176-3, and grants 2019/10173-6, 2013/08216-2), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Coordination for the Improvement of Higher Education Personnel, Finance Code 001, scholarship to I.F., and grant 88887.513530/2020-00), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, The National Council for Scientific and Technological Development, scholarship to M.P. No. 307184/2020-0, W.M. No. 309207/2020-7, and E.A. 309399/2021-1). W.M. acknowledges funding support from Fundação de Amparo à Pesquisa do Estado do Amazonas (PAPAC 005/2019, PRO-ESTADO and POSGRAD calls).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil Extracellular Traps Kill Bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 2.Papayannopoulos V., Zychlinsky A. NETs: A New Strategy for Using Old Weapons. Trends Immunol. 2009;30:513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan M.J., Radic M. Neutrophil Extracellular Traps: Double-Edged Swords of Innate Immunity. J. Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorch S.K., Kubes P. An Emerging Role for Neutrophil Extracellular Traps in Noninfectious Disease. Nat. Med. 2017;23:279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 5.Papayannopoulos V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 6.Chapman E.A., Lyon M., Simpson D., Mason D., Beynon R.J., Moots R.J., Wright H.L. Caught in a Trap? Proteomic Analysis of Neutrophil Extracellular Traps in Rheumatoid Arthritis and Systemic Lupus Erythematosus. Front. Immunol. 2019;10:423. doi: 10.3389/fimmu.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dömer D., Walther T., Möller S., Behnen M., Laskay T. Neutrophil Extracellular Traps Activate Proinflammatory Functions of Human Neutrophils. Front. Immunol. 2021;12:636954. doi: 10.3389/fimmu.2021.636954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giaglis S., Hahn S., Hasler P. “The NET Outcome”: Are Neutrophil Extracellular Traps of Any Relevance to the Pathophysiology of Autoimmune Disorders in Childhood? Front. Pediatr. 2016;4:97. doi: 10.3389/fped.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao S., Fang H., Dang E., Xue K., Zhang J., Li B., Qiao H., Cao T., Zhuang Y., Shen S., et al. Neutrophil Extracellular Traps Promote Inflammatory Responses in Psoriasis via Activating Epidermal TLR4/IL-36R Crosstalk. Front. Immunol. 2019;10:746. doi: 10.3389/fimmu.2019.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khandpur R., Carmona-Rivera C., Vivekanandan-Giri A., Gizinski A., Yalavarthi S., Knight J.S., Friday S., Li S., Patel R.M., Subramanian V., et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Romo G.S., Caielli S., Vega B., Connolly J., Allantaz F., Xu Z., Punaro M., Baisch J., Guiducci C., Coffman R.L., et al. Netting Neutrophils Are Major Inducers of Type I IFN Production in Pediatric Systemic Lupus Erythematosus. Sci. Transl. Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., Schneider A.H., Caetité D., Tavares L.A., Paiva I.M., et al. SARS-CoV-2–Triggered Neutrophil Extracellular Traps Mediate COVID-19 PathologySARS-CoV-2 Directly Triggers ACE-Dependent NETs. J. Exp. Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li R.H.L., Tablin F. A Comparative Review of Neutrophil Extracellular Traps in Sepsis. Front. Vet. Sci. 2018;5:291. doi: 10.3389/fvets.2018.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bordon K.d.C.F., Cologna C.T., Fornari-Baldo E.C., Pinheiro-Júnior E.L., Cerni F.A., Amorim F.G., Anjolette F.A.P., Cordeiro F.A., Wiezel G.A., Cardoso I.A., et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020;11:1132. doi: 10.3389/fphar.2020.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calixto J.B. The Role of Natural Products in Modern Drug Discovery. An. Acad. Bras. Ciênc. 2019;91:e20190105. doi: 10.1590/0001-3765201920190105. [DOI] [PubMed] [Google Scholar]
- 16.Angeletti A., Volpi S., Bruschi M., Lugani F., Vaglio A., Prunotto M., Gattorno M., Schena F., Verrina E., Ravelli A., et al. Neutrophil Extracellular Traps-DNase Balance and Autoimmunity. Cells. 2021;10:2667. doi: 10.3390/cells10102667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Oliveira I.S., Pucca M.B., Wiezel G.A., Cardoso I.A., Bordon K.d.C.F., Sartim M.A., Kalogeropoulos K., Ahmadi S., Baiwir D., Nonato M.C., et al. Unraveling the Structure and Function of CdcPDE: A Novel Phosphodiesterase from Crotalus Durissus Collilineatus Snake Venom. Int. J. Biol. Macromol. 2021;178:180–192. doi: 10.1016/j.ijbiomac.2021.02.120. [DOI] [PubMed] [Google Scholar]
- 18.Dhananjaya B.L., D’Souza C.J.M. An Overview on Nucleases (DNase, RNase, and Phosphodiesterase) in Snake Venoms. Biochem. Mosc. 2010;75:1–6. doi: 10.1134/S0006297910010013. [DOI] [PubMed] [Google Scholar]
- 19.de Oliveira I.S., Pucca M.B., Ferreira I.G., Cerni F.A., da Silva Jacob B.d.C., Wiezel G.A., Pinheiro-Júnior E.L., Cordeiro F.A., Bordon K.d.C.F., Arantes E.C. State-of-the-Art Review of Snake Venom Phosphodiesterases (SvPDEs) Toxicon. 2022;217:121–130. doi: 10.1016/j.toxicon.2022.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Mitra J., Bhattacharyya D. Phosphodiesterase from Daboia Russelli Russelli Venom: Purification, Partial Characterization and Inhibition of Platelet Aggregation. Toxicon. 2014;88:1–10. doi: 10.1016/j.toxicon.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim N.M., Salama W.H., Hakim A.E.E. Phosphodiesitrase Activity of Some Egyptian Snake Venoms: Biochemical and Immunological Characteristics and Effect on Blood Coagulation of Phosphodiesterase Enzyme from Naja Nigricollis Venom. J. Chem. Pharm. Res. 2016;8:11. [Google Scholar]
- 22.Peng L., Xu X., Shen D., Zhang Y., Song J., Yan X., Guo M. Purification and Partial Characterization of a Novel Phosphodiesterase from the Venom of Trimeresurus Stejnegeri: Inhibition of Platelet Aggregation. Biochimie. 2011;93:1601–1609. doi: 10.1016/j.biochi.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Sittenfeld A., Raventós H., Cruz R., Gutiérrez J.M. DNase Activity in Costa Rican Crotaline Snake Venoms: Quantification of Activity and Identification of Electrophoretic Variants. Toxicon. 1991;29:1213–1224. doi: 10.1016/0041-0101(91)90194-V. [DOI] [PubMed] [Google Scholar]
- 24.Sales P.B.V., Santoro M.L. Nucleotidase and DNase Activities in Brazilian Snake Venoms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008;147:85–95. doi: 10.1016/j.cbpc.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 25.McInnes I.B., Gravallese E.M. Immune-Mediated Inflammatory Disease Therapeutics: Past, Present and Future. Nat. Rev. Immunol. 2021;21:680–686. doi: 10.1038/s41577-021-00603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi H., Yang S., Zhang L. Neutrophil Extracellular Traps and Endothelial Dysfunction in Atherosclerosis and Thrombosis. Front. Immunol. 2017;8:928. doi: 10.3389/fimmu.2017.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakkim A., Fürnrohr B.G., Amann K., Laube B., Abed U.A., Brinkmann V., Herrmann M., Voll R.E., Zychlinsky A. Impairment of Neutrophil Extracellular Trap Degradation Is Associated with Lupus Nephritis. Proc. Natl. Acad. Sci. USA. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czaikoski P.G., Mota J.M.S.C., Nascimento D.C., Sônego F., Castanheira F.V.e.S., Melo P.H., Scortegagna G.T., Silva R.L., Barroso-Sousa R., Souto F.O., et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE. 2016;11:e0148142. doi: 10.1371/journal.pone.0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colón D.F., Wanderley C.W., Franchin M., Silva C.M., Hiroki C.H., Castanheira F.V.S., Donate P.B., Lopes A.H., Volpon L.C., Kavaguti S.K., et al. Neutrophil Extracellular Traps (NETs) Exacerbate Severity of Infant Sepsis. Crit. Care. 2019;23:113. doi: 10.1186/s13054-019-2407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.